Abstract
A highly sensitive method is proposed for the simultaneous determination of dopamine and tyrosine using a Ti3C2 nano layer modified screen printed electrode. The electro-oxidation of dopamine at the surface of the modified electrode was studied using cyclic voltammetry, chrono-amperometry, and differential pulse voltammetry. Under the optimized conditions, the differential pulse voltammetric peak current of dopamine increased linearly with dopamine concentrations in the ranges of 0.5–600.0 µM, and the detection limit of 0.15 µM was obtained for dopamine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Dopamine (DA) and tyrosine (Tyr) are well known as an extracellular chemical messenger called neurotransmitter (NTM) and an essential amino acid in biological systems, respectively. DA plays an important physiological role in the functioning of central nervous, renal, hormonal, and cardiovascular systems as an NTM [1–4], and it is an immune regulator under septic conditions [5]. The loss of an NTM may result in some serious diseases, such as Parkinson’s disease and schizophrenia [6, 7].
Tyr is a vital constituent of proteins, which is indispensable in human nutrition for establishing and maintaining a positive nitrogen balance [8]. Addition of Tyr to dietary and food products or pharmaceuticals is sometimes necessary due to its scarcity in some food materials. Trace-levels of Tyr can modulate and control acetylcholine receptors metabolic stability in muscle cells [9]. A high concentration of Tyr in a culture medium results in an increased sister chromatid exchange, while the absence of Tyr could lead to the impairing of protein synthesis, thus it causes development of imbalance in biological systems. In pharmaceutical industry, Tyr can be converted to DA using enzymatic reaction. To control the reaction progress, a sensor may be needed to obtain the necessary amounts of the end product (DA) and of the substrate (Tyr).
Thus, various commonly usable analytical methods for DA and Tyr have been developed. Some examples of these methods are the ultrahigh performance liquid chromatography tandem mass spectrometry [10], chemiluminescence [11], spectroscopy [12], and spectrofluorimetry [13].
All these techniques, however, require a compressing system, temperature controlling systems, separation systems, and other spectro-photometric or electric detection systems. The electrochemical methods have received special attention due to their unique qualities such as a simple pretreatment procedure, less time-consumption, better selectivity, good sensitivity, and low cost [14–21]. In this sense, electrochemical detection of DA and Tyr has received much interest due to their importance for the central nervous system. However, a significant problem for DA and Tyr determination is the fouling effect due to the accumulation of reaction products on the electrode surface [22]. Thus, a promising approach to overcome problems arising from fouling of the biological substrate is the use of chemically modified electrodes [23–30]. Recently, MXenes (of the formula Mn + 1XnTx, where M is a transition metal, X is C and/or N, and Tx denotes –OH, –F, and =O surface groups), a novel family of two-dimensional metal carbides, have been used which can be produced by the selective etching of the A-group (generally group IIIA and IVA elements) layers from the MAX phases [31–33]. MXenes have already demonstrated their potential as promising electrode materials for Li-ion batteries [34], supercapacitors [35], and sensors [36, 37] because of their high electrical conductivity, a large surface area, a layered structure, remarkable chemical stability, and environmentally friendly characteristics [38, 39].
A screen-printed electrode (SPE) is an attractive alternative choice due to its miniaturized size, low cost, easiness in fabrication, rapid responses, and disposability, which makes it especially suitable for an on-site analysis [40–45].
In this study, a highly sensitive electrochemical method for the simultaneous determination of DA and Tyr using Ti3C2 modified SPE (Ti3C2/SPE) is reported. The proposed method is simple, sensitive, easy, and economical for a routine analysis. The authors consider that the proposed method would be a potential step forward in the simul-taneous electrochemical determination of DA and Tyr in biological fluids.
EXPERIMENTAL
Apparatus and Chemicals
The electrochemical measurements were performed with an Autolab potentiostat/galvanostat (PGSTAT 302N, Eco Chemie, the Netherlands). The experimental conditions were controlled with the General Purpose Electrochemical System software. The SPE used (Drop Sens, Oviedo, Spain) consisted of three main parts: a graphite counter electrode, a silver pseudo-reference electrode, and a graphite working electrode.
All solutions were freshly prepared with double distilled water. DA, Tyr, and all of the other reagents were of the analytical grade and were used as obtained from Merck (Darmstadt, Germany). The buffer solutions (PBSs) were prepared from orthophosphoric acid and its salts in the pH range of 2.0–9.0.
Synthesis of Ti3C2 Nano Layers
In the first step, titanium powder, aluminum powder, and graphite powder with molar ratios of 3, 1.1, and 1.9 were injected into a pellet mill over a period of 360 min, at 600 rpm to synthesize the nano layers. MXene layers were inserted, and MXene was prepared in the powder form with Ti3AlC2 formula.
Next, 60 mL of 40% purity hydrogen fluoride solution was added to 0.2 g of Ti3AlC2 powder and heated at 25°C for 20 h. Afterwards, to prepare MXene with Ti3C2 formula, the prepared suspension was centrifuged and washed with deionized water, and the precipitate was dried at 55°C [46].
Preparation of Modified Electrode
To prepare Ti3C2/SPE a bare SPE was coated with Ti3C2 nano layers as follows: 1 mg of Ti3C2 nano layers was dispersed in 1 mL distilled water and ultrasonicated for about 30 min. Next, 5 μL of this suspension was coated on the surface of the SPE and dried at room temperature.
Preparation of Real Samples
One milliliter of DA from an ampoule was diluted to get 10 mL with 0.1 M PBS (pH 7.0); then, different volumes of the diluted solution were transferred into each of a series of 25 mL volumetric flasks and diluted to the mark with PBS. The DA content was analyzed by the proposed method using the standard addition method.
Urine samples were stored in a refrigerator immediately after collection. Ten milliliters of the samples were centrifuged for 15 min at 2000 rpm. The supernatant was filtered out by using a 0.45 µm filter. Next, different volumes of the solution were transferred into a 25 mL volumetric flask and diluted to the mark with PBS (pH 7.0). The diluted urine samples were spiked with different amounts of DA and Tyr. The DA and Tyr contents were analyzed by the proposed method by using the standard addition method.
RESULTS AND DISCUSSION
Electrochemical Behavior of DA on Ti3C2/SPE
Due to the fact that the electrochemical behavior of DA is pH-dependent, optimizing the pH of the solution is necessary for obtaining the best results. Hence, the evaluations were performed at different pH values ranging from 2.0–9.0, and the results showed that the best results during the electro-oxidation of DA at the surface of the modified electrodes could be obtained at pH 7.0.
Figure 1 illustrates the cyclic voltammograms (CV) of a 700.0 μM DA using the Ti3C2/SPE (curve a) and an unmodified SPE (curve b). As it can be easily noticed, the maximum oxidation of DA occurs at 210 mV in the case of Ti3C2/SPE, which is around 60 mV more negative than that observed in the case of the unmodified SPE. The peak currents are also much higher in the case of the analyses performed with Ti3C2/SPE, thus indicating that the modification of the SPEs with Ti3C2 nano layers greatly enhances the electrochemical behaviour of the electrode for the analysis of DA.
Effect of Scan Rate
Increasing the scan rate leads to enhanced oxidation peak currents according to the obtained results from the study of the effect of potential scan rates on the oxidation currents of DA (Fig. 2). In addition, there is a linear relationship between Ip and the square root of the potential scan rate (v1/2), which demonstrates that the oxidation procedure of the analyst is in control of diffusion [47].
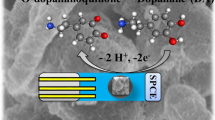
The oxidation process of DA is related to the conversion of the catechol group in DA into o-quinone. The electrochemical oxidization process of DA follows the two-electron/two-proton mechanism. The reaction mechanism of DA on the modified electrode surface is in Scheme 1.

Scheme 1 . Possible reaction of DA on the electrode.
Chronoamperometric Measurements
Chronoamperometric measurements of DA at Ti3C2/SPE were carried out by setting the working electrode potential at 0.25 V for the various concentrations of DA in PBS (pH 7.0) (Fig. 3). For chrono-amperometric analysis of electroactive materials under mass transfer limited conditions, the Cottrell equation was used as borrowed from [47]:
where D is the diffusion coefficient (cm2 s–1), and Cb is the bulk concentration (mol cm–3). The best fitting of I vs. t−1/2 plots based on the experimental was found for different DA samples, and the slopes of the straight lines were next plotted against the concentrations of DA. Using the resulting slope and the Cottrell equation, the mean value of D was found to be 2.9 × 10–5 cm2/s.
Chronoamperograms obtained at Ti3C2/SPE in 0.1 M PBS (pH 7.0) for different concentrations of DA. Numbers 1–5 correspond to 0.1, 0.25, 0.5, 1.0, and 1.5 mM of DA. Insets: (a) Plots of I vs. t −1/2 obtained from chronoamperograms 1–5 and (b) Plot of the slope of the straight lines against DA concentrations.
Calibration Curve
Based on the resulting peak currents of DA when using Ti3C2 nano layers/SPE, the quantitative analysis of DA was done in water solutions. The Ti3C2/SPE, as working electrode, was used in a range of DA concentrations in 0.1 M PBS via differential pulse voltammetry (DPV) due to the advantages of DPV including the improved sensitivity and better performance in analytical applications (Fig. 4). According to the results, there is a linear relationship between the peak currents and concentrations of DA within the concentration range of 0.5–600.0 µM, with the correlation coefficient of 0.9992. The detection limit was obtained to be 0.15 µM. Table 1 presents a comparison of Ti3C2/SPE analytical performance of this work with other electrochemical sensors involved in the DA analysis studied elsewhere [48–51]. As can be seen, the obtained here results are comparable with those reported by others.
Simultaneous Determination of Dopamine and Tyrosine
To the best of the authors’ knowledge, there is on the modified Ti3C2/SPE for the simultaneous determination of DA and Tyr, and this is the first report on this theme.
The electrochemical determination of DA using bare electrodes suffers from the interference by Tyr, because the oxidation potential for Tyr is fairly close to that of DA. The determination of those two compounds was performed by simultaneously changing the concentrations of DA and Tyr, and recording the DPV data (Fig. 5). The voltammetric results showed well-defined anodic peaks at potentials of 200 and 700 mV, corresponding to the oxidation of DA and Tyr, respectively, indicating that the simultaneous determination of these compounds is feasible at the Ti3C2 nano layers/SPE as shown in Fig. 5. The sensitivity of the modified electrode towards the oxidation of DA was found to be 0.0458 µA µM–1. This is very close to the value obtained in the absence of Tyr (0.046 µA µM–1, see Fig. 4), indicating that the oxidation processes of these compounds at Ti3C2/SPE are independent, and, therefore, the simultaneous determination of their mixtures is possible without significant interferences.
DPVs of Ti3C2/SPE in 0.1M PBS (pH 7.0) containing different concentrations of DA and Tyr: (from inner to outer) mixed solutions of 5.0 + 0.5, 20.0 + 3.0, 50.0 + 7.5, 100.0 + 40.0, 200.0 + 125.0, and 400.0 + 275.0, respectively, in which the first value is concentration of DA in micromolar, and the second value is concentration of Tyr in micro molar. (a) Plot of peak currents as a function of DA concentrations, and (b) plot of the peak currents as a function of Tyr concentrations.
Analysis of Real Samples
To assess the applicability of the application of the modified electrode for the determination of DA and Tyr in real samples, the described method was applied in DA ampoule and urine samples. For the purpose of the analysis the standard addition method was used, and the results are given in Table 2. The observed recoveries of DA and Tyr were satisfactory, and the reproducibility of the results was demonstrated based on the mean relative standard deviation (R.S.D.).
CONCLUSIONS
This work reports the construction of Ti3C2/SPE and its application in the simultaneous determination of DA and Tyr. The modified electrode exhibited excellent electrocatalytic activity towards the detection of DA and Tyr co-oxidized at the electrode with a wide potential difference. Thus, the electrode could electrochemically discriminate the sensing of DA and Tyr. To sum up, simultaneous as well as independent electrochemical determinations of DA and Tyr are possible without electrochemical interference from each other.
REFERENCES
Kang, T.F., Shen, G.L., and Yu, R.Q., Voltammetric behavior of dopamine at nickel phthalocyanine polymer modified electrodes and analytical applications, Anal. Chim. Acta, 1997, vol. 354, p. 343.
Tahernejad-Javazmi, F., Shabani-Nooshabadi, M., and Karimi-Maleh, H., Analysis of glutathione in the presence of acetaminophen and tyrosine via an amplified electrode with MgO/SWCNTs as a sensor in the hemolyzed erythrocyte, Talanta, 2018, vol. 176, p. 208.
Damier, P., Hirsch, E.C., Agid, Y., and Graybiel, A.M., The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease, Brain, 1999, vol. 122, p. 1437.
Dhiman, P., Sharma, S., Kumar, A., Shekh, M., et al., Rapid visible and solar photocatalytic Cr(VI) reduction and electrochemical sensing of dopamine using solution combustion synthesized ZnO–Fe2O3 nano heterojunctions: mechanism elucidation, Ceram. Int., 2020, vol. 46, p. 12255.
Beck, G., Hanusch, C., Brinkkoetter, P., Rafat, N., et al., Effekte von Dopamin auf die zellulare und humorale Immunantwort von Patienten mit Sepsis, Anaesthesist, 2005, vol. 54, p. 1012.
Singh, A., Kandimala, G., Dewey, R.B., Jr., and O’Suilleabhain, P., Risk factors for pathologic gambling and other compulsions among Parkinson’s disease patients taking dopamine agonists, J. Clin. Neurosci., 2007, vol. 14, p. 1178.
Vinoth, S., Ramaraj, R., and Pandikumar, A., Facile synthesis of calcium stannate incorporated graphitic carbon nitride nanohybrid materials: A sensitive electrochemical sensor for determining dopamine, Mater. Chem. Phys., 2020, vol. 245, art. ID 122743.
Jin, G.P. and Lin, X.Q., The electrochemical behavior and amperometric determination of tyrosine and tryptophan at a glassy carbon electrode modified with butyrylcholine, Electrochem. Commun., 2004, vol. 6, p. 454.
Sava, A., Barisone, I., Di Mauro, D., Fumagalli, G., et al., Modulation of nicotinic acetylcholine receptor turnover by tyrosine phosphorylation in rat myotubes, Neurosci. Lett., 2001, vol. 313, p. 37.
Gottas, A., Ripel, A., Boix, F., Vindenes, V., et al., Determination of dopamine concentrations in brain extracellular fluid using microdialysis with short sampling intervals, analyzed by ultra high performance liquid chromatography tandem mass spectrometry, J. Pharm. Toxicol. Methods, 2015, vol. 74, p. 75.
Duan, H., Li, L., Wang, X., Wang, Y., et al., A sensitive and selective chemiluminescence sensor for the determination of dopamine based on silanized magnetic graphene oxide-molecularly imprinted polymer, Spectrochim. Acta, Part A, 2015, vol. 139, p. 374.
Edelhoch, H., Spectroscopic determination of tryptophan and tyrosine in proteins, Biochemistry, 1967, vol. 6, p. 1948.
Wang, F., Qing, Y., and Ci, Y.X., Spectrofluorimetric determination of the substrates based on the fluorescence formation with the peroxidase-like conjugates of hemie with proteins, Anal. Lett., 1992, vol. 25, p. 1469.
Karimi-Maleh, H., Karimi, F., Alizadeh, M., and Sanati, A.L., Electrochemical sensors, a bright future in the fabrication of portable kits in analytical systems, Chem. Rec., 2020, vol. 20, p. 682.
Vinay, M.M. and Nayaka, Y.A., Iron oxide (Fe2O3) nanoparticles modified carbon paste electrode as an advanced material for electrochemical investigation of paracetamol and dopamine, J. Sci. Adv. Mater. Devices, 2019, vol. 4, p. 442.
Cong, C., Bian, K., Zhang, X., Luo, L., et al., Sensitive measurement of tumor markers somatostatin receptors using an octreotide-directed Pt nano-flakes driven electrochemical sensor, Talanta, 2020, vol. 208, art. ID 120286.
Karimi-Maleh, H., Karimi, F., Orooji, Y., Mansouri, G., et al., A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin, Sci. Rep., 2020, vol. 10, art. ID 11699.
Chen, X., Zhang, G., He, Y., Shi, L., et al., A sensitive electrochemical sensor based on Au@Pd hybrid nanorods supported on B-doped graphene for simultaneous determination of acetaminophen, dopamine and tyrosine, Int. J. Electrochem. Sci., 2020, vol. 15, p. 5927.
Karimi-Maleh, H., Karimi, F., Malekmohammadi, S., Zakariae, N., et al., An amplified voltammetric sensor based on platinum nanoparticle/polyoxometalate/two-dimensional hexagonal boron nitride nanosheets composite and ionic liquid for determination of N-hydroxysuccinimide in water samples, J. Mol. Liq., 2020, vol. 310, art. ID 113185.
Anuar, N.S., Basirun, W.J., Shalauddin, M., and Akhter, S., A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode, RSC Adv., 2020, vol. 10, art. ID 17336.
Karimi-Maleh, H., Cellat, K., Arıkan, K., Savk, A., et al., Palladium–nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing, Mater. Chem. Phys., 2020, vol. 250, art. ID 123042.
Lane, R.F. and Blaha, C.D., Detection of catecholamines in brain tissue: Surface-modified electrodes enabling in vivo investigations of dopamine function, Langmuir, 1990, vol. 6, p. 56.
Miraki, M., Karimi-Maleh, H., Taher, M.A., Cheraghi, S., et al., Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa, J. Mol. Liq., 2019, vol. 278, p. 672.
Manjunatha, J.G., Kumara Swamy, B.E., Mamatha, G.P., Chandra, U., et al., Cyclic voltammetric studies of dopamine at lamotrigine and TX-100 modified carbon paste electrode, Int. J. Electrochem. Sci., 2009, vol. 4, p. 187.
Bijad, M., Karimi-Maleh, H., and Khalilzadeh, M.A., Application of ZnO/CNTs nanocomposite ionic liquid paste electrode as a sensitive voltammetric sensor for determination of ascorbic acid in food samples, Food Anal. Methods, 2013, vol. 6, p. 1639.
Karimi-Maleh, H., Sheikhshoaie, M., Sheikhshoaie, I., Ranjbar, M., et al., A novel electrochemical epinine sensor using amplified CuO nanoparticles and an-hexyl-3-methylimidazolium hexafluorophosphate electrode, New J. Chem., 2019, vol. 43, p. 2362.
Mazloum Ardakani, M., Talebi, A., Naeimi, H., Nejati Barzoky, M., et al., Fabrication of modified TiO2 nanoparticle carbon paste electrode for simultaneous determination of dopamine, uric acid, and L-cysteine, J. Solid State Electrochem., 2009, vol. 13, p. 1433.
Alavi-Tabari, S.A.R., Khalilzadeh, M.A., and Karimi-Maleh, H., Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle, J. Electroanal. Chem., 2018, vol. 811, p. 84.
Liu, J., Sun, L., Li, G., Hu, J., et al., Ultrasensitive detection of dopamine via electrochemical route on spindle-like α-Fe2O3 mesocrystals/rGO modified GCE, Mater. Res. Bull., 2021, vol. 133, art. ID 111050.
Baghizadeh, A., Karimi-Maleh, H., Khoshnama, Z., Hassankhani, A., et al., A voltammetric sensor for simultaneous determination of vitamin C and vitamin B6 in food samples using ZrO2 nanoparticle/ionic liquids carbon paste electrode, Food Anal. Methods, 2015, vol. 8, p. 549.
Naguib, M., Mochalin, V.N., Barsoum, M.W., and Gogotsi, Y., 25th anniversary article: MXenes: A new family of two-dimensional materials, Adv. Mater., 2014, vol. 26, p. 992.
Wang, F., Yang, C., Duan, C., Xiao, D., et al., An organ-like titanium carbide material (MXene) with multilayer structure encapsulating hemoglobin for a mediator-free biosensor, J. Electrochem. Soc., 2014, vol. 162, p. B16.
Come, J., Naguib, M., Rozier, P., Barsoum, M.W., et al., A non-aqueous asymmetric cell with a Ti2C-based two-dimensional negative electrode, J. Electrochem. Soc., 2012, vol. 159, p. A1368.
Sun, D., Wang, M., Li, Z., Fan, G., et al., Two-dimensional Ti3C2 as anode material for Li-ion batteries, Electrochem. Commun., 2014, vol. 47, p. 80.
Wang, X., Kajiyama, S., Iinuma, H., Hosono, E., et al., Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors, Nat. Commun., 2015, vol. 6, p. 1.
Liu, H., Duan, C., Yang, C., Shen, W., Wang, F., and Zhu, Z., A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2, Sens. Actuators, B, 2015, vol. 218, p. 60.
Wang, F., Yang, C., Duan, M., Tang, Y., et al., TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances, Biosens. Bioelectron., 2015, vol. 74, p. 1022.
Peng, Q., Guo, J., Zhang, Q., Xiang, J., et al., Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide, J. Am. Chem. Soc., 2014, vol. 136, p. 4113.
Mashtalir, O., Lukatskaya, M.R., Kolesnikov, A.I., Raymundo-Pinero, E., et al., The effect of hydrazine intercalation on the structure and capacitance of 2D titanium carbide (MXene), Nanoscale, 2016, vol. 8, p. 9128.
Shi, W., Li, J., Wu, J., Wei, Q., et al., An electrochemical biosensor based on multi-wall carbon nanotube-modified screen-printed electrode immobilized by uricase for the detection of salivary uric acid, Anal. Bioanal. Chem., 2020, vol. 412, p. 7275.
Motia, S., Bouchikhi, B., Llobet, E., and El Bari, N., Synthesis and characterization of a highly sensitive and selective electrochemical sensor based on molecularly imprinted polymer with gold nanoparticles modified screen-printed electrode for glycerol determination in wastewater, Talanta, 2020, vol. 216, art. ID 120953.
Li, G., Zeng, J., Zhao, L., Wang, Z., et al., Amperometric cholesterol biosensor based on reduction graphene oxide–chitosan–ferrocene/platinum nanoparticles modified screen-printed electrode, J. Nanopart. Res., 2019, vol. 21, p. 162.
Jirakunakorn, R., Khumngern, S., Choosang, J., Thavarungkul, P., et al., Uric acid enzyme biosensor based on a screen-printed electrode coated with Prussian blue and modified with chitosan-graphene composite cryogel, Microchem. J., 2020, vol. 154, art. ID 104624.
Knežević, S., Ognjanović, M., Nedić, N., Mariano, J.F., et al., A single drop histamine sensor based on AuNPs/MnO2 modified screen-printed electrode, Microchem. J., 2020, vol. 155, art. ID 104778.
Zhan, Z., Zhang, H., Niu, X., Yu, X., et al., Microliter sample insulin detection using a screen-printed electrode modified by nickel hydroxide, ACS Omega, 2020, vol. 5, p. 6169.
Alhabeb, M., Maleski, K., Anasori, B., Lelyukh, P., et al., Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene), Chem. Mater., 2017, vol. 29, p. 7633.
Bard, A.J. and Faulkner, L.R., Electrochemical Methods: Fundamentals and Applications, New York: Wiley, 2000, 2nd ed.
Li, Y., Lin, H., Peng, H., Qi, R., et al., A glassy carbon electrode modified with MoS2 nanosheets and poly (3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid, Microchim. Acta, 2016, vol. 183, p. 2517.
Yang, B., Wang, H., Du, J., Fu, Y., et al., Direct electrodeposition of reduced graphene oxide on carbon fiber electrode for simultaneous determination of ascorbic acid, dopamine and uric acid, Colloids Surf. A, 2014, vol. 456, p. 146.
Wang, X., Zhang, F., Xia, J., Wang, Z., et al., Modification of electrode surface with covalently functionalized graphene oxide by l-tyrosine for determination of dopamine, J. Electroanal. Chem., 2015, vol. 738, p. 203.
Yao, Z., Yang, X., Niu, Y., Wu, F., et al., Voltammetric dopamine sensor based on a gold electrode modified with reduced graphene oxide and Mn3O4 on gold nanoparticles, Microchim. Acta, 2017, vol. 184, p. 2081.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Navid Arbabi, Hadi Beitollahi Ti3C2 Nano Layer Modified Screen Printed Electrode as a Highly Sensitive Electrochemical Sensor for the Simultaneous Determination of Dopamine and Tyrosine. Surf. Engin. Appl.Electrochem. 58, 13–19 (2022). https://doi.org/10.3103/S1068375522010082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375522010082