Abstract
The crystal transformation method using ethanol as a dehydration medium is a new method which can create a porous crystal. In this study α-cyclodextrin (α-CD) hexahydrate was transformed by this method and porous ethanol dihydrate crystal was obtained. The pore volume measured was 0.25 mL/g and the median pore diameter was 0.11 μm. Dissolution rate of this crystal was several times higher than that of (α-CD) hexahydrate or anhydrate obtained by drying.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An overall view of the literature results provides various hints toward a better understanding of amorphous and glassy states, and of their role in many technologically relevant processes, for example, in pharmaceutics [1]. Physical properties of amorphous carbohydrates (either in the anhydrous or in the low-moisture state) have always been of great interest [2].
Crystalline sugars typically exist in anhydrate and hydrated forms such as lactose and lactose monohydrates, trehalose and trehalose dihydrates, α-, β- and γ-cyclodextrins (CDs) and hexa-, dodeca- and higher hydrates. The forms are mutually converted through a dehydration process performed by heating (drying method) and moisture absorption [3]. The abundant literature on trehalose has also concerned several aspects of molecular mobility of the trehalose–water systems and of structural transitions between crystalline (hydrate and anhydrate) forms and amorphous forms, particularly for its role in many phenomena related to biologically important events such as bioprotection. Moreover, recently a unique method of dehydration was developed through which dihydrate trehalose dehydrated to anhydrate trehalose with fine porous structure using ethanol as a dehydration medium (ethanol method) [4]. Furthermore, stable anhydrate form of lactose could be obtained via a nucleation and growth process in ethanol [5]. Dehydration of hydrate crystal with solvent is often described as a soft dehydration technique. On the other hand, thermal dehydration by heating is often described as a hard dehydration [6].
While using ethanol to induce phase transformations of hydrates, it has been reported that needle- or whisker-like crystals, with an expanded surface area, can be obtained [7, 8]. Rehydration leads only to a partial reduction of the surface area.
CDs are cyclic maltooligosaccharides consisting of six or more glucose units linked by α-(1→4) glycosidic bonds. CDs form the inclusion complexes of various organic substances by the fact that the central cavity is hydrophobic and are applied to various uses such as stabilization, masking, powderization, emulsification and solubility improvement of incorporated compound. The α-cyclodextrin (α-CD) consisting of six glucose units usually exists as a nonhygroscopic hexahydrate under the environmental condition. An anhydrate crystal of this compound can be easily prepared by drying the α-CD hexahydrate (α-CD·6H2O) under reduced or atmospheric pressure. The determination of the crystal structure of the α-CD·6H2O reveals that four of the six hydrating water molecules are located outside the α-CD and the two remaining water molecules are located in the central cavity [9–11]. In this study, the crystal transformation induced by ethanol method was applied to the α-CD·6H2O and new porous ethanol dihydrate crystals were obtained.
Experimental
Materials
The α-CD·6H2O was recrystallized from water using marketed product from Ensuiko Sugar Refining Co. (Yokohama, Japan). The α-cyclodextrin anhydrate was obtained by drying α-CD·6H2O in a stainless container at 100 °C for 20 h with a ventilation drier. Ethanol (purity above 99%) was obtained from Nihon Alcohol Distribution Co., Ltd. (Tokyo, Japan).
Physical methods
Transformation by the ethanol method
The crystal transformation was carried out in a glass reactor without the reflux column and equipped with an agitator in a water bath with temperature control unit. Ethanol of 1,000 mL was preheated to 70 °C. The α-CD·6H2O of 100 g were added, and agitated at about 170 rpm at 70 °C. About 30–50 mL of the reaction mixture was aspirated into a sampling vessel and separated with the basket type centrifuge immediately. Wet crystals were dried for 60 min in a ventilation drier at 50 °C in order to remove the adhesive ethanol.
Rehydration of the transformed crystal
α-CD hexahydrate, ethanol dihydrate and anhydrate by drying method at 100 °C for 20 h were incubated at a constant temperature and inside a humidity box (LHU-113, ESPEC, Tokyo, Japan). 1.5 g of each crystal were placed on petri dishes (ϕ75 × h20 mm) and incubated at 50 °C and relative humidity of 80%. Water content was measured at different times.
Moisture and ethanol content analysis
Moisture content was determined using a Karl Fischer titrator (Model MKS-510 N, Kyoto Electronics Manufacturing Co., Kyoto, Japan). Ethanol content was determined from the difference between the weight loss on heating and moisture content. Weight loss on heating was measured with Halogen Moisture Analyzer (Model HB43-S, Mettler Toledo International Inc., Switzerland). The ethanol content was also measured by gas chromatography (GC-17A, Shimazu, Kyoto, Japan) with a capillary column HR-1 (Shinwa Chemical Ind. Ltd). Gas chromatography conditions were as follows: injection temperature, 130 °C; column temperature, 100 °C; and carrier gas, He.
X-ray powder diffractometry (XRD)
The α-CD crystals were pounded in a mortar and filled in a sample holder of an aluminum plate for exposure to Cu-Kα radiation in a powder X-ray diffractometer (Model RAD-II B, Rigaku Co., Tokyo, Japan). Samples were scanned at a scanning speed of 3°/min over a diffraction angular range from 3 to 140°.
Scanning electron microscopy (SEM)
Scanning electron microscopy (Model JSM 6060, JEOL Co., Ltd., Tokyo, Japan) was used to investigate the microstructural properties of the α-CD crystals. The α-CD crystals were placed on a double-sided adhesive carbon tape (Nisshin EM Co., Ltd., Tokyo, Japan) adhered to a SEM stub. All the samples were analyzed at an acceleration voltage of 2.0 kV without deposition.
Pore size analysis
Pore size distribution, pore diameter and intrusion volume were measured by a mercury porosimeter (Autopore 9520 mercury porosimeter, Georgia, USA). About 0.2 g of sample was set in the measurement cell and mercury filling pressure was started from 7 kPa.
Solubility rate
30 ml of distilled water was poured into the 50 ml beaker in the 25 °C water bath. The crystals of 1.5 g was added and stirred at 150 rpm with stirring bar. The sample was taken out in 5, 15, 45 and 60 s, and concentration was analyzed by optical density method.
Results and discussion
Crystal transformation with the ethanol method
Figure 1 shows the crystal transformation behavior of dehydration of the α-CD·6H2O at 70 °C with the ethanol method (Fig. 1a) and rehydration at 50 °C under 80% relative humidity (RH) (Fig. 1b). The moisture content decreased rapidly within 5 min using the ethanol dehydrating reaction. After that, the moisture content had a constant value around 4%. On the other hand, the ethanol content showed the opposite behavior of the moisture content. The value was increasing during 5 min and subsequently had a constant value about 7%. In the case of rehydration, the moisture content rapidly increased early in the course of reaction then gradually increased. As well as dehydration, the ethanol content showed the reverse change from the moisture content. As shown in Fig. 2, a satisfactory linear correlation could be established between the molar ratio of ethanol to α-CD and the molar ratio of water to α-CD during the dehydration by the ethanol method and rehydration reaction. This suggests the displacement of four water molecules to two ethanol molecules might have occurred. The dehydrating crystal transformation from hexahydrate to ethanol dihydrate inclusion complex crystal is shown as follows,
It is known that the α-CD hexahydrate has two water molecules inside the cavity and four outside [9]. By this presence of different water types, diffusion of water molecules from α-CD on dehydration and adsorption had a stepwise behavior [3, 12]. Bettinetti et al. [13] reported that two moles of water molecules included in one mol of α-CD was released in the last step on the microwave drying. Berbenni et al. studied the dehydration process using conventional and high-resolution thermogravimetric analysis and demonstrated that the process is constituted by four dehydration stages [14]. Stage 1 is due to surface water molecules release. Stages 2 and 3 correspond to the water molecules which are included in the interstices. Stage 4 individuates the water molecules inside the α-CD cavity. From the investigation of the crystal structure of the α-CD methanol pentahydrate [15] and the β-CD ethanol inclusion complex [16–18] crystallized from solution, the methanol or ethanol molecule found in the CD cavity and most water molecules are in interstices of CD molecules. In the case of ethanol method, the four molecules of water in the interstices might be swapped by two ethanol molecules and the two molecules of included water in the α-CD cavity remained as they were. Various solid-states of α-CD are reported, i.e., a number of hydrates both polymorphs and of different stoichiometry, a dehydrated-hydrate crystal form and an amorphous form [19, 20]. In this experiment, the crystal of α-CD ethanol dihydrate could be obtained. Perkkalainen et al. obtained 1-O-α-d-glucopyranosyl-d-mannitol-ethanol as ethanol containing crystals using anhydrate ethanol as a solvent [21]. We investigated also the crystal transformation of dihydrate trehalose to anhydrate trehalose using ethanol [4]. However, maltose and trehalose did not involve ethanol in the crystal obtained. The reversible displacement of four molecules of water in α-CD to ethanol is a very interesting crystal transformation reaction.
Characteristics of the particle structure
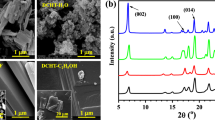
Figure 3 shows the X-ray diffraction patterns of hexahydrate (A), ethanol dihydrate by the ethanol method (B) and anhydrate by drying at 100 °C for 20 h (C). Angles of characteristic diffraction peaks which are shown in Fig. 3, were identified as 14.2° for hexahydrate, 11.3° for ethanol dihydrate and 12.5° for anhydrate crystal, respectively. The diffraction pattern of hexahydrate (A) is similar to the pattern of the hexahydrate cage structure [22] and the anhydrate resembles the pattern that Nakai et al. reported [3]. The diffraction intensity of ethanol dihydrate (B) is low but this particle is crystalline unlike hexahydrate or anhydrate crystal.
Figure 4 shows the comparison of SEM photographs. The hexahydrate has a smooth surface (A), but shallow cracks can be seen on the surface by raising magnification (D). Concerning the anhydrate obtained by drying, the crystal is similar to hexahydrate in morphology (C). The cracks are very big and deep (F) and some crystals are broken. In the case of ethanol dihydrate, the sides and edges are round and small crystals seem to be aggregated since the small particles can not be confirmed (B). The surface was covered with fine crystals and formed porous structure that may extend to the inside of the crystal (E). Figure 5 shows the pore size distribution and differential intrusion of ethanol dihydrate measured with a mercury porosimeter. The pore volume measured for an intrusion volume less than 1 μm was 0.25 mL/g and the median pore diameter was 0.11 μm. In the case of trehalose anhydrate transformed by ethanol method, the pore volume was 0.28 mL/g and the median pore diameter was 0.21 μm [4]. The CD obtained had a more microscopic structure in comparison with the trehalose. Nordhoff and Ulrich indicated the dendritic growth of the stable phase in term leads to needle- or whisker-like crystals, which have the desired larger specific surface area in comparison to the initial crystalline substance [8]. A solvent-mediated phase transformation from a metastable (higher solubility) to stable (lower solubility) form can take place when a solid is in contact with a solvent [23].
Comparison of dissolution rate
The physicochemical properties of pharmaceuticals, including solubility and dissolution rate can be influenced by the degree of crystallinity, solvation state, and crystal form. At room temperature, the anhydrate forms of ampicillin, theophylline, and glutethimide have a higher dissolution rate than their corresponding hydrates [24]. This difference in dissolution rate can be attributed to the difference in the free energy of hydration. Therefore, dissolution rates of each α-CD crystal were investigated. Important physical properties, such as solubility, dissolution rate, powder flow and tableting behavior depend on the crystal structure. Figure 6 shows the comparison of the solution (dissolution) rate of α-CD crystal. Ethanol dihydrate crystal could be dissolved into water quickly compared with hexahydrate and anhydrate crystals. By observation with the microscope, it was found that the crystals collapsed at a moment to come in contact with water. The porous structure obtained, and the diffusion of the crystallized ethanol to the water solution, increased the surface area of the crystal and thus the solution rate was higher. Kneading process is commonly used for preparation of inclusion complex [25] and Yoshii et al. reported the effective formation of inclusion complex with ethanol using this process [26]. Application of an inclusion complex, by mixing or kneading α-CD ethanol dihydrate directly with organic materials is expected.
Abbreviations
- α-CD:
-
α-Cyclodextrin
- α-CD·6H2O:
-
α-Cyclodextrin hexahydrate
- CDs:
-
Cyclodextrines
- RH:
-
Relative humidity
- XRD:
-
X-ray powder diffraction
- SEM:
-
Scanning electron microscopy
References
Cui Y (2007) Int J Pharm 339:3–18
Magazu S, Migliardo F, Mondelli C, Vadala M (2005) Carbohydr Res 340:2796–2801
Nakai N, Yamamoto K, Terada K, Kajiyama A, Sasaki I (1986) Chem Pharm Bull 34:2178–2182
Ohashi T, Yoshii H, Furuta T (2007) Carbohydr Res 342:819–825
Garnier S, Petit S, Coquerel G (2002) Therm Anal Calori 68:489–502
Kirka JH, Danna SE, Blatchfordb CG (2007) Int J Pharm 334:103–114
Berlin E, Anderson BA, Pallansch MJ (1972) J Dairy Sci 55:1396–1399
Nordhoff S, Ulrich J (1999) J Therm Anal Calori 57:181–192
Manor PC, Saenger W (1974) J Am Chem Soc 96:3630–3639
Klar B (1980) Acta Cryst B36:1154–1165
Nascimento CS Jr, Dos Santos HF, De Almedia WB (2004) Chem Phys Lett 397:422–428
Tanada S, Nakamura T, Kawasaki N, Kurihara T, Umemoto Y (1996) J Colloid Interface Sci 181:326–330
Bettinetti GP, Giordano F, Rillosi M, Mura P (1995) Proc 1st World Meeting APGI/APV, Budapest, May
Berbenni V, Marini A, Bruni G (1998) Thermochim Acta 322:137–151
Hingerty B, Saenger W (1975) J Am Chem Soc 98:3357–3365
Tokuoka R, Abe M, Fujiwara T, Tomita K, Saenger W (1980) Chem Lett 9:491–494
Aree T, Chaichit N (2003) Carbohydr Res 338:1581–1589
Aree T, Chaichit N (2008) Carbohydr Res 343:2285–2291
Chacko KK, Saenger W (1981) J Am Chem Soc 103:1708–1715
Novak C, Bettinetti GP, Sorrenti M, Negri A, Sztatisz J (1997) Proceedings of the calorimetry experimental thermodynamics and thermal analysis conference, CETTA 1997:8–13 Zakopane, 55
Perkkalainen P, Pitkanen I, Huuskonen J (1999) J Molec Struct 510:179–189
Hunt MA, Rusa CC, Tonelli AE, Balik CM (2004) Carbohydr Res 339:2805–2810
Cardew PT, Davey R (1985) J Proc R Soc Lond A398:415–428
Shefter E, Higuchi T (1963) J Pharm Sci 52:781–791
Gil A, Chamayou A, Leverd E, Bougaret J, Baron M, Couarraze G (2004) Eur J Pharm Sci 23:123–129
Yoshii H, Kometani T, Furuta T, Watanabe Y, Linko YP (1998) Biosci Biotechnol Biochem 62:2166–2170
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohashi, T., Verhoeven, N., Okuda, D. et al. Formation of porous α-CD ethanol dihydrate by crystal transformation method. Eur Food Res Technol 230, 195–199 (2009). https://doi.org/10.1007/s00217-009-1150-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-009-1150-9