Abstract
Chemiluminescent analysis indicates that the polysaccharide extracts from Se-enriched G. lucidum (Se-GLPs) could protect DNA from hydroxyl radical oxidative damage in dose dependent manner. Moreover, Se-GLPs exhibited higher activities of scavenging superoxide and hydroxyl radicals than its analog from common G. lucidum extract as suggested by EPR measurement, indicating that Se plays an important role in increasing the antioxidant activities of the polysaccharide extracts. This was confirmed by spin-trapping experiments showing that at the same polysaccharide concentration, the activities of all the Se-GLPs samples in scavenging hydroxyl radical increased with the increase of Se content. Additionally, all Se-GLPs samples showed stronger activities of attenuating the production of superoxide radical than that of hydroxyl radical.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species produced by sunlight, ultraviolet, ionizing radiation, chemical reactions and metabolic processes have a wide variety of pathological effects, such as causing DNA damage, carcinogensis and cellular degeneration related to aging [1, 2]. Superoxide and hydroxyl radicals are inevitably formed in almost all aerobic organisms. In cellular oxidation reactions, superoxide radical is normally formed first, and its effects can be magnified by production of other kinds of cell-damaging free radicals and oxidizing agents. However, the damaging action of the hydroxyl radical seems to be the strongest among free radicals due to its highest reactivity [3, 4]. Although almost the all organisms have evolved a variety of complex defense and repair systems that have evolved to protect them against oxidative damage, only these systems are insufficient to entirely prevent the damage [5]. Fortunately, antioxidant supplements containing antioxidants can help human body prevent or reduce the oxidative damage caused by these toxic radicals.
Selenium is an essential element for human body and can only be obtained from food or other source of supplementation. A high incidence of cancer and other disease is attributed to a lower selenium level in the body [6]. The biological role of selenium has received considerable attention since early times. Its biological functions are at least in part associated with its antioxidant activity [7–9]. As known, many biological molecules such as glutathione peroxidase, thioredoxin reductasaes, as well as numerous small organic molecules show promising antioxidant activity.
However, to date, the antioxidant activities of polysaccharides containing Se, especially effects of Se on antioxidant activity of biological molecules, have received little attention.
On the other hand, G. lucidum (GL) has been used as a traditional Chinese medicine since ancient times. It has been proved to have extensive physiological effects on chronic hepatopathy, hypertension, bronchitis, arthritis, neurasthenia, neoplasin, tumorigenic diseases and so on [10–12]. Polysaccharide is considered as one of the most important bioactive components of GL, which also exhibits antitumor, immuno-modulating, liver protective, hypoglycemic and platelet aggregation-inhibiting activities [13]. Se-enriched G. lucidum (Se-GL) has been successfully cultivated in our group [14]. Previous studies showed that G. lucidum could absorb 20–30% of inorganic selenium in substrate and transformed most of them into organic Se. Moreover, 11.2–18.0% of the organic Se was found in the polysaccharide fraction [15]. We have found that selenium can pronouncedly increase the antioxidative activity of the protein extracts from the Se-GL [16]. These raises questions of whether selenium in polysaccharide extracts from Se-GL can have the same function with expectation to find a component with higher bioactivity.
Based on these thinking, the antioxidant activity of polysaccharide extracts from Se-GL and common GL was determined, respectively, by chemiluminescence method [17–19] and electron paramagnetic resonance (EPR) in conjunction with spin trapping techniques. The polysaccharide extracts from Se-GL showed stronger antioxidant activity than those from common GL, implying that selenium in the polysaccharide extracts plays a positive role in enhancing antioxidant activity of polysaccharide extracts.
Materials and methods
Materials
All solvents/chemicals used were of analytical grade. Hypoxanthine (HPX) and xanthine oxidase (XOD) were obtained from Sigma Chemical Co. (Beijing, People’s Republic of China). 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was obtained from Labtec (Tokyo, Japan), diethylenetriamine-N, N, N′, N′′, N′′-pentaacetic acid (DTPA), FeSO4, and H2O2 were purchased from Beijing Chemical Co. (Beijing, People’s Republic of China). The cultivation method of Se-enriched G. lucidum (Se-GL) was basically the same as that of G. lucidum (GL) [20]. The only difference was that a series of 100, 200, and 250 μg of Se in the form of sodium selenite per gram of substrate was added to the substrate for obtaining Se-enriched G. lucidum samples 1 (Se-GLPs 1), 2 (Se-GLPs 2), and 3 (Se-GLPs 3), respectively [14].
Preparation of polysaccharides from GL and Se-GL
Freeze-dried and powdered GL or Se-GL (10 g) was stirred into 100 mL of 1.0 M NaOH at 4 °C. After 4 h, the supernatant was obtained by filtration. The protein in the supernatant was removed using the Sevag method [21]. Ethanol was added to the supernatant until 75% (v/v) of ethanol was obtained. The resultant precipitate was collected by centrifugation, and dissolved in 5.0 mL of distilled water. This solution was then filtrated and dialyzed against distilled water at 4 °C three times. After 24 h, the solution was lyophilized and stored at −20 °C for use [22].
Determination of selenium and polysaccharide contents
The selenium concentration of tested samples was determined by atomic fluorescence spectrophotometry (AFS-1201, Haiguang Analytical Co., China) as reported recently [16]. The Se content was given by the Se concentration detected by AFS over the exact weight of lyophilized samples.
The polysaccharide concentration was determined by the phenol-sulfate method [23]. The polysaccharide content was obtained by the polysaccharide concentration over the exact weight of lyophilized polysaccharide extracts.
Evaluation of antioxidant capacity of GLPs and Se-GLPs by chemiluminescence
The CuSO4-Phen-Vc-DNA solution was prepared with acetate buffer (pH = 5.5) as described previously [19]. The final concentrations of Cu2+, Phen, Vc, and DNA were 50 μM, 350 μM, 350 μM, and 1.0 μg/mL, respectively. Exactly 0.8 mL of the above solution was added to a 1 mL cuvette of the Ultra Weak Chemiluminescence Analyzer (BPCL-G-K, Inst. of Biophysics, Academia Sinica, Beijing, People’s Republic of China). Then each 0.2 mL solution of polysaccharides, concentrations indicated above was injected into the cuvette. The same volume of distilled water in place of the polysaccharide solution was used as control. After 1 min, 20 μL H2O2 (3%) was added to the cuvette while the kinetic curve of chemiluminescence was measured at 22.0 ± 0.1 °C [19]. The depression rate of chemiluminescence (CL) due to both DNA damage and Phen oxidation was calculated using the following formula: depression rate (%) = (C − C 1)/C × 100%, where C is the value of photon count of CL for control and C 1 is the value of photon count of CL for test samples.
Evaluation of activity of scavenging hydroxyl radical and superoxide radical of GLPs and Se-GLPs by the EPR method
EPR was used to monitor HO• and O2 •− formation by using DMPO as a spin trapping reagent. EPR spectra were recorded on an EPR spectrometer (model JES-TE200, JEOL Co., Tokyo, Japan). Samples (1-mm-i.d.) of quartz capillaries were placed in a TE102 cavity for measurement at room temperature. Typical spectrometer parameters for monitoring HO• were: central field, 336.5 mT; microwave power, 10.0 mW; modulation frequency, 9.44 GHz; scanning width, 10 mT; modulation amplitude, 0.2 mT; time constant, 0.1 s; and scanning period, 120 s. All spectra were recorded exactly 2.0 min after addition of the last reagent. The concentrations of reactants are indicated in the figure captions. The scavenging effects of GLPs and Se-GLPs on hydroxyl radical were calculated by E (the scavenging effect) = [(h o − h x)/h o] × 100%, where h o and h x are the amplitude of EPR signal of control containing DTPA + DMPO + Fe (II) + H2O2 and samples containing DTPA + DMPO + Fe (II) + different polysaccharide extracts + H2O2, respectively [24].
Superoxide anion radicals were generated from an HPX–XOD reaction system in 10 mM phosphate buffer solution (pH 7.4) [24, 25]. Briefly, 5 μL of 1.0 M DMPO, 5 μL of 6 mM HPX, 5 μL of 4 mM DTPA and 5 μL of tested sample were put into a test tube. The amount of DMPO-O2 − formed was determined exactly 80 s after the addition of 5 μl of 50 mU/mL XOD using the EPR spectrometer. The EPR spectrometer conditions were central field, 336.3 mT; microwave power, 10.0 mW; modulation frequency, 9.44 GHz; scanning width, 5.0 mT; modulation amplitude, 0.079 mT; time constant, 0.1 s; and scanning period, 120 s. The scavenging activity was denoted as the percentage inhibition of the peak intensity of the control in which 5 μL of phosphate buffer solution was used instead of sample solution [26]. The scavenging effects of GLPs and Se-GLPs were calculated the same way for superoxide radical as for hydroxyl radical.
Statistics analysis
The data were analyzed using the statistical analysis system (SAS 9.0) package software for the analysis of variance and Duncan’s test. All experiments were carried out in triplicate. The significance was established at p ≤ 0.05.
Results and discussion
Protective effects of Se-GLPs on DNA and Phen against oxidative damage by free radicals
The protective effects of Se-GLPs on DNA and Phen from oxidative damage by free radicals were assayed with a CuSO4-Phen-Vc-H2O2-DNA chemiluminescent system [19]. The kinetic traces of inhibition of Se-GLPs to chemiluminescence induced by both DNA damage and Phen oxidation are shown in Fig. 1 and have the same general profile as were reported for inhibition of selenium-enriched protein extracts and green tea polyphenols to chemiluminescence, respectively [16, 19]. There are two peaks on the spectra; the first peak represents the chemiluminecence due to Phen possibly oxidized by O2 •− and the second peak corresponds to the chemiluminescence of DNA damage likely induced by HO• [17, 19]. Compared with control (curve A), the time of appearance of the second peak was delayed, accompanied by a decrease of its intensity upon the addition of Se-GLPs to the chemiluminescent system. The increase of the Se-GLPs concentration, from 0.0514 mg/mL (curve B), 0.103 mg/mL (curve C), and 0.206 mg/mL (curve D), to 0.410 mg/mL (curve E) caused an increase in the inhibiting effect of Se-GLPs on the chemiluminescence due to DNA damage (peak 2) and Phen oxidation (peak 1). Moreover, the chemiluminescence due to DNA damage (peak 2) can hardly be detected within experiment time when the concentration of Se-GLPs was up to 1.030 mg/mL (curve F), indicating that 1.030 mg/mL of Se-GLPs could totally quench the producing of hydroxyl radicals which lead to the oxidative damage of DNA. Additionally, it can also be observed that the inhibiting effect of Se-GLPs (expressed in depression rate and delay time) on the chemiluminescence due to DNA damage is more pronounced than that on the chemiluminescence due to Phen oxidation (p < 0.05) (Table 1, Fig. 1). On the whole, Se-GLPs exhibited DNA protective effects from oxidative damage in dose dependent manner, though the delay time of Phen did not show the dose-dependency significantly. This is similar to the result we have recently reported on the protein extracts from Se-enriched G. lucidum [16].
Inhibition of Se-GLPs to chemiluminescence due to both DNA damage (peak 2) and Phen oxidation (peak 1) in CuSO4-Phen-Vc-H2O2-DNA-Se-GLPs system. Curve A distilled water alone. Curve B 0.0514 mg/mL Se-GLPs. Curve C 0.103 mg/mL Se-GLPs. Curve D 0.206 mg/mL Se-GLPs. Curve E 0.410 mg/mL Se-GLPs. Curve F 1.03 mg/mL Se-GLPs. Conditions: 50 μM Cu2+, 350 μM Phen, 350 μM Vc, 14.7 mM H2O2, and 1.0 μg/mL DNA, 22 °C
The contribution of all the Se-GLPs samples to prevent DNA from oxidative damage by hydroxyl radical possibly comes from two parts, namely, polysaccharides itself and selenium moiety in the polysaccharides. Polysaccharides might act as hydrogen donors by providing hydrogen atom from HO group in polysaccharide chains to free radical such as peroxy radicals (ROO•), forming a new polysaccharide radical. The producing polysaccharide radical can react with the peroxy radicals, producing non-radical as following [19, 27, 28].
where P-OH is the polysaccharide, n is chemically calculating factor of antioxidant.
However, another possibility cannot be excluded that hydroxyl group (-OH) in polysaccharide ring could form coordinate with free metal ions such as copper and iron ions, which are necessary for free radical production [27, 28]. Resulting complexes are inactive for the production of free radicals.
The contribution of the selenium moiety in the Se-GLPs samples to antioxidant activity probably also stems from, in part, the ability to complex the free metal ion. It was proposed that selenium possibly exists in the polysaccharides containing Se in the form of selenyl group (-SeH) or seleno-acid ester [29]. The new groups may be able to complex Cu+ or Cu2+, thereby inhibiting the generation of hydroxyl radicals (HO•) and superoxide radicals (O2 •−) in CuSO4-Phen-Vc-DNA system. However, the real mechanism of Se-GLPs’ protecting effect of DNA from oxidative damage is beyond the scope of the present study and need further testifying.
The role of selenium in Se-GLPs in scavenging hydroxyl radical
Electron paramagnetic resonance (EPR) is a powerful tool for detection of all kinds of radical. In order to characterize the role of selenium in Se-GLPs, EPR in conjunction with spin trap technique was used to determine the ability of GLPs and Se-GLPs with different Se content to attenuate hydroxyl radical production from the Fenton reaction. The results are shown in Tables 2 and 3; Figs. 2 and 3. Spectrum A of Fig. 2 is that of the DMPO-OH adduct in a control experiment adding reagents in the sequence DTPA + DMPO + Fe (II) + H2O2. The one-electron oxidation of Fe (II) by H2O2 is an efficient generator of hydroxyl radical [24] and an intense spectrum from trapped HO• was observed (spectrum A). With Se-GLPs 3, a weakest EPR signal was obtained (spectrum B).
EPR spectra of the DMPO-OH. The EPR spectrum was recorded 2.0 min after the addition of H2O2. Curve A control (DTPA + DMPO + Fe (II) + H2O2). Curve B DTPA + DMPO + Fe (II) +1.03 mg/mL Se-GLPs 3+ H2O2. Conditions: 0.8 mM H2O2, 20 μM Fe2+, 0.8 mM DTPA, and 100 mM DMPO in 10 mM phosphate buffer (pH 7.4)
Scavenging effects of GLPs, Se-GLPs 1, Se-GLPs 2, and Se-GLPs 3 on hydroxyl radical generated from the Fenton reaction. Values are mean ± SD of three independent experiments. Conditions: 0.8 mM H2O2, 20 μM Fe2+, 0.8 mM DTPA, 100 mM DMPO in 10 mM phosphate buffer (pH 7.4), and polysaccharide of 1.03 mg/mL for all samples
It is evident that the polysaccharide contents of GLPs (86.5%), Se-GLPs 1 (85.9%), Se-GLPs2 (86.3%), and Se-GLPs 3 (87.1%) are nearly same (p > 0.05), the activity of scavenging HO• increases from 34.7%, 44.1%, 47.7% to 52.5%, respectively (Fig. 3) as the content of Se increase in all the samples, and following the order GLPs (1.45 μg selenium/g polysaccharide extract) < Se-GLP 1 (20.53 μg selenium/g polysaccharide extract) < Se-GLPs 2 (49.62 μg selenium/g polysaccharide extract) < Se-GLPs 3 (60.50 μg selenium/g polysaccharide extract). Because the polysaccharide concentration was kept constant, the different activity of GLPs and Se-GLPs stems from the different Se-content contained in the Se-GLPs, and not from the polysaccharide itself, a finding indicating that Se plays an important role in enhancing the antioxidant capacity of the polysaccharide extracts. The higher activity of the Se-enriched polysaccharide extracts in scavenging HO• comes from the higher reactivity of the selenium moiety including possibly formed selenyl group (-SeH) [29] in the Se-GLPs as compared to hydroxyl group (-OH) in the regular analogues. A similar result was obtained with protein extracts from the same Se-enriched G. lucidum (Se-GL) in this section of experiment [16]. This implies that the antioxidation mechanism of protein extracts from Se-GL might be similar to that of the polysaccharide extracts from the same source, namely, Se-contained moiety in both kinds of extracts plays an important role in attenuating the hydroxyl radical.
Comparison of the antioxidant activity of sodium selenite, GLPs, GLPs plus sodium selenite, and Se-GLPs
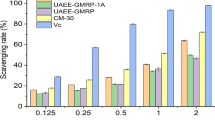
In order to determine whether there is a synergetic effect between selenium and Se-GLPs, the activities of scavenging free radicals (O2 •− and HO•) of selenite, GLPs, GLPs plus selenite and Se-GLPs 3 were compared by spin trapping experiments and the results are shown in Table 4. All samples reduced the amount of both O2 •− and HO• trapped by DMPO, a result consistent with the above observation that Se-GLPs had the ability to prevent DNA damage and Phen oxidation from hydroxyl and superoxide radicals, respectively (Fig. 1, Table 1). When the Se concentration of Na2SeO3, GLPs + Na2SeO3, and Se-GLPs 3 stayed constant, Se-GLPs 3 showed the strongest activities of attenuating O2 •− and HO• production by 82.0% and 73.3%, respectively, agreeing with the above observation that Se could increase the antioxidant activity of Se-GLPs. However, the sum of the scavenging effect of Na2SeO3 (31.0%) on HO• and that of GLPs (51.0%) on HO• was approximate 10% bigger than that of Se-GLPs 3 on HO• (73.3%); similarly, the sum of the scavenging effect of Na2SeO3 (13.4%) on O2 •− and that of GLPs (71.2) on O2 •− was also a little higher than that of Se-GLPs 3 on O2 •− (82.0%) (Table 4), suggesting that there may be no synergetic effect between Se in the Se-GLPs and the polysaccharide itself on scavenging free radicals. In contrast, an obvious synergetic effect between the protein extracts from Se-enriched G. lucidum and their Se components has been observed [16]. Furthermore, the radical scavenging activities of Se-GLPs is significantly higher than that of GLPs + Na2SeO3 (p < 0.05) (Table 4), a result implying that the chemical form of selenium seems also be crucial for polysaccharide’s antioxidative activities, which is similar to what we have found in the protein extracts from Se-enriched G. lucidum [16]. In addition, it is of interest to note that except for Na2SeO3, other three samples with polysaccharide exhibited stronger activities of attenuating the production of superoxide radical than hydroxyl radical. This might be due to the inhibition of polysaccharides on activity of the XOD enzyme, which catalyzes the formation of superoxide radical [30], besides their activity against superoxide radical. Illustrating the mechanism of the free radical scavenging activities of Se-enriched polysaccharide extracts and their analogs are under investigation.
Conclusions
The polysaccharide extracts from Se-enriched G. lucidum (Se-GLPs) exhibited DNA protective effects from hydroxyl radical oxidative damage in dose dependent manner. Moreover, at the same polysaccharide concentration, the polysaccharide extracts from Se-enriched G. lucidum showed much stronger properties against hydroxyl and superoxide radicals than those from unenriched G. lucidum as suggested by spin-trapping experiment. These results demonstrate that selenium in polysaccharide extracts plays an important role in enhancing their antioxidant activities. The idea was further confirmed by the observation that with increasing the Se content in the polysaccharide extracts, the antioxidant activities of all the Se-GLPs samples increased. However, unlike their protein analogues [16], there is no synergetic effect observed between Se moiety and polysaccharide itself in antioxidant activity.
References
Marx JL (1987) Science 235:529–531
Sarafian TA, Bredesen DE (1994) Free Radic Res 21:1–8
Liu F, Ooi VEC, Chang ST (1997) Life Sci 60(10):763–771
Nunoshiba T, Obata F, Boss AC, Oikawa S, Mori T, Kawanishi S, Amamoto K (1999) J Biol Chem 274:34832–34837
Simic MG (1988) Mutat Res 202:377–386
Clark LC, Combs GF, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongard A, Lesher JL, Park HK, Sanders B, Smith CL, Taylor JR (1996) J Am Med Assoc 276:1957–1963
Ip C (1998) J Nutr 128:1845–1854
Tapiero H, Townsend DM, Tew KD (2003) Biomed Pharmacother 57:134–144
Chen J, Berry MJ (2003) J Neurochem 86:1–12
Chang R (1994) Effective dose of Ganoderma in humans. In: Proceedings of contributed symposium 59A; 5th international mycological congress, Vancouver, Canada, pp 117–121
Mizuno T (1996) In: Mizuno T, Kim BK (eds) Ganoderma lucidum. II-Yang Pharm. Co. Ltd, Seoul, pp 101–106
Wasser SP, Weis AL (1999) Int J Med Mushroom 1:31–62
Lien EJ (1990) Progress in drug research. Basel, Birkhauser, pp 125–134
Zhao L, Zhao Z, Tong J, Hu X (2004) Personal Communication. DAPR Laboratory, College of Food Science & Nutritional Engineering, China Agricultural University, Beijing, China, pp 30–45
Zhao L, Zhao G, Zhao Z, Chen P, Tong J, Hu X (2004) J Agirc Food Chem 52(12):3954–3959
Zhao L, Zhao G, Hui B, Zhao Z, Tong J, Hu X (2004) J Food Sci 69:183–187
Fedorova OS, Olkin SE, Berdnikov VM (1982) Z Phys Chemie, Leipzig 263:529–549
Hara T, Tsukagoshi K, Imaki M (1987) Bull Chem Soc Jpn 60:1537–1539
Zhang J, Qin J, Cao E, Zhang Z, Zhen Y (1996) Acta Biochemica Biophysica Sinica (in Chinese) 12:691–695
Zhao QX (1995) Biol Bull (in Chinese) 30:47–47
Whistler LR (1965) Removal of moteln: sevag medical in carbohydrate chemistry. Academic, New York, pp 76–82
Yu S, Gao D, Li G (1998) J South China Univ Technol (Nat Sci) (in Chinese) 26:123–127
Dubois M, Gilles KA, Hamilton JK (1956) J Annu Chem 28(3):350–356
Finkelstein E, Rosen GM, Rauckman EJ (1980) Arch Biochem Biophys 200:1–16
Grady JK, ChenY, Chasteen ND, Harris DC (1989) J Biol Chem 264:20224–20229
Finkelstein E, Rosen GM, Rauckman EJ, Paxton J (1979) Mol Pharmacol 16:675–685
Chen CY, DingYQ, Elmahadi EA, Zhou JY, Li Y, Xu HB (1998) Chin J Biochem Mol Biol 14(4):422–426
Deng CH, Yang XL, Wang Y, Gu XM, Zhou JY, Xu HB (2001) Chin J Biochem Pharm 22(1):1–4
Huang KX (1994) In: Xu HB (ed) Selenium: its chemistry, biochemistry and application in life science. Huazhong Science and Technology University Press, Wuhan, pp 104–145
Xiong QB, Kadota S, Tani T, Namba T (1996) Biol Pharm Bull 19:1580–1585
Acknowledgments
This work was supported by Program for New Century Excellent Talents in University (NCET) in 2004 in China and China postdoctoral funding (2005037399).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, L., Zhao, G., Du, M. et al. Effect of selenium on increasing free radical scavenging activities of polysaccharide extracts from a Se-enriched mushroom species of the genus Ganoderma . Eur Food Res Technol 226, 499–505 (2008). https://doi.org/10.1007/s00217-007-0562-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0562-7