Abstract
A new electrochemical sensor, based on NdFeO3 nanoparticles as electrocatalytic material, was proposed here for the detection of dopamine (DA). NdFeO3 nanoparticles were first synthesized by a simple thermal treatment method and subsequent annealing at high temperature (700 °C). The prepared electrocatalytic material has been characterized in detail by SEM-EDX, XRD, and Raman techniques. Characterization results display its sheet-like morphology, constituted by a porous network of very small orthorhombic NdFeO3 nanoparticles. NdFeO3 electrocatalytic material was then used to modify the working electrode of screen-printed carbon electrodes (SPCEs). Electrochemical tests demonstrated that NdFeO3– modified screen-printed carbon electrode (NdFeO3/SPCE) exhibited a remarkable enhancement of the dopamine electrooxidation, compared to the bare SPCE one. The analytical performance of the developed sensor has been evaluated for the detection of this analyte by means of the square-wave voltammetry (SWV) technique. The modified electrode showed two linear concentration ranges, from 0.5 to 100 μM and 150 to 400 μM, respectively, a limit of detection (LOD) of 0.27 μM (at S/N = 3), and good reproducibility, stability, and selectivity. Additionally, we also report an attempt made to propose the modified sensor for the simultaneous detection of dopamine and uric acid (UA). The procedure was also applied for the determination of dopamine in spiked real samples. So, this paper reports for the first time the use of a modified NdFeO3 screen-printed electrode for developing an electrochemical sensor for the quantification of important biomolecules.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA) is a neurotransmitter, i.e., a complex chemical messenger that coordinates communication between neurons, which in turn affect every cell, tissue, and system in the body and that is associated with feelings of pleasure and satisfaction [1]. It is also associated with addiction, movement, motivation, and natural behaviors, such as with eating and sex, or unnatural, as with drug addiction. Knowing neurotransmitter levels can help to correct and/or prevent problems from occurring in the future. An imbalance in DA levels in the body will have complications [2,3,4]. For example, dramatically elevated levels, the so-called dopamine storm, can be associated with delusions, agitation, mania, and frank psychosis. Low DA levels can drive us to use drugs (self medicate), alcohol, smoke cigarettes, gamble, and/or overeat.
In the past years, different analytical methods for the detection of DA were used, such as chromatography [5], electrochemiluminescence [6], and fluorometry [7]. Expensive and intricate instruments and time consuming are major weaknesses of the mentioned methods; then, introducing an inexpensive and easy method for DA determination on small-size devices is very valuable. Among these latter, electrochemical techniques have unique features and can give, in addition to specifications mentioned, fast response with high accuracy and sensitivity [8]. Recently in order to increase the sensitivity and selectivity of electrochemical sensors, researchers are using novel electrocatalytic materials at the nanometer scale such as metal, metal chalcogenide, metal oxide, or their composites as electrode modifiers [9,10,11]. These nanostructures are today widely investigated to replace enzymes as electroactive tags and catalytic centers, providing large surface area, high conductivity, and electrocatalytic activity, which results in the development of more robust and low-cost devices with higher thermal and chemical stability [12].
In recent years, perovskite materials possessing an ABO3 structure have been considered for their catalytic and electrocatalytic properties [13,14,15]. NdFeO3 is an important compound of this class of materials, with applications in gas sensing and spin memory devices [16,17,18,19,20]. We guess that the redox couple FeIII /FeIV could be an important factor in favoring electrocatalytic properties; nevertheless, pure NdFeO3 has not been so far reported for electrochemical DA sensing application. To our knowledge, only one paper described the use of NdFeO3 for determination of metoclopramide. However, the sensing layer is a composite with NdFeO3 nanoperovskite and 2-chloro-1,3-dimethyl-imidazolinium hexafluorophosphate ionic liquid crystal in the presence of sodium dodecyl sulfate [21]. In this research, a new method for synthesis NdFeO3 nanocrystals, i. e., the thermal treatment method, was used. This preparation method offers the advantages of simplicity, low cost, and low reaction temperatures; in addition, it is environment-friendly as it produces no by-product effluents with a periodic time of 48 h (from preparation until calcination) [22, 23]. NdFeO3 nanoparticles produced after annealing at high temperature (700 °C) were used to modify screen-printed carbon electrode (SPCE) in order to detect dopamine. Here, we used a commercial SPCE electrochemical platform, which is more suitable to develop sensors for practical and in field use, than the conventional glassy carbon electrode-based platform.
The electrochemical and analytical performances of the developed modified NdFeO3 electrode have been largely investigated. Notwithstanding the growing role of rare earth perovskite in various sectors of electrochemistry and in the fabrication of electrochemical sensors, this is the first report on the development of a modified NdFeO3 screen-printed electrode for electroanalytical applications. Results of this study demonstrated its good sensitivity, reproducibility, stability, and selectivity for detection of dopamine. Further, the modified electrode exhibited negligible interference from uric acid (UA), so in addition, here, we report the attempt done for the simultaneous detection of dopamine and uric acid.
Experimental
Synthesis of NdFeO3
NdFeO3 nanoparticles have been synthesized by a thermal treatment method. Metal nitrates were used as precursors, polyvinyl pyrrolidone (PVP) was used as the capping agent, and deionized water was used as the solvent. Iron nitrate, Fe(NO3)3 × 9H2O, and neodymium nitrate, Nd(NO3)3 × 6H2O, were purchased from Acros Organics with purities exceeding 99%. An aqueous solution of PVP was prepared by dissolving the polymer in 100 ml of deionized water at 90 °C; then, 0.1 mmol of iron nitrate and 0.1 mmol of neodymium nitrate (Fe:Nd = 1:1) were placed in the polymer solution, which was stirred for 2 h using a magnetic stirrer. At the end of the 2-h period, the solution was poured into a glass Petri dish and heated in an oven at 100 °C for 24 h to evaporate the water. The solid product formed was crushed, ground in a mortar to form a soft powder, and, finally, annealed at 700 °C (in air for 3 h) to decompose organic compounds.
Characterization
The morphology of NdFeO3 sample was investigated using a Zeiss 1540XB FE SEM (Zeiss, Germany) instrument operating at 10 kV. The crystalline structure of synthesized materials was investigated using X-ray powder diffraction (XRD) by means of a Bruker D8 Advance A 25 X-ray diffractometer operating at 40 kV and in the range 20–80° (2-theta), with an increasing rate of 0.01°/s. The average crystallite size, d, has been calculated using the Scherrer’s formula:
where λ = 1.5418 Å is the X-ray wavelength, θB is the maximum of the Bragg diffraction peak (in radians), and B is the full width at half maximum (FWHM) of the XRD peak.
Raman spectra were carried out, in air at room temperature, using a Horiba XploRA spectrometer. Spectra were excited using the 532-nm line from a solid-state laser and integrated for 10 s, using a × 50 long working distance microscope objective.
Fabrication of the NdFeO3-modified screen-printed electrode
For the preparation of the modified sensor, 10 mg of NdFeO3 nanoparticles was dispersed in 1 mL of double-distilled water and then sonicated until a homogenous suspension was obtained. The commercial screen-printed carbon electrodes (SPCEs), purchased from DropSens, Llanera (Asturias), Spain, consist of a planar substrate equipped with a 4-mm-diameter (0.125 cm2) carbon working electrode, a carbon counter electrode, and a silver pseudo-reference electrode. The SPCE electrode was modified using a wet impregnation method, dropping 5 μL of the above prepared suspension on the working electrode surface. Finally, the modified NdFeO3/SPCE sensor was dried at room temperature and stored in ambient conditions until use.
Electrochemical tests
Electrochemical experiments were performed with a DropSens μStat 400 potentiostat/galvanostat. The electrochemical behavior of the prepared sensor was investigated by cyclic voltammetry (CV) in 0.1 M phosphate buffered saline (PBS) solution at pH 7, in the presence and absence of dopamine (DA), in the − 0.2 to 0.5-V potential region, and at a scan rate between 10 and 70 mV s−1. Amperometric measurements, recording the current at a fixed optimal potential during successive additions of DA, and square-wave voltammetry (SWV) were also carried out to assess the viability of these techniques for performing the quantitative determination of dopamine. The same tests were also carried out with uric acid as analyte and with DA+ UA mixtures for exploiting the simultaneous determination of both analytes.
The statistical analysis and curve fitting were performed using Origin software (Northampton, MA, USA). Reproducibility of the data was confirmed by repeating the experiments at least three times. Analyses of the same concentration in a single run and in three separate runs three times were achieved to examine the intra-day and inter-day precisions, respectively. The limit of detection (LOD) was obtained from S/N = 3 and confirmed by the formula: LOD = 3Sb/q where Sb is the standard deviation of the blank solution and q is the slope of the calibration plot [24, 25].
Results and discussion
NdFeO3 synthesis and characterization
NdFeO3 nanoparticles were synthesized by the thermal treatment method. The chosen method is simpler, cheaper, and can operate at lower reaction temperatures compared to more conventional synthesis methods [21, 26]. The morphological and microstructural characteristics of synthesized NdFeO3 after annealing at 700 °C are reported below. Figure 1 shows SEM images of the NdFeO3 sample taken at different magnifications.
On a large scale, the sample appears constituted by very small nanoparticles having a spherical-like shape and a narrow size distribution, assembled to form wide sheets. At a higher magnification, we can clearly observe the fine grains of the sample and its highly porous structure. These findings also demonstrate that the synthesized NdFeO3 display high stability against thermal grain growth and coalescence.
The microstructure of the prepared electrocatalytic material has been investigated in detail by XRD and micro-Raman techniques. The XRD pattern of the NdFeO3 sample is shown in Fig. 2a. The pattern shows the reflection planes (101), (111), (121), (220), (022), (042), (242), and (430), which confirm the presence of a single-phase NdFeO3 with a pure orthorhombic phase (JCPDs reference code: 00025-1149). The average crystallite size, computed by using the Scherrer’s formula, was found to be 28 nm, in fair agreement with SEM data and suggesting that particles obtained are nanocrystals.
The micro-Raman spectrum (see Fig. 2b) bands during 100–200 cm−1 can be attributed to the stretching modes of the Nd–O bond. Peaks between 200 and 400 cm−1 are related to bending and stretching modes of FeO6 octahedral, whereas the band centered at 465 cm−1 is due to the bending mode of the O–Fe–O bond, which confirm the perovskite structure of the synthesized material.
Electrochemical tests on NdFeO3/SPCE
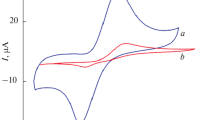
First, the electrochemical behavior (see Fig. 3) of the bare SPCE (curve a) and NdFeO3 modified SPCE electrode (curve b) in PBS (pH 7.0) and in the absence of dopamine is compared. In the investigated potential range, no peak was noted on both electrodes. However, on the modified electrode, the cycle shows a large hysteresis, which is likely related to the elevated surface area of the highly porous working electrode.
Figure 3 shows also CV cycles obtained in the presence of dopamine (50 μM DA in pH 7.0 PBS) at the bare SPCE (curve c) and NdFeO3/SPCE electrode (curve d). The modified electrode shows a negative shift of oxidation peak potential, from 0.14 to 0.06 V with respect to the bare electrode. In addition, the anodic peak corresponding to DA oxidation exhibits an improved peak current. CV results demonstrate clearly that NdFeO3 nanoparticles behave as a good electrocatalyst for dopamine electrooxidation. Further, as it is well known that the catalytic activity is influenced by the surface roughness of the electrode catalytic surface [27], we can suppose that the nanoscale surface features introduced by NdFeO3 provide to increase the effective surface area of modified NdFeO3 working electrode and consequently the electrocatalytic activity. It can be also hypothesized that NdFeO3 nanoparticles can act as essential center sites for the electrostatic interaction of dopamine with the working electrode. This factor favors the diffusion of DA to the electrode and promote the electron transfer pathway between DA and electrode due to a specific adsorption orientation of DA molecules [28].
The effect of scan rate (υ) on the redox peak currents of 50 μM DA at the NdFeO3/SPCE were recorded at different scan rates in the range of 0.01–0.07 V s−1 (Fig. 4a, b). The redox peak current linear relationship with the scan rate indicates that the electrochemical reaction surface is controlled by the adsorption phenomenon. The linear regression equations were deduced as ipa = 74.8 υ (V s−1) + 0.57, R2 = 0.996 and ipc = − 66.1 υ (V s−1) + 0.45, R2 = 0.998, where R2 is the determination coefficient.
Quantification of dopamine with NdFeO3/SPCE
To evaluate the efficiency of the developed electrode toward detection of dopamine in aqueous solutions, the influence of the concentration of dopamine at the NdFeO3 NPs modified sensor in 0.1 M of phosphate buffer solution, using the square-wave voltammetry technique, is reported in Fig. 5. Specifically, Fig. 5a shows that SWV responses of DA and anodic peak current increased over a wide concentration range from 0.5 to 400 μM. The calibration curve for DA, constructed by plotting the current (μA) vs. concentrations of DA (μM), is shown in Fig. 5b. Linear regression analysis was performed to calculate the slope and intercept using the calibration curve data. The proposed sensor exhibited linear responses to DA in the ranges of 0.5–100 and 150–400 μM, respectively. The linear regression equations for the two ranges of concentration are:
The limit of detection (LOD) was obtained from S/N = 3 and is as low as 270 nM, which is of the same order of magnitude of that obtained for previous DA sensors based on perovskites reported in the literature so far [13, 14, 29,30,31]. The LOD obtained here lies within the limit of physiological levels of dopamine (10−5 to 10−3 mM) in the human body [31].
In Table 1, the overall performances (electrochemical method used for the dopamine detection, linear range, and LOD) of the proposed NdFeO3 NP modified sensor are compared with previous perovskite-based DA sensors.
To evaluate the reproducibility of the NdFeO3 NPs modified sensor for the quantification detection of DA, according to SWV procedure above reported, a solution of 50 μM of this analyte has been examined, collecting the current for a total of 5 times. A relative standard deviation (RSD) of less than 3.4% has been determined. To assess the stability of NdFeO3 NPs modified sensor, the CV current response was collected, cycling the sensor 20 times in the presence of 50 μM dopamine in PBS solution (see Fig. S1 in the Electronic Supplementary Material (ESM)). After 25 successive runs, the peak currents showed only a slight decrease (less than 5.3%). The results obtained demonstrate that this electrode is little subjected to surface fouling by the products of DA oxidation.
Simultaneous determination of dopamine and uric acid
Uric acid is the primary end product of purine metabolism. It is present in physiological fluids such as blood and causes several diseases such as hyperuricemia when present at abnormal levels [32]. Preliminary, SWV tests (not shown) have been performed for ascertaining the possibility for the NdFeO3 NP modified sensor to detect UA. A clear anodic peak appeared in both electrochemical methods at around 230 mV in the presence of this analyte in 0.1 M of phosphate buffer solution. It is interesting to note that, so far, no report is present in the literature describing the detection of uric acid by this perovskite nanostructure. The influence of the concentration of UA shows that the anodic peak current increased linearly in the concentration range from 50 to 400 μM. The following linear regression equation has been obtained:
Since UA coexist with DA in blood, it is important to determine simultaneously these two analytes [33]. Here, the neodymium iron oxide modified sensor was applied for that by using square-wave voltammetry in phosphate buffer solution at pH 7. Figure 6a shows the SWV recorded starting from a mixture containing 50 μM of UA and increasing the concentration of DA from 0 to 50 μM. Meanwhile, a similar experiment was conducted with UA in the presence of DA. In Fig. 6b, the SWV recorded starting from a mixture containing 50 μM of both DA and UA and increasing the concentration of UA from 50 to 750 μM is represented.
SWV response of NdFeO3/SPEC in presence of both DA and UA. a 50 μM UA and increased concentration of DA (1–50 μM) in PBS solution. b 50 μM DA and increased concentration of UA (50–750 μM) in PBS solution. c Comparison between calibration curves at different DA concentrations (1–50 μM) without UA and in the presence of UA (50 μM). Error bars are standard errors from triplicate measurements
As clearly seen, the difference in the oxidation peak potentials for DA and UA is large enough for separation and simultaneous determination of DA and UA in a mixture. In fact, the peak separation of oxidation for both DA and UA was estimated to be ∼ 0.15 V.
Figure 6 a and b also demonstrate that the oxidation currents of UA show small changes when DA concentration increases and vice-versa, indicating that the addition of one of these analytes does not affect the determination of the other. To confirm this statement, SWV measurements for DA previously obtained with the same sensor and in the same experimental conditions in the absence of UA are compared in Fig. 6c with the SWV measurements for DA in the presence of UA. All experiments were performed in triplicate. The data are fitted by the same straight line, confirming further that the addition of UA does not affect the determination of DA.
Epinephrine (EP), another neurotransmitter of the family of cathecolamines, has been also tested for evaluating the possibility of selectively detect DA and EP [34, 35]. However, in this case, we noted a superposition of peak related to the oxidation process involving both analytes at similar potential, so precluding this dual determination.
Selectivity of neodymium-based electrode and real sample analysis
In order to study the applicability of the developed sensor in real media, the selectivity of the sensor was evaluated by an amperometric method at a fixed potential of 0.1 V. In fact, some other biological molecules such as glucose, uric acid (UA), and folic acid (AF) coexist with dopamine in physiological samples and can potentially interfere with the electrochemical dopamine measurement. Figure 7 shows the detection by amperometric method of DA in the presence of the above biological molecules and electrolytes (K+, Na+).
It has been evidenced that there is no significant change in the current response for 0.2 mM DA in the presence of 0.1 mM of K+, Na+, glucose, uric acid, and folic acid, demonstrating that the fabricated electrode is selective toward the determination of DA in the presence of other potential interferents at physiological concentrations or more.
At last, we performed the analysis of dopamine in a urine sample by using the standard addition method. As electrode fouling and/or other unwanted effects can occur when performing tests with pure real samples, urine sample was diluted ten times, then a known quantity of dopamine was added. The recovery of the spiked samples ranged between 97.7 and 102.7%, indicating that the detection of dopamine by this procedure is free from interferences present in the urine sample matrix. The relative standard deviation (n = 3) is below 3.7%, which can be considered compatible with a practical use of the sensor for real samples analysis.
Conclusion
We have presented here a very simple electrochemical sensor, consisting of a commercial three electrode planar platform modified by depositing NdFeO3 NPS on the working electrode. The fabrication of the sensor ensures reliable and fast production. From the electroanalytical results, NdFeO3 modified electrode shows better performances with respect to the bare electrode and to other perovskite-based sensors reported in the literature. The NdFeO3 modified sensor shows also significant effectiveness toward the simultaneous determination of DA and UA. In summary, we demonstrated the utility of the proposed NdFeO3 perovskite material as a modifier for fabricating enzyme-free electrochemical sensors, which could have significant advantages looking at their electroanalytical applications for monitoring biochemical substances.
References
Kurian MA, Gissen P, Smith M, Heales SJ, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721.
Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73.
Castro SS, Mortimer RJ, De Oliveira MF, Stradiotto NR. Electrooxidation and determination of dopamine using a nafion®-cobalt hexacyanoferrate film modified electrode. Sensors. 2008;8:1950.
Mazloum-Ardakani M, Rajabi H, Beitollahi H, Mirjalili B, Akbari A, Taghavinia N. Voltammetric determination of dopamine at the surface of TiO2 nanoparticles modified carbon paste electrode. Int J Electrochem Sci. 2010;5:147.
Guan CL, Ouyang J, Li QL, Liu BH, Baeyens WRG. Simultaneous determination of catecholamines by ion chromatography with direct conductivity detection. Talanta. 2000;50:1197.
Li L, Liu HY, Shen YY, Zhang JR, Zhu JJ. Electrogenerated chemiluminescence of Au nanoclusters for the detection of dopamine. Anal Chem. 2011;83:661.
Wu HP, Cheng TL, Tseng WL. Phosphate-modified TiO2 nanoparticles for selective detection of dopamine, levodopa, adrenaline, and catechol based on fluorescence quenching. Langmuir. 2007;23:7880.
Jackowska K, Krysinski P. New trends in the electrochemical sensing of dopamine. Anal Bioanal Chem. 2013;405:3753.
Zhao D, Yu G, Tian K, Xu C. A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosens Bioelectron. 2016;82:119.
Cheng M, Zhang X, Wang M, Huang H, Ma J. A facile electrochemical sensor based on well-dispersed graphene-molybdenum disulfide modified electrode for highly sensitive detection of dopamine. J Electroanal Chem. 2017;786:1–7.
Pandiselvi K, Thambidurai S. Chitosan–ZnO/polyaniline nanocomposite modified glassy carbon electrode for selective detection of dopamine. Int J Bio Macromolecules. 2014;67:270.
Blanco-López MC, Rivas M. Anal Bioanal Chem. 2019. https://doi.org/10.1007/s00216-019-01680-x.
Wang G, Sun J, Zhang W, Jiao S, Fang B. Simultaneous determination of dopamine, uric acid and ascorbic acid with LaFeO3 nanoparticles modified electrode. Microchim Acta. 2009;164:357.
Thirumalairajan S, Girija K, Ganesh V, Mangalaraj D, Viswanathan C, Ponpandian N. Detection of the neurotransmitter dopamine by a glassy carbon electrode modified with self-assembled perovskite LaFeO3 microspheres made up of nanospheres. RSC Adv. 2014;4:25957.
Thirumalairajan S, Girija K, Ganesh V, Mangalaraj D, Viswanathan C, Ponpandian N. Cryst Growth Des. 2013;13:291.
El-Ads EH, Atta NF, Galal A, Eid NA. Novel synthesis of LaFeO3 nanostructure dendrites: a systematic investigation of growth mechanism, properties, and biosensing for highly selective determination of neurotransmitter compoundsInt. J Electrochem Sci. 2018;13:1452.
Atta NF, Binsabt MH, El-Ads E. EH, Galal a. synthesis of neodymium-iron nanoperovskite for sensing applications of an antiallergic drug. Turk J Chem. 2017;41:476.
Singh S, Singh A, Yadav BC, Dwivedi PK. Electrochemically reduced graphene oxide and Nafion nanocomposite for ultralow potential detection of organophosphate pesticide. Sensors Actuators B Chem. 2013;177:730–9.
Xinshu N, Weimin D, Weiping D, Kai J. Preparation and gas-sensing properties of NdFeO3 nanocrystalline. J Rare Earths. 2003;21:630–2.
Lou X, Jia X, Preparation XJ. Gas sensing property for C2H5OH detection of perovskite-type NdFeO3. J Rare Earths. 2005;23:328–31.
Atta NF, El-Ads EH, Galal A. Evidence of core-shell formation between NdFeO3 nano-perovskite and ionic liquid crystal and its application in electrochemical sensing of metoclopramide. J Electrochem Soc. 2016;163:B325–34.
Naseri MG, MajlesAra M, Saion E, Shaari A. Superparamagnetic magnesium ferrite nanoparticles fabricated by a simple, thermal-treatment method. J Magn Magn Mater. 2014;350:141.
Naseri MG, Halimah MK, Dehzangi A, Kamalianfar A, Saion E. A comprehensive overview on the structure and comparison of magnetic properties of nanocrystalline synthesized by a thermal treatment method. J Phys Chem Solids. 2014;75:315.
Mocak J, Bond A, Mitchell S, Scollary G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: application to voltammetric and stripping techniques. Pure Appl Chem. 1997;69:297–328.
Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry, 5th ed. Essex: Pearson Education; 2005. p. 121–3.
Navarro MC, Pannunzio-Miner EV, Pagola S, Gomez MI, Carbonio R. Structural refinement of Nd[Fe(CN)6]·4H2O and study of NdFeO3 obtained by its oxidative thermal decomposition at very low temperatures. J Solid State Chem. 2005;178:847.
Li H, Wang J, Liu M, Wang H, Su P, Wu J, et al. A nanoporous oxide interlayer makes a better Pt catalyst on a metallic substrate: nanoflowers on a nanotube bed. Nano Res. 2014;7:1007.
Fazio E, Spadaro S, Bonsignore M, Lavanya N, Sekar C, Leonardi SG, et al. Molybdenum oxide nanoparticles for the sensitive and selective detection of dopamine. J Electroanal Chem. 2018;814:91.
Atta NF, Shimaa MA, El-Ads HE, Galal A. Nano-perovskite carbon paste composite electrode for the simultaneous determination of dopamine, ascorbic acid and uric acid. Electrochim Acta. 2014;128:16.
Priatharshni S, Tamilselvan A, Viswanathan C, Ponpandian N. LaCoO3 nanostructures modified glassy carbon electrode for simultaneous electrochemical detection of dopamine, ascorbic acid and uric acid. J Electrochem Soc. 2017;164:B152–8.
Vijayaraghavan T, Sivasubramanian R, Hussain S, Ashok A. A facile synthesis of LaFeO3-based perovskites and their application towards sensing of neurotransmitters. Chem Select. 2017;2:5570.
Grassi D, Ferri L, Desideri G, Di Gioiosa P, Cheli P, Del Pinto R, et al. Chronic hyperuricemia, uric acid deposit and cardiovascular risk. Curr Pharm Des. 2013;19:2432.
Dong J, Hu Y, Zhu S, Xu J, Xu Y. A highly selective and sensitive dopamine and uric acid biosensor fabricated with functionalized ordered mesoporous carbon and hydrophobic ionic liquid. Anal Bioanal Chem. 2010;396:1755.
Lavanya N, Fazio E, Neri F, Bonavita A, Leonardi SG, Neri G, et al. Simultaneous electrochemical determination of epinephrine and uric acid in the presence of ascorbic acid using SnO2/graphene nanocomposite modified glassy carbon electrode. Sensors Actuators B. 2015;221:1412–22.
Lavanya N, Leonardi SG, Sekar C, Ficarra S, Galtieri A, Tellone E, et al. Detection of catecholamine neurotransmitters by nanostructured SnO2-based electrochemical sensors: a review of recent progress. Mini-Rev Org Chem. 2018;15:382–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection New Developments in Biosensors with guest editors Francesco Baldini and Maria Minunni.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 153 kb)
Rights and permissions
About this article
Cite this article
Anajafi, Z., Naseri, M., Marini, S. et al. NdFeO3 as a new electrocatalytic material for the electrochemical monitoring of dopamine. Anal Bioanal Chem 411, 7681–7688 (2019). https://doi.org/10.1007/s00216-019-01975-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01975-z