Abstract
Many researchers have studied the biological effects of carotenoids and the more appropriate procedure for extracting them from vegetable sources. In this work we propose a rapid and low-cost procedure to extract lycopene from tomato in order to by-pass the problems related to the high cost of this molecule. Following this procedure we have obtained over 95% pure all-trans-lycopene checked by DAD-HPLC coupled with mass-spectrometer equipped with APCI source and by UV–Vis spectroscopy. Moreover, in order to evaluate the effectiveness of this procedure, we have assayed the capacity of the extracted lycopene to inhibit proliferation in T-lymphocyte jurkat J32 cells in comparison with authentic standard all-trans-lycopene. On this cellular line both standard lycopene and extracted lycopene tended to be dose-dependent but this latter seems to be more active even at lowest concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lycopene (Fig. 1) is the pigment principally responsible for the characteristic deep-red color of ripe tomato fruits and tomato products. It has attracted attention due to its biological and physicochemical properties, especially related to its effects as a natural antioxidant. Although it has no provitamin A activity, lycopene does exhibit a physical quenching rate constant with singlet oxygen almost twice as high as that of beta-carotene [1]. This makes its presence in the diet of considerable interest. Epidemiological studies have shown an inverse relationship between the intake of fruits and vegetables and the risk of several types of cancer [2] and such effects of fruits and vegetables have been attributed to carotenoids [3]. Both in vitro and in vivo studies have produced supportive results to this theory.
Tomatoes and related tomato products are the major source of lycopene compounds, and are also considered an important source of carotenoids in the human diet, in fact a considerable body of epidemiological evidences demonstrates an association of tomato consumption with reduced prostate cancer risk [4]. Recent data demonstrate that consumption of some nutrients confers protection against sporadic prostate cancer [5]. In the Health Professionals follow up study, Giovannucci et al. [6] showed that estimated intake of lycopene (in form of tomato based products), was linked to lower prostate cancer risk. Further investigations consolidated the epidemiological evidence for a role of lycopene in prostate cancer prevention by showing that in addition to lycopene intake also lycopene blood levels are inversely correlated with prostate cancer risk [7–10].
General trend of recent scientific literature [11–13] proposes new evidences related to the role of each single carotenoid, for this reason it is important to define a procedure for the extraction of individual carotenoids from the matrix. Since the lycopene results the molecule with the highest biological/pharmacological value respect to the other carotenoids [14–17], many researchers have proposed various methods for its extraction [18–21].
In this paper, a new rapid method for lycopene extraction from tomato in order to produce pure lycopene using a low-cost procedure is proposed. To define the strict relation among the effectiveness of this method, the purity of the extracted molecule and its biological properties, the capacity of extracted lycopene to inhibit proliferation in T-lymphocyte jurkat J32 cells in comparison with authentic standard lycopene was assayed.
From the literature [22], it is clear that total recovery of lycopene is not possible even using high performance method such as supercritical fluid extraction (SFE). Recently, the use of supercritical fluid extraction is often applied with alternate success [23, 24], however, the capital costs necessary for the extraction setup are high and this limits the application of this technique both for research and industrial scale-up. In fact from an industrial point of view, the separation of components from, for example, solid materials by means of a supercritical fluid as a solvent under high pressure is troublesome and requires high capital costs for high-pressure extraction equipment.
Materials and methods
All solvents (analytical, uvasol and HPLC grade) were purchased by Sigma-Chemical Co. (St Louis, MO, USA). Crystalline all-trans-lycopene (ψ,ψ-carotene) from tomato (>90% pure, as indicated in the label) was also provided by Sigma-Chemical Co., the purity of lycopene standard was checked by spectrophotometry and resulted 91.2%. Iodine resublimed, 99.9% pure, was purchased by Carlo Erba Reagenti (Milan, Italy).
Cell culture and reagents
The human Jurkat T cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were mantained in IMDM medium supplemented with 10% FBS 2 mM l-glutamine (Invitrogen, Carlsbad, CA, USA). Cells were passaged three times weekly. Cells used for all experiments were in the logarithmic growth phase, and the medium used for experiments had the same constituents as that used for cell passage, unless otherwise indicated.
Methods
Extraction of lycopene
The tomato samples were protected from the action of both light and oxygen in the air in order to prevent them from damaging the dye.
All steps were performed in subdued lighting at room temperature. Tomatoes were purchased in a grocery store. Individual tomatoes were sliced and all parts of the fruit were utilized. The fruit tissue was cut into approximately 1.5 cm cubes. Fresh tomato samples (500 g) were minced in a high speedy mill until chunks were less then 4 mm (∼5 min). Fresh tomatoes were diluted 1:1 (w:v) in deionized water before blending. In this way a well-homogenized tomato sauce was obtained, then this product was heated at boiling for 10 min in the darkness. In order to separate the most polar fraction and make tomato product more suitable for successive steps of extraction, the hot suspension was submitted to treatment with Extractor Naviglio® (Nuova Estrazione sas, Napoli, Italy), a dynamic solid–liquid extractor working under pressure, eight bars for 5 min using water (2 L) as solvent [25]. In particular the suspension was placed in a special sack built with synthetic like-nylon fiber and hermetically sealed. This fiber is permeable to water but is able to keep tomato suspension. This device discharges the water automatically at the end of the extraction yielding a product with a ∼50% of moisture. After removing water by under vacuum filtration, obtaining a tomato paste (moisture about 16%), the next step in the extraction procedure was to homogenize the mixture and filtrate the resulting carotenoid extract. First the tomato paste residue was transferred in waring blender using acetone as solvent (1:1 w/v). The extraction with acetone was performed within 45 s for three times. Then the extract was filtered by means of an under vacuum system. First two steps with acetone removed the water almost totally, while the third step removed the residue moisture. The acetone filtrates (yellow coloured) were discarded while the solide residue was submitted to extraction with diethyl ether (1:1 w/v). The aspect of the residue was of powder–granulous consistance and was red-violet colored. Also this extraction was performed for three times. The extraction time using diethyl ether must be within 30 s for each step. At the end, the aspect of the residue was powder–granulous consistance and white colored. The red coloured ether filtrates were combined and the organic phase was removed and evaporated under reduced pressure setting T at 30 °C. The presence of a low percent of oil from tomato seeds in the final extract was removed for absorption on filter paper for 10′, three times at darkness and low temperature (∼15 °C). Finally the red residue was submitted at nitrogen flow for 10 min and liophylized for 2 h to remove totally the solvents (yield 0.2131 g). This material was immediately stocked at −20 °C until its utilization (Scheme 1).
The experiment was carried out in duplicate and the result represents the mean value.
Chromatography
High performance liquid chromatography, of our “home made” lycopene by DAD-HPLC apparatus (Varian 9012 pump, on-line degasser SCM1000 and UV6000LP, Spectra system, Thermo separation product, UK) was performed using a modified method described in literature [26]. All the spectra were recorded by DAD in the range from 380 to 700 nm and acquired by Excalibur software system (ThermoQuest Corporation, Manchester, UK). Mobile phase was constituted by (a) n-hexane/2-propanol 99:1 (v/v), and (b) 2-propanol. The gradient, 0% B for 10′, 0–5% B in 4′, 5% B for 6′, 5–0% B in 4′, 0% B for 6′. Eluents A and B were used without addition of triethylamine.
Column: μ-porasil 3.9 × 300 mm 10 μm (Waters, Milford, MA, USA). Flow rate, 1.2 mL/min. Injection volume, 20 μl. Chromatogram and spectrum of lycopene extracted from tomato is shown in Fig. 2. Identification of the carotenoid was based on retention times and comparison with an authentic standard as well as co-chromatography with added standard of pure all-trans-lycopene.
The purity of lycopene was calculated as the area of the all-trans lycopene as a percentage of the total chromatographic area. Necessary precautions to avoid carotenoid degradation during analysis were taken, such as working under subdued light, use of amber glassware or protection with aluminium foil, and use of N2.
To assess the isomeric purity of the extracted carotenoid, a mixture of cis-isomers was generated in laboratory in order to check that they did not co-elute with all-trans-lycopene. A iodine solution was prepared dissolving 6 mg of iodine resublimed (99.9% pure) in 4 mL of CH2Cl2 and 10 μL were added to 100 mL of a solution of lycopene 0.01% in hexane, prepared dissolving 10 mg of lycopene in 100 mL of hexane. The resultant mixture was exposed to light from an incandescent lamp for 1 min and then directly injected in HPLC apparatus using the same analytical conditions above reported.
Mass spectrometry
Mass spectrum was monitored in the mass range m/z 200–700 on a Finnigan LC AQA mass spectrometer equipped with APCI interface (Finnigan, Manchester, UK). The APCI vaporizer temperature was held at 450 °C, the corona discharge voltage was optimized to 4.5 kV. The spectrometer has been tuned to optimize the signal of m/z 537([M + H]⊕) of β-carotene. Detection was performed in positive mode.
Spectrophotometric analysis
All of the solutions were prepared under subdued light and kept under a nitrogen atmosphere at −20 °C. An UV–Vis spectrophotometer Jasco 7850 (Jasco Inc., Easton, MD, USA) was used for UV–Vis spectroscopy. A concentrated solution of lycopene from tomato was prepared by dissolving 15–20 mg of crystals in 10 ml dichloromethane and diluting to 100 ml with n-hexane. The sample was vortexed vigorously for 1 min to dissolve; from this, a 1:100 dilution in n-hexane was prepared and used for the spectrophotometric measurement at 472 nm. Generally, the concentration of carotenoids is calculated from their extinction coefficients in hexane [27], defined as the absorbance of 1% w/v solution in 1 cm path cuvette at defined wavelength. In particular percentage of lycopene in tomato was calculated using the following relation:
being A absorbance at 472 nm, P weight of sample (in g), 3,450 extinction coefficient of lycopene in n-hexane (E 1%1cm ).
MTT assay
Triplicates samples consisting of 4 × 104 viable Jurkat cells/0.1 mL−1/well were cultured in 96-well flat bottom plates in the presence or absence of different concentrations of lycopene for 48 h. Lycopene authentic standards and lycopene extracted in accordance with our procedure, were dissolved in THF [28] and solubilized in cell culture medium. The final concentration of THF in cell culture medium was 0.5%. This vehicle did not affect the parameters measured in the presented experiment as assessed by high cell viability (>95%). At the conclusion of the culture period, 25 μl of a 5 mg/mL MTT stock solution were added to each culture well and the plates were incubated at 37 °C for an additional 2 h. The dark-colored crystals produced by viable cells were solubilized with a 30% SDS solution. Plates were centrifuged and the supernatant removed. Absorbance of the supernatant was read at 540 nm in a microplate reader.
Results and discussion
The aim of this work was to obtain lycopene as pure molecule. Lycopene in fresh tomato fruits occurs essentially in the all-trans configuration [29] and using this method of extraction lycopene at its natural status can be recovered, in fact the results from HPLC-APCI-MS were consistent with authentic standard lycopene in all-trans configuration. The recommended method for extracting carotenes from freeze-dried vegetables [30] is labor-intensive, uses toxic solvents, and requires overnight saponification. Although saponification gives excellent extraction efficiencies and is useful for quantitative measurements of total carotenoids, it is less successful for determining individual carotenoids because carotenoids are degraded and isomerized by saponification conditions. Developing a simpler, faster, less toxic method for routine extraction of carotenoids and in particular of lycopene from tomato and tomato-based products, would be useful for scientists and public health workers attempting to use not only fruits and vegetables but pure carotenoids to combat several diseases related to an oxidative stress [31–33].
A low-cost procedure to produce pure lycopene, a very expensive carotenoid, was carried out and this is remarkable because it should be impossible to produce drugs, dietary supplements or functional foods based on pure lycopene for the final cost of these products. In our study a novel method of extraction that allows a complete recovery of lycopene from tomato fruits was reported. The fundamental step for obtaining lycopene from tomatoes is the breaking of cells: if breaking of tomato cells is not complete, only a partial extraction of yellow, yellow–orange carotenoids and in particular a mixture of α, β and γ carotene and lutein can be obtained, while the matrix remains red coloured. It is very difficult to break vegetable cells, specially those of Lycopersicon esculentum, for this reason the extraction was performed using a sequence of mechanical, physical and chemical actions. The important role is played by altering of mechanical action by waring blender and Naviglio Extractor® and physical action by temperature: only at the end of this sequence a complete cell disruption can be obtained. Naviglio extractor represents a technological innovation in the field of solid–liquid extraction, it is based on a suction effect, generated by a compression of extracting solvent on solids at a pressure of eight bars for a determinate time, and followed by an immediate decompression at a pressure of about 0.1–1 bar. Rapid release of extracting liquid from the inside of a solid matrix, because of pressure gradient, transports mechanically the extractable compounds contained in the solid matrix towards the outside. In this case distilled water as solvent was used. After this step a double advantage was obtained: on one hand a complete cell disruption for the application of the pressure on extractable tomato material, on the other hand the removing of most polar fraction (low and high water-soluble substances such as sugars, soluble fiber, proteins, minerals). The removing of water-soluble subtances has simplified a lot the tomato paste; in fact the successive extractions with organic solvents resulted easier and complete. This is relevant because a faster process (without many repetition of the same step) avoids dangerous pigment degradation. The successive steps, extraction with organic solvents performed in waring blender, represent the final phase of the procedure of carotenoids extraction. The contact with the first solvent, acetone, allows the removing of the water and more polar carotenoids and β-carotene, but it is not able to extract lycopene that remains into the matrix. It is important to perform three times the extraction with acetone to remove completely the water and consequentially all the polar and medium-polar carotenoids. The time of extraction is even more relevant; it is necessary to perform the extraction for max 45 s in each step because a longer time produces loss of lycopene. In fact if the acetone extraction is performed over 45 s, in particular in the last of three acetone extractions, a partial extraction of lycopene begins. In the successive phase we used diethyl ether, for three times; after the first ether extraction we removed about the 80% of red carotenoids, while the second and last ether extractions allowed a complete recovery of red carotenoids. In this case the extraction was performed for 30 s in each step because it was the minimum time able to extract lycopene. The time of extraction must be respected in order to avoid a too long time of contact with the solvent, because this promoves trans–cis isomerization. After this procedure the matrix had a fibrous aspect (not soluble fiber) and remained white coloured: all carotenoids were extracted from it. Most extraction methods [34–36] use organic solvents such as hexane, ethanol, acetone, methanol, tetrahydrofuran, and petroleum ether. As indicated by Barth et al. [37] that propose the SFE method, traditional solvent extraction of carotenoids is time-consuming, requires multiple steps, and consumes large amounts of organic solvents. The amount and the price of organic solvents directly influence the total costs of producing an acceptable extract/product. Moreover, when the final product is used as a food ingredient, it is absolutely necessary to remove all potentially toxic solvents. Using our method we have resolved the occurrences above mentioned, in fact the exposed procedure requires about 30 min for the total extraction of lycopene and further 30 min for complete purification (removing of seeds oil). After this extraction the lyophylisation at least for 2 h and the immediate storage at −20 °C can help to preserve the extracted lycopene against degrading processes. The removing of solvent from the residue in the various steps of extraction normally occurs by means of under vacuum filtration at room temperature and then the solvents are re-distilled at low temperature (∼35 °C). Under these conditions the recovery of solvents is very high (over 80%) allowing a reduction of costs. Furthermore, this method can be applied on dried ripe tomato skins and seeds, also for the tomato industry by-products. In fact the by-products of the food industry, such as wastes from the production of peeled tomatoes and tomato concentrate could be an excellent source, especially of lycopene. This could be a relevant help to reduce the total costs of extraction.
Acetone and diethyl ether are most volatile solvents and then under the conditions above exposed are removed completely from the extract. Specific tests (GC–MS) for the presence of potentially toxic solvents were performed (data not shown). The extracts resulted absolutely sure for human health. From literature we have known that some procedures such as SFE with the addition of co-solvent (i.e., chloroform) showed the presence of traces of this latter in the final extract [38].
UV–Vis spectroscopy and HPLC-APCI-MS were carried out to confirm the identity of carotenoid extracted. In Fig. 2 the DAD-HPLC chromatogram and the spectrum of the peak at RT 2.82 min are shown. The spectrum recorded in the region 380–700 nm showed three main peaks of absorption at 444, 472 and 503 nm. The absence of functional groups was demonstrated by the chromatographic behavior (t r = 2.82). These data were absolutely consistent with those recorded for the authentic standard of lycopene (ψ,ψ-carotene) under the same conditions and in accordance with previous literature [39]. The λ max at 442, 468, 500 nm corresponded to an acyclic carotenoid with 11 conjugated double bonds [39]. Moreover, the UV–visible light spectrum in hexane, with the maximum wavelength (λ max) at 444, 472, and 503 nm (Fig. 2), is also described in literature as characteristic of all-trans-lycopene chromophore [40, 41].
The purity of lycopene calculated by DAD-HPLC was 98.5%.
It is known that a natural, all-trans carotenoid is partially converted by iodine into a mixture of its cis–trans isomers [42, 43]. Simultaneously a decrease in the intensities and wavelengths of the extinction maxima takes place in the visible spectral region. In fact hypsochromic shifts of 6 nm were shown for λ max values of all-trans-lycopene. These observations are in accordance with the theoretical conclusions that among all stereoisomers of a polyene the all-trans form, possessing the extended coplanar structure, must have the greatest colour intensity. Moreover, the bending of the molecule does not necessarily involve a decrease in the extinction in every spectral region. On the contrary, such a spatial change, brought about by iodine catalysis, causes the appearance of a new marked maximum which we call the “cis-peak.” The latter is located somewhere within the region 320–380 nm e.g., at 362 nm for lycopene in hexane. The results of the experiment of iodine isomerization, reported in Fig. 3, showed the presence of a second relevant peak (RT 3.30 min) after the treatment of all-trans lycopene with iodine. This is very important because confirms that the HPLC method proposed is able to separate the all-trans-lycopene (RT 2.82 min) from other cis-isomers (RT 3.30 min). The relative UV–Vis spectra of two chromatographic peaks are consistent with the structure of all-trans-lycopene (Fig. 3a) and cis-lycopene (Fig. 3b), respectively. In fact the absorption spectrum of cis-lycopene has a cis-peak at 360 nm and λ max at wavelengths slightly lower than those of all-trans-lycopene (ψ,ψ-carotene).
The UV–Vis spectroscopy remains a technique of first choice for the analysis of carotenoids because it is a rapid, reproducible, unambiguous analysis. In fact, using this technique it is possible to distinguish different carotenoids in accordance with the literature [40, 41]. For this reason the high purity of lycopene was checked using the specific coefficient of extinction (3,450 using hexane as solvent) and recording UV spectrum at 472 nm (maximum of absorption for lycopene). The purity degree for lycopene resulted over 95%.
Mass spectrometry (MS) has been widely used in the elucidation of the structures of carotenoids. In our study APCI-MS to determine the structure of final product was employed. APCI-MS spectrum showed the characteristic fragmentation of lycopene. In Fig. 4 the detection of the carotenoid in positive-ion APCI-MS by extraction of the protonated molecule, [M + H]⊕ (top) and the computer reconstructed mass chromatogram of the same (bottom) are shown. The base peak at m/z 537 belongs to the protonated molecule, [M + H]⊕. Moreover, also the molecular radical ion [M]⊕ with the mass m/z 536 appears with high intensity. The other ions recorded at m/z 444 and m/z 430, identified as [M-92] and [M-106], are typical fragments for lycopene and β-carotene. In particular the peak [M-92] is formed by free radical fragmentation from the radical cation [M]⊕ and it represents the loss of toluene that derives from the chain arrangement, while [M-106] represents the loss of xylene [44]. The ion recorded at m/z 467 [M-69]⊕ is characteristic for lycopene and it is absent in mass spectra of β-carotene and other carotenoids. In fact it represents the lack of ψ end group of lycopene, –CH2–CH=C(CH3)2. This characteristic fragmentation pattern, and in particular the peaks at 467 [M-69]⊕ and 536 [M]⊕ are consistent with the structure C40H56 and then identify lycopene unambiguously (Fig. 3).
Lycopene obtained following this procedure was tested on Jurkat T-cells.
Antiproliferative effects of lycopene on Jurkat T cells
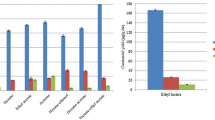
To asses the antiproliferative effects of lycopene on the growth of a tumor cell line, Jurkat T cells were incubated in the presence of different concentrations of extracted lycopene or standard lycopene. Cell proliferation and survival was directly assessed by measuring changes in cell density using the colorimetric MTT assay. The exposure of the cells to increasing concentrations of the two carotenoids resulted in a dose dependent inhibition of cell proliferation (Fig. 5). Figure 5 confirms that Jurkat cells were highly sensitive to both extracted and standard lycopene, in that a significant decrease in MTT incorporation was observed at the lowest concentration (0.37 μM) but the latter were less sensitive to the first, in fact at least a concentration two times higher of the standard was required to obtain the same antiproliferative effect induced by extracted lycopene. It is known that lycopene at physiological concentrations can inhibit human cancer cell growth by interfering with growth factor receptor signalling and cell cycle progression [45]. From this point of view it is possible to think that the different purity of the two substances is determinant for the biological activity. The results shown in Fig. 5 suggest that the activity observed at higher concentrations could be attributed to a toxic and aspecific effect of the two molecules on the target cells. Collectively the data presented in Fig. 5 suggest that low micromolar concentrations of lycopene are able to induce an antiproliferative effect on Jurkat J32 cells and that the quality, purity and extraction method can affect the remarkable biological/pharmacological role of this molecule. Only using a pure high-quality lycopene it is possible to have a good biological response, for this reason is important to define an extraction method able to produce a low-cost lycopene. Pure high-quality lycopene can easily be extracted and recovered from tomato using the proposed method.
Effect of extracted lycopene or authentic standard lycopene on the growth of T lymphocyte jurkat J32 cells. Cells (4 × 104 viable Jurkat cells/0.1 ml/well) were treated with various concentrations of both lycopenes and measured for viability by MTT assay at 48 h after treatment. The absorbance (optical density) was read at 540 nm. The values represent the mean ± SD of three independent experiments
References
Di Mascio PD, Kaiser S, Sies H (1989) Arch Biochem Biophys 274:532–538
Astrog P (1997) Food Sci Tech 8:406–413
Peto R, Doll R, Buckley JD, Sporn MB (1981) Nature 290:201–208
Mills PK, Beeson WL, Phillips RL, Fraser G (1989) Cancer 64:598–604
McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, Wilkinson GS, Graham S (2005) Nutr Cancer 53:33–41
Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC (1995) J Natl Cancer Inst 87:1767–1776
Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC (2002) J Natl Cancer Inst 94:391–398
Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, Stampfer MJ (1999) Cancer Res 59:1225–1230
Giovannucci E (1999) J Natl Cancer Inst 91:317–331
Tang L, Taiyi J, Zeng X, Wang JS (2005) J Nutr 135:287–290
Tapiero H, Townsend DM, Tew KD (2004) Biomed Pharmacother 58:100–110
Yaping Z, Suping Q, Wenli Y, Zheng X, Hong S, Side Y, Dapu W (2002) Food Chem 77:209–212
Martin KR, Wu D, Meydani M (2000) Atherosclerosis 150:265–274
Narisawa T, Fukaura Y Hasebe M, Ito M, Aizawa R, Murakoshi M, Uemura S, Khachik F, Nishino H (1996) Cancer Lett 107:137–142
Nagasawa H, Mitamura T, Sakamoto S, Yamamoto K (1995) Anticancer Res 15:1173–1178
Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, Sharoni Y (1995) Nutr Cancer 24:257–66
Böhm V, Puspitasari-Nienaber NL, Ferruzzi MG, Schwartz SJ (2002) J Agric Food Chem 50:221–226
P´ol J, Hy¨otyläinen T, Ranta-Aho O, Riekkola M.L (2004) J Chromatogr A 1052:25–31
Vasapollo G, Longo L, Rescio L, Ciurlia L (2004) J Supercritical Fluids 29:87–96
Davis AR, Fish WW, Perkins-Veazie P (2003) Postharvest Biol Technol 28:425–430
Fish WW, Perkins-Veazie P, Collins JKA (2002) J Food Compost Anal 15:309–317
Rozzi NL, Singh RK, Vierling RA, Watkins BA (2002) J Agric Food Chem 50:2638–2643
Cadoni E, De Griorgi MR, Medda E, Poma G (2000) Dyes Pigm 44:27–32
Topalu H, Sasaki M, Goto M, Hayakawa K (2006) J Agric Food Chem 54:5604–5610
Naviglio D (2003) Anal Lett 36:1645–1657
Psomiadou E, Tsimidou M (1998) J Agric Food Chem 46:5132–5138
Scott KJ, Finglas PM, Scale R, Hart DJ, De Froidmont-Görtz I (1996) Food Chem 57:85–90
Bertran US, Pung A, Churley M, Kappock TJIV, Wilkins LR, Cooney RV (1991) Carcinogenesis 11:671–678
Chandler LA, Schwartz SJ (1987) J Food Sci 52:669–672
Williams S (ed) (1984) Official methods of analysis of the Association of Official Analytical Chemists, 14th edn. p 835
Rao AV, Shen H (2002) Nutr Res 22:1125–1131
Atessahin A, Yilma S, Karahan I, Ceribasi AO, Karaoglu A (2005) Toxicology 212:116–123
Kokias S, Gordon MH (2003) Eur J Clin Nutr 57:1135–40
Tan B (1988) J Food Sci 53:954–959
Heinonen MI, Ollilainen V, Linkola EK, Varo PT, Koivistoinen PE (1989) J Agric Food Chem 37:655–659
Tonucci LH, Holden JM, Beecher GR, Khachik F, Davis CS, Mulokozi G (1995) J Agric Food Chem 43:579–586
Barth MM, Zhou C, Kute KM, Rosenthal GA (1995) J Agric Food Chem 43:2876–2878
Baysal T, Ersus S, Starmans DAJ (2000) J Agric Food Chem 48:5507–5511
Azevedo-Meleiro CH, Rodriguez-Amaya DB (2004) J Food Compost Anal 17:385–396
Britton G (1995) UV/visible spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids vol 1B: spectroscopy. Birkäuser, Basel, pp 13–62
Britton G (1991) Carotenoids, in methods in plant biochemistry, vol 7. Academic, London, pp 473–518
Polgár A, Zechmeister L (1942) J Am Chem Soc 64:1856–1861
Zechmeister L, Polgár A (1943) J Am Chem Soc 65:1522–1527
Enzell CR, Back S (1995) Mass spectrometry. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids, vol 1B: spectroscopy. Birkäuser, Basel, pp 261–320
Heber D, Qing-Yi L (2002) Exp Biol Med 227:920–923
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montesano, D., Fallarino, F., Cossignani, L. et al. Innovative extraction procedure for obtaining high pure lycopene from tomato. Eur Food Res Technol 226, 327–335 (2008). https://doi.org/10.1007/s00217-006-0541-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0541-4