Abstract
The fluorescent microsphere has been increasingly used as detecting label in immunoassay because of its stable configuration, high fluorescence intensity, and photostability. In this paper, we developed a novel lateral flow fluorescent microsphere immunoassay (FMIA) for the determination of sulfamethazine (SMZ) in milk in a quantitative manner with high sensitivity, selectivity, and rapidity. A monoclonal antibody to SMZ was covalently conjugated with the carboxylate-modified fluorescent microsphere, which is polystyrene with a diameter of 200 nm. Quantitative detection of SMZ in milk was accomplished by recording the fluorescence intensity of microspheres captured on the test line after the milk samples were diluted five times. Under optimal conditions, the FMIA displays a rapid response for SMZ with a limit of detection of as low as 0.025 ng mL−1 in buffer and 0.11 μg L−1 in milk samples. The FMIA was then successfully applied on spiked milk samples and the recoveries ranged from 101.1 to 113.6 % in the inter-batch assay with coefficient of variations of 6.0 to 14.3 %. We demonstrate here that the fluorescent microsphere-based lateral flow immunoassay (LFIA) is capable of rapid, sensitive, and quantitative detection of SMZ in milk.

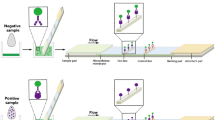
Schematic illustration of the strategy for sulfamethazine detection using a lateral flow fluorescent microsphere immunoassay
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoassay is one of the most popular screening methods for the detection of target molecules in a variety of fields, such as medical diagnosis, environmental monitoring, and food safety [1–3]. Currently, the most often used labels in immunoassays are enzymes (e.g., horseradish peroxidase), fluorescein (e.g., fluorescein isothiocyanate), colloidal gold particles, etc. In order to meet the requirement of sensitive detection, recently, some new materials with superior properties have been synthesized to facilitate high-performance detection for advanced immunoassay, such as fluorescent microsphere, quantum dot, carbon nanoparticles, etc. [4–6].
The fluorescent microsphere, which has a stable configuration, high fluorescence intensity, and photostability, has become a novel fluorescent label and is already employed in biomedical, genomics, and medical diagnosis fields [5, 7–9]. It also presents a narrow distribution of fluorescence intensity and size along with extensive selection of colors available, thus, it is potentially more accurate and diverse than the current diagnostic test systems in use and can be quantitatively analyzed, thus providing an excellent tool for novel detection methods.
Veterinary drug residues in food stuffs have caused very serious problems and pose a potential hazard to consumers, including toxicity risks, antibacterial resistance, and carcinogenic presumption [10–12]. Thus, rapid, sensitive, and specific approaches are imperative in detecting veterinary drug residues of animal products. Until now, no report on the detection of veterinary drug residues using fluorescent microsphere as label has been published. Sulfamethazine (SMZ) belongs to the sulfonamides, which is one of the most frequently utilized individual sulfonamides in veterinary medicine for prevention and treatment of infection throughout the world [13]. Some regulations have been established in many countries and international institutions to control the use of SMZ. In the European Union and China, the maximum residue limits (MRLs) for SMZ in edible animal tissues is set at 100 μg kg−1 [14, 15], whereas MRLs established by Codex Alimentarius Commission is much lower at 20 μg kg−1 in cattle milk [16].
In previous reports, lateral flow immunoassays (LFIA) using colloidal gold as label for the detection of SMZ have been accomplished [17–20]. O'Keeffe et al. developed a LFIA for the detection of SMZ with a cut-off value at 6.3 μg L−1, while, the LFIA developed by Li et al. presented 8 μg L−1 in swine urine [17, 18]. For pig muscle, milk, and fish, a colloidal gold immunoassay for the determination of SMZ reported by Wang et al. showed a cut-off value at 20 μg kg−1 [19]. Recently, Guo et al. developed a three test-line LFIA for simultaneous detection of SMZ, sulfadiazine, and sulfaquinoxaline residues in egg and chicken muscle with a cut-off value at 80 μg kg−1 [20]. Li et al. described a LFIA for the detection of sulfadiazine in eggs and chickens with a cut-off level at 5 μg kg−1 [21]. Compared with the conventional strategies like enzyme-linked immunosorbent assay (ELISA), the LFIA technology utilizing colloidal gold as label outstands in both scientific and industrial realm because of its rapid and on-site exploitation, which make it possible to detect SMZ with high speed, acceptable sensitivity, and specificity in most cases. However, LFIAs generally cannot render results in quantitative manner in most cases, and sometimes lack of sensitivity to meet the high requirement for more strictly supervision of SMZ in animal-origin food.
In the present paper, a reliable method of lateral flow immunoassay using fluorescent microsphere as label was proposed. The fluorescent microsphere immunoassay (FMIA) is simple, rapid, and highly sensitive for the detection of SMZ in milk samples. It takes less than 10 min to complete the test and the result can be quantitative analyzed by an ESE-Quant LFR Fluorescence reader. Compared with previous study, this method exhibits high sensitivity and can be quantitatively analyzed.
Materials and methods
Reagents and materials
SMZ and other sulfonamides, bovine serum albumin (BSA), ovalbumin (OVA), goat anti-mouse IgG, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), and N-hydroxysuccinimide (NHS) were provided by Sigma-Aldrich (St. Louis, MO). FluoSpheres® Carboxylate-Modified Microspheres, 0.1 μm, Red Fluorescent (580/605), 2 % solids, were purchased from Invitrogen (Carlsbad, CA). 2-(4-morpholino)-ethanesulfonic acid (MES) was purchased from Aladdin Chemistry CO. Ltd (Shanghai, China). Dextran 4000 was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). HiTrap protein A Sepharose 4 Fast Flow was purchased from Pharmacia Corporation (Uppsala, Sweden). Purified water was obtained using a Milli-Q water purification system (Millipore, Bedford, MA). The nitrocellulose (NC) membrane (Millipore, HF13520s25) was purchased from Millipore Corporation (Millipore, Bedford, MA). The sample pad (CH37K) and the absorbance pad (SB08) were supplied by Shanghai Liangxin Co. Ltd. (Shanghai, China). Monoclonal antibody (MAb) to SMZ was previously prepared in our laboratory and purified by using Protein A before use [22]. The coating antigen was prepared by conjugating SMZ to OVA using the diazotization method as described in our previous work [23].
Apparatus
The NanoDrop ND-1000 spectrophotometer was purchased from Gene Company Ltd. (Hong Kong, China). The ZX1000 Dispensing Platform and the CM4000 Guillotine Cutting Module used to prepare the LFIA were purchased from BioDot Inc. (Irvine, CA). The ESE-Quant LFR Fluorescence reader was purchased from QIAGEN (Dusseldorf, Germany). The UV spectrometer was provided by Qiangyun Co. (Shanghai, China).
MAb purification
The MAb was dialyzed against PBS buffer (0.01 M, pH = 7.4) at 4 °C for 3 days, and then was purified by using a HiTrap MabSelect SuRe (GE Healthcare (Little Chalfont, UK)) and a HiTrap HIC column (GE Healthcare (Little Chalfont, UK)) orderly referring to the instruction. The concentration of purified MAb was determined with the spectrophotometer at 280 nm and diluted to 1 mg mL−1 with deionized water and stored at 4 °C and finished within 2 days.
Fluorescent microsphere-MAb conjugates
The MAb was conjugated to fluorescent microspheres according to the protocol for the carboxylate-modified microspheres from Invitrogen with slightly modifications. Briefly, 15 μg purified MAb was dissolved of in 400 μL MES buffer (0. 05 M, pH = 6.5) in a glass centrifuge tube. After adding 5 μL of a 2 % aqueous suspension of carboxylate-modified microsphere, 2 μg of EDC and 2 μg of NHS to the centrifuge, the mixture was blended by vortexing and the pH was adjusted to 6.5 with dilute NaOH. The reagents were incubated on an orbital shaker for 2 h at room temperature. An amount of 300 μg glycine was added to quench the reaction for 30 min at room temperature. After centrifugation at 12,000 rpm at 4 °C for 15 min, the protein-labeled microsphere particles were separated from unreacted protein. The sediment was re-suspended in 100 μL solution (0.01 M phosphate buffer (PB) containing 1 % BSA, 0.4 % dextran 4000, and 0.2 % Tween 20). The suspension was dispersed and blended by supersonic vibration for 10 min. FM-MAb conjugates were stored at 4 °C until needed in the assay.
LFIA preparation
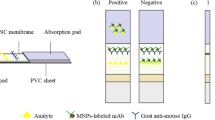
The strip of LFIA is consisted of three sections as follows: sample pad, NC membrane, and absorbent pad. The sample pad was made from glass fiber and treated with 0.01 M PBS buffer (pH = 7.4) containing 0.1 % Tween 20 as surfactant and then dried at 37 °C for 3 h. Meanwhile, the SMZ-OVA antigen and goat anti-mouse IgG were separately diluted to 0.3 and 1 mg mL−1 with coating buffer (0.02 M carbonate buffer, pH = 9.6), and then sprayed onto the NC membrane by the ZX1000 dispensing platform as the test and control lines, respectively. The desired volumes were 0.7 μL/mm for the test line and 0.8 μL/mm for the control line. The NC membrane was dried at 37 °C for 1 h and stored under dry conditions at room temperature until needed. Then, all of the parts were assembled on a plastic adhesive backing card using the Batch Laminating System LM5000, and each part overlapped 2 mm to ensure the solution covered the entire strip during the assay. A desired volume (1 μL) of FM-MAb conjugates was dispensed onto the sample pad using an Eppendorf dispenser. Finally, the strip was cut with a 3-mm width using Guillotine Cutting System CM 4000 for the following assay.
LFIA procedure
When a sample (about 70 μL) is placed on the sample pad, the FM-MAb conjugates are dissolved and then moved up along the membrane with the liquid by capillary action. When no SMZ is present in the sample, FM-MAb conjugates would be trapped by the immobilized SMZ-OVA at the test-line on the NC membrane to form an intense red-colored fluorescent band. The unreacted FM-MAb conjugates migrated further and could be trapped by immobilized goat-anti-mouse IgG, forming the control line. The presence of two fluorescent lines indicates a negative result. Conversely, if the sample contains SMZ, it would compete with the SMZ-OVA at the test line for the limited amount of FM-MAb. When a sufficient amount of SMZ is in the sample, the SMZ would bind to all the FM-MAb and fully prevent the binding of FM-MAb to SMZ-OVA. Therefore, at the test line position, the fluorescent band would not be seen. In any assay, the control line should always reveal to ensure the system is working properly. Quantitative analysis can be realized by reading the fluorescence intensity of test line with the fluorescence reader.
Standard curves and specificity
To establish the calibration curves of the LFIA, the standard samples were prepared by diluting a 2 mg mL−1 SMZ stock solution in anhydrous DMF in both PBS buffer (0.01 M, pH = 7.4) and pretreated milk sample to different levels. The ultimate concentration of SMZ in both PBS and diluted milk samples were 0, 0.01, 0.1, 1, 10, and 100 μg L−1. The fluorescence intensity for each standard sample was determined five times (n = 5) using the fluorescence reader, and the mean intensity calculated. The standard curve was established using fluorescence intensity and log10 of their corresponding SMZ concentration. The linear regression equation was calculated and the relationship between fluorescence intensity and log10 of SMZ concentration was assessed by the correlation coefficient of determination (r 2).
To evaluate the specificity of the LFIA, several sulfonamide compounds including sulfamethazine, N 4-acetyl sulfamethazine, sulfadimethoxine, sulfamerazine, sulfamethoxazole, sulfamonomethoxine, sulfadiazine, sulfathiazole, sulfamethopyrazine, sulfamethizole, sulpfaquinoxaline, and sulfabromomethazine were evaluated at different concentrations (0.1, 1, 10, 50, 100, 200, and 1,000 μg L−1). The cross-reactivity values were calculated using the following equation:
Spiked sample and recovery
SMZ-free skimmed milk was supplied by the National Reference Laboratory for Veterinary Drug Residues (Beijing, China). The milk was spiked with a 2-μg L−1 SMZ stock solution in anhydrous DMF to different concentrations, was blended on a vortex mixer for 20 s, and left to stand at room temperature for 15 min. One milliliter of sample was diluted with 4 mL sample diluents (0.01 M PB, pH = 7.4, containing 0.05 % Tween 20).
To examine the repeatability and accuracy of the test, milk samples spiked with SMZ to the concentration of 0.5, 2.5, 12.5, and 62.5 μg L−1 were pretreated and analyzed three times (n = 3) for the intra-batch assay precision and three successive batches for the inter-batch assay precision. The recovery and coefficient of variation (CV, in percent) were calculated.
Results and discussion
Covalent coupling optimization
The readout of fluorescent intensity is directly influenced by the covalent coupling of fluorescent microspheres to MAb. The efficiency of the coupling is closely related to the purity of the MAb, whereas ascites normally contains some impurities such as lipid and unrelated protein, which will reduce the ratio of coupling of MAb and fluorescent microsphere, resulting in a low fluorescent intensity. In the present study, we selected the HiTrap MabSelect SuRe protein purification column to filter the unspecific substances. Figure 1 shows the fluorescent intensity before and after MAb purification and the accretion of fluorescent intensity being obvious after the purification. The purification of MAb increases the efficiency of coupling, indicating that the purification procedure of ascites before coupling is necessary for effectively coupling of fluorescent microsphere to antibody.
In order to attain the optimal covalent coupling condition, we also carried out a series experiment to determine the coupling ratio of fluorescent microspheres to MAb. Since the fluorescent intensity kept growing with the increase of the concentration of MAb (see Fig. 1), it indicated that even a high concentration of MAb (to 30 μg) would not completely bind the limited amount of microsphere (5 μL), whereas the sensitivity of the test would decline when the concentration of MAb is higher. Balancing these two factors, 15 μg MAb binding to 5 μL microspheres was used as the optimum amount to ensure both a high coupling ratio and the sensitivity of the assay.
The time required, however, for the coupling of fluorescent microspheres with MAb was evaluated from 1 h to overnight at 37 °C (Fig. 2). As a result, incubating the reaction mixture for 2 h at 37 °C showed high stability and the best fluorescent intensity development.
According to the instruction for the fluorescent microspheres from Invitrogen, EDC was always used to activate the surface carboxyl groups on the microsphere particles in the past study [24]. In our study, only using EDC resulted in the polymerization and sedimentation of the microspheres, which leaded to the remains of FM-MAb on the sample pad and NC membrane, thus had negative effects on the accuracy and repeatability of the LFIA. The reaction system still appeared more stable when EDC and NHS were used together. It is probably because NHS created a stable amine-reactive product thus increased coupling efficiency and stability [25, 26]. Several different proportions of EDC and NHS were evaluated (see Table 1) to compare the result of the reaction and the residue of FM-MAb on the conjugate pad and NC membrane. Finally, 2 μg EDC/2 μg NHS was selected as the optimal condition according to the results shown in Table 1.
LFIA optimization
An optimal pH of the buffer is of great significance for the development of fluorescent intensity. Both a higher and lower pH values will make the charge groups on the microspheres be neutralized, leading to the agglomeration and reduction of fluorescent intensity. The influence of pH of MES buffer on the fluorescent intensity is shown in Fig. 3. The maximum fluorescent intensity was achieved when the assay buffer was at pH 6.5.
Subsequently, two coating buffers, namely, carbonate buffer (CB, 0.05 M, pH 9.6) and PB (0.01 M, pH 7.4) were evaluated and compared with immobilize SMZ-OVA and goat anti-mouse IgG on the NC membrane. The color development and stability of the LFIA was better when using CB than PB.
Assay performance was evaluated eventually with respect to the pretreatment of the sample pad. Previous study showed that when sample pad was pretreated with dilution buffer containing the surfactants, such as Tween 20 or PEG, the flow rate of the sample solution was accelerated, the background was eliminated, and the sensitivity and stability of the LFIA were improved [27]. In our study, we evaluated PBS buffer containing different concentration of Tween 20 ranging from 0.01 to 0.15 % and finally selected pretreatment solution consisting of 0.01 M PBS (pH 7.4) containing 0.1 % (v/v) Tween 20.
Sensitivity and specificity
According to the principle described above, the fluorescence intensity on the test line is inversely proportional to the concentration of SMZ in the samples. When the concentration of SMZ was more than 12.5 μg L−1, the color of test line cannot be distinguished by the naked eye under UV light (Fig. 4). The fluorescent signal of the test line can be quantitatively detected by using an ESE-Quant LFR Fluorescence reader even when the concentration of SMZ was 100 μg L−1.
Detection of SMZ standard samples using the LFIA. The strips A–D were for strips loaded with buffer containing 0, 0.1, 2.5, and 12.5 ng mL−1 of SMZ, respectively. The upper is the fluorescence imaging of LFIA under UV light at 365 nm. The more SMZ present in the buffer, the weaker the test line color. The bottom curves are the readout of fluorescence intensity of test lines in the upper strip using the ESE-Quant reader (excitation wavelength, 580 nm and emission wavelength, 605 nm)
The fluorescent intensity decreased as the concentration of SMZ increased in the standard samples (see Fig. 5). There was a reasonable linear relationship between fluorescent density and the log10 of the concentration of SMZ in the range of 0.01–100 μg L−1 in PBS (r 2 = 0.9984). The IC50 and limit of detection of the developed LFIA for SMZ were 1.03 and 0.025 μg L−1.
Table 2 shows the cross-reactivity results obtained by the LFIA. The results show that the LFIA produced significant cross-reactivity with the sulfamethazine (100 %), N 4-acetyl sulfamethazine (65.6 %), sulfamerazine (6.2 %), and sulfabromomethazine (8.1 %) but negligible cross-reactivity (<0.1 %) with sulfadimethoxine, sulfamethoxazole, sulfamonomethoxine, sulfadiazine, sulfathiazole, sulfamethopyrazine, sulfamethizole, and sulfaquinoxaline. The cross-reactivity values of MAb in the LFIA are similar to that observed in the ELISA using the same MAb by He and Liu et al. [22].
Spiked samples analysis
Milk, containing high level of lipids, sugar, and proteins, is a relatively complex matrix, which may have an influence on the development of the immunoassay. In this study, a simple and rapid pretreatment was performed to minimize the matrix interference. The SMZ-free skimmed milk was provided by the National Reference Laboratory for Veterinary Drug Residues (Beijing, China) and then undertaken a fivefold dilution with the PBS diluents (containing 0.05 % Tween 20) to diminish the exhibition of the fluorescent signal on the background. As seen in Fig. 5, the standard curve that was constructed in milk samples was nearly indistinguishable from that which was constructed with the PBS buffer. Consequently, recovery studies were executed with the milk samples spiked with known concentrations of SMZ and the results were summarized in Table 3. Recovery of SMZ in milk samples ranged from 94.6 to 108.3 % in the intra-batch assay determination with CVs of 5.7 to 14.1 and 101.1 to 113.6 % in the inter-batch assay with CVs of 6.0 to 14.3 %, which indicated that this LFIA could successfully measure SMZ in milk samples.
Conclusions
The present study reported a lateral flow fluorescent microsphere immunoassay for rapid and sensitive detection of SMZ in milk. Under optimal conditions, this LFIA is capable of detecting a minimum of 0.11 μg L−1 SMZ in milk sample within 10 min. The IC50 of the assay is 5.58 μg L−1 in milk sample, and the recoveries of SMZ from spiked milk samples ranged from 101.1 to 113.6 % in the inter-batch assay with CVs of 6.0 to 14.3 %. Overall, the novel lateral flow FMIA is a rapid, simple, and sensitive analytical method, which is highly effective for the detection of SMZ residue in milk. The results indicated that fluorescent microsphere could provide powerful probes for the detection of veterinary residues in animal-originating food.
References
Posthuma-Trumpie GA, Korf J, van Amerongen A (2009) Anal Bioanal Chem 393(2):569–582
Josko D (2012) Clin Lab Sci 25(3):179–184
Van Emon JM (2001) J AOAC Int 84(1):125–133
Baptista PV, Doria G, Quaresma P, Cavadas M, Neves CS, Gomes I, Eaton P, Pereira E, Franco R (2011) Prog Mol Biol Transl Sci 104:427–488
Langenhorst RJ, Lawson S, Kittawornrat A, Zimmerman JJ, Sun Z, Li Y, Christopher-Hennings J, Nelson EA, Fang Y (2012) Clin Vaccine Immunol 19:180–189
Posthuma-Trumpie GA, Wichers JH, Koets M, Berendsen LB, Amerongen A (2012) Anal Bioanal Chem 402:593–600
Chen R, Bi Y, Yang G, Liu Z, Zeng B, Tong T (2010) Diagn Mol Pathol 19:172–179
Eucker SA, Hoffman BD, Natesh R, Ralston J, Armstead WM, Margulies SS (2010) Brain Res 1326:128–134
Balasuriya UB, Shi PY, Wong SJ, Demarest VL, Gardner IA, Hullinger PJ, Ferraro GL, Boone JD, De Cino CL, Glaser AL, Renshaw RW, Ledizet M, Koski RA, MacLachlan NJ (2006) J Vet Diagn Investig 18:392–395
De Liguoro M, Di Leva V, Gallina G, Faccio E, Pinto G, Pollio A (2010) Chemosphere 81:788–793
Skold O (2001) Vet Res 32:261–273
Poirier LA, Doerge DR, Gaylor DW, Miller MA, Lorentzen RJ, Casciano DA, Kadlubar FF, Schwetz BA (1999) Regul Toxicol Pharmacol 30(3):217–222
Kuenzel WJ (2009) Poult Sci 88(4):824–831
European Commission, Off J Eur Union, 60, 16. http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500015931.pdf
Regulation No. 235 Ministry of Agriculture of the People’s Republic of China, 2002
Codex Alimentarius Commission Homepage (2007). Available from http://www.codexalimentarius.net/vetdrugs/data/vetdrugs/details.html?id=57. Accessed 9 April 2013
O'Keeffe M, Crabbe P, Salden M, Wichers J, Van Peteghem C, Kohen F, Pieraccini G, Moneti G (2003) J Immunol Methods 278:117–126
Wang L, Wang S, Zhang J, Liu J, Zhang Y (2008) Anal Bioanal Chem 390:1619–1627
Guo Y, Ngom B, Le T, Jin X, Wang L, Shi D, Wang X, Bi D (2010) Anal Chem 82:7550–7555
Wang X, Li K, Shi D, Jin X, Xiong N, Peng F, Peng D, Bi D (2007) J Chromatogr B 847:289–295
Li X, Zhang G, Liu Q, Feng C, Wang X, Yang Y, Xiao Z, Yang J, Xing G, Zhao D, Cai S, Chen H (2009) Food Addit Contam A 26:314–325
He J, Shen J, Suo X, Jiang H, Hou X (2005) J Food Sci 70:113–117
Wang Z, Zhang J, Zhang S, Shen J (2011) Food Agr Immunol 22:115–124
East DA, Mulvihill DP, Todd M, Bruce IJ (2011) Langmuir 27:13888–13896
Grabarek Z, Gergel J (1990) Anal Biochem 185:131–135
Staros JV, Wright RW, Swingle DM (1986) Anal Biochem 156:220–222
Li X, Luo P, Tang S, Beier RC, Wu X, Yang L, Li Y, Xiao X (2011) J Agric Food Chem 59:6064–6070
Acknowledgments
This work is supported by grants from Trans-Century Training Programme Foundation for the Talents by the Ministry of Education (NCET-12-0529), Key Projects in the National Science and Technology Pillar Program during the 12th Five-Year Plan Period (2011BAK10B01, 2012BAK17B00), and Sino-Russian International Scientific and Technological Cooperation (2011DFR30470).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, R., Li, H., Zhang, H. et al. Development of a lateral flow fluorescent microsphere immunoassay for the determination of sulfamethazine in milk. Anal Bioanal Chem 405, 6783–6789 (2013). https://doi.org/10.1007/s00216-013-7150-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7150-4