Abstract
An online ultra-high-performance-liquid chromatography-triple quadrupole tandem mass spectrometry (UHPLC-MS/MS) method for detection and quantification of natural and synthetic estrogens and their conjugates in aqueous matrices was developed. Target compounds include the natural estrogen estradiol (E2) and its main metabolites estrone (E1) and estriol (E3), the synthetic estrogens ethinylestradiol (EE2) and diethylstilbestrol (DES) and their conjugates estrone 3-sulfate (E1-3S), estriol 3-sulfate (E3-3S), estradiol 17-glucuronide (E2-17G), estrone 3-glucuronide (E1-3G), and estriol 16-glucuronide (E3-16G). After pH adjustment, sample filtration and addition of internal standards (IS), water samples (5 mL) were preconcentrated on a Hypersil GOLD aQ column after which chromatographic separation was achieved on a Kinetex C18 column using methanol and water as a mobile phase. The experimental parameters, such as sample loading flow rate, elution time, the percentage of organic solvent in the aqueous-organic eluent mixture, pH, and volume of analyzed samples, were optimized in detail. The benefits of the method compared to previously published methods include minimum sample manipulation, lower detection limits, reduced total analysis time, and overall increased method accuracy and precision. Method detection limits (MDLs) are in subnanogram per liter, complying with the requirements of the EC Decision 2015/495 (Watch list) for hormones listed therein. Applicability of the developed method was confirmed by analysis of river and raw wastewater samples taken directly from urban sewerage systems before being discharged into the river.

Sheme of online SPE-UHPLC-MS/MS system
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endocrine-disrupting compounds (EDCs) have become a public health concern in modern times because of their detrimental effects on the human endocrine system [1, 2]. Among the various substances with reported endocrine-disrupting properties, natural and synthetic estrogens are of particular interest due to their high estrogenic potency [3,4,5] and the effects that they could cause to aquatic organisms even at below nanogram per liter concentrations [6, 7]. The three major naturally occurring estrogens in women are 17β-estradiol (E2) and its main metabolites estrone (E1) and estriol (E3). They are also used as contraceptives or medication for menopausal women, i.e., E2 and the synthetic estrogen 17β-ethinylestradiol (EE2) are active ingredients in a number of drugs used in physiological hormonal replacement therapies, treatment of prostate and breast cancer, and hair lotions for contrasting alopecia [8]. Both natural and synthetic steroids, in either conjugated (as glucuronides and sulfates, principally) or unconjugated form, are excreted in the urine of mammalians and enter the aquatic environment via wastewater treatment plant (WWTP) effluents or untreated discharges.
Estrogens have been detected by a variety of analytical procedures in influents and effluents of WWTPs and in surface water, groundwater, and drinking water [9,10,11,12,13,14,15,16]. The complexity of the environmental matrices and the very low environmental concentrations require the use of highly sensitive and selective methods to monitor these EDC compounds in water. Although a number of approaches for the detection of estrogens have already been published, their determination is still a difficult analytical task. New requirements in the field of water policy by the European Community Water Framework Directive have become of scientific interest and a new challenge in the analytical world [17]. According to the EC Decision 2015/495, estrogen compounds such as E1, E2, and EE2 are included in a novel Watch list of emerging pollutants, among other substances for monitoring in the European Union [18]. The maximum acceptable method detection limits (MDLs) required for EE2 and E1/E2 are 0.035 and 0.4 ng/L, respectively. However, achieving such low MDL in real environmental samples (i.e., surface water) is an extremely difficult achievement that requires either large volume off-line solid-phase extraction (SPE) or specifically optimized online approach combined with high sensitivity detection using the last generation mass spectrometry (MS/MS) instruments [19]. While several authors reported MDLs for E1 and E2 in the range of the required 0.4 ng/L [20, 21], none of the methods described in the literature achieve detection of EE2 at the required 0.035 ng/L making the online approach particularly suitable and necessary for such a demanding task.
Nowadays, the technique of choice for the analysis of this group of compounds of different polarities in complex matrices is liquid chromatography-tandem mass spectrometry (LC-MS/MS), as less demanding method than gas chromatography (GC-MS), since it does not require an additional step such as derivatization or hydrolysis, which may cause losing information about hormone conjugates (e.g., sulfate and glucuronide) [22]. According to published papers, estrogen glucuronides and sulfates have received less attention, because of their low estrogenic potency, and only a few papers took into account their environmental distribution and behavior [23,24,25,26,27,28]. However, their monitoring is especially relevant for WWTP samples, where deconjugation may occur resulting in an increase of free form [5, 23, 29, 30]. Many reported methods are based on off-line SPE [31,32,33,34], which makes them time-consuming and requires a large volume of samples (250–1000 mL) in order to achieve low MDLs. Other drawbacks of the off-line SPE procedures are that they often need many steps before reaching an extract concentration suitable for instrumental analysis, of which only a small portion is actually injected onto the chromatographic column. In recent years, labor-saving and cost-effective online SPE has become more popular as a sample preparation technique for water analysis due to the small amounts of solvents necessary and low sample volume required (mL range), as well as a remarkable decrease in the total analysis time and improvement of precision, accuracy, and sensitivity [21, 35,36,37,38,39]. Furthermore, the additional sample contamination and analyte losses, which may occur during the off-line SPE sample pretreatment, are avoided. Although currently available techniques could generally yield MDLs in the subnanogram per liter range, further improvements are demanded and the applicability of the proposed methodologies must be tested on real water samples. MDLs and method quantification limits (MQLs) were estimated with standard solutions and rarely in real matrices, so they were probably often underestimated [40]. It should be noted that in some cases, the MQLs reported [41, 42] were calculated by statistical extrapolations, and estrogen concentrations are quantified up to four orders of magnitude lower than the investigated linearity range [37].
In this context, the purpose of this work was to develop and validate a completely automated, reliable, and cost-effective method based on online SPE and ultra-high-performance-liquid chromatography mass spectrometry (UHPLC-MS/MS) for the determination of the most active and environmentally relevant estrogens at below the nanogram per liter levels in water thus complying with the required maximum acceptable MDLs set up in EC Decision 2015/495 [18]. Target compounds included the natural estrogen E2, its main metabolites E1 and E3, the synthetic estrogens EE2 and diethylstilbestrol (DES), and the conjugates estrone 3-sulfate (E1-3S), estriol 3-sulfate (E3-3S), estradiol 17-glucuronide (E2-17G), estrone 3-glucuronide (E1-3G), and estriol 16-glucuronide (E3-16G). The method was validated in real water matrices, such as raw wastewater and river water receiving municipal and industrial discharges.
Material and methods
Chemicals and materials
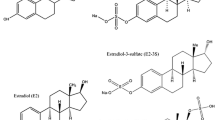
All pure standards of the target estrogens E2, E1, E3, EE2, DES, E1-3S, E3-3S, E2-17G, E1-3G, and E3-16G were purchased from Sigma-Aldrich (Germany). Isotopically labeled compounds, used as internal standards (IS), were E1-d4, E2-d2, EE2-d4, and E1-3S-d4 obtained from CDN Isotopes Pointe-Claire, Quebec, Canada. The chemical structures and physicochemical properties of target-free and conjugated estrogens are shown in the Electronic Supplementary Material (ESM) in Table S1. Both individual stock standard and isotopically labeled internal standard solutions were prepared on a weight basis in methanol at a concentration of 1000 mg/L. After preparation, standards were stored at −20 °C. Working standard solutions, containing all estrogens, were prepared in water and were renewed before each analytical run by mixing appropriate amounts of the intermediate solutions. The standard mixtures were used as spiking solutions for the preparation of the standard curve and in the recovery study. Separate mixtures of isotopically labeled internal standards were obtained in methanol and further dilutions in water for online analysis. Samples for off-line analysis were prepared in methanol:water (10:90) corresponding to the initial conditions of the chromatographic run. Methanol and water were of HPLC grade purity from Fisher Scientific (Whitby, ON, Canada). Ammonium and sodium hydroxide were obtained from Scharlab SL (Barcelona, Spain). Glass microfiber filters GF/F (0.7 μm) were purchased from Whatman (Fairfield, CT, USA) and nylon membrane syringe filters (0.45 μm), used for sample filtration before HPLC analysis, were provided from Sigma-Aldrich (Germany).
Sample collection and preparation
The method was optimized using three types of water matrices, namely river water (Onyar, located in Girona, Catalonia, Spain), WWTP effluent, and influent water (WWTP Quart, Catalonia, Spain). The applicability of the developed method was tested on river (S1–S15) and raw wastewater samples (WW1–WW15) collected at 15 sampling sites in the north of Serbia (see Fig. S1 in the ESM). River water samples were taken from Danube Basin and its major tributaries Tisa and Sava, downstream of the discharges of raw wastewater. Sites affected by the discharges of urban and agricultural wastes were selected along the rivers as sampling points. Raw wastewater samples were taken directly from urban sewerage systems before discharge into the receiving rivers without any treatment. Information regarding the sampling sites is provided in the ESM Table S2. All samples were collected as grab samples in prewashed amber glass bottles and transported back to the laboratory under cooled conditions (4 °C) and immediately frozen at −20 °C until analysis. Before analysis, water samples were defrosted and filtered through 0.7-μm glass microfiber filter. Water samples were pH adjusted to 11 with sodium hydroxide and were filtered through 0.45-μm nylon membrane syringe filters. Finally, samples were placed in amber glass SPE vials (10 mL), and prior to HPLC analysis, a mix of IS (E1-d4, E2-d2, EE2-d4, and E1-3S-d4) was added to achieve a final concentration of 50 ng/L.
Analytical method
Chromatographic analysis was performed using an automated online preconcentration system EQuan™ coupled to a TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher Scientific) equipped with an electrospray (ESI) source. The base of the EQuan™ system is a high-end HPLC system with a high-performance injector that handles sample volumes from 10 μL up to 5 mL fully automated. The system consists of a PAL autosampler (CTC Analysis) and two quaternary pumps: a loading pump (Accela™ 600 pump) and an elution pump (Accela™ 1250 pump) both from Thermo Fisher Scientific, and a three-valve switching device unit with a six-port valve. A divert valve was programmed to control loading and elution of the two LC columns. The first column was used for preconcentration of the sample (20 × 2.1 mm, 12-μm particle size Hypersil GOLD aQ, Thermo Fisher Scientific) and the C18 reversed-phase column for chromatographic separation (50 × 2.1 mm, 1.7-μm particle size Kinetex, C18, Phenomenex) (see Fig. S2 in the ESM which shows the different valve positions of the online system during analysis).
UHPLC-MS/MS analysis conditions
After testing different volumes (1 to 5 mL), the injection volume was set to 5 mL for all types of matrices. The flow rate through the loading column was 1.75 mL/min during the charge step. In the transfer step, the flow rate was set at 0.5 mL/min, while during the analysis time, the flow rate through the eluting column was 0.4 mL/min. After the charging step, the six-port switching valve was activated and the analytes were transferred from the preconcentration column to the analytical column. When the transfer was finished, the switching valve was activated and the analytes were separated. Simultaneously, the preconcentration column was rinsed and conditioned. Chromatographic separation was performed under gradient elution conditions using methanol and water. The initial conditions were 50% methanol and they were held during 3.5 min. Then, the gradient was linearly increased to 70% methanol during 1 min. Afterwards, the gradient was linearly increased to 100% methanol in 3 min and kept isocratic for 1 min. Finally, at the end of the run, the initial conditions were reached. The duration of the whole analytical procedure was 10.5 min and all the steps were performed automatically. The overall chromatography conditions are shown in Table 1.
Off-line mode was only used in the optimization procedure to optimize chromatographic separation and to assess the relative recovery of the online extraction. Relative recovery was calculated by comparing the peak areas obtained in the online analyses of spiked HPLC samples with those obtained from the injection of standard mixture in off-line mode. The optimized conditions for the off-line analysis were also obtained using methanol and water as a mobile phase. The elution gradient was as follows: 10% methanol held isocratically during the first 2.5 min, linear increase to 100% methanol in 2.5 min, isocratic elution at 100% methanol during the next 1.5 min, and return to the initial conditions at 6 min (see Table S3 in the ESM). The total elution time of the method was 6 min.
Mass spectrometry
The detection was carried out on a TSQ Vantage triple quadrupole (QqQ) mass spectrometer (Thermo Fisher Scientific), equipped with an ESI turbo spray ionization source. The optimized parameters which showed higher response for the majority of the compounds were as follows: spray voltage 2500 V, sheath gas pressure 40 (N2), auxiliary gas pressure 20 (N2), ion sweep gas pressure 0.5 (N2), and with capillary and vaporizer temperature set at 300/350 °C, respectively. Natural and synthetic estrogens and conjugates were separated under negative ionization (NI) conditions as [M−H]−. The cycle time was adjusted to 0.250 s, giving a minimum of 12 points per peak, and the first and third quadrupole (Q1 and Q3) were operated at unit resolution (0.7 Da FWHM) and with the second quadrupole (Q2) collision gas pressure set at 1.2 mTorr.
Quantitative analysis was performed using selected reaction monitoring (SRM) mode, for each compound, and the two most abundant transitions were monitored: the most intense one used for quantification and the other for confirmation of the chemical identity. In order to maximize sensitivity and peak reproducibility, data acquisition was performed under time-scheduled conditions. The optimized MS/MS parameters and time-scheduled conditions for SRM analysis of individual target compounds are shown in Table 2. The entire system was controlled via Xcalibur 2.2 software and data were processed using TraceFinder 3.1 (Thermo Fisher Scientific).
Method validation
The method was validated by using HPLC water and three environmental matrices, spiked with a standard mixture of target analytes and IS, which were added prior to analysis. Each of the experimental conditions evaluated was run in triplicate.
The relative recovery (RE) for the online method was evaluated by spiking HPLC grade water, confirming the efficiency of the analyte transfer to the online system. In order to allow the comparison, the same mass of mix standard of analytes was injected in the off-line and online system (0.5 ng injected on-column) [43]. The peak area ratio of the selected estrogen of a direct injection (20 μL) was compared with those of the online volume injection (5 mL). RE was calculated by using following equation:
Where A online/AIS online is the peak area ratio of analyte to internal standard (IS) measured online, while A off − line/AIS off − line is the peak area ratio of analyte to IS measured off-line. Relative recoveries are indicated in Fig. S3 in the ESM. In general, most of the target compounds were recovered at more than 80% efficiency. Calculated standard deviation of all compounds was low, indicating a good precision of the online extraction.
Throughout the recovery evaluation, wastewater influent and effluent were spiked in triplicate at 50 ng/L and surface water at 5 ng/L (considering as much cleaner matrix). Concentrations obtained after online SPE procedure, calculated by internal standard calibration, were compared by the theoretical initial spiking levels. All the values shown in the “Results and discussion” section represent the mean of the triplicate measurements. Along the analysis, quality control standards (QCs) were injected in order to ensure a good performance of the analysis. QCs were daily prepared in two levels 5 and 50 ng/L with a mixture of target analytes and IS and were measured after every 10 injections during the analysis. Intraday and interday precision were determined from six repeated injections of a 5-ng/L standard mixture during the same day (repeatability) and in four successive days (reproducibility).
For quantification of the analytes, a calibration curve was constructed on the basis of seven points in the concentration range of 0.5–100 ng/L, whereas the corresponding IS was added at 50 ng/L. The IS were used to correct matrix effects and recovery of extraction. In the absence of appropriate isotopically labeled analogues, for some of the compounds, quantification was performed with the closely eluting IS (see Table 2). According to the Commission Decision 2002/657/CE, for positive identification, the following criteria were adopted: (1) LC chromatographic retention time agreement within 2%; and (2) relative abundance of the two transitions, selected as precursor ion and product ion, fall within a range ±20% [44]. Identification of the target analytes was accomplished by comparing the retention time and UHPLC-MS/MS signals of the target compounds in the samples with those of standards analyzed under the same conditions. Complete elution of analytes and absence of carryover were checked injecting blank samples after each batch of samples. Throughout the whole determination procedure, contamination of blanks (water and methanol) was never observed.
Results and discussion
Optimization of online procedure and LC-LC operational parameters
Optimization of the online procedure was done by a series of tests to achieve the optimum extraction performance. Throughout the study, the following key parameters have been tested as essential in the development of an online procedure: sample loading flow rate, elution time, the percentage of organic solvent in the aqueous-organic eluent mixture, pH, and volume of analyzed samples.
A comparative study employing three types of online columns with different chemical modifications (i.e., Hypersil GOLD™ aQ, Hypersil GOLD™ PEP, Hypersil GOLD™ PFP) was previously reported by Gorga et al. [21] for the analysis of endocrine disruptors including some natural and synthetic estrogens. In our approach, the Hypersil GOLD™ aQ provided good extraction recoveries for most of the analytes, including the E3 as the least hydrophobic one.
Different loading flow rates from the injection loop to the SPE column can cause great differences in the preconcentration efficiency, making its optimization a necessary step. After testing loading flow rates in the range from 1000 to 3000 μL/min, results showed that the preconcentration efficiency was increasing when flow rate was faster than 1000 μL/min because the free hormones were strongly retained in the cartridge, especially the less polar ones (i.e., E2, E1, EE2, and DES). However, an additional increase of flow rate produced losses in the chromatographic area response for E2, EE2, and DES (see Fig. S4 in the ESM). A similar behavior was observed for all conjugated estrogens. For the compound that elutes first in the chromatographic analysis (i.e., E3), a slight initial increase was observed by increasing flow rate to 1750 μL/min, but considerable analyte losses of this compound were observed at higher flow rates. Since conjugated forms of estrogens are less demanding compounds generally yielding the lowest MDL values, the negative impact of peak area response was considered acceptable. Therefore, a flow rate of 1750 μL/min was selected as a compromise in order to decrease sample loading time while allowing better interactions between the analytes in the water samples and the surface of the sorbent, giving the highest analyte peak response for the analyzed compounds.
The elution time is determined as the time required for target compounds to be completely eluted from the SPE column to the analytical column. A short elution time may lead to incomplete elution, while a long elution time may be time-consuming and increase the possibility of eluting the sample matrix [38]. In our study, chromatographic separation was performed under gradient elution conditions using methanol and water and the effect of elution time was checked for values of 5.25–6.75 min. As shown in Fig. S5 in the ESM, the chromatographic peak areas of estrogens proportionally increased with the elution time, with the effect being relevant especially for the less polar compounds which are strongly retained in the SPE column. Nevertheless, too slow elution from SPE preconcentration column results in peak broadening, which may cause a decrease in sensitivity [45]. Therefore, after running the experiments applying different elution times, the optimal elution time of 5.5 min was selected accordingly, whereupon all analyzed analytes could be eluted from the SPE column.
The mobile phase in the elution step was optimized to ensure the absolute desorption of the all analyzed estrogens and to obtain baseline separation of the analytes. The influence of methanol percentage ranging from 10 to 75% on chromatographic response has been investigated showing the best chromatographic response at methanol percentage of 50%. The additional increase in the methanol percentage up to 75% showed lower values in the mean chromatographic area for EE2, E2, E3, DES, and E1. In contrast, a different trend of chromatographic response was only observed for E3-3S. Similar results by using a higher ratio of methanol have been reported by Wang et al. [36] and Ciofi et al. [37] indicating that some of the estrogens such as E2, E1, and DES could not be baseline separated.
Influence of sample pH on the adsorption efficiency
Sample pH is reported as an important parameter that may influence greatly the extraction efficiency of the SPE procedure [36, 46, 47]. A majority of target analytes, mostly free estrogen compounds, have relatively high pKa vales (9–10) (see ESM Table S1) [48], so basic conditions should favor obtaining better results in the extraction process. Studies of Guo et al. [38] and Iparraguirre et al. [49] have shown that the addition of ammonia and basic conditions in mobile phases in ESI result in an improved ionization efficiency and consequently lower limits of detection of E1, E2, and EE2. Our preliminary results showed that using ammonia as modifier in the mobile phase yielded poor peak shape and overall not satisfactory results. However, increasing pH of the sample resulted in a remarkable improvement in terms of higher chromatographic response and signal to noise (S/N) (see Fig. S6 in the ESM). We compared both sodium and ammonium hydroxide to adjust sample pH, in the range between pH 8 and 11, and the first one provided better recoveries and response for the vast majority of estrogens analyzed. The results of the comparative study of different pH values of the samples are summarized in Fig. 1. As shown, an increase in pH to 11 resulted in the best ion response (peak area) for compounds requiring extremely low MDLs, i.e., E1, E2, and EE2.
However, there was a concern that such a high pH may potentially affect the performance of the column, either in terms of extraction efficiency or in terms of chromatographic response. Also, there was an issue of premature aging of the column that may cause the pressure buildup and ultimate clogging when the online SPE column is coupled with an analytical column [50]. Thus, the column lifetime was considered as an important parameter and special attention was paid to evaluate the potential negative effect of using such high pH. However, no negative effect occurred, and it is worth mentioning that throughout all the optimization study, the same SPE column was used, and no apparent increase of column pressure, no remarkable changes on peak shapes, and decrease of preconcentration efficiency were observed, even after thousands of injections. Thus, the pH of the samples was adjusted to pH 11 for further analysis.
Sample volume
In order to evaluate the influence of the loading volume on the method detection limits, three different volumes (2, 4, and 5 mL) were tested. Both peak areas and the S/N response of analytes were monitored as a function of injection volumes. Results shown in Fig. 2 indicate that the peak areas for eight of ten analytes increased nearly proportionally with the introduction of higher sample volumes (5 mL), meaning that higher volumes are beneficial and needed to obtain low MDLs. Nevertheless, it was noted that the introduction of a larger volume of the sample caused an increase of unwanted interfering compounds, due to the presence of co-extracted substances in the environmental water that may differently affect the signal variability of each analyte. A strong impact of the interferences was observed for E3 and DES, whose peaks were accompanied by another peak caused by the presence of interferences, thus making the quantification of these compounds difficult (see Fig. S7 in the ESM). However, in this case, careful selection of retention time windows improved S/N and facilitated the quantification. Data acquisition performed under scheduled SRM showed that interferences were nearly eliminated and applied conditions show higher S/N ratios for the analyzed estrogens. Still, with increasing sample volumes, notable losses were observed for E3 and E3-3S, as previously observed by Naldi et al. [51]. Hence, a trade-off between higher sensitivity for all analytes and somehow higher MDLs for E3 and E3-3S was made.
Method performance evaluation
The matrix effect is a common problem in UHPLC-MS/MS, especially when using an ESI, which can have a significant impact on analyte signal and can influence the reproducibility, linearity, and accuracy of the method. It should be specifically evaluated, since it is strongly dependent on the sample type and is unpredictable [52].
Herein, the effects of sample matrix interferences on the online extraction were evaluated together with the matrix effects on efficiency of ionization and estimated as process efficiency (PE). As reported by other authors, process efficiency represents the combination between the matrix effect and the recovery of the sample [53,54,55]. PE was calculated by comparing the mean peak areas for target compounds in standard solution, with the mean peak area of the matrix spiked at the same concentration of the standard. The following equation was applied in the three evaluated matrices (i.e., river water, effluent, and influent):
Where A spiked matrix is the average peak area of the analyte measured in the spiked sample matrix (n = 3), A unspiked matrix is the average peak area of the unspiked sample matrix (n = 3), and A standard represents the average peak area of the standard solution spiked in HPLC grade water at the same concentration of the standard (n = 3). Target compounds for PE evaluation were spiked at 5 ng/L (Fig. 3).
The values lower or greater than 100% indicate signal suppression or enhancement in the samples, respectively. As shown in Fig. 3, the results for river water evidenced high signal suppression for conjugated estrogens, while the signal enhancement was observed for the majority of the free estrogens. In more complex matrices, such as WWTP effluent and especially in WWTP influent, all compounds showed consequential ion suppression. As reported previously [56], signal suppression is strongly dependent on the amount of dissolved organic carbon (DOC), such as surfactants, volatile compounds, and co-eluting analytes present in the matrix [37]. Therefore, it is of crucial importance to account for the matrix effect in the analyte quantification. Herein, we used deuterated standards to overcome the matrix effect. Figure 3 shows that matrix components in river water, effluent, and influent wastewater samples had no considerable effect on signal responses of the target compounds after internal standard corrections since these labeled compounds have similar suppression/enhancement as the target compounds. This proves that using appropriate deuterated internal standards helps to overcome the matrix effect and to reliably determine estrogens at environmentally relevant concentrations.
The method performance was evaluated through estimation of the linearity, sensitivity (by calculating instrumental detection limits (IDL), MDL, and MQL), intraday (repeatability), and interday precision (reproducibility). Results for analytical method validation parameters obtained in HPLC water are summarized in Table 3, whereas Table 4 shows the parameters determined for each matrix (surface, effluent, and influent).
The chromatographic response demonstrated good linearity in the range from 0.5 to 100 ng/L, with excellent determination coefficients (r 2) of linear regressions greater than 0.9990 for all analyzed compounds. Excellent recoveries were obtained after spiking HPLC water in triplicate at 5 ng/L, with values over 87% and precision calculated as relative standard deviation (RSD, %) with not higher than 5%. IDL were calculated as S/N = 3 for the lowest point of the calibration curve constructed in HPLC grade water. Obtained IDL values ranged from 0.01 to 0.36 pg injected. These IDL values are in a similar range to those previously reported by Gorga et al. [21]. Repeatability and reproducibility were also acceptable showing %RSD values below 7.8 and 18.1%, respectively. The majority of obtained values fall below 7%, therefore evidencing the good precision of the method (Table 3).
MDLs and MQLs were experimentally estimated as the minimum detectable amount of analyte with a S/N of 3 and 10, respectively, in different matrices tested (Table 4). As mentioned before, the main analytical challenge in the analysis of hormones in environmental samples is the need to detect below nanogram per liter levels. Consequently, the excellent robustness and the capability to detect such low concentrations are essential in the analysis of estrogen compounds in real water samples. The MDLs and MQLs were matrix dependent with values ranging from 0.02 to 0.07 ng/L and 0.07 to 0.22 ng/L in river water, from 0.02 to 0.12 ng/L and 0.08 to 0.97 ng/L in WWTP effluent, from 0.03 to 0.3 ng/L and 0.10 to 1 ng/L in WWTP influent, respectively. It should be highlighted that the obtained MDL values obtained for river water comply with the required maximum acceptable method detection limit for surface waters (0.035 ng/L for EE2 and 0.4 ng/L for E1 and E2) set in the Watch list of the European Commission [18]. In river water, WWTP influent and effluent satisfactory recoveries with values over 73% and below 120% were obtained for the majority of compounds. The overall method precision, calculated as RSD (%), was also satisfactory and ranged from 1 to 15% for all compounds and matrices tested, showing higher RSD values for most compounds in wastewater (Table 4). The online SRM chromatogram obtained for all the compounds spiked at 5 ng/L in a real river water sample is shown in the ESM in Fig. S8.
Comparison with previously published online SPE-UHPLC methods
The online SPE-UHPLC-MS/MS proposed herein for estrogen analysis in surface and wastewater was compared to the ones previously reported in the literature (Table 5). To the best of our knowledge, the lowest MDLs currently reported for estrogens are obtained by Gorga et al. [21], who investigated estrogens together with other related EDCs. Using a sample volume of 5 mL, the authors achieved MDLs for E1, E2, and EE2 of 0.05, 0.037, and 0.14 ng/L, respectively, for surface water. In the case of wastewater, injection volume was set at 2 mL and MDLs for compounds E1, E2, and EE2 of 0.14, 0.59, and 3.8 ng/L and 0.14, 5.4, and 4.2 ng/L were obtained for effluent and influent samples, respectively. Other authors also reported MDLs lower than the nanogram per liter level. For example, Guo et al. [38] developed a simple and suitable procedure for the simultaneous analysis of estrogen and androgen compounds in water samples by using 50 mL of sample reaching MDLs in the range of 0.5–2, 0.5–1, and 0.5–2 ng/L, respectively, for surface water, WWTP effluent, and influent. All other online methods reported much higher MDLs [37, 43, 51, 57].
By adjusting the pH of the sample to 11 and by optimizing all operational parameters, we have succeeded to obtain a robust method yielding noticeable lower MDLs complying therefore with the requirements of the EC Decision 2015/495 [18]. Figure 4 shows one of the online chromatograms obtained at 5 ng/L for E2, E1, and EE2 in surface water sample. As seen, the S/N depends on the compound and matrix complexity, but still high enough to meet the required criteria. As mentioned before, two SRM transitions were monitored for each compound. The first transition was used for quantification purposes, whereas the second one was used to confirm the identity of the target compounds, including the isotopically labeled internal standards (see Fig. S9 in the ESM). Furthermore, our method provides quantitative measurements of both free estrogens and conjugates in a single run for each sample.
Method application on real samples
As a part of the validation procedure, the method developed was applied to the analysis of the target analytes in real environmental water samples. The established method was successfully applied to assess the concentration of the estrogens in river water and raw wastewater collected in northern Serbia. The results are summarized in Table 6. Of the ten compounds investigated, eight estrogens were detected in raw wastewater, while six of them were detected in river water. Despite the very low MDL achieved with the proposed methodology, none of synthetic estrogens, such as EE2 and DES, were found in the analyzed samples.
Levels detected in raw wastewater were similar in all analyzed samples and were in the high nanogram per liter range. Although the dilution could be significant in river water, the levels observed in wastewater discharged directly into the river without treatment are of potential concern and may result in river concentrations sufficiently high to induce estrogenic activity in aquatic species. The mean concentrations of estrogens detected in river water and wastewater samples are summarized and given in the ESM on Tables 4Sa and 4Sb, respectively.
Figure 5 shows the SRM chromatograms obtained in the online analysis of the target analytes under the optimized conditions in two of the analyzed real samples of wastewater. Generally, the levels found in our study are in a similar range as those recently reported by other authors [21, 37, 38, 43]. However, it should be emphasized that this sampling campaign was not designed as an environmental monitoring of the analyzed compounds but only to test the applicability of the developed methodology. Thus, spatial and temporal variability was not taken into account.
Conclusions
A fully automatic online method was developed and validated for the analysis of natural and synthetic estrogens and their conjugates in both river and wastewater samples. By using the tandem mass spectrometry detection instrument, UHPLC-MS/MS has substantially improved the performance of chromatographic methods by reducing MDLs and aiding analyte identification. The integrated system improved analytical performance (analyte reliability and repeatability of the method), increased sample throughput, and reduced operating costs and contamination risks. The methodology also ensures cost efficiency, saves time of analysis, and minimizes errors from manual manipulation. This proposed method represents an improvement in terms of lower detection limits and higher selectivity, compared to the previously published online methods. An effective compromise was achieved by pH adjustment of the samples. The negative ion intensity was remarkably improved when pH was set at basic conditions into the samples, and a remarkable increase in the average recoveries was observed for most of the analyzed compounds. The proposed methodology is suitable to comply with the requirements for monitoring of hormones at the European level reaching low MDL set up in the EC Decision 2015/495. The applicability of the method was demonstrated on real samples by confirming widespread occurrence of free and conjugated estrogens in environmental waters.
References
Allner B, von der Gönna S, Griebeler EM, Nikutowski N, Weltin A, Stahlschmidt-Allner P. Reproductive functions of wild fish as bioindicators of reproductive toxicants in the aquatic environment. Environ Sci Pollut Res Int. 2010;17(2):505–18.
Jobling S, Beresford N, Nolan M, Rodgers-Gray T, Brighty GC, Sumpter JP, Tyler CR. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol Reprod. 2002;66(2):272–81.
Sumpter JP, Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995;103(7):173–8.
Thorpe KL, Cummings RI, Hutchinson TH, Scholze M, Brighty G, Sumpter JP, Tyler CR. Relative potencies and combination effects of steroidal estrogens in fish. Environ Sci Technol. 2003;37(6):1142–9.
Ternes TA, Stumpf M, Mueller J, Haberer K, Wilken RD, Servos M. Behavior and occurrence of estrogens in municipal sewage treatment plants—I. Investigations in Germany, Canada and Brazil. Sci Total Environ. 1999;225(1–2):81–90.
Hansen PD, Dizer H, Hock B, Marx A, Sherry J, McMaster M, Blaise C. Vitellogenin a biomarker for endocrine disruptors. TrAC Trends Anal Chem. 1998;17(7):448–51.
Larsson DGJ, Adolfsson-Erici M, Parkkonen J, Pettersson M, Berg AH, Olsson PE, Förlin L. Ethynilestradiol an undesired fish conctraceptive? Aquat Toxicol. 1999;45(2–3):91–7.
Kim J-H, Lee SY, Lee H-J, Yoon N-Y, Lee W-S. The efficacy and safety of 17α-estradiol (Ell-Cranell® alpha 0.025%) solution on female pattern hair loss: single center, open-label, non-comparative, phase IV study. Ann Dermatol. 2012;24(3):295–305.
Kuch HM, Ballschmiter K. Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC–(NCI)–MS in the picogram per liter range. Environ Sci Technol. 2001;35(15):3201–6.
Kim SD, Cho J, Kim IS, Vanderford BJ, Snyder SA. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007;41(5):1013–21.
Matějíček D, Kubáň V. Enhancing sensitivity of liquid chromatographic/ion-trap tandem mass spectrometric determination of estrogens by on-line pre-column derivatization. J Chromatogr A. 2008;1192(2):248–53.
Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ Sci Technol. 2009;43(3):597–603.
Pedrouzo M, Borrull F, Pocurull E, Marcé RM. Estrogens and their conjugates: determination in water samples by solid-phase extraction and liquid chromatography–tandem mass spectrometry. Talanta. 2009;78(4–5):1327–31.
López-Roldán R, de Alda ML, Gros M, Petrovic M, Martín-Alonso J, Barceló D. Advanced monitoring of pharmaceuticals and estrogens in the Llobregat River basin (Spain) by liquid chromatography–triple quadrupole-tandem mass spectrometry in combination with ultra performance liquid chromatography–time of flight-mass spectrometry. Chemosphere. 2010;80(11):1337–44.
Sodré FF, Pescara IC, Montagner CC, Jardim WF. Assessing selected estrogens and xenoestrogens in Brazilian surface waters by liquid chromatography–tandem mass spectrometry. Microchem J. 2010;96(1):92–8.
Chang H, Wan Y, Wu S, Fan Z, Hu J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: comparison to estrogens. Water Res. 2011;45(2):732–40.
Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off J Eur Commun. L327/1 (2000) 1–73.
Commission Implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council (notified under document C (2015) 1756).
Guedes-Alonso R, Montesdeoca-Esponda S, Sosa-Ferrera Z, Santana-Rodríguez JJ. Liquid chromatography methodologies for the determination of steroid hormones in aquatic environmental systems. Trends Environ Anal Chem. 2014;3-4:14–27.
Kuster M, López de Alda MJ, Hernando MD, Petrovic M, Martín-Alonso J, Barceló D. Analysis and occurrence of pharmaceuticals, estrogens, progestogens and polar pesticides in sewage treatment plant effluents, river water and drinking water in the Llobregat river basin (Barcelona, Spain). J Hydrol. 2008;358:112.
Gorga M, Petrovic M, Barceló D. Multi-residue analytical method for the determination of endocrine disruptors and related compounds in river and waste water using dual column liquid chromatography switching system coupled to mass spectrometry. J Chromatogr A. 2013;1295:57–66.
Vega-Morales T, Sosa-Ferrera Z, Santana-Rodríguez JJ. Determination of various estradiol mimicking-compounds in sewage sludge by the combination of microwave-assisted extraction and LC–MS/MS. Talanta. 2011;85(4):1825–34.
Belfroid AC, Van der Horst A, Vethaak AD, Schäfer AJ, Rijs GBJ, Wegener J, Cofino WP. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Sci Total Environ. 1999;225(1–2):101–8.
D’ascenzo G, Di Corcia A, Gentili A, Mancini R, Mastropasqua R, Nazzari M, Samperi R. Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci Total Environ. 2003;302(1–3):199–209.
Gentili A, Perret D, Marchese S, Mastropasqua R, Curini R, Di Corcia A. Analysis of free estrogens and their conjugates in sewage and river waters by solid-phase extraction then liquid chromatography-electrospray-tandem mass spectrometry. Chromatographia. 2002;56(1):25–32.
Isobe T, Shiraishi H, Yasuda M, Shinoda A, Suzuki H, Morita M. Determination of estrogens and their conjugates in water using solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2003;984:195–202.
Labadie P, Budzinski H. Determination of steroidal hormone profiles along the Jalle d’Eysines River (near Bordeaux, France). Environ Sci Technol. 2005;39(14):5113–20.
Mouatassim-Souali A, Tamisier-Karolak SL, Perdiz D, Cargouet M, Levi Y. Validation of a quantitative assay using GC/MS for trace determination of free and conjugated estrogens in environmental water samples. J Sep Sci. 2003;26(1–2):105–11.
Johnson AC, Belfroid A, Di Corcia A. Estimating steroid oestrogen inputs into activated sludge treatment works and observations on their removal from the effluent. Sci Total Environ. 2000;256(2–3):163–73.
Baronti C, Curini R, D’Ascenzo G, Di Corcia A, Gentili A, Samperi R. Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ Sci Technol. 2000;34(24):5059–66.
Al-Odaini NM, Zakaria MP, Yaziz MI, Surif S. Multi-residue analytical method for human pharmaceuticals and synthetic hormones in river water and sewage effluents by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2010;1217:6791–806.
Huerta-Fontela M, Galceran MT, Ventura F. Fast liquid chromatography–quadrupole-linear ion trap mass spectrometry for the analysis of pharmaceuticals and hormones in water resources. J Chromatogr A. 2010;1217:4212–22.
Liu S, Ying G-G, Zhao J-L, Chen F, Yang B, Zhou L-J, Lai H-J. Trace analysis of 28 steroids in surface water, wastewater and sludge samples by rapid resolution liquid chromatography–electrospray ionization tandem mass spectrometry. J Chromatogr A. 2011;1218:1367–78.
Tomšíková H, Aufartová J, Solich P, Nováková L, Sosa-Ferrera Z, Santana-Rodríguez JJ, Nováková L. High-sensitivity analysis of female-steroid hormones in environmental samples. Trends Anal Chem. 2012;34:35–58.
Viglino L, Aboulfadl K, Prévost M, Sauvé S. Analysis of natural and synthetic estrogenic endocrine disruptors in environmental waters using online preconcentration coupled with LC-APPI-MS/MS. Talanta. 2008;76:1088–96.
Wang S, Huang W, Fang G, He J, Zhang Y. On-line coupling of solid-phase extraction to high-performance liquid chromatography for determination of estrogens in environment. Anal Chim Acta. 2008;606(2):194–201.
Ciofi L, Fibbi D, Chiuminatto U, Coppini E, Checchini L, Del Bubba M. Fully-automated on-line solid phase extraction coupled to high-performance liquid chromatography–tandem mass spectrometric analysis at sub-ng/L levels of selected estrogens in surface water and wastewater. J Chromatogr A. 2013;1283:53–61.
Guo F, Liu Q, Qu G-b, Song S-j, Sun J-t, Shi J-b, Jiang G-b. Simultaneous determination of five estrogens and four androgens in water samples by online solid-phase extraction coupled with high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2013;1281:9–18.
Valsecchi S, Polesello S, Mazzoni M, Rusconi M, Petrovic M. On-line sample extraction and purification for the LC–MS determination of emerging contaminants in environmental samples. Trends Environ Anal Chem. 2015;8:27–37.
Miège C, Bados P, Brosse C, Coquery M. Method validation for the analysis of estrogens (including conjugated compounds) in aqueous matrices. TrAC Trends Anal Chem. 2009;28(2):237–44.
Qin F, Zhao YY, Sawyer MB, Li XF. Column-switching reversed phase-hydrophilic interaction liquid chromatography/tandem mass spectrometry method for determination of free estrogens and their conjugates in river water. Anal Chim Acta. 2008;627(1):91–8.
Vuillet E, Wiest L, Baudot R, Grenier-Loustalot M-F. Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A. 2008;1210(1):84–91.
Fayad PB, Prévost M, Sauvé S. On-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry optimized for the analysis of steroid hormones in urban wastewaters. Talanta. 2013;115:349–60.
European Commission. Council Regulation (EEC)-OJEC L 224-Annex 11990.
Soriano JM, Jiménez B, Redondo MJ, Moltó JC. Comparison of different sorbents for on-line liquid-solid extraction followed by high-performance liquid chromatographic determination of nitrogen-containing pesticides. J Chromatogr A. 1998;822(1):67–73.
Quintana JB, Carpinteiro J, Rodríguez I, Lorenzo RA, Carro AM, Cela R. Determination of natural and synthetic estrogens in water by gas chromatography with mass spectrometric detection. J Chromatogr A. 2004;1024(1–2):177–85.
Rodriguez-Mozaz S, Lopez de Alda MJ, Barceló D. Picogram per liter level determination of estrogens in natural waters and waterworks by a fully automated on-line solid-phase extraction-liquid chromatography-electrospray tandem mass spectrometry method. Anal Chem. 2004;76(23):6998–7006.
Lewis KM, Archer RD. pK values of estrone, 17β-estradiol and 2-methoxyestrone. Steroids. 1979;34(5):485–99.
Iparraguirre A, Navarro P, Rodil R, Prieto A, Olivares M, Etxebarria N, Zuloaga O. Matrix effect during the membrane-assisted solvent extraction coupled to liquid chromatography tandem mass spectrometry for the determination of a variety of endocrine disrupting compounds in wastewater. J Chromatogr A. 2014;1356:163–70.
Souverain S, Rudaz S, Veuthey JL. Restricted access materials and large particle supports for on-line sample preparation: an attractive approach for biological fluids analysis. J Chromatogr B. 2004;801(2):141–56.
Naldi AC, Fayad PB, Prévost M, Sauvé S. Analysis of steroid hormones and their conjugated forms in water and urine by on-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry. Chem Cent J. 2016;10:30.
Antignac JP, de Wasch K, Monteau F, De Brabander H, Andre F, Le Bizec B. The ion suppression phenomenon in liquid chromatography–mass spectrometry and its consequences in the field of residue analysis. Anal Chim Acta. 2005;529(1–2):129–36.
Buhrman DL, Price PI, Rudewiczcor PJ. Quantitation of SR 27417 in human plasma using electrospray liquid chromatography-tandem mass spectrometry: a study of ion suppression. J Am Soc Mass Spectrom. 1996;7(11):1099–105.
Trufelli H, Palma P, Famiglini G, Cappiello A. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom Rev. 2011;30(3):491–509.
Farré MJ, Insa S, Mamo J, Barceló D. Determination of 15 N-nitrosodimethylamine precursors in different water matrices by automated on-line solid-phase extraction ultra-high-performance-liquid chromatography tandem mass spectrometry. J Chromatogr A. 2016;1458:99–111.
EPA Method 539 Document No. 815-B-10-001 (2010) Determination of hormones in drinking water by solid phase extraction (SPE) and liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS).
Vega-Morales T, Sosa-Ferrera Z, Santana-Rodríguez JJ. Development and optimisation of an on-line solid phase extraction coupled to ultra-high-performance liquid chromatography-tandem mass spectrometry methodology for the simultaneous determination of endocrine disrupting compounds in wastewater samples. J Chromatogr A. 2012;1230:66–76.
Acknowledgements
This work was supported by the Spanish Ministry of Economy and Competitiveness through the coordinated project TransformCoast (CGL2014-56530-C4-4-R) and by the Generalitat de Catalunya (Consolidated Research Groups 2014SGR291-ICRA). M. Čelić acknowledges for the grant received from the Spanish Ministry of Economy and Competitiveness (BES-2015-072297). Partial financial support from the Ministry of Education, Science and Technological Development of the Republic of Serbia, through the project no. 172050, is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Advances in LC-MS/MS Analysis with guest editors Damià Barceló and Mira Petrovic.
Electronic supplementary material
ESM 1
(PDF 380 kb)
Rights and permissions
About this article
Cite this article
Čelić, M., Insa, S., Škrbić, B. et al. Development of a sensitive and robust online dual column liquid chromatography-tandem mass spectrometry method for the analysis of natural and synthetic estrogens and their conjugates in river water and wastewater. Anal Bioanal Chem 409, 5427–5440 (2017). https://doi.org/10.1007/s00216-017-0408-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0408-5