Abstract
Infectious diseases are still a worldwide important problem. This fact has led to the characterization of new biomarkers that would allow an early, fast and reliable diagnostic and targeted therapy. In this context, Pseudomonas aeruginosa can be considered one of the most threatening pathogens since it causes a wide range of infections, mainly in patients that suffer other diseases. Antibiotic treatment is not trivial given the incidence of resistance processes and the fewer new antibiotics that are placed on the market. With this scenario, relevant quorum sensing (QS) molecules that regulate the secretion of virulence factors and biofilm formation can play an important role in diagnostic and therapeutic issues. In this review, we have focused our attention on phenazines, as possible new biomarkers. They are pigmented metabolites that are produced by diverse bacteria, characterized for presenting unique redox properties. Phenazines are involved in virulence, competitive fitness and are an essential component of the bacterial QS system. Here we describe their role in bacterial pathogenesis and we revise phenazine production regulation systems. We also discuss phenazine levels previously reported in bacterial isolates and in clinical samples to evaluate them as putative good candidates to be used as P. aeruginosa infection biomarkers. Moreover we deeply go through all analytical techniques that have been used for their detection and also new approaches are discussed from a critical point.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa is an opportunistic nosocomial human pathogen, which causes a broad spectrum of acute and chronic infections such as bloodstream infections in intensive care units (ICU), burn and chronic dermal wound infections, surgical site infections, hospital-acquired pneumonia and respiratory and urinary tract infections. P. aeruginosa has also been identified as the cause of bacterial outbreaks in neonatal intensive care units (NICUs) [1]. All these infections occur mainly in patients with other diseases or injuries, just as severe burn wounds, AIDS, lung cancer, chronic obstructive pulmonary disease (COPD), bronchiectasis and cystic fibrosis (CF) [2] or as nosocomial infections caused by biofilm contamination of medical devices (i.e. catheter related infections) [3]. Given its pathogenic potential and ubiquitous environmental presence, it is surprising that P. aeruginosa rarely infects healthy community individuals. Nonetheless, infections caused by this bacterium can be devastating in hospitalized or sick patients [2].
Clinically, P. aeruginosa infections are usually classified into ‘acute’ and ‘chronic’ even though the difference between them is not always clear. Hence, in some diseases such as CF, P. aeruginosa may colonize the lung with little new clinical evidence of disease [4]. In this group of patients, ‘exacerbations’ of these chronic infections may clinically resemble a new acute infection, when they are not. Thus, in the absence of previous microbiological culture data these chronic infections may be mistakenly classified as acute. Because of this panorama, some authors prefer to categorize the respiratory infections of P. aeruginosa into ‘transient’ and ‘persistent’ to emphasize the importance of the microbiological status over the symptoms [2].
This is due to the fact that P. aeruginosa infections typically evolve to a pattern of persistence (chronicity). This strategy allows the microorganism to survive for long periods under the challenging selective pressure forced by the immune system and antibiotic treatment, thanks to a biofilm mode of growth [5] and adaptive evolution mediated by genetic variation [6].
It has been shown that hypermutability plays an important role on this adaptive evolution. In this sense, P. aeruginosa strains that have survived for example in the lung of CF patients for more than 30 years turn into very well adapted pathogens. Thus, long-term adaptations in P. aeruginosa are likely brought about by an increased mutability of its genome while in the biofilm [6,7,8]. Accumulation of mutations leads to phenotypic changes such as increased alginate production (in CF isolates) which is a polysaccharide overproduced in the case of mucoid variants (see Fig. 1), loss of QS, loss of motility, loss of effector proteins of the type III secretion system (see next section), loss of the O-antigen components of the lipopolysaccharide (LPS), reduced virulence, reduced capacity for in vitro biofilm formation and increased antibiotic resistance [2, 9,10,11]. All these changes result in greater difficulty or even impossibility of pathogen eradication, eliciting a major inflammatory response and promoting accelerated dysfunction and poorer prognosis. For this reason, eradication therapies for P.aeruginosa infections at early stages are recommended in order to prevent chronicity, since at this stage strains are more susceptible to antimicrobials. However, despite the growing knowledge on this type of infections, early diagnostic is still a challenge.
Models of Pseudomonas aeruginosa infection establishment. In (A) P. aeruginosa is equipped with a full arsenal of virulence traits including pili, flagella, type 3 secretion systems (T3SS) and secreted virulence factors. Epithelial cell binding occurs via flagella and pili to various structures including asialoGM1. Toxin production injures the surrounding host tissue. Epithelial injury also results in loss of mechanical clearance mechanisms and establishment of the pseudomonal biofilm leading to a persistent infection. In (B) P. aeruginosa infects an already inflamed surface with a defective mucociliary elevator. The infecting organisms may or may not be piliated, and may already exist in a ‘biofilm’ state if acquired from another patient. The infection occurs strictly in the mucous layer where ‘nests’ of pseudomonads bind to cell debris and extracellular DNA rather than to the epithelial surface. Adapted from Williams et al.2
Moreover, the wide array of clinical manifestations of P. aeruginosa infections can be mainly associated to two issues: the first one relates with the high adaptability of this pathogen which translates in severe pathogenicity and the second one has to do with chronicity and with the important relevance of the underlying host defect, which can be quite significant, especially in lung infections [12]. In addition, the emergence of multiple drug resistance processes has become a major threat for current medical treatment of these infections around the world [13, 14]. Hence, a high percentage of resistant strains are being reported in different surveillance studies conducted in different countries and continents [15,16,17].
Thus, in this context, rapid, easy-to-use and efficient diagnostic tools to control these infections seem to be a clear need. The development of new methods requires the identification of specific infection biomarkers. This review focuses on phenazines as potential targets to be detected in P. aeruginosa infections. To demonstrate their utility as putative good biomarkers, first we describe all different P. aeruginosa virulence traits, we explain the role of phenazines in the pathogenesis caused by this bacterium, and we analyze the levels of these compounds already reported from the analysis of isolates and directly from clinical samples. Finally, we focus on different analytical approaches for measuring these pigments presenting them from a critical perspective.
Pseudomonas aeruginosa VIRULENCE
P. aeruginosa expresses an arsenal of virulence traits that are powerful weapons damaging host cells, which can be classified in two categories (see Fig. 2).
Cell-associated determinants (or surface-bound virulence determinants)
Flagella and pili, bacterial surface appendages, are not only needed for their motility but are also involved in other biological functions. Thus, in case of wounds in the CF airway, P. aeruginosa uses flagella-driven swimming to reach epithelial cells located closed to the damaged area plus amino acid sensor-driven chemotaxis. In addition, pili are utilized for binding to epithelial cells near wounds [18]. Moreover, the binding of these appendages to certain target molecules such as Toll-like receptor 5 (TLR5) can induce a strong proinflammatory response [19, 20]. On the other side P. aeruginosa LPS, major component of the outer surface membrane in gram negative bacteria, has been found to elicit an immunogenic response on the host and also to show the ability of binding to Cystic Fibrosis Transmembrane Regulator (CFTR). CFTR is a membrane protein that acts as chloride channel in vertebrates. Mutations in the gene that encodes CFTR cause dysregulation of epithelial fluid transport resulting in CF. LPS binding to CFTR facilitates pathogen invasion into the epithelial cells, which could explain the higher susceptibility of CF patients to suffer infections by this microorganism [21]. Furthermore, LPS seems to interact directly with the outer membrane protein H (oprH), increasing the structural stability and also reducing permeability of the bacterial outer membrane justifying P. aeruginosa significant antibiotic resistance [22]. In any case, the contribution of LPS to pathogenesis and host immunity depends on LPS isoform (variations are found mainly in the lipid A component) and on the structural variation in the O-antigen side chain. Furthermore, LPS effect relies also on the underlying patient basis for increased susceptibility to infection [23].
Secreted virulence factors
Secreted virulence factors comprise a series of cytotoxins such as those of the type 3 secretion system (T3SS) which include several protein toxins that play important roles on the pathogenesis of P. aeruginosa, particularly at the initial stages of the infection (see Hauser [24] for a review on this system). The expression of many of these virulence factors is regulated by the QS network. This system is controlled and synchronized by RhlR, LasR and PQS regulators, which are involved in a series of events related to the production of biofilm, toxins and pigments, as well as to the development of antimicrobial resistance [25,26,27]. This group of QS related compounds (distinct L-homoserine lactones and 2-alkyl-4 quinolones) can be detected in the sputum of infected patients [28, 29].
Exotoxin A is another secreted virulence factor that inhibits host protein synthesis resulting in apoptosis, superoxide production and mitochondrial dysfunction. It is found in CF sputum and its levels are higher during periods of ‘exacerbation’ and lower during periods of relative stability [30, 31].
Phenazines (i.e. pyocyanin, PYO) are pigmented bacterial metabolites that have a function on microbial competition and virulence and are secreted in high amounts during the early colonization phase to ensure the establishment of the infection. Phenazines exert a large number of effects on host cells [32] including direct damage mainly triggered through formation of reactive oxygen species, alteration of cytokine production, ciliary motion inhibition in human nasal ciliated epithelium and interruption of cell signaling. In addition, they have a role on apoptosis [33], cell cycle arrest [34] and they induce premature senescence [35]. Thus, phenazines play an important role on P.aeruginosa virulence, as it has been found in PYO deficient mutants that cause attenuated acute and chronic lung infection in mouse, with respect to wild-type P. aeruginosa strains [36]. Moreover, phenazines can exert a toxic effect on other cells but instead benefit their producers by mediating extracellular electron transfer and survival in anoxic environments [37].
Finally, P. aeruginosa secretes a number of non specific proteases and phospholipases, which display a vast array of cytotoxic effects on cell surfaces and intracellular matrices but their role during human infections, is still unclear. Recently, the number of novel virulence factors is being extended by using comparative genomics, transcriptomics and proteomics as a tool. After putative virulence factors are identified their functions and modes of action may be further studied by mutagenesis, structural biology or biochemical methods [38] .
PHENAZINES IN Pseudomonads and thier cellular effects
Phenazines constitute a large class of natural antibiotics that are produced by diverse bacteria and exhibit unique redox properties and broad-spectrum antibiotic activity [39]. Moreover, they are involved in biofilm formation [40] and act as cell signals regulating patterns of gene expression [41, 42]. They have also been greatly studied given their biotechnological applications such as plant disease management [43].
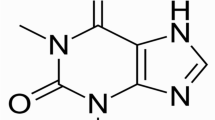
About 50 pigmented, heterocyclic nitrogen-containing secondary metabolites are synthesized by some strains of fluorescent Pseudomonas spp. and a few other bacterial genera. Fluorescent pseudomonads are the best studied phenazine producing microorganisms, including strains of P. fluorescens, P. chlororaphis (previously classified as P. aureofaciens), and P. aeruginosa. Except for P. fluorescens, which produces only the yellow compound phenazine-1-carboxylic acid (PCA) (see Table 1), Pseudomonas spp typically synthesizes two or more phenazines, with the relative amount of each one strongly influenced by growth conditions. Therefore, in addition to PCA, P. aureofaciens produces the orange and brick-red compounds 2-hydroxyphenazine-1-carboxylic acid (2-OHPCA) and 2-hydroxyphenazine (2-OHphz), whereas P. chlororaphis produces PCA and the green compound phenazine-1-carboxamide (PCN). P. aeruginosa, is the first phenazine producing microorganism that was reported in the literature [44, 45] and its characteristic blue-green pigment PYO (5-N-methyl-1-hydroxyphenazine) probably is the first described phenazine. PYO production is associated with a high percentage of P. aeruginosa isolates [46]. In 1981 Reyes et al. [47] tested 835 consecutive isolates of P. aeruginosa, all of them isolated from clinical samples obtained from University of Utah Medical Center and found that 98% of the strains produced PYO. Nevertheless, some divergences have been reported concerning PYO detection in clinical samples. Thereby, Wilson et al. [48] identified by HPLC 2 phenazine pigments, PYO and 1-hydroxyphenazine (1-OHphz), in the sputum of 9 from 13 (70%) CF and bronchiectasis patients colonized by P. aeruginosa. Concerning infected burn patients, Muller et al. [35] examined 7 samples of wound dressings detecting PYO presence just in 4 of them (57%).
P. aeruginosa also produces other phenazines including the above mentioned 1-OHphz, PCA, PCN, aeruginosin A (5-methyl-7-amino-1-carboxymethylphenazinium betaine) and aeruginosin B (5-methyl-7-amino-1-carboxy-3-sulfophenazinium betaine). Though little is known about the fine tuning of the timing of expression and the relative quantities of the different phenazines produced, it is well established that P. aeruginosa is the only microorganism that synthesizes PYO, for which reason this molecule is used as biomarker for phenotypic identification of this bacterium [49].
The most studied P. aeruginosa phenazines are PYO, 1-OHphz and PCA. Bioactivities of these compounds are linked to their ability to redox-cycle [42], which leads to generation of reactive oxygen species that cause host cell damage. The zwitterionic character of PYO readily makes it suitable to penetrate biological membranes [36] triggering a broad spectrum of harm to cultured epithelia cells [11], alteration of epithelial cytokine production [50, 51], modulation of cellular signaling pathways [52] and ultimately resulting in the induction of premature cellular senescence in human fibroblasts [35] and apoptosis in neutrophils [53, 54] (see Fig. 3).
In this regard, PYO is considered the most potent virulence factor secreted by P. aeruginosa [49]. This pigment alters the physiology and homeostasis of host cells (see [10, 32, 55]), interfering with the (i) electron transport dynamics, (ii) cellular respiration (by diminishing the levels of both NAPH and glutathione), (iii) energy metabolism (by decreasing ATP levels and by disrupting the calcium homeostasis), (iv) gene expression (e.g., several genes are induced in response to PYO, including mucins, inflammatory cytokines, chemokines and oxidative stress-responsive genes), (v) innate immune mechanisms (e.g., acceleration of neutrophil apoptosis) and (iv) redox balance status (by enhancing the reactive oxygen species) [10, 32, 56]. As an immunomodulator, PYO has been found to impair ciliary beat [57], which is an important defence mechanism of the upper and lower respiratory tract against inhaled particulate matter and bacteria, due to a decrease on intracellular c-AMP and ATP caused by these toxins. PYO and 1-OHphz have been reported to slow mucociliary clearance in vivo at concentrations similar to those detected from clinical samples [58]. Moreover, PYO inhibits nitric oxide production by macrophages and endothelial cells [59], prostacyclin production by endothelial cells [60], oxidation of leukotriene B4 by neutrophils [61] and eicosanoid metabolism by platelets [62]. Due to its redox properties, this blue phenazine enhances superoxide production [63], increases apoptosis in neutrophils [54] and inactivates α1-protease inhibitor [64]. In addition, PYO increases calcium signaling in human airway epithelial cells [65], stimulates interleukin (IL)-8 release [66] and inhibits the regulation of the normal activation of monocyte and T cell expressed and secreted chemoattractant protein-1 release in human epithelial cells [67, 68] (for additional information on PYO effects please read [32, 69]).
Regulation of PHENAZINE production
Regulation of phenazine biosynthesis is critical given their ecological properties on the biocontrol of some plant pathogens [70, 71] and from a clinical perspective since they play an important role on the virulence process during acute and chronic infections [36, 69, 72].
Phenazine production is regulated by complex networks (see Fig. 4). Focusing on pseudomonads, an operon structure containing 7 genes is involved in the biosynthesis of these compounds. Among these bacteria, P. aeruginosa strains are the unique organisms that show in their genomes two phenazine biosynthetic operons (phz1 and phz2) with identical DNA sequences and organization. A complex crosstalk exists between them to keep the homeostatic balance of phenazine production [73]. In Pseudomonads the phenazine synthetic core genes are highly conserved whereas in other bacterial species this operon structure is more variable [74]. These “core” operons encode for 5 enzymes that convert chorismic acid to PCA and/or phenazine-1,6-dicarboxylic acid (PDC) plus other genes that exert functions related with regulation, transport, resistance and conversion of PCA/PDC to strain-specific phenazine products [74].
Biosynthesis regulation and signaling system of phenazines production in Pseudomonas aeruginosa. Quorum Sensing cascade, small RNAs and environmental factors control phenazine synthesis by regulating phz1 and phz2 operons activity. Moreover, PhzA-G proteins are involved on transforming chorismic acid into phenazine-1-carboxylic acid (PCA) or into phenazine-1,6-dicarboxylic acid (PDC) which will be transformed into strain specific phenazine products also through enzymes encoded as well by genes included in phz operons which in addition exert other functions such as regulation, transport and resistance processes
Phenazine operon expression control is exerted by two different systems, homoserine lactone (HSL)-mediated QS and small noncoding RNAs (sRNAs) along with environmental factors [75]. In P. aeruginosa, QS includes 2 hierarchical HSL-based systems, Las and RhI, which in turn are modulated by a third QS circuit known as Pseudomonas quinolone signal (PQS) [76]. In this context, it has been reported that molecules with “Quorum Quenching” ability could inhibit PYO production in P. aeruginosa [77]. Moreover, it has been demonstrated that in this complex regulation system, phenazines themselves can act as signaling molecules too [52].
During chronic infections, P. aeruginosa populations undergo a characteristic adaptation process that usually includes genetic changes that eventually can alter phenazine synthesis as well. Two of the genes that are frequently mutated in isolates obtained from CF patients are lasR and mucA (mucA22 mutant or mucoid phenotype) which are associated with the phenotypic switch to mucoid strain and loss of QS, considered hallmarks of chronic virulence. These mutations, particularly the one located in lasR gene, occur at early stages of the infection and are related to entering into the stationary phase or to slow-growth conditions [78, 79]. LasR mutants are associated to reduced virulence factor production under laboratory conditions, even though it has been reported that some isolates of the Liverpool cystic fibrosis epidemic strain (LES) (lasR mutants) continuously and strongly overproduce the important virulence factor PYO while, under the same conditions, wild type cells do not [80]. The prevalent occurrence of this uncommon phenotype suggests that it may exert an essential role in relation with the success of this epidemic strain. Besides this, the bacterial mucA22 phenotype is associated to an important inflammatory response that causes acceleration on lung functional loss and a greater difficulty in eradicating the pathogen [81]. This happens together with a down-regulation of PYO synthesis upon entry into early stationary phase but a continued production of it when this growth phase is extended in comparison to wild type strains [82]. In addition, genes encoding for products involved in phenazine biosynthesis, have been identified as positive regulators of the mucoid phenotype [83].
PHENAZINE levels in clinical samples and clinical interpretation
Despite the interest in knowing if phenazines can be considered as good biomarkers of infection, only few studies do report research on the levels of such compounds in biological fluids. Thus, most of the works already available describe PYO measures carried out by analyzing clinical isolates of P. aeruginosa. The analysis of clinical samples requires culturing them in selective media to be able to choose bacterial isolates that will be grown again before proceeding with genotyping/phenotyping studies, including determination of phenazines in culture media. Concerning direct measures in clinical samples, most of the existing data come from CF patient’s samples or from patients suffering other respiratory tract infections. Conversely, very little information is available about phenazine levels in P. aeruginosa infections affecting other tissues or organs such as the urinary tract or the skin (burn wounds) among others.
Focusing on the assessment of PYO as a good biomarker of P. aeruginosa infections Mowat et al. [84] described a correlation between PYO production and periods of pulmonary exacerbation in CF patients. They demonstrated that P. aeruginosa populations are not only diverse but dynamic since they accumulate multiple mutations in chronic infections which could facilitate antimicrobial resistance, and contributing to virulence processes. In addition, changes in the CF lung during exacerbations may provide an environment that favors PYO overproducing isolates. In any case, the role of PYO in the development of clinical symptoms was not clear but high levels of morbidity seemed to be linked with PYO overproducing bacterial phenotypes. Later on, Rada et al. [10], described a mechanistic model to explain how PYO alters the function of the airway epithelium that fits with the clinical symptoms observed in patients infected with P. aeruginosa. Briefly, PYO produced by these bacteria enters into the cytosol of the respiratory epithelial cells and produce reactive oxygen species (ROS). Among other effects, these molecules inhibit ciliary beat frequency impairing P. aeruginosa clearance. In addition, reduced NADPH levels hinder antibacterial functions and PYO inactivation. Moreover, PYO long-term effects include changes in host gene expression such as the ones that cause the inhibition of the antioxidant catalase and the induction of inflammatory cytokines. The proinflammatory effects lead to aberrant neutrophil recruitment and also induce mucin hypersecretion which obstructs the airways and promotes bacterial entrapment.
Focusing on chronic infections and unlike acute ones or exacerbation periods, other mutations have been reported that result in a decreased expression of virulence factors such as exotoxin A, T3SS, quinolones and PYO [6, 12, 85]. In this way, bacteria evade the host immune system, since it recognizes numerous virulence factors and finally attempts to kill those cells that express them.
As previously mentioned, the most important aspect of P. aeruginosa is its capability to quickly adapt and accumulate traits/mutations that allow bacterial infection to persist. Pseudomonas has the ability to change its transcriptional profile rapidly to avoid being killed by host defences [86]. In this sense, P. aeruginosa can survive on a stationary state for years. Adaptation may include downregulation of the surface and secreted virulence traits described above and conversion to a mucoid phenotype. Ryall et al. [82] have performed phenotypic and gene expression studies of the stationary phase physiology of mucA22 mutants demonstrating complex and subtle changes in virulence factor production, including PYO, that result in their downregulation upon entry into stationary phase but, and in contrast to wild type strains, continued production in prolonged infections. These findings indicate that mucA22 mutation provides a second mechanism, in addition to the commonly occurring lasR mutation associated to long-term infection in CF patients, inhibiting QS during chronic infection. Despite this, many authors have reported that secretion of virulence factors is diminished after long-term colonization [86, 78], which may seem to contradict the results from Ryall et al. In this context, the data reported by Cabeen [87] supports the above mentioned results given that he showed that during stationary phase or slow growth conditions (in vitro conditions), lasR cells also continuously produce PYO while wild type cells do not.
Furthermore, little is known about the fluctuations within P. aeruginosa populations during very short periods of intense selective pressure, such as when intravenous antibiotic therapy is administered during exacerbations. P. aeruginosa concentrations are high during an exacerbation and decrease with antibiotic treatment, reducing symptomatology. Interestingly, recent data suggests that the majority of exacerbations are not due to acquisition of new strains of Pseudomonas but a clonal expansion of existing strains [88, 89]. According to some authors, PYO levels could change during exacerbation periods as a result of antibiotic treatment. Fothergill et al. [80] analyzed PYO concentration in 40 sputum isolates of 3 patients chronically infected by LES. Samples were taken (A) first at the beginning of the exacerbation, before intravenous antibiotic treatment had started; (B) the second during the exacerbation, whilst antibiotic therapy was being carried out; (C) the third at the end of the exacerbation when symptoms had resolved. They found that, for patients I and II, there was an increase in the prevalence of isolates exhibiting PYO overproduction during exacerbation period, while isolates from patient III showed overproduction just at the start of the exacerbation period with a decrease trend. The study was not sufficiently representative as the number of patients was very low and the antibiotic treatment received was different for patients I and II (meropenem + colistin) than for patient III (ceftazidime+ colistin). In addition to this, Suzuki et al. [90], working with bacterial cultures, reported that exposure to low doses of macrolides decreases elastase and PYO production just after 2 months of treatment, suggesting that long term treatment with some antibiotics may inhibit in a time-dependent manner the production of multiple virulence factors.
Phenazine levels produced by isolates selected from clinical samples of Pseudomonas aeruginosa infected patients
In these studies PYO has always been found to be secreted at significant concentrations [69, 80, 84, 91]. In fact, PYO overproduction in P. aeruginosa clinical isolates has been frequently reported. In this sense, Caldwell et al. [69] showed that 9 from 13 CF P. aeruginosa isolates were found to produce PYO levels at higher concentration than those produced by wild type strain (PA01) under the same conditions. Moreover, PYO has been detected in most clinical isolates obtained from catheters, sputa, urine and other clinical samples from infected patients. Thus, Silva et al. [92] reported PYO secretion levels of strains collected from patients hospitalized in intensive treatment units of Brazilian hospitals between 1999 and 2010, compared with the reference P. aeruginosa strain ATCC 27853, used as control. Samples were taken from rectum, tracheal aspirate, mouth, blood, urine, venous catheter, pleural secretion, eschar, CF lungs, sputum and nasal secretion. All P. aeruginosa strains were able to produce and secrete PYO to the extracellular environment, although in different amounts. Overall, the mean production was 9.61 ± 4.32 μg/mL (ranging from 1.29 to 25.94 μg/mL), even though those isolated from urine had the highest PYO levels (mean of 20.15 ± 5.65 μg/mL), while the strains isolated from sputum showed the lowest production (mean of 3.80 ± 0.39 μg/mL) of this virulence factor. It should be noticed that P. aeruginosa is one of the most frequent agents of urinary tract infections especially in patients with indwelling urethral catheters. Concerning this, Tielen et al. [93] reported a genotype/phenotype study of 30 isolates from urinary tract infections, and found similarities with the behavior of strains from lung infections.
One of the last works published about P. aeruginosa isolates (isolated from CF patients sputum) included 338 samples selected from the major CF patient cohort in Italy [94]. In this case, pulse-field gel electrophoresis was used to analyse the corresponding genotypes identifying 43 profiles shared by two or more patients and 214 profiles exclusive to individual patients. From the 4 prevalent clusters they carried out a phenotype characterization, evaluating swimming and twitching capacities, proteases, siderophore and PYO secretion, mutation frequency and lasR mutant phenotype. Just for one of the prevalent profiles, they could find positive results for all hallmarks of bacterial adaptation. They concluded that CF patients of this cohort shared common pulsotypes, but given the phenotypic heterogeneity observed, they could not determine specific traits associated to P. aeruginosa genotypic prevalence.
Focusing on P. aeruginosa burn infections, Khadim and Marjani [95] have recently published a study which compares 37 isolates obtained from burn patients with suspected P. aeruginosa infection with isolates obtained from other types of infected samples (wound swab, fluids, ear…). The results of this analysis revealed a high prevalence of PYO producers (59%) and biofilm formation (37% formed moderate biofilms and 11% formed strong ones) in P. aeruginosa isolates from burn infections.
Studies on phenazine levels in clinical samples from Pseudomonas aeruginosa infected patients
In 1988, Wilson et al. reported for the first time measurement of phenazines in sputum and their effect on ciliary beating [48]. They collected sputum from colonized patients (n = 13, 8 bronchiectasis and 5 CF) and not colonized (5) with P. aeruginosa, plus pulmonary secretions from airways of CF lungs removed at transplantation. PYO and 1-OHphz were analyzed by HPLC-UV after sample extraction and purification by solid-phase extraction (SPE). Interestingly, despite the complexity of the analytical procedure, PYO and 1-OHphz were detected in a significant number of sputa samples (9 from 13) from P. aeruginosa infected patients, with concentration levels ranging from 0.2 to 27.3 μg/mL (compared with the volume of sputum solid phase) for PYO and between 0.1–3.5 μg/mL for 1-OHphz. Previously, these authors demonstrated that in in vitro conditions 16 μg/mL of PYO cause a gradual slowing of the ciliary beating frequency (CBF) over 4 h associated with epithelial disruption, that 4 μg/mL causes similar changes and that 32 μg/mL of 1-OHphz exert an immediate action producing ciliary slowing and dyskinesia [96]. No phenazines were detected in the samples from the control group.
PYO has also been quantified by HPLC-UV on ear secretions analyzing samples from patients with different type of infections. The detected concentrations ranged from 3 to 2714 nmol/g [97].
Regarding lung infections, although the data obtained by Wilson et al. [48] is relevant, the number of patients is limited to extract conclusions regarding the value of PYO as biomarker. In this sense, there is a need to perform well conceived clinical studies using larger patient cohorts. Unfortunately, to our knowledge no other study addressing direct detection of this phenazine in clinical samples has been reported until more recently by Hunter at al. [72]. They have addressed this question by measuring concentrations of PYO and PCA in expectorated sputum samples of 45 CF patients and extracted interesting conclusions from their studies, but they are now under revision given that they have detected a technical problem on the procedure used for these measurements [98]. Thus, they collected sputa from a well characterized group of patients (available data about age, CFTR genotype, antibiotic treatments etc.…) and after an appropriate sample treatment, phenazine content was quantified by HPLC-UV. In the same way, the homogenized sputa samples were used for bacterial isolation on agar plates, cultured on lysogeny broth and the phenazine content of the corresponding supernatants was also quantified by measuring the absorbance at λ = 691 (PYO). According to their initial results, phenazine levels negatively correlate with pulmonary function and positively associate with the rate of pulmonary function decline. Moreover, PYO concentration in sputum showed a strong statistically dependence on P. aeruginosa bacterial load (CFUs, colony forming units). According to Hunter’s data [72], the concentration of PYO, PCA and total phenazines in CF sputum correlate with the disease severity, which points to the potential use of PYO as a disease biomarker, providing additional information related to the severity of infection. This is a very important and challenging statement that needs revision and confirmation. Moreover, they mentioned that PYO concentration levels in sputa would depend on PYO producing capability of the strains, but also on P. aeruginosa CFU load, which has also been found to correlate with the disease severity (higher CFU/sputum g when more serious is the infection process). Furthermore, these data also raise the fact that in vivo analysis of PYO levels could provide much more realistic information than quantifying just PYO production from clinical isolates.
In this respect, our group has obtained very interesting results when studying PYO levels produced by P. aeruginosa bacterial isolates which are now being validated analyzing directly a broad spectrum of clinical samples (unpublished results).
Potential analytical approaches for measuring PYO as potential infection biomarker
The most frequently used methods for phenazine analysis are the measurement of their characteristic absorbance spectra or HPLC-DAD (or MS). More recently other approaches have been reported (electrochemical sensors, Surface-enhanced Raman Spectroscopy (SERS), but there are still not many papers describing in vitro or in vivo (animal models or human patients) studies carried out with them. Following, there is a description on the performance of these techniques.
Absorbance
Most analytical approaches reported for phenazine quantification do exploit their optical properties, mainly measuring PYO absorbance under particular pH conditions. Thus, PYO is considered to be a blue pigment that is produced and secreted into the medium by stationary phase cultures of P. aeruginosa. At pH 7.0, PYO exists predominantly in its non protonated form (pKa = 4.9), as a zwitterion. Under these conditions, PYO solution is blue with a maximal absorption at 691, 381, 312, and 238 nm. PYO quantification in culture media is usually performed measuring the absorbance at 690 nm by calculating the concentration in μmols using the extinction coefficient for this wavelength (ɛ = 4.31 × 103 M − 1 cm − 1 at pH 7) [99]. However, the color and the characteristic PYO absorption spectrum are pH sensitive and solvent dependent [100]. Thus, its strong blue color detected at neutral pH turns red at acidic pH, with maxima at 520, 366 and 281 nm. Hence, acidified media is then measured at 520 nm. For this purpose, some authors do extract first with chloroform and then again with 0.2 N HCl, and quantify PYO using the formula: [PYO, μg/mL] = Abs520 × 17.072 [101,102,103]. The detectability accomplished is in the μM range.
HPLC-UV-vis (DAD) or MS
This is the most accurate method to quantify phenazines. For this purpose usually reverse-phase columns (5 μm particle size, Waters Symmetry C18, Bondapak C18) are used. Mobile phase contains mixtures of organic solvent (propan-2-ol, acetonitrile, and methanol) and aqueous acidic solution (0.1% TFA, 5% acetic acid or 25 mM ammonium acetate) and the elution of the phenazines is made on a gradient mode (for more information see [48, 104, 105]). Detection is performed either by UV-Vis (DAD) or by MS. In the first case it is advisable to record the whole spectra in order to observe the multiple absorbances corresponding to PYO and to the rest of phenazines existing at acidic pH. Moreover, MS allows unequivocal identification of phenazines by combining retention time and recording the specific mass (i.e. PYO M + H+ m/z 211/212) [48, 106]. The detectability accomplished by UV-Vis is in the low μM range (i.e. 2 μM for PYO and PCA and about 20 μM for 1-OHphz), while for MS using single-ion-monitoring (SIM) detectability can be in the low nM. Nevertheless, in order to accomplish reliable measurements, samples usually have to be treated before by solvent extraction and eventually purified by reverse-phase solid-phase extraction (SPE), hindering the use of this approach for routine high throughput screening or clinical diagnostic applications.
Electrochemical systems
Due to its redox properties, PYO is able to accept and donate electrons, which allows its direct electrochemical detection. While many molecules can be reduced or oxidized, the majority of biologically relevant small molecules are oxidized at positive voltages vs. Ag/AgCl. PYO is unique on this respect since it is oxidized at a negative voltage (approximately −250 mV vs. Ag/AgCl reference). At these voltages, the only detected chemicals are mainly phenazines and folate. The detection of PYO in buffered solutions and supernatants from cultured P. aeruginosa has been accomplished using large carbon electrodes [107] and microfabricated electrochemical sensors [108, 109]. Moreover, last works on this field reported the development of very sensitive voltammetric and amperometric [110, 111] sensors reaching very low limits of detection (LOD) down to nM range. Thus, recently this approach has also been used to detect PYO in spiked biological samples, reaching detectability values in the low μM range [111, 112]. Furthermore, wound fluid exudate samples obtained from patients with chronic wounds have been directly evaluated for the presence of Pseudomonas by using a voltammetric sensor [113]. The electrochemical results were compared with 16S rRNA sequencing. This analysis yielded 9 correct matches, 3 false positives and 2 false negatives which translate on a sensitivity of 71% and specificity of 57% for Pseudomonas. Later on, the same group validated this electrochemical detection strategy by measuring PYO in 94 clinical strains belonging to different patient cohorts: hospital-acquired infection patients and patients suffering CF [114]. The results obtained showed that all isolates produced PYO and the concentrations measured correlated with patient symptoms and comorbidity. Besides that, Yang et al. [115] have developed an electrochemical system that allows measuring PYO in human fluids by using a redox reactivation module that amplifies the signal about 405 times reaching an extremely low LOD of 47 ± 1 pM. More recently, a paper-based electrochemical sensor has been described by Alatraktchi et al. [116] to detect PYO. This approach achieves an LOD of 95 nM when working with bacterial cultures, does not require a pretreatment of the sample and is a fast and cheap alternative given that is based on electrode printing on paper.
Thus, electrochemical methods show important advantages for the development of point of care (PoC) devices to be used for diagnosis purposes. This is due to the fact that they usually have low cost, they avoid time-consuming traditional plate culture procedures, showing usually a fast speed of operation. In addition, they do not require any sample pretreatment. Nevertheless, some constraints still need to be solved in terms of possible interferents [114] together with the inherent limitations of the electrochemical sensors by themselves which could be improved by using other electrode types (micro or nanofabricated electrodes) which would increase the costs. In this sense, Elkhawaga et al. [117] lately have described a PYO sensor that improves voltammetric output measures by modifying ITO electrodes with polyaniline/gold nanoparticles reaching a LOD in the nM range and presenting high selectivity for PYO traces when working with matrices containing different interferents such as uric acid and glucose.
Surface-enhanced Raman spectroscopy (SERS)
SERS-based techniques have been used to identify bacteria, examining the spectra of the cells [118], but detection of bacterial biomarkers emerges as a more specific approach. In this context, Wu et al. [119] reported SERS determination of PYO. To perform this assay, samples require a pretreatment that includes an extraction step with an organic solvent (i.e. chloroform) before being added on top of a clean (free of possible environment contaminants) silver nanorod substrate. This reported platform can detect PYO in the nM range and its performance has been evaluated in aqueous solutions, culture media and spiked clinical sputum samples. More recently, a combination of SERS imaging and principal component analysis (PCA) has come up as a robust method for in situ spatiotemporal mapping of PYO in heterogeneous biological systems containing P. aeruginosa communities. Using these techniques, Polisetti et al. [120] reported that the production of PYO depends both on the growth carbon source and on each strain of P. aeruginosa studied. Furthermore, a novel SERS platform for label-free, rapid and sensitive PYO detection has been fabricated based on the use of biodegradable gold coated zein films that further enhance Raman signal [121]. Implemented as a PoC device it allows PYO detection in drinking water in 30 min without the need of complex pretreatment. Spiked samples have been measured and the LOD calculated (25 μM). These results, offer the possibility of protecting the environment from nonbiodegradable materials together with the advantage that this technology offers as a lab-on-a chip sensor.
Immunoanalytical techniques
Antibodies for 1-OHphz have recently been reported (PCT/ES2014070161). 1-OHphz is the main metabolite of PYO and shows almost the same profile of activities. Polyclonal antibodies raised have been used to develop a microplate-based ELISA able to measure PYO with a LOD of 0.01 ± 0.01 nM in buffer. At present, the assay takes about 90 min and approximately 100 samples can be measured in parallel, using small sample volumes (100–200 μL). Prior to the assay, the sample is divided in two parts, one for direct 1-OHphz determination and the other for PYO quantification by previous conversion to 1-OHphz in just 20 min. As a result of this assay, both PYO and 1-OHphz levels can be quantified simultaneously in the same microplate [122].
Preliminary experiments performed with sputa samples from control patients not infected with P. aeruginosa pointed to the possibility of using this assay for the analysis of PYO and 1-OHphz in complex samples without the need of previous extraction or purification of the sample. Moreover, the detectability accomplished is below the reported levels of these phenazines in clinical samples (see above), for which reason apparently there would be no need of previous sample concentration. So far, this is the first immnochemical method already reported for the detection of PYO [122] that could represent an important improvement for the diagnosis of infectious diseases caused by P. aeruginosa.
Recently, the same group has been able to raise monoclonal antibodies showing very high affinites for both molecules 1-OHphz and PYO (unpublished results). This fact has allowed the setting up of an ELISA assay that quantifies PYO without the need of a previous transformation to 1-OHphz. Thus, by using both immunoreagents, poly and monoclonal PYO antibodies, both compounds have been already detected in bacterial isolates.
Therefore, these assays are quite fast (they take less than 90 min), reliable (they are very specific and robust), present very high sensitivity down to the low nM range, they show highthroughput capabilities and according previous preliminary experiments with different matrices, they should allow to measure different kinds of biological samples by performing simple treatments or not treatment at all. Moreover, their implementation as PoC devices should be also feasible.
Concluding remarks
P. aeruginosa is a multidrug resistant pathogen that causes disease in immune-compromised patients. Thus, is one of the most commonly isolated microorganisms in healthcare associated and community acquired infections. It affects different areas of the body such as: ears, skin, lungs, soft tissues and blood. Moreover, in CF, P. aeruginosa is the main respiratory pathogen establishing chronic infections and being the leading determinant of morbidity and mortality due to pulmonary complications. Thus, in this context, detection of P. aeruginosa at early colonization stages could help on prescribing more appropriate and specific antibiotic treatment while reducing the appearance of resistant strains and therefore avoiding chronic infection.
Eventhough extensive studies at basic and clinical level have been reported there is still a lack of fast and reliable diagnostic tools. Currently, the most commonly used methods for the detection of this bacterium include culture plate techniques which imply a relatively long time delay on confirming the infection. Besides that, quantitative PCR is gaining ground but requires expensive equipment, previous nucleic acid purification steps, highly trained personnel and detailed validation of the results for an accurate clinical interpretation.
The ability of P. aeruginosa to raise disease depends on the production of virulence factors. This fact points them as potential biomarkers of infection. Moreover, the concentration of these molecules is found in direct proportion to the bacterial load. In this context, PYO emerges as a very interesting target for developing new detection techniques. PYO is a specific virulence factor of P. aeruginosa and can also be considered as well an important QS signalling molecule [36, 123]. Thus, this pigment alters crucial cellular functions. For instance, it is involved in the process of lung destruction during chronic infections contributing to the accelerated decline in lung function in CF patients [69] and remains an important cause of serious wound infections and mortality in burn patients [113]. With this in mind, an important challenge arises on the field of developing new technologies for a rapid and reliable detection of novel biomarkers allowing an early infection diagnosis. The implementation of new methodologies such as nanotechnology and biosensing combined with specific immunoreagents could significantly improve the management of infectious diseases and this would translate on a better quality of life of the patient, faster initiation of the therapy and a more specific use of antibiotics avoiding resistance processes development. Lately in this field there have been noticeable advances but still these technologies have not been launched to the market due to the lack of international regulatory guidelines required to evaluate the safety of the different nanomaterials and their possible toxicity. Moreover, it still needs to be assessed if nanotechnology-based biosensors are reproducible, robust, reliable and low-cost enough to satisfy the current market requirements and to prove that they are better than the conventional technologies already used.
References
Jefferies JMC, Cooper T, Yam T, Clarke SC. Pseudomonas aeruginosa outbreaks in the neonatal intensive care unit – a systematic review of risk factors and environmental sources. J Med Microbiol. 2012;61(Pt 8):1052–61. https://doi.org/10.1099/jmm.0.044818-0.
Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonas aeruginosa: host defence in lung diseases. Respirology (Carlton, Vic). 2010;15(7):1037–56. https://doi.org/10.1111/j.1440-1843.2010.01819.x.
Olejnickova K, Hola V, Ruzicka F. Catheter-related infections caused by Pseudomonas aeruginosa: virulence factors involved and their relationships. Pathog Dis. 2014;72(2):87–94. https://doi.org/10.1111/2049-632X.12188.
Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183(3):444–52. https://doi.org/10.1086/318075.
Høiby N, Johansen HK, Moser C, Ciofu O. Clinical relevance of Pseudomonas aeruginosa: a master of adaptation and survival strategies. In Pseudomonas model organism, pathogen, cell factory. Weinheim: Wiley-VCH GmbH & Co.; 2008.
Mena A, Smith EE, Burns JL, Speert DP, Moskowitz SM, Perez JL, et al. Genetic adaptation of Pseudomonas aeruginosa to the Airways of Cystic Fibrosis Patients is Catalyzed by Hypermutation. J Bacteriol. 2008;190(24):7910–7. https://doi.org/10.1128/jb.01147-08.
Boles BR, Thoendel M, Singh PK. Genetic variation in biofilms and the insurance effects of diversity. Microbiology. 2005;151(9):2816–8. https://doi.org/10.1099/mic.0.28224-0.
Boles BR, Singh PK. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc Natl Acad Sci U S A. 2008;105(34):12503–8. https://doi.org/10.1073/pnas.0801499105.
Ciofu O, Mandsberg LF, Bjarnsholt T, Wassermann T, Hoiby N. Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants. Microbiology. 2010;156(Pt 4):1108–19. https://doi.org/10.1099/mic.0.033993-0.
Rada B, Leto TL. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013;21(2):73–81. https://doi.org/10.1016/j.tim.2012.10.004.
Lau GW, Hassett DJ, Britigan BE. Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol. 2005;13(8):389–97. https://doi.org/10.1016/j.tim.2005.05.011.
Faure E, Kwong K, Nguyen D. Pseudomonas aeruginosa in chronic lung infections: how to adapt within the host? Front Immunol. 2018;9:2416. https://doi.org/10.3389/fimmu.2018.02416.
Mudau M, Jacobson R, Minenza N, Kuonza L, Morris V, Engelbrecht H, et al. Outbreak of multi-drug resistant Pseudomonas aeruginosa bloodstream infection in the haematology unit of a south African academic hospital. PLoS One. 2013;8(3):e55985. https://doi.org/10.1371/journal.pone.0055985.
Muraki Y, Kitamura M, Maeda Y, Kitahara T, Mori T, Ikeue H, et al. Nationwide surveillance of antimicrobial consumption and resistance to Pseudomonas aeruginosa isolates at 203 Japanese hospitals in 2010. Infection. 2013;41(2):415–23. https://doi.org/10.1007/s15010-013-0440-0.
Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, et al. International nosocomial infection control consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control. 2012;40(5):396–407.
Xiao M, Wang Y, Yang QW, Fan X, Brown M, Kong F, et al. Antimicrobial susceptibility of Pseudomonas aeruginosa in China: A review of two multicentre surveillance programmes, and application of revised CLSI susceptibility breakpoints. Int J Antimicrob Agents. 2012;40(5):445–9. https://doi.org/10.1016/j.ijantimicag.2012.07.002.
Marra AR, Camargo LFA, Pignatari ACC, Sukiennik T, Behar PRP, Medeiros EAS, et al. Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. J Clin Microbiol. 2011;49(5):1866–71. https://doi.org/10.1128/JCM.00376-11.
Schwarzer C, Fischer H, Machen TE. Chemotaxis and binding of Pseudomonas aeruginosa to scratch-wounded human cystic fibrosis airway epithelial cells. PLoS One. 2016;11(3):e0150109. https://doi.org/10.1371/journal.pone.0150109.
Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, et al. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66(1):43–51.
Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol. 2004;30(5):627–34. https://doi.org/10.1165/rcmb.2003-0260OC.
Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci U S A. 1997;94(22):12088–93.
Kucharska I, Liang B, Ursini N, Tamm LK. Molecular interactions of lipopolysaccharide with an outer membrane protein from Pseudomonas aeruginosa probed by solution NMR. Biochemistry. 2016;55(36):5061–72. https://doi.org/10.1021/acs.biochem.6b00630.
Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol. 2007;297(5):277–95. https://doi.org/10.1016/j.ijmm.2007.03.012.
Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7(9):654–65. https://doi.org/10.1038/nrmicro2199.
Winstanley C, Fothergill JL. The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosa infections. FEMS Microbiol Lett. 2009;290(1):1–9. https://doi.org/10.1111/j.1574-6968.2008.01394.x.
Jakobsen TH, Bjarnsholt T, Jensen PO, Givskov M, Hoiby N. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors. Future Microbiol. 2013;8(7):901–21. https://doi.org/10.2217/fmb.13.57.
Williams P, Camara M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol. 2009;12(2):182–91. https://doi.org/10.1016/j.mib.2009.01.005.
Middleton B, Rodgers HC, Camara M, Knox AJ, Williams P, Hardman A. Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol Lett. 2002;207(1):1–7. https://doi.org/10.1111/j.1574-6968.2002.tb11019.x.
Collier DN, Anderson L, McKnight SL, Noah TL, Knowles M, Boucher R, et al. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol Lett. 2002;215(1):41–6. https://doi.org/10.1111/j.1574-6968.2002.tb11367.x.
Morlon-Guyot J, Mere J, Bonhoure A, Beaumelle B. Processing of Pseudomonas aeruginosa exotoxin A is dispensable for cell intoxication. Infect Immun. 2009;77(7):3090–9. https://doi.org/10.1128/iai.01390-08.
Jaffar-Bandjee MC, Lazdunski A, Bally M, Carrere J, Chazalette JP, Galabert C. Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa. J Clin Microbiol. 1995;33(4):924–9.
Hall S, McDermott C, Anoopkumar-Dukie S, McFarland AJ, Forbes A, Perkins AV et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins (Basel). 2016;8(8). doi:https://doi.org/10.3390/toxins8080236.
Bell SC, De Boeck K, Amaral MD. New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. Pharmacol Ther. 2015;145:19–34. https://doi.org/10.1016/j.pharmthera.2014.06.005.
Mossine VV, Waters JK, Chance DL, Mawhinney TP. Transient Proteotoxicity of bacterial virulence factor Pyocyanin in renal tubular epithelial cells induces ER-related Vacuolation and can be efficiently modulated by Iron chelators. Toxicol Sci. 2016;154(2):403–15. https://doi.org/10.1093/toxsci/kfw174.
Muller M, Li Z, Maitz PK. Pseudomonas pyocyanin inhibits wound repair by inducing premature cellular senescence: role for p38 mitogen-activated protein kinase. Burns. 2009;35(4):500–8. https://doi.org/10.1016/j.burns.2008.11.010.
Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10(12):599–606. https://doi.org/10.1016/j.molmed.2004.10.002.
Costa KC, Bergkessel M, Saunders S, Korlach J, Newman DK. Enzymatic degradation of Phenazines can generate energy and protect sensitive organisms from toxicity. mBio. 2015;6(6):e01520–15. https://doi.org/10.1128/mBio.01520-15.
Wu HJ, Wang AH, Jennings MP. Discovery of virulence factors of pathogenic bacteria. Curr Opin Chem Biol. 2008;12(1):93–101. https://doi.org/10.1016/j.cbpa.2008.01.023.
Mavrodi DV, Blankenfeldt W, Thomashow LS. Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation. Annu. Rev. Phytopathol. 2006;44:417–45. https://doi.org/10.1146/annurev.phyto.44.013106.145710.
Ramos I, Dietrich LE, Price-Whelan A, Newman DK. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res Microbiol. 2010;161(3):187–91. https://doi.org/10.1016/j.resmic.2010.01.003.
Du X, Li Y, Zhou Q, Xu Y. Regulation of gene expression in Pseudomonas aeruginosa M18 by phenazine-1-carboxylic acid. Appl Microbiol Biotechnol. 2015;99(2):813–25. https://doi.org/10.1007/s00253-014-6101-0.
Dietrich LE, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321(5893):1203–6. https://doi.org/10.1126/science.1160619.
Dowling DN, O'Gara F. Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends Biotechnol. 1994;12(4):133–41. https://doi.org/10.1016/0167-7799(94)90091-4.
Laursen JB, Nielsen J. Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem Rev. 2004;104(3):1663–86. https://doi.org/10.1021/cr020473j.
Pierson LS III, Pierson EA. Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol. 2010;86(6):1659–70. https://doi.org/10.1007/s00253-010-2509-3.
Fordos M. Recherches sur les matieres colorantes des suppurations bleues, pyocyanine et pyoxanthose. CR Hebd Seances Acad Sci. 1863;56:1128–31.
Reyes EA, Bale MJ, Cannon WH, Matsen JM. Identification of Pseudomonas aeruginosa by pyocyanin production on tech agar. J Clin Microbiol. 1981;13(3):456–8.
Wilson R, Sykes DA, Watson D, Rutman A, Taylor GW, Cole PJ. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988;56(9):2515–7.
Penesyan A, Kumar SS, Kamath K, Shathili AM, Venkatakrishnan V, Krisp C, et al. Genetically and phenotypically distinct Pseudomonas aeruginosa cystic fibrosis isolates share a Core proteomic signature. PLoS One. 2015;10(10):e0138527. https://doi.org/10.1371/journal.pone.0138527.
Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol. 1999;104(1):72–8.
Moura-Alves P, Fae K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512(7515):387–92. https://doi.org/10.1038/nature13684.
Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61(5):1308–21. https://doi.org/10.1111/j.1365-2958.2006.05306.x.
Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, et al. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol. 2002;168(4):1861–8. https://doi.org/10.4049/jimmunol.168.4.1861.
Allen L, Dockrell DH, Pattery T, Lee DG, Cornelis P, Hellewell PG, et al. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J Immunol. 2005;174(6):3643–9. https://doi.org/10.4049/jimmunol.174.6.3643.
Rada B, Leto TL. Redox warfare between airway epithelial cells and Pseudomonas: dual oxidase versus pyocyanin. Immunol Res. 2009;43(1–3):198–209. https://doi.org/10.1007/s12026-008-8071-8.
Liu GY, Nizet V. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 2009;17(9):406–13. https://doi.org/10.1016/j.tim.2009.06.006.
Kanthakumar K, Taylor GW, Cundell DR, Dowling RB, Johnson M, Cole PJ, et al. The effect of bacterial toxins on levels of intracellular adenosine nucleotides and human ciliary beat frequency. Pulm Pharmacol. 1996;9(4):223–30. https://doi.org/10.1006/pulp.1996.0028.
Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect Immun. 2004;72(7):4275–8. https://doi.org/10.1128/IAI.72.7.4275-4278.2004.
Shellito J, Nelson S, Sorensen RU. Effect of pyocyanine, a pigment of Pseudomonas aeruginosa, on production of reactive nitrogen intermediates by murine alveolar macrophages. Infect Immun. 1992;60(9):3913–5.
Kamath JM, Britigan BE, Cox CD, Shasby DM. Pyocyanin from Pseudomonas aeruginosa inhibits prostacyclin release from endothelial cells. Infect Immun. 1995;63(12):4921–3.
Muller M, Sorrell TC. Leukotriene B4 omega-oxidation by human polymorphonuclear leukocytes is inhibited by pyocyanin, a phenazine derivative produced by Pseudomonas aeruginosa. Infect Immun. 1992;60(6):2536–40.
Muller M, Sztelma K, Sorrell TC. Inhibition of platelet eicosanoid metabolism by the bacterial phenazine derivative pyocyanin. Ann N Y Acad Sci. 1994;744:320–2. https://doi.org/10.1111/j.1749-6632.1994.tb52752.x.
Muller M, Sorrell TC. Modulation of neutrophil superoxide response and intracellular diacylglyceride levels by the bacterial pigment pyocyanin. Infect Immun. 1997;65(6):2483–7.
Britigan BE, Railsback MA, Cox CD. The Pseudomonas aeruginosa secretory product pyocyanin inactivates alpha1 protease inhibitor: implications for the pathogenesis of cystic fibrosis lung disease. Infect Immun. 1999;67(3):1207–12.
Denning GM, Railsback MA, Rasmussen GT, Cox CD, Britigan BE. Pseudomonas pyocyanine alters calcium signaling in human airway epithelial cells. Am J Phys. 1998;274(6 Pt 1):L893–900. https://doi.org/10.1152/ajplung.1998.274.6.L893.
Denning GM, Wollenweber LA, Railsback MA, Cox CD, Stoll LL, Britigan BE. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect Immun. 1998;66(12):5777–84.
Denning GM, Iyer SS, Reszka KJ, O'Malley Y, Rasmussen GT, Britigan BE. Phenazine-1-carboxylic acid, a secondary metabolite of Pseudomonas aeruginosa, alters expression of immunomodulatory proteins by human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L584–92. https://doi.org/10.1152/ajplung.00086.2003.
Look DC, Stoll LL, Romig SA, Humlicek A, Britigan BE, Denning GM. Pyocyanin and its precursor phenazine-1-carboxylic acid increase IL-8 and intercellular adhesion molecule-1 expression in human airway epithelial cells by oxidant-dependent mechanisms. J Immunol. 2005;175(6):4017–23. https://doi.org/10.4049/jimmunol.175.6.4017.
Caldwell CC, Chen Y, Goetzmann HS, Hao Y, Borchers MT, Hassett DJ, et al. Pseudomonas aeruginosa exotoxin Pyocyanin causes cystic fibrosis airway pathogenesis. Am J Pathol. 2009;175(6):2473–88. https://doi.org/10.2353/ajpath.2009.090166.
Mavrodi DV, Parejko JA, Mavrodi OV, Kwak YS, Weller DM, Blankenfeldt W, et al. Recent insights into the diversity, frequency and ecological roles of phenazines in fluorescent Pseudomonas spp. Environ Microbiol. 2013;15(3):675–86. https://doi.org/10.1111/j.1462-2920.2012.02846.x.
Haas D, Defago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3(4):307–19. https://doi.org/10.1038/nrmicro1129.
Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, Newman DK. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol. 2012;47(6):738–45. https://doi.org/10.1165/rcmb.2012-0088OC.
Cui Q, Lv H, Qi Z, Jiang B, Xiao B, Liu L, et al. Cross-regulation between the phz1 and phz2 operons maintain a balanced level of Phenazine biosynthesis in Pseudomonas aeruginosa PAO1. PLoS One. 2016;11(1):e0144447. https://doi.org/10.1371/journal.pone.0144447.
Mavrodi DV, Peever TL, Mavrodi OV, Parejko JA, Raaijmakers JM, Lemanceau P, et al. Diversity and evolution of the phenazine biosynthesis pathway. Appl Environ Microbiol. 2010;76(3):866–79. https://doi.org/10.1128/aem.02009-09.
Sakhtah HP-WA, Dietrich LEP. Regulation of Phenazyne biosynthesis. In: Chincholkar SaT L, editor. Microbial Phenazines. Heidelberg: Springer-Verlag; 2013. p. 19–42.
Dubern JF, Diggle SP. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol BioSyst. 2008;4(9):882–8. https://doi.org/10.1039/b803796p.
Subramaniyan S, Divyasree S, Sandhia GS. Phytochemicals as effective quorum quenchers against bacterial communication. Recent Pat Biotechnol. 2016;10(2):153–66.
D'Argenio DA, Wu M, Hoffman LR, Kulasekara HD, Deziel E, Smith EE, et al. Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol Microbiol. 2007;64(2):512–33. https://doi.org/10.1111/j.1365-2958.2007.05678.x.
Hoffman LR, Kulasekara HD, Emerson J, Houston LS, Burns JL, Ramsey BW, et al. Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J Cyst Fibros. 2009;8(1):66–70. https://doi.org/10.1016/j.jcf.2008.09.006.
Fothergill J, Panagea S, Hart C, Walshaw M, Pitt T, Winstanley C. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol. 2007;7(1):45. https://doi.org/10.1186/1471-2180-7-45.
Firoved AM, Boucher JC, Deretic V. Global genomic analysis of AlgU (sigma(E))-dependent promoters (sigmulon) in Pseudomonas aeruginosa and implications for inflammatory processes in cystic fibrosis. J Bacteriol. 2002;184(4):1057–64. https://doi.org/10.1128/jb.184.4.1057-1064.2002.
Ryall B, Carrara M, Zlosnik JE, Behrends V, Lee X, Wong Z, et al. The mucoid switch in Pseudomonas aeruginosa represses quorum sensing systems and leads to complex changes to stationary phase virulence factor regulation. PLoS One. 2014;9(5):e96166. https://doi.org/10.1371/journal.pone.0096166.
Damron FH, Barbier M, McKenney ES, Schurr MJ, Goldberg JB. Genes required for and effects of alginate overproduction induced by growth of Pseudomonas aeruginosa on Pseudomonas isolation agar supplemented with ammonium metavanadate. J Bacteriol. 2013;195(18):4020–36. https://doi.org/10.1128/JB.00534-13.
Mowat E, Paterson S, Fothergill JL, Wright EA, Ledson MJ, Walshaw MJ, et al. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am J Resp Crit Care. 2011;183(12):1674–9. https://doi.org/10.1164/rccm.201009-1430OC.
Bohn YS, Brandes G, Rakhimova E, Horatzek S, Salunkhe P, Munder A, et al. Multiple roles of Pseudomonas aeruginosa TBCF10839 PilY1 in motility, transport and infection. Mol Microbiol. 2009;71(3):730–47. https://doi.org/10.1111/j.1365-2958.2008.06559.x.
Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A. 2006;103(22):8487–92. https://doi.org/10.1073/pnas.0602138103.
Cabeen MT. Stationary phase-specific virulence factor overproduction by a lasR mutant of Pseudomonas aeruginosa. PLoS One. 2014;9(2):e88743. https://doi.org/10.1371/journal.pone.0088743.
Aaron SD, Kottachchi D, Ferris WJ, Vandemheen KL, St Denis ML, Plouffe A, et al. Sputum versus bronchoscopy for diagnosis of Pseudomonas aeruginosa biofilms in cystic fibrosis. Eur Respir J. 2004;24(4):631–7. https://doi.org/10.1183/09031936.04.00049104.
Goss CH, Burns JL. Exacerbations in cystic fibrosis · 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–7. https://doi.org/10.1136/thx.2006.060889.
Suzuki S, Mano Y, Fujitani K, Furuya N. Effects of long- term, low-dose macrolide treatment on Pseudomonas aeruginosa PAO1 virulence factors in vitro. Arch Clin Microbiol. 2017;8(4):50. https://doi.org/10.4172/1989-8436.100050.
El-Fouly MZ, Sharaf AM, Shahin AAM, El-Bialy HA, Omara AMA. Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J Radiat Res Appl Sci. 2015;8(1):36–48. https://doi.org/10.1016/j.jrras.2014.10.007.
Silva LV, Galdino AC, Nunes AP, Dos Santos KR, Moreira BM, Cacci LC, et al. Virulence attributes in Brazilian clinical isolates of Pseudomonas aeruginosa. Int J Med Microbiol. 2014;304:990–1000. https://doi.org/10.1016/j.ijmm.2014.07.001.
Tielen P, Narten M, Rosin N, Biegler I, Haddad I, Hogardt M, et al. Genotypic and phenotypic characterization of Pseudomonas aeruginosa isolates from urinary tract infections. Int J Med Microbiol. 2011;301(4):282–92. https://doi.org/10.1016/j.ijmm.2010.10.005.
Cigana C, Melotti P, Baldan R, Pedretti E, Pintani E, Iansa P, et al. Genotypic and phenotypic relatedness of Pseudomonas aeruginosa isolates among the major cystic fibrosis patient cohort in Italy. BMC Microbiol. 2016;16(1):142. https://doi.org/10.1186/s12866-016-0760-1.
Khadim MM, Al Marjani MF. Pyocyanin and biofilm formation in Pseudomonas aeruginosa isolated from burn infections in Baghdad. Iraq Jordan J Biol Sci. 2019;12:31–5.
Wilson R, Pitt T, Taylor G, Watson D, MacDermot J, Sykes D, et al. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987;79(1):221–9. https://doi.org/10.1172/jci112787.
Reimer Å. Concentrations of the Pseudomonas aeruginosa toxin Pyocyanin in human ear secretions. Acta Otolaryngol. 2000;120(543):86–8. https://doi.org/10.1080/000164800454062.
Retraction: Phenazine Content in the Cystic Fibrosis Respiratory Tract Negatively Correlates with Lung Function and Microbial Complexity. Am. J. Respir. Cell Mol Biol 2019;60(1):134-. doi:https://doi.org/10.1165/rcmb.601retraction.
Reszka KJ, O'Malley Y, McCormick ML, Denning GM, Britigan BE. Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide. Free Radical Bio Med. 2004;36(11):1448–59. https://doi.org/10.1016/j.freeradbiomed.2004.03.011.
Cheluvappa R. Standardized chemical synthesis of Pseudomonas aeruginosa pyocyanin. MethodsX. 2014;1:67–73. https://doi.org/10.1016/j.mex.2014.07.001.
El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shaaban MI. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog. 2014;74(0):25–32. https://doi.org/10.1016/j.micpath.2014.07.008.
Essar DW, Eberly L, Hadero A, Crawford IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1991;172(2):884–900. https://doi.org/10.1128/jb.172.2.884-900.1990.
Kurachi M. Studies on the biosynthesis of Pyocyanine (II): isolation and determination of Pyocyanine. Bull Inst Chem Res. 1958;36(6):174–87.
Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, Newman DK. Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J Bacteriol. 2011;193(14):3606–17. https://doi.org/10.1128/jb.00396-11.
Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183(21):6454–65. https://doi.org/10.1128/jb.183.21.6454-6465.2001.
Watson D, MacDermot J, Wilson R, Cole PJ, Taylor GW. Purification and structural analysis of pyocyanin and 1-hydroxyphenazine. Eur J Biochem. 1986;159(2):309–13.
Sharp D, Gladstone P, Smith RB, Forsythe S, Davis J. Approaching intelligent infection diagnostics: carbon fibre sensor for electrochemical pyocyanin detection. Bioelectrochemistry. 2010;77(2):114–9. https://doi.org/10.1016/j.bioelechem.2009.07.008.
Webster TA, Sismaet HJ, Goluch ED. Amperometric detection of pyocyanin in nanofluidic channels. Nano LIFE. 2013;03(01):1340011. https://doi.org/10.1142/S1793984413400114.
Webster TA, Goluch ED. Electrochemical detection of pyocyanin in nanochannels with integrated palladium hydride reference electrodes. Lab Chip. 2012;12(24):5195–201. https://doi.org/10.1039/C2LC40650K.
Alatraktchi FA, Johansen HK, Molin S, Svendsen WE. Electrochemical sensing of biomarker for diagnostics of bacteria-specific infections. Nanomedicine (Lond). 2016;11(16):2185–95. https://doi.org/10.2217/nnm-2016-0155.
Alatraktchi FA, Andersen SB, Johansen HK, Molin S, Svendsen WE. Fast Selective Detection of Pyocyanin Using Cyclic Voltammetry. Sensors (Basel). 2016;16(3). doi:https://doi.org/10.3390/s16030408.
Webster TA, Sismaet HJ, Conte JL, Chan IJ, Goluch ED. Electrochemical detection of Pseudomonas aeruginosa in human fluid samples via pyocyanin. Biosens Bioelectron. 2014;60(0):265–70. https://doi.org/10.1016/j.bios.2014.04.028.
Sismaet HJ, Banerjee A, McNish S, Choi Y, Torralba M, Lucas S, et al. Electrochemical detection of Pseudomonas in wound exudate samples from patients with chronic wounds. Wound Repair Regen. 2016;24(2):366–72. https://doi.org/10.1111/wrr.12414.
Sismaet HJ, Pinto AJ, Goluch ED. Electrochemical sensors for identifying pyocyanin production in clinical Pseudomonas aeruginosa isolates. Biosens Bioelectron. 2017;97:65–9. https://doi.org/10.1016/j.bios.2017.05.042.
Yang Y, Yu YY, Wang YZ, Zhang CL, Wang JX, Fang Z, et al. Amplification of electrochemical signal by a whole-cell redox reactivation module for ultrasensitive detection of pyocyanin. Biosens Bioelectron. 2017;98:338–44. https://doi.org/10.1016/j.bios.2017.07.008.
Alatraktchi FA, Noori JS, Tanev GP, Mortensen J, Dimaki M, Johansen HK, et al. Paper-based sensors for rapid detection of virulence factor produced by Pseudomonas aeruginosa. PLoS One. 2018;13(3):e0194157. https://doi.org/10.1371/journal.pone.0194157.
Elkhawaga AA, Khalifa MM, El-Badawy O, Hassan MA, El-Said WA. Rapid and highly sensitive detection of pyocyanin biomarker in different Pseudomonas aeruginosa infections using gold nanoparticles modified sensor. PLoS One. 2019;14(7):e0216438. https://doi.org/10.1371/journal.pone.0216438.
Rusciano G, Capriglione P, Pesce G, Abete P, Carnovale V, Sasso A. Raman spectroscopy as a new tool for early detection of bacteria in patients with cystic fibrosis. Laser Phys Lett. 2013;10(7):075603. https://doi.org/10.1088/1612-2011/10/7/075603.
Wu X, Chen J, Li X, Zhao Y, Zughaier SM. Culture-free diagnostics of Pseudomonas aeruginosa infection by silver nanorod array based SERS from clinical sputum samples. Nanomedicine. 2014. https://doi.org/10.1016/j.nano.2014.04.010.
Polisetti S, Baig NF, Morales-Soto N, Shrout JD, Bohn PW. Spatial mapping of Pyocyanin in Pseudomonas aeruginosa bacterial communities using surface enhanced Raman scattering. Appl Spectrosc. 2017;71(2):215–23. https://doi.org/10.1177/0003702816654167.
Jia F, Barber E, Turasan H, Seo S, Dai R, Liu L, et al. Detection of Pyocyanin using a new biodegradable SERS biosensor fabricated using gold coated Zein nanostructures further decorated with gold nanoparticles. J Agric Food Chem. 2019;67(16):4603–10. https://doi.org/10.1021/acs.jafc.8b07317.
Pastells C, Pascual N, Sanchez-Baeza F, Marco MP. Immunochemical determination of Pyocyanin and 1-Hydroxyphenazine as potential biomarkers of Pseudomonas aeruginosa infections. Anal Chem. 2016;88(3):1631–8. https://doi.org/10.1021/acs.analchem.5b03490.
Karatuna O, Yagci A. Analysis of quorum sensing-dependent virulence factor production and its relationship with antimicrobial susceptibility in Pseudomonas aeruginosa respiratory isolates. Clin Microbiol Infect. 2010;16(12):1770–5. https://doi.org/10.1111/j.1469-0691.2010.03177.x.
Acknowledgements
This work has been funded by the Spanish government through the project QS4CF (RTI2018-096278-B-C21), by Fundació La Marató de TV3 (201825-30-31) and by the program Marie Sklodowska Research Fellowship through the project New Diagnostics for Infectious Diseases (ND4ID, 675412-ND4ID-H2020-MSCA-ITN-2015). The Nb4D group (formerly Applied Molecular Receptors group, AMRg) is a consolidated research group (Grup de Recerca) of the Generalitat de Catalunya and has support from the Departament d’Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya (expedient: 2014 SGR 1484). CIBER-BBN is an initiative funded by the Spanish National Plan for Scientific and Technical Research and Innovation 2013-2016, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions are financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund. The ICTS “NANBIOSIS”, and particularly the Custom Antibody Service (CAbS, IQAC-CSIC, CIBER-BBN), is acknowledged for the assistance and support related to some immunoreagents mentioned in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Female Role Models in Analytical Chemistry.
Rights and permissions
About this article
Cite this article
Vilaplana, L., Marco, MP. Phenazines as potential biomarkers of Pseudomonas aeruginosa infections: synthesis regulation, pathogenesis and analytical methods for their detection. Anal Bioanal Chem 412, 5897–5912 (2020). https://doi.org/10.1007/s00216-020-02696-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02696-4