Abstract
With the establishment by CODEX of a 200 ng/g limit of inorganic arsenic (iAs) in polished rice grain, more analyses of iAs will be necessary to ensure compliance in regulatory and trade applications, to assess quality control in commercial rice production, and to conduct research involving iAs in rice crops. Although analytical methods using high-performance liquid chromatography-inductively coupled plasma-mass spectrometry (HPLC-ICP-MS) have been demonstrated for full speciation of As, this expensive and time-consuming approach is excessive when regulations are based only on iAs. We report a streamlined sample preparation and analysis of iAs in powdered rice based on heated extraction with 0.28 M HNO3 followed by hydride generation (HG) under control of acidity and other simple conditions. Analysis of iAs is then conducted using flow-injection HG and inexpensive ICP-atomic emission spectroscopy (AES) or other detection means. A key innovation compared with previous methods was to increase the acidity of the reagent solution with 4 M HCl (prior to reduction of As5+ to As3+), which minimized interferences from dimethylarsinic acid. An inter-laboratory method validation was conducted among 12 laboratories worldwide in the analysis of six shared blind duplicates and a NIST Standard Reference Material involving different types of rice and iAs levels. Also, four laboratories used the standard HPLC-ICP-MS method to analyze the samples. The results between the methods were not significantly different, and the Horwitz ratio averaged 0.52 for the new method, which meets official method validation criteria. Thus, the simpler, more versatile, and less expensive method may be used by laboratories for several purposes to accurately determine iAs in rice grain.

Comparison of iAs results from new and FDA methods
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a result of the establishment of a CODEX maximum limit of 200 ng/g inorganic arsenic (iAs) in milled (white) rice and 350 ng/g iAs in husked (brown) rice grain [1], a large number of rice samples will need to be analyzed for iAs worldwide. Analytically, iAs consists of the sum of As3+ and As5+, or each can be measured individually, as opposed to total As which includes all forms of As. Rice contains variable levels of dimethylarsinic acid (DMA) and traces of monomethylarsonic acid (MMA) depending on soil management during rice production. For risk assessment purposes to help set the CODEX limit, fundamental studies required analytical methods that could sensitively determine the full speciation of As composition in rice and other foods in human diets (and other forms of exposure) [2,3,4,5,6,7]. Studies have shown that organoarsenical forms of As were not toxicologically significant enough at the levels found in rice grain to be included in the CODEX limit [8]. Thus, analytical and screening methods for As in rice need only test for relatively higher concentrations of iAs for a variety of purposes, including quality control checks of harvested rice, regulatory and trade monitoring, and agronomic investigations intended to reduce iAs levels in rice.
By focusing only on iAs, the costs of materials, instruments, time, and labor can be reduced in comparison to methods that entail full speciation of As. Speciation is ordinarily conducted using high-performance liquid chromatography (HPLC) followed by As measurement with inductively coupled plasma (ICP)-mass spectrometry (MS) [2,3,4,5,6,7, 9,10,11]. Such instrumentation is very expensive to purchase, operate, and maintain, including the need for highly qualified analysts. Elegant ICP-MS methods have been reported [3,4,5,6,7, 10], including recent improvements in speed [9,10,11], but for current needs, we considered use of older well-developed methods to determine iAs [12] as well as others recently proposed [13,14,15,16]. Although methods using solid-phase extraction to separate the As from the sample extracts have been reported [14,15,16], we did not find these as useful in higher sample throughput applications. Thus, we sought to develop a new streamlined method for the laboratory that could quickly and accurately monitor iAs in dozens of samples in a batch.
After grinding of the rice grain, the next step in the analysis involves extraction of iAs, which has been the subject of many previous reports [17]. Several extraction methods have been shown to be effective in releasing the iAs from powdered rice samples, and with respect to maximizing throughput, the heating of powdered rice with 0.28 M HNO3 for 90 min has been accepted as a reliable method to release the iAs [6]. Adding H2O2 to this solution oxidizes the As3+ to As5+, which is followed by filtering and analysis by different techniques. This approach is considered more reliable than measuring both As3+ and As5+ separately. ICP-MS (or MS/MS) is one option, but we reasoned that hydride generation (HG) of arsine from the As3+ in pre-reduced filtered rice extracts would lead to the determination of iAs more simply and cheaply by ICP-atomic emission spectroscopy (AES), atomic fluorescence spectroscopy (AFS) [15], or atomic absorption spectroscopy (AAS) [16].
The practical quantification limit of iAs in rice grain using HG is about 20 ng/g dry rice using ICP-AES or AAS, and lower detection limits can be achieved by AFS or ICP-MS to measure the arsine-As. This easily meets the CODEX standard for use of analytical methods that achieve a limit of quantification at least 1/5 of the MRL. Different HG devices and conditions can also affect detection limits by providing different dilution factors of the arsine produced during HG.
Feldmann’s group [13, 14] described a straight-forward method that uses HG of arsine gas and analysis of the As in the arsine by the different techniques. By increasing the concentration of HCl in the HG system, interferences from DMA, the major organic form of As in rice, were almost completely rejected in the determination of iAs. In the HG-ICP-AES analysis of many rice samples, with various As levels, including National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 1568b—rice flour, we examined the contribution of DMA interfering in the iAs result. Using the US Food and Drug Administration (FDA) extraction method [6], we found that inclusion of 4 M HCl in the pre-reduction solution minimized the DMA interference, yet still protected the equipment from excessive HCl exposure.
We have analyzed thousands of rice samples to further evaluate the reliability of the new method in routine use. In our in-house validation experiments, high accuracy was achieved for samples consisting of many varieties of rice and iAs levels. We felt that the method would be highly beneficial to others and sought to transfer this method for wider implementation.
The aim of this study was to conduct an inter-laboratory validation of the new streamlined method to determine if it would meet international acceptability standards for the common purposes listed above. We invited many interested collaborators experienced in the analysis of iAs in rice to participate in the study and sent blind duplicate rice powder test portions for them to extract and analyze using the HG protocol, which permitted use of different analytical techniques. Some of the participants also analyzed the samples by different techniques, including full speciation by the HPLC-ICP-MS method.
Materials and methods
Rice samples with a wide range of expected iAs concentrations were obtained from Anna McClung of the USDA-ARS Dale Bumpers National Rice Research Center in Stuttgart, AR, or purchased from local markets. Table 1 lists the rice samples by their source code names, types, and the blind duplicate test sample numbers in the study. Rice grown with alternate wetting and drying irrigation contains substantially lower iAs and total As than rice grown using traditional continuous flood methods because the soil is aerobic enough to limit formation of arsenite needed for high absorption of As by rice [18].
The rice samples were ground to a fine powder using a UDY (Fort Collins, CO, USA) Cyclone Sample Mill with a 0.8-mm stainless steel screen. Ground rice samples were stored at 4 °C to preserve As speciation. The rice samples were apportioned (~ 20 g) into sets of 20-mL polyethylene vials numbered no. 1–12 in accordance with Table 1 (unknown to the participating labs). Also, all labs were provided ~ 10-g portion of NIST (Gaithersburg, MD, USA) SRM 1568A rice flour, which was known to the participants. The test sample sets were shipped by overnight shipping to arrive to the labs as soon as possible, and the labs were provided with the method protocols, a reporting form, and 2 months to perform the analyses. Enough material was provided for at least 25 replicate extractions for each test sample, if needed, and the labs reported analyzing 2–6 replicates as indicated in the Electronic Supplementary Material (ESM).
The protocol is provided in the ESM. In brief, 0.70-g (dry weight) rice powder test portions were weighed into 50-mL screw-cap polyethylene (PE) vials (e.g., DigiTUBES from SCP Science; Baie D’Urfé, Québec, Canada) and extracted by the participants using the standard US FDA “HotBlock” method [6] with 10 mL of 0.28 M HNO3 at 95 °C for 90 min. After allowing the tubes to reach room temperature, the extracts were filtered into graduated 50-mL PE collection tubes through Whatman 40 filter paper fitted into PE funnels (pre-rinsed with uncollected extraction solvent). The filters were rinsed twice with 0.28 M HNO3, and the filtrate (extract) was diluted with 0.28 M HNO3 to the 20-mL mark on the tubes.
The participants were instructed to use the specific HG conditions in the protocol for analysis of iAs, but they were permitted to modify operating conditions to suit their HG devices and analytical instruments. The list of participants and their instrumental approaches appears in the “Acknowledgments.” One lab compared both HG-ICP-MS and HG-AAS for the same set of test samples, and another lab compared iAs results for the test samples determined by the HG-ICP-MS method and the HPLC-ICP-MS full speciation method [6]. Four labs used HG-ICP-AES, one lab used HG-AFS, and three other participants only analyzed the test portions following the HPLC-ICP-MS full speciation method protocol [6].
Results and discussion
Method optimization
In initial studies, we investigated the simple HotBlock extraction HG-ICP-AES method to measure iAs in rice based on research by Feldmann’s group [13, 14]. Their use of a higher HCl concentration in rice extracts subjected to HG appeared to limit DMA conversion to an arsine that would interfere in the ICP-AES result for iAs. They had not pre-reduced sample arsenate generated by H2O2 (which was included in the extraction solvent) before conducting HG, while we had been following protocols that called for pre-reduced arsenate prior to the HG step.
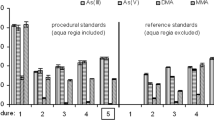
Therefore, we decided to study the effect of HCl concentration on measurements of iAs vs. DMA in rice extracts. Milled and husked rice with substantially different levels of iAs and organoarsenicals were extracted using the FDA HotBlock method, and varied DMA and HCl concentrations were added to the extracts. As shown in Fig. 1, we confirmed the finding of Pétursdóttir et al. [14] in a simpler approach to include HCl in the pre-reduction reagent solution. When using 4 M HCl, the contribution of DMA in the iAs result was reduced to about 4% of the As present in the form of DMA in the sample, which is less than the typical measurement uncertainty in HPLC-ICP-MS analysis of rice. If the measured iAs in the new method approaches the CODEX limit of 200 ng/g in milled rice (or 350 ng/g in husked rice), then the iAs result can be subtracted from total As to yield the contribution from DMA (and trace MMA). The correct iAs concentration is then calculated by subtracting 4% of the As contributed by the organoarsenical species.
Effect of HCl concentration in the pre-reduction solution on the measurement of iAs contributed from DMA in HG-ICP-AES analysis of rice extracts. Blank, flood, and aerobic rice with different iAs and DMA concentrations were spiked with 0, 200, 500, and 1000 μg/L DMA. Ultimately, 4 M HCl was selected in the final method, which led to ≈ 4% of the DMA present contributing to the iAs result
The updated method using HotBlock extraction, filtration, and flow-injection HG-ICP-AES analysis was further simplified as much as practicable to minimize costs without reducing accuracy in the results. For HG, it is not necessary to use centrifugation and membrane filtration of extracts as in HPLC-ICP-MS [6]; simple filtration with filter paper in a funnel yields extracts ready for HG analysis. This change reduces sample preparation time and cost of supplies.
Inter-laboratory validation
Unknown to the participants, the set of rice test samples followed the blind duplicate experimental design used in AOAC International collaborative studies (Table 1). We purchased and otherwise obtained bulk rice samples and analyzed them for total As and iAs to obtain a diverse range of rice varieties and As levels encountered in real-world applications. Sample lots selected for the study included specific samples containing low, medium, and high levels of iAs in both milled and brown rice. We invited more than 20 labs internationally, known to conduct As analysis of rice samples, to participate in the study, and others also asked to participate after hearing about the study. Ultimately, the sets of test samples were sent to 16 labs worldwide, and 14 labs returned results of their analyses: 12 labs used the new streamlined protocol and 4 labs used the HPLC-ICP-MS full speciation method [6].
Reported iAs results were entered into an Excel spreadsheets, including one developed by AOAC International for inter-laboratory collaborative studies, and calculations were made to determine within-laboratory repeatability (RSDr), among laboratory reproducibility (RSDR), and Horwitz ratios (HorRat) [19]. The ESM provides tables of the reported iAs results. Although 12 labs returned results using the new streamlined method protocol, results from participating labs no. 11 and no. 12 were very late, and their reports contained obvious errors, such as no detection of iAs in test sample no. 10 by lab no. 11 and reported iAs concentration in the NIST SRM by lab no. 12 that was 106 ng/g greater than the certified value of 92 ng/g. For these reasons, their results were excluded from the evaluation. Even so, the much higher RSDr and biases in the results from these two labs nearly always made them Cochran and/or (double) Grubbs outliers if they were included. No other results were found to be statistical outliers and excluded in the study.
Two labs were each counted twice, one of which compared HG-ICP-MS and HG-AAS and the other compared the new method with the HPLC-ICP-MS full speciation method. In each case, the labs re-extracted different test portions from the test samples they were provided. Their results are shown in Fig. 2, and in both cases, no significant differences were observed in the different analyses. This demonstrates that the same test samples in the same labs yield essentially indistinguishable results by the same sample preparation method with different analytical tools (Fig. 2A) or the two different methods altogether (Fig. 2B). Figure 2A also shows that the lab using HG-AFS also yielded insignificantly different results from the other detection methods.
Table 2 is a compilation of the reported iAs results in the blind duplicates for each lab using the new method sorted into the different analytical techniques, and Table 3 gives the reported results from the labs using the HPLC-ICP-MS method. The %RSDr for each lab indicates the precision in their analyses of the test samples. Overall, the new method independent of the analytical technique averaged 5.0% RSDr (n = 10), which was nearly identical to the RSDr of 5.1% for the HPLC-ICP-MS method (n = 4).
The HG-ICP-MS and HG-ICP-AES results among the labs listed in Table 2 were further compiled and compared as shown in Fig. 3. On average, the RSDr was 3.3% (n = 4) for the labs using HG-ICP-AES vs. 6.4% (n = 4) for the labs using HG-ICP-MS. The HG-AAS results from lab no. 1 similarly averaged RSDr of 6.6% in the iAs analysis of the test samples, and HG-AFS for lab no. 6 yielded average RSDr of 5.5%. Not enough labs participated using different analytical techniques to make a valid comparison, but the small data set indicates that HG-ICP-AES may provide slightly more precise results than the other approaches. Lab no. 9 in particular yielded merely 1.5% RSDr by HG-ICP-AES, demonstrating exceptional performance using the method.
Figure 3 also shows the exceptional consistency in average iAs concentrations reported among the different labs using the same sample preparation and flow-injection HG method with different means of determination. A rather consistent bias of − 21% in the test sample results from lab no. 3 led the RSDR for HG-ICP-MS to reach 13%. Otherwise, RSDR would have averaged 8% (n = 3), equaling the RSDR of 8% (n = 4) among the labs using HP-ICP-AES.
Table 4 compiles the iAs results using the new method for 10 labs in five countries in comparison with the results from 4 labs in two countries using the HPLC-ICP-MS full speciation method. These results are also plotted in Fig. S1 (see ESM). The new streamlined method averaged a consistent − 8 to − 13% bias vs. the HPLC-ICP-MS method, but F test and T test statistical treatments showed no differences in the test sample results exceeding the 95% confidence level. The observed bias was likely just an artifact in the measurement due to the small data set of only four labs using the HPLC-ICP-MS method. As shown in Fig. 2B, when the same lab compared the two methods using the same test samples, the bias disappeared.
RSDR averaged 11.3% for the new streamlined method vs. 10.4% for the HPLC-ICP-MS method, which led to nearly identical average HorRat values of 0.52 and 0.49, respectively. HorRat is calculated by dividing the observed RSDR from the predicted RSDR calculated from the Horwitz equation, 2C−0.15, in which C is the analyte’s mass fraction concentration in the sample (e.g., g/g). HorRat of 1.0 is the average among hundreds of AOAC International collaborative studies conducted over decades [19], and HorRat < 2.0 is generally deemed acceptable for methods validated among at least eight labs to achieve AOAC International Official Method status. Predicted RSDR ranged from 20 to 23% for the 80–280 ng/g measured iAs concentrations in the rice test samples, and HorRat < 0.67 in this study demonstrates that the new streamlined method yields significantly better than average performance among collaboratively validated analytical methods.
One of the weaknesses in validations based solely on the HorRat is that only precision of the method is taken into account, not trueness. An underlying assumption when conducting a multi-laboratory study is that acceptable method precision is also indicative of acceptable analytical trueness. Determination of analyte recoveries using spiked test samples is a common approach to assess trueness, but analytes tend to be more readily extracted from spiked matrices than incurred matrices due to stronger analyte-matrix interactions in the latter case. Moreover, matrix blanks are not always available into which the analyte(s) can be spiked, such as iAs in rice. Arsenic is ubiquitous in soil and water, and perhaps As-free rice grain could be grown in highly controlled conditions, but this is not feasible. Thus, only incurred rice test samples could be evaluated, and although incurred samples are more desirable than spiked samples, the actual iAs concentration in the test samples needs to be known to assess method trueness.
In this study, using incurred samples, we evaluated trueness of the new method in two ways: (1) comparison of iAs results with the previously validated HPLC-ICP-MS full speciation method and (2) inclusion of NIST SRM 1568b rice flour as one of the test samples. As already mentioned in the first case, no significant difference in results occurred between the different methods. In the second case, the NIST Certificate of Analysis for SRM 1568b states that iAs = 92 ± 10 ng/g, DMA = 180 ± 12 ng/g (as As), MMA = 11.6 ± 3.5 ng/g (as As), and total As = 285 ± 14 ng/g. These certified concentrations were determined from multi-laboratory analyses using different validated methods of many 1-g test portions from different vials.
The average iAs concentration determined by the 10 labs using the new streamlined method was 108 ± 12 ng/g, which equaled the 108 ± 14 ng/g result from the 4 labs using the full speciation method. This 17% bias is higher than that desirable for both methods, but the average iAs concentration becomes 100.3 ± 6.2 ng/g (9% bias) if the “suspicious” 132 ng/g result from lab no. 3 using the full speciation method is excluded from its data set. As observed in Fig. 1, the new method is expected to yield a high bias of 4% equivalent As contribution from DMA (and MMA) in the iAs determination. In the case of SRM 1568b, the DMA and MMA contribution equals 7.7 ng/g (4% of 192 ng/g), which yields a corrected iAs concentration of 100.3 ng/g with the new method, which exactly matches the 100.3 ng/g iAs concentration using the HPLC-ICP-MS method (n = 3).
Conclusions
Due to regulation of only iAs in rice products (as opposed to total As or full speciation), large numbers of samples will need to be analyzed for iAs. Common methods for full speciation of As in rice samples are expensive, and a simpler method for iAs analysis was optimized and evaluated in this study. We showed that the use of 4 M HCl in the pre-reduction reagent solution prior to flow-injection HG ICP-AES limited the contribution of As from DMA in rice to 4% in the iAs result. An inter-laboratory validation of the final method among 10 labs using different detection techniques for both milled and brown rice with varied levels of iAs and DMA yielded HorRat < 0.67 for all test samples. The iAs determinations were not significantly different from the full speciation method used by four other labs to analyze the test samples. In these respects, the new streamlined method may be used to meet analytical needs for a variety of purposes.
References
Codex Alimentarius. 2017a. General standard for contaminants and toxins in food and feed. CODEX STAN 193–1995. (CSX_193e.pdf).
D’Amato M, Forte G, Caroli S. Identification and quantification of major species of arsenic in rice. J AOAC Int. 2004;87:238–43.
de la Calle MB, Emteborg H, Linsinger TPJ, Montoro R, Sloth JJ, Rubio R, et al. Does the determination of inorganic arsenic in rice depend on the method? Trends Anal Chem. 2011;30:641–51.
Heitkemper DT, Vela NP, Stewart KR, Westphal CS. Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. J Anal Atom Spectr. 2001;16:299–306.
Heitkemper DT, Kubachka KM, Halpin PR, Allen MN, Shockey NV. Survey of total arsenic and arsenic speciation in US-produced rice as a reference point for evaluating change and future trends. Food Addit Contam B. 2009;2:112–20.
Kubachka KM, Shockey NV, Hanley TA, Conklin SD, Heitkemper DT. Elemental analysis manual Section 4.11: Arsenic speciation in rice and rice products using high performance liquid chromatography-inductively coupled plasma-mass spectrometric determination. Version 1.1, 2012. (www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm328363.htm).
Llorente-Mirandes T, Calderon J, Lopez-Sanchez JF, Centrich F, Rubio R. A fully validated method for the determination of arsenic species in rice and infant cereal products. Pure Appl Chem. 2012;84:225–38.
European Food Safety Agency. Scientific opinion on arsenic in food. EFSA J. 2009;7:1351.
Jackson BP. Fast ion chromatography-ICP-QQQ for arsenic speciation. J Anal Atom Spectrom. 2015;30:1405–7.
Gray PJ, Tanaba CK, Ebeler SE, Nelson J. A fast and fit-to-purpose arsenic speciation method for wine and rice. J Anal Atom Spectrom. 2017;32:1031–4.
Narukawa T, Chiba K, Sinaviwat S, Feldmann J. A rapid monitoring method for inorganic arsenic in rice flour using reversed phase - high performance liquid chromatography - inductively coupled plasma mass spectrometry. J Chromatogr A. 2017;1479:129–36.
Onken BM, Hossner LR. Determination of arsenic species in soil solution under flooded conditions. Soil Sci Soc Am J. 1996;60:1385–92.
Musil S, Pétursdóttir ÁH, Raab A, Gunnlaugsdóttir H, Krupp E, Feldmann J. Speciation without chromatography using selective hydride generation: inorganic arsenic in rice and samples of marine origin. Anal Chem. 2014;86:993–9.
Pétursdóttir ÁH, Friedrich N, Musil S, Raab A, Gunnlaugsdóttir H, Krupp EM, et al. Hydride generation ICP-MS as a simple method for determination of inorganic arsenic in rice for routine biomonitoring. Anal Methods. 2014;6:5392–6.
Chen G, Chen T. SPE speciation of inorganic arsenic in rice followed by hydride-generation atomic fluorescence spectrometric quantification. Talanta. 2014;119:202–6.
Rasmussen RR, Qian Y, Sloth JJ. SPE HG-AAS method for the determination of inorganic arsenic in rice—results from method validation studies and a survey on rice products. Anal Bioanal Chem. 2013;405:7851–7.
Welna M, Szymczycha-Madeja A, Pohl P. Comparison of strategies for sample preparation prior to spectrometric measurements for determination and speciation of arsenic in rice. Trends Anal Chem. 2015;65:122–36.
Linquist BA, Anders MM, Adviento-Borbe MA, Chaney RL, Nalley LL, da Rosa EF, et al. Reducing greenhouse gas emissions, water use and grain arsenic levels in rice systems. Glob Chang Biol. 2015;21:407–17.
Horwitz W, Albert R. The Horwitz ratio (HorRat): a useful index of method performance with respect to precision. J AOAC Int. 2006;89:1095–109.
Acknowledgments
We thank Anna McClung for providing the rice samples grown with traditional flood irrigation vs. alternative wetting and drying irrigation for the study. We also thank Amy Poet and Julie Napolitano for their extensive and careful work which was critical to the project. We very much appreciate the scientists and their laboratory colleagues who participated in the inter-laboratory validation study: (analysis by HG-ICP-AES) Amy Poet and Julie Napolitano, Adaptive Cropping Systems Laboratory, USDA-ARS, Beltsville, MD, USA; Tomohito Arao and Koji Baba, National Institute for Agro-Environmental Sciences, Tskuba, Ibaraki, Japan; Philip Moore, USDA-ARS, University of Arkansas, Fayetteville, AR, USA; Trenton Roberts, Dept. of Crop, Soil, and Environmental Sciences, Fayetteville, AR, USA; Eri Matsumoto, Tama Laboratory, Japan Food Research Laboratories, Tokyo, Japan; (by HG-AFS) Fangjie Zhao, College of Resources and Environmental Sciences, Nanjing Agricultural University, Nanjing, China; (by HG-ICP-MS) Michael Bukowski (and by HG-AAS), USDA-ARS-GFHNRL, Grand Forks, ND, USA; Jörg Feldmann and Andrea Raab (by HPLC-ICP-MS), Trace Element Speciation Laboratory, University of Aberdeen, Scotland, UK; Anitha Kunhikrishnan, Won-Il Kim, and Ji-Hyock Yoo, Dept. of Agro-Food Safety, National Institute of Agricultural Sciences, Republic of Korea; Cheryl Stephenson and Marvin Boyd, Jr., Eurofins Central Analytical Laboratories, New Orleans, LA, USA; Yong-Guan Zhu and Guo-Xin Sun, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, China; (by HPLC-ICP-MS) Sean Conklin, Chemical Contaminants Branch, US Food and Drug Administration, Center for Food Safety and Applied Nutrition, College Park, MD, USA; Kent Lanclos and James Krest, Technology and Science Division, USDA Grain Inspection, Packers, and Stockyards Administration, National Grain Center, Kansas City, MO, USA; and Andrew Meharg and Manus Carey, Institute for Global Food Security, Queen’s University Belfast, Northern Ireland, UK.
Funding
Funding for this research was provided by The Rice Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Food Safety Analysis with guest editor Steven J. Lehotay.
Disclaimer: The use of trade, firm, or corporation names does not constitute an official endorsement or approval by the USDA of any product or service to the exclusion of others that may be suitable.
Electronic supplementary material
ESM 1
(PDF 496 kb)
Rights and permissions
About this article
Cite this article
Chaney, R.L., Green, C.E. & Lehotay, S.J. Inter-laboratory validation of an inexpensive streamlined method to measure inorganic arsenic in rice grain. Anal Bioanal Chem 410, 5703–5710 (2018). https://doi.org/10.1007/s00216-018-1075-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1075-x