Abstract
The present paper describes the development, validation and application of a method for inorganic arsenic (iAs) determination in rice samples. The separation of iAs from organoarsenic compounds was done by off-line solid-phase extraction (SPE) followed by hydride generation atomic absorption spectrometry (HG-AAS) detection. This approach was earlier developed for seafood samples (Rasmussen et al., Anal Bioanal Chem 403:2825–2834, 2012) and has in the present work been tailored for rice products and further optimised for a higher sample throughput and a lower detection limit. Water bath heating (90 °C, 60 min) of samples with dilute HNO3 and H2O2 solubilised and oxidised all iAs to arsenate (AsV). Loading of buffered sample extracts (pH 6 ± 1) followed by selective elution of arsenate from a strong anion exchange SPE cartridge enabled the selective iAs quantification by HG-AAS, measuring total arsenic (As) in the SPE eluate. The in-house validation gave mean recoveries of 101–106 % for spiked rice samples and in two reference samples. The limit of detection was 0.02 mg kg−1, and repeatability and intra-laboratory reproducibility were less than 6 and 9 %, respectively. The SPE HG-AAS method produced similar results compared to parallel high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry (ICP-MS) analysis. The SPE separation step was tested collaboratively, where the laboratories (N = 10) used either HG-AAS or ICP-MS for iAs determination in a wholemeal rice powder. The trial gave satisfactory results (HorRat value of 1.6) and did not reveal significant difference (t test, p > 0.05) between HG-AAS and ICP-MS quantification. The iAs concentration in 36 rice samples purchased on the Danish retail market varied (0.03–0.60 mg kg−1), with the highest concentration found in a red rice sample.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a trace element present in the environment and consequently in various food items. The total As content in food products of plant origin is usually low, but rice and rice-based products are exceptions [2]. Rice contains most often three forms of arsenic; inorganic arsenic (iAs) and the methylated species monomethylarsonic acid (MA) and dimethylarsinic acid (DMA) [3, 4]. As has two oxidation states and the two forms of iAs: arsenite (AsIII) and arsenate (AsV), which can interconvert during extraction [5]. Dietary intake of iAs is of special concern due to its carcinogenicity to humans [6], whereas DMA and MA are considered of less toxicological importance [7]. Rice grains and rice-based products are staple foods in many countries and is one of the major contributors to the iAs exposure according to the European Food Safety Authority (EFSA). Since the total dietary intake of iAs for some European consumer groups are close to or even above the recommended margins of exposure (0.3–8 μg (kg bw)−1 day−1 Benchmark Dose Level), the possibility of a risk to these consumers groups cannot be excluded [7]. Rice contains typically between 0.05 and 0.20 mg kg−1 iAs [8], but concentrations up to 1–2 mg kg−1 in specific rice products have been reported [9]. Inorganic As represents very variable percentages of total As (10–90 %) in rice [10] and therefore selective detection methods for iAs are required for accurate exposure assessment. Arsenic in foodstuffs is currently not regulated in the European legislation [11], and China seems to be the only country with a maximum level (ML) for iAs in rice (0.15 mg kg−1) [8]. The Codex Alimentarius Committee on contaminants in food, established by FAO and WHO, proposed in 2012 a draft MLs for iAs in raw and polished rice (0.3 and 0.2 mg kg−1, respectively) [8], but this proposal has not been adopted yet. Internationally accepted validated method(s) are needed before establishment of MLs as emphasised by EFSA (2009) [7], WHO (2011) [12] and Codex (2012) [8]. Currently, two European standards for the determination of iAs have been published: EN 15517:2008 for iAs in seaweed by hydride generation atomic absorption spectrometry (HG-AAS) [13] and EN 16278:2012 for iAs in animal feedingstuffs of marine origin by solid-phase extraction (SPE) HG-AAS [14]. Furthermore, China has a national standard for determination of abio-arsenic in foods, where the (abio-)As extracted with 6 M hydrochloric acid is detected by HG atomic fluorescence spectrometry (AFS) [15].
A wide range of sample pretreatment methods (extraction into water, acidic extraction with different acids, alkaline extraction, enzymatic digestion, etc.) and instrumental set-ups can be used for As speciation analysis, as thoroughly reviewed by Francesconi and Kuehnelt [16]. Recently, the European Union Reference Laboratory for Heavy Metals in Feed and Food organised a proficiency test on the determination of iAs in rice, where the participants (N = 32) used various analytical techniques of their own choice. The main conclusion from the study was that, among seven expert laboratories, the determination of iAs in rice did not depend on the applied analytical method [17]. High-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry (HPLC-ICP-MS) is often favoured for arsenic speciation analysis, due to the low limits of detection and the high selectivity of this detector even when analysing real sample matrices. Separation of As species, by anion exchange chromatography exploiting their different pKa values and charges, is widely used [17, 18]. However, the ICP-MS detector is not available in all food control laboratories since its purchase and running costs are higher compared to other elemental detection techniques like e.g. the AAS and AFS.
The aim of the present work was to develop a method for the specific determination of iAs in rice samples based on anion exchange SPE separation and HG-AAS detection, using the same principles as previously applied for seafood samples [1, 14]. The method was further optimised in order to achieve a lower detection limit and an easy-to-use high-throughput sample extraction approach. The current study presents the method performance characteristics obtained from an in-house validation study and the results from a collaborative trial of the SPE separation approach. Furthermore, the method was applied to a range of different rice samples (N = 36) obtained in the Danish retail shops.
Experimental
Chemicals and reagents
The following chemicals and reagents were used to prepare the solutions used for the experimental work, including extraction of samples, the SPE and HPLC separations as well as the HG-AAS and ICP-MS detection procedures. Methanol (MeOH) was HPLC grade (Rathburn, Walkerburn, Scotland, UK) and acetic acid (CH3COOH) pro analysis quality (Merck, Darmstadt, Germany). The 67–69 % nitric acid (HNO3) and 34–37 % hydrochloric acid (HCl) were Plasma Pure grade (SCP Science, The Netherlands), 30 % hydrogen peroxide (H2O2) and 25 % ammonia (NH3) were Suprapure grade (Merck), sodium hydroxide (NaOH) was Ph Eurgrade (Merck) and ammonium carbonate ((NH4)2CO3) was Puratronic grade (Alfa Aesar, Karlsruhe, Germany). Sodium tetrahydroborate (NaBH4) (Fluka, Buchs, Switzerland), l-ascorbic acid (Fluka) and potassium iodide (KI) (Merck) were all pro analysis quality. The 30 % silicone antifoam emulsion in water was obtained from Sigma-Aldrich (Steinheim, Germany). Water was ultrapurified using a Millipore system (Molsheim, France). Serial dilutions of arsenate (AsV) standard stock solution (1,000 ± 3 mg l−1) from (CPI Peak Performance, USA) were prepared daily in water.
Samples: preparation, extraction and analysis
Various types of rice products, including white (N = 11), brown (N = 10), parboiled (N = 1), black rice (N = 5), red rice (N = 2) and rice crackers (N = 7) were obtained from local retail shops in the Copenhagen area. Minimum of 10 g of each of the samples was homogenised and ground to a fine powder using a commercial coffee grinder (Tefal Simply Invents, China) and stored at room temperature (20 °C) until analysis. Two natural incurred rice reference materials, IMEP-107 rice (Institute for Reference Materials and Measurements Geel, Belgium) containing 0.107 ± 0.014 mg kg−1 iAs and 0.172 ± 0.018 mg kg−1 total arsenic [19] and the Standard Reference Material NIST1568a rice flour (National Institute of Standards and Technology, Maryland, USA) certified for 0.29 ± 0.03 mg kg−1 total arsenic, were included. Though no certified iAs value exists for NIST1568a rice flour, a literature consensus value of approximately 0.096 mg kg−1 iAs has been reported [20].
Subsamples (approx. 0.5 g dry weight) were extracted with 10 ml of a diluted acidic mixture (0.1 M HNO3 and 3 % H2O2) in 13-ml round bottomed polypropylene tubes assisted by water bath heating (90 °C ± 2) for 60 min. After 10 min centrifugation (2,500×g, 10 °C), the sample extracts were transferred to clean plastic tubes, before aliquots were analysed by SPE HG-AAS (as described below). External calibration standards matching the final sample solvent composition were included twice: in the beginning and at the end of each sequence, for quantification. Linear calibration curves were drawn by the least squares method points by plotting the response of the analyte against the concentration.
The rice sample for the collaborative trial was obtained as a fine powdered rice meal in a retail shop and was homogenised by thorough shaking before being distributed into dark glass bottles (N = 30; approx. 10 g/bottle). A homogeneity test carried out following the requirements set by the ISO 13528 [21] demonstrated that the test sample was sufficiently homogeneous for the collaborative trial. The samples were dispatched to the participants in May 2012 and results were obtained by the end of July 2012. Sample extraction with diluted HCl and SPE separation was done according to EN16278:2012 [14] and both the HG-AAS approach and determination by ICP-MS was applied for the iAs detection in the SPE eluates.
SPE HG-AAS
Inorganic arsenic was selectively separated from other arsenic compounds using a silica-based strong anion exchange SPE (Strata SAX, Phenomenex, 6 ml, 500 mg 55 μm, 70 Å). The SPE procedure followed the approach previously reported by Rasmussen et al. [1] with slight modifications in solvent composition (Fig. 1). The sample extract was buffered with a 70 mM (NH4)2CO3 buffer (1+1) and pH adjusted if outside the 5.0–7.5 range. Following loading of the buffered sample extract on the SPE column (step 3, Fig. 1), a washing step with 0.5 M acetic acid (step 4, Fig. 1) removed MA and DMA from the SPE. In the final elution step, the SPE eluates (step 5, Fig. 1) containing iAs were collected and pre-reduced prior to determination of the (total) arsenic concentration by HG-AAS. Pre-reduction included mixing of 0.8 ml SPE eluate with 5.6 ml reduction solution (30 mM KI, 28 mM l-ascorbic acid, 0.1 % (v/v) silicone, 3 M HCl), which was allowed to react for 1 h at room temperature. Subsequently, 4.8 ml of 3 M HCl was added and it was left to react for another hour at room temperature prior to HG-AAS measurement. The AsV standards and the SPE sample eluates were pre-reduced simultaneously. The HG-AAS (Thermo Fisher Scientific, Cambridge, UK) was operated as previously described [1]. The gaseous arsenic hydrides (AsH3) generated with NaOH/NaBH4 (0.5 % w/v) and HCl (4.7 M) were transported by argon gas through a dryer tube (610 × 22 mm id, Perma pure model MD-110-24F-4, Toms River, USA) to reduce vapour precipitation, which may decrease the sensitivity. The six external calibration standards matching the solvent composition of the samples were 0 and in the range 0.25–4.98 μg l−1 corresponding to 0 and 0.044–0.87 mg kg−1 in a rice sample.
Method validation
In-house validation data for SPE HG-AAS were collected in three series (white rice, brown rice and rice crackers), which were analysed on three separate days by two different laboratory technicians. Each validation series comprised 24 samples, i.e. rice samples spiked with AsV at four levels (0, 0.3, 0.55 and 0.80 mg kg−1) each analysed in triplicates, one reagent blank, two reference materials (NIST1568a and IMEP-107) analysed in duplicates and single determinations of unspiked retail rice samples. Additional retail samples were analysed in an extra series, where also one reagent blank and two reference materials (NIST 1658a and IMEP-107) were included. The spiked samples had a varying natural content of iAs with white rice, brown rice and rice crackers, containing 0.05, 0.15 and 0.28 mg kg−1 (N = 3), respectively. AsV were added prior to the sample extraction step in order to reach the desired spike levels.
Inorganic arsenic SPE separation was also collaboratively tested in 10 German trace element laboratories, handing in 11 data sets for iAs determination in a rice sample. Only two of the participants (laboratory ID 3 and 4) had previous experience with the SPE technique. A 2-day theoretical iAs speciation and hands-on SPE training was given to the laboratory staff beforehand the trial.
Data processing
Statistical evaluation of the data followed ISO 5725-2 [22] and was carried out in Microsoft Excel 2010 spreadsheets following the procedure described by the Nordic Committee on Food Analysis [23]. The within-day and between-day data were used for the determination of the in-house relative standard deviation of repeatability (RSDir) and reproducibility (RSDIR), respectively. The limit of detection (LOD) and limit of quantification (LOQ) were determined as three times and six times the standard deviation at in-house validation conditions (SDIR) divided by the recovery, respectively, both based on results obtained from the analysis of the NIST1568a rice flour with relative low iAs content (∼0.096 mg kg−1). In the collaborative trial, the repeatability and reproducibility RSDs were calculated from within-laboratory (RSDr) and between-laboratory (RSDR) data. The obtained relative standard derivations (RSDobtained) were compared with predicted values (RSDpredicted) calculated by the Horwitz equation (2(1–0.5·log C)) for the specific mass fraction (C in gram per gram) and the HorRat value (RSDobtained/RSDpredicted) was calculated. From the laboratory mean, the overall mean (X obs) and associated standard derivation (u obs) was calculated using data from compliant labs. Outlying results for laboratory precision (variance) were identified by the Cochran test and outliers for laboratory replicates or laboratory means were identified by the Grubbs test [22].
Results and discussion
Optimization of the analytical procedure
The original extraction procedure developed for seafood samples [1] was in the present work further optimised to achieve a lower detection limit by increasing the sample to solvent ratio by a factor 2.5 (from 0.2 to 0.5 g sample per 10 ml solvent), which is within the typical ratio applied by other iAs speciation laboratories for rice samples [24]. The weak acidic aqueous solvent was exchanged from HCl (0.06 M) to HNO3 (0.1 M) in order to reduce the Cl− content, which is strongly retained on anion exchange columns and can potentially create argon chloride interference (40Ar35Cl+ on 75As+) in the ICP-MS detection. Application of extraction by water bath heating (90 °C, 60 min) instead of the previously used microwave-assisted extraction (90 °C, 25 min + cooling) approach enabled a higher sample throughput because of a higher capacity of the water bath and the use of single-use tubes for the extraction. H2O2 (3 % v/v) was added for quantitative oxidation of AsIII to AsV for stronger retention on the anion SPE cartridge as demonstrated previously [18].
The separation of iAs from MA and DMA in an extract of the NIST1568a rice sample is illustrated in Fig. 2, which shows overlaid HPLC-ICP-MS chromatograms from the loading-, washing- and elution steps, respectively, of the SPE separation. AsV eluted exclusively in elution step, whereas the other arsenic species eluted in the fractions from the loading and washing steps.
Overlaid HPLC-ICP-MS chromatogram of three SPE fractions (load, wash and eluate) of a rice sample (NIST1568a) containing both inorganic and organoarsenic species. Asterisk, this peak was also observed in reagent blank eluate and is attributed to an artefact signal caused by the many non-retained compounds eluting at the column void time
In-house method performance characteristics
The SPE HG-AAS method was in-house validated using spiked (levels 0.3: 0.55 and 0.80 mg kg−1) and naturally incurred rice samples. A summary of the in-house validation results are shown in Table 1. Mean recoveries of 101–106 % were obtained for the spiked and reference rice samples (Table 1). Good agreement with the consensus values for the reference materials IMEP-107 and NIST1568a and the results obtained in this study: 0.108 ± 0.008 and 0.100 ± 0.007 mg kg−1 (mean ± standard deviation), respectively, were achieved.
The method complies with the required performance criteria for methods to be used for official food control purposes as decided by the European Commission [25]. Low values for the in-house repeatability for replicate measurements (RSDir 3–6 %) were obtained and also the intra-laboratory reproducibility was satisfactory since the RSDIR values (7–9 %) did not exceed the Horwitz equation values (RSDHorwitz) for the specific concentration level [26] as also requested by the European Commission [25]. The performance was within the same range for low, medium and high spike and for white rice, brown rice and rice crackers, which were analysed in three different analytical series. The LOD and LOQ were estimated at 0.02 and 0.04 mg kg−1, respectively.
A linear response for external standards was obtained up to 4.98 μg l−1 (corresponding to 0.87 mg kg−1), with correlation coefficients (R 2) ≥ 0.994. Matrix effects were not seen since spiking experiments gave quantitative recoveries (105–106 %, Table 1). Plastic containers were applied whenever possible to avoid AsV contamination from glassware and the iAs content in reagent blanks was at a low level (corresponding to <0.002 mg kg−1), indicating that no contamination were introduced during the extraction and separation procedures. At 2.5 times, LOQ level acceptable values for trueness (101–103 %) and reproducibility (7 %) were obtained for the natural incurred reference materials (NIST 1568a and IMEP-107) containing approximately 0.1 mg kg−1 (Table 1). The method working range defined as LOQ to the highest calibration standard was from 0.04–0.87 mg kg−1.
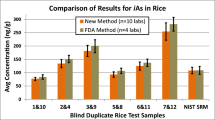
All rice sample extracts (from both naturally incurred, spiked and reference material) were analysed by both SPE HG-AAS and a HPLC-ICP-MS reference method [1]. A comparison of the results from the two methods (Fig. 3) showed good agreement although a slightly higher (3 % on average) and statistical significantly different (p < 0.05, paired t test) results were obtained compared to the HPLC-ICP-MS results. No significant differences between the two methods were observed for seafood samples as documented in a previous study [1]. An overestimation of iAs by SPE HG-AAS could indicate presence of organo-As compounds in the eluate; however, the HPLC chromatograms did not support this suspicion (Fig. 2), since the peak around 2 min also was present in the reagent blank. The 3 % difference between the two methods has no practical implications for control purposes since measurements between laboratories typical vary much more.
Collaborative testing of the method
The SPE separation method was furthermore tested in a collaborative trial on a rice meal sample. The rice sample was analysed by 10 laboratories handing in 11 datasets (Fig. 4). One laboratory was a Cochran outlier (ID 1) and was excluded from the calculations, whereas two Cochran stragglers (lab 3 and 5) having only medium variance within the four replicates were included. No significant difference (p = 0.05, t test) between the use of HG-AAS (n = 7) or ICP-MS (n = 3) for the arsenic determination following the SPE separation was observed. The laboratory, which applied both ICP-MS and HG-AAS (laboratory ID 7 and 8 in Fig. 4) as detectors, obtained similar results for the two detection approaches (Fig. 4).
The overall mean (X obs) at 0.16 mg kg−1 is in fair agreement with results obtained at DTU at 0.19 mg kg−1 from the homogeneity testing, indicating satisfactory accuracy. For the precision, values at 19.6 % for RSDr and 34.2 % for RSDR were obtained, respectively. These values are at the same level as the values from the IMEP-32 study on marine animal feedingstuffs using EN16278:2012 [14] (RSDr, 5.4–17.5 % and RSDR, 13.2–31.9 %) and are evaluated as satisfactory. A HorRat value at 1.6 was obtained, which is satisfactory according to the requirements in EU directive 333/2007 [27]. The target standard deviation (σ) for the collaborative trial was fixed to 15 % reflecting the state-of-the-art in this field of analysis [17]. Seven out of 11 participants (64 %) had mean values within the target interval (X obs ± 2 · σ), which is at the same level as reported (75 % within the target interval, N = 32) in another iAs trial on rice [17].
Retail rice samples
The SPE HG-AAS method was applied on 36 rice samples from the Danish retail market (Fig. 5 and Electronic Supplementary Material Table S1). White rice (N = 11) on average contained a lower amount of iAs (median value 0.09 mg kg−1) compared to other rice types. Median values for black (N = 5) and brown rice (N = 10) were also at relatively low concentration levels (both 0.14 mg kg−1); however, single samples of these types contained up to 0.3–0.4 mg kg−1. The rice crackers (N = 7) was the sample type with the highest iAs concentration (median 0.28 mg kg−1 and up to 0.57 mg kg−1). Red (N = 2) and parboiled rice (N = 1) were also analysed but here the samples were too few to give general remarks.
The amounts are within the ranges previously reported for samples of rice and rice products [8]. Both type of rice as well as iAs environmental contamination is of importance for the plant accumulation of iAs [28, 29]. The iAs levels detected in rice and rice products were for 6 of 36 samples above the MLs for raw rice (0.3 mg kg−1) proposed by Codex [8].
Conclusion
A SPE HG-AAS method for the determination of iAs in rice was optimised and subsequently successfully validated. In-house average recoveries in the range of 101–106 % and repeatability and reproducibility RSD values at 3–6 and 7–9 %, respectively, were in accordance with previous results obtained for marine samples. Due to optimization of the extraction procedure, a lower LOD was obtained (0.02 mg kg−1) which is well below the proposed maximum levels (0.2–0.3 mg kg−1). Collaborative testing of the SPE separation method on a wholemeal rice meal sample gave a satisfactory HorRat value of 1.6. The method was applied to a range of rice and rice products samples (n = 36) and concentrations from 0.03 to 0.60 mg kg−1 of iAs were found. Rice crackers were the sample type with the highest iAs contents, whereas white rice generally had the lowest content. The SPE HG-AAS method presented here enables iAs determination by inexpensive instrumentation (HG-AAS) and is fit for purpose as a candidate method for future control of rice and rice products.
References
Rasmussen RR, Hedegaard RV, Larsen EH, Sloth JJ (2012) Development and validation of an SPE HG-AAS method for determination of inorganic arsenic in samples of marine origin. Anal Bioanal Chem 403:2825–2834
Schoof R, Yost L, Eickhoff J, Crecelius E, Cragin D, Meacher D, Menzel D (1999) A market basket survey of inorganic arsenic in food. Food and Chem Toxicol 37:839–846
Pizarro I, Gómez M, Palacios MA, Cámara C (2003) Evaluation of stability of arsenic species in rice. Anal Bioanal Chem 376:102–109
Williams P, Price A, Raab A, Hossain S, Feldmann J, Meharg A (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39:5531–5540
Abedin MJ, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J (2002) Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ Sci Technol 36:962–968
IARC (International Agency for Research on Cancer) (1987) IARC monographs on the evaluation of the carcinogenic risks to humans. Suppl. 7. Overall evaluations of carcinogenicity: an updating of IARC monographs, vol 1–42
EFSA (European Food Safety Authority) (2009) Scientific opinion on arsenic in food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J 7(10):1351, 1–199
Codex (2012) Proposed draft maximum levels for arsenic in rice (at step 3). http://usriceproducers.com/files/362_2012.01_WHO.maximumlevelsforarsenic.pdf. Accessed 4 Feb 2013
Sun GX, Williams PN, Carey AM, Zhu YG, Deacon C, Raab A, Feldmann J, Islam RM, Meharg AA (2008) Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environ Sci Technol 42:7542–7546
Heitkemper DT, Vela NP, Stewart KR, Westphal CS (2001) Determination of total and speciated arsenicin rice by ion chromatography and inductively coupled plasma mass spectrometry. J Anal At Spectrom 16:299–306
Commission EC (2006) Commission regulation (EC) no. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Commun L364:5–24
WHO (World Health Organization) (2011) < br />Evaluation of certain contaminants in food: seventy-second meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 959
European Standard (EN 15517:2008) Determination of trace elements—determination of inorganic As in seaweed by hydride generation atomic absorption spectrometry (HG-AAS) after acid digestion
European Standard (EN 16278:2012) Animal feeding stuffs—determination of inorganic arsenic by hydride generation atomic absorption spectrometry (HG-AAS) after microwave extraction and separation by solid phase extraction (SPE)
GB/T5009.11-2003. Determination of total arsenic and abio-arsenic in foods.
Francesconi KA, Kuehnelt D (2004) Determination of arsenic species: a critical review of methods and applications, 2000–2003. Analyst 129:373–395
de la Calle MB, Emteborg H, Linsinger TPJ, Montoro R, Sloth JJ, Rubio R, Baxter MJ, Feldmann J, Vermaercke P, Raber G (2011) Does the determination of inorganic arsenic in rice depend on the method? TrAC 30:641–651
Raber G, Stock N, Hanel P, Murko M, Navratilova J, Francesconi KA (2012) An improved HPLC-ICPMS method for determining inorganic arsenic in food: application to rice, wheat and tuna fish. Food Chem 134(1):524–532
de la Calle MB, Linsinger T, Emteborg H, Charaud-Got J, Verbist I (2010) Report of the Seventh interlaboratory comparison organized by the European Union-Reference Laboratory for Heavy Metals in Feed and Food, IMEP-107: total and inorganic As in rice. JRC-IRMM, Geel, Belgium EUR 24314 EN
Raab A, Baskaran C, Feldmann J, Meharg AA (2009) Cooking rice in a high water to rice ratio reduces inorganic arsenic content. J Environ Monit 11:41–44
ISO (2005) 13528: statistical methods for use in proficiency testing by interlaboratory comparisons. ISO, Geneva
ISO (1994) 5725-2: accuracy (trueness and precision) of measurement methods and results. Part 2: basic method for the determination of repeatability and reproducibility of a standard measurement method. ISO, Geneva
NMKL (Nordic Committee on Food Analysis) (2009) Validation of chemical analytical methods. NMKL procedure 4:ed. 3
de la Calle M, Baer I, Robouch P, Cordeiro F, Emteborg H, Baxter M, Brereton N, Raber G, Velez D, Devesa V (2012) Is it possible to agree on a value for inorganic arsenic in food? The outcome of IMEP-112. Anal Bioanal Chem 404(8):2475–2488
Commission EC (2002) Commission decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun L221:8–36
Horwitz W (1982) Evaluation of analytical methods used for regulation. J Assoc Off Anal Chem 65:525–530
Commission EC (2007) Commission regulation (EC) no 333/2007 of 28 March 2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo(a)pyrene in foodstuffs. Off J Eur Commun L88:29–38
Williams P, Islam M, Adomako E, Raab A, Hossain S, Zhu Y, Feldmann J, Meharg A (2006) Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ Sci Technol 40:4903–4908
Jorhem L, Åstrand C, Sundström B, Baxter M, Stokes P, Lewis J, Grawé KP (2008) Elements in rice from the Swedish market: 1. Cadmium, lead and arsenic (total and inorganic). Food Addit Contam 25:284–292
Acknowledgments
Funding for this study was provided by the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 211326. A special thanks to German VDLUFA laboratories who participated in the collaborative trial.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Rapid Detection in Food and Feed with guest editors Rudolf Krska and Michel Nielen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 67 kb)
Rights and permissions
About this article
Cite this article
Rasmussen, R.R., Qian, Y. & Sloth, J.J. SPE HG-AAS method for the determination of inorganic arsenic in rice—results from method validation studies and a survey on rice products. Anal Bioanal Chem 405, 7851–7857 (2013). https://doi.org/10.1007/s00216-013-6936-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6936-8