Abstract
To determine sulphamethazine (SMZ) residues in edible animal foods (pig muscle, chicken muscle, egg, fish, milk and liver), a competitive direct enzyme-linked immunosorbent assay (ELISA) and a colloidal gold immunoassay were established. The limits of detection of the ELISA and the colloidal gold immunoassay were 0.02 and 0.5 μg kg−1, respectively. The specificity of the ELISA developed to the SMZ was high according to the results of cross-reactivity testing with 14 kinds of sulphonamides. To obtain a more sensitive immunoassay, buffer solution (30 mmol L−1 phosphate-buffered saline with 0.05% Tween 20, pH 8.5) was optimized through the whole test procedure. A simple and efficient extraction method for the rapid detection of SMZ residues in foods was developed, with recoveries between 74 and 117.5%. Matrix effects can be avoided by 1:10 dilution of pig muscle, chicken muscle, egg, fish, milk and liver with optimal buffer. The detection limit of SMZ was 5 μg kg−1 in liver and 2 μg kg−1 in the other five samples. For the validation of the ELISA tests, sample extracts were analysed by ELISA and high-performance liquid chromatography. The results obtained by these two methods showed a good correlation (r 2) which was greater than 0.9. The colloidal gold immunoassay presented in this assay was successfully applied to determine SMZ in pig muscle, milk and fish below or equal to the maximum residue level (20 μg kg−1).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of stock raising, sulphonamides, a group of antimicrobial agents, have been used widely because of their high effectiveness and low mammalian toxicities. Sulphamethazine (SMZ) is one kind of sulphonamide which is widely used to treat bacterial diseases in human and veterinary medicine and to promote growth in cattle, sheep, pigs and poultry [1, 2]. The chemical structure of SMZ is shown in Fig. 1. SMZ was tested by oral administration in mice and in rats. It produced thyroid follicular-cell adenomas in mice and follicular-cell adenomas and carcinomas in rats. There is inadequate evidence in humans for the carcinogenicity of SMZ; however, it still is a potential menace to human health. To protect consumers from risks related to SMZ residues, maximum residue levels (MRLs) have been established by law. In Europe, Canada and the USA, the MRL of SMZ in edible tissues is 100 μg kg−1 (valid for the EU and the USA) [3]. In Japan, the MRL is 20 μg kg−1.
Traditionally, the detection techniques for SMZ residues include gas chromatography (GC), high-performance liquid chromatography (HPLC) [1], bioassays, thin-layer chromatography (TLC) and liquid chromatography (LC) with mass spectrometry [4]. The bioassays and TLC have poor sensitivity, and GC and HPLC methods require highly qualified personnel and extensive sample cleanup as well as expensive equipment. Moreover, they are not suitable for screening large amounts of samples. Development of immunochemical approaches has led to simpler and rapider methods to monitor and quantify SMZ residues in edible foods [5].
Till now, several groups have developed immunoassays for SMZ detection [1, 2, 6–9]; most of the assays rely on the direct enzyme-linked immunosorbent assay (ELISA) to determine SMZ residues in animal tissues, plasma and milk. Euna et al. [1] developed a direct competitive ELISA for screening SMZ in pork tissues with a detection limit of 10 ng g−1. Dixon-Holland and Katz [6] developed a direct ELISA for SMZ residues in milk, swine urine and muscle tissue with a detection limit of 10–20 ng g−1. Renson et al. [8] detected SMZ in muscle tissue and liver by ELISA and the detection limits were 4.8 and 5.1 ng g−1, respectively. Martlbauer et al. [10] developed an ELISA for the detection of SMZ in milk with a detection limit of 1.3 ng g−1. In the present study, the accuracy, sensitivity, specificity, matrix effects and utility of ELISA for detecting SMZ in six edible foods are described. A colloidal gold immunoassay with two formats, flow through and lateral flow, was also developed to determine SMZ in pig muscle, milk and fish.
The aims of this study were (1) to develop a simple and rapid extraction procedure that could significantly reduce matrix effects, (2) to select an optimal buffer solution that to be used in direct ELISA, (3) to compare the effect of homologous assay and heterologous assay on the sensitivity and specificity of the ELISA method, (4) to compare the ELISA results with those obtained by a traditional HPLC method and (5) to compare the effect of a direct competitive ELISA test (microwell plate) and colloidal gold immunoassay (membrane-based).
Experimental
Reagents
Keyhole limpet haemocyanin (KLH), ovalbumin (OVA), fish skin gelatin (FG) and Freund’s complete and incomplete adjuvants were purchased from Sigma (St. Louis, MO, USA). Bovine serum albumin (BSA) was obtained from Merck (Darmstadt, Germany). Reagent grade 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) (EDC), hydrogen peroxide and 3,3′,5,5′-tetramethylbenzidine (TMB) were from Sigma (St. Louis, MO, USA). Protein A–Sepharose 4B was purchased from Amersham Biosciences (Uppsala, Sweden).
Materials and instruments
Enzyme-linked immunosorbent assay
Polystyrene 96-well microwell plates were from Nunc (Rockilde, Denmark), and the microplate washer was from Bio-Rad (Hercules, CA, USA). Immunoassay absorbance was read with a Multiskan Spectrum instrument purchased from Thermo Labsystems (Vantaa, Finland) in dual-wavelength mode (450–650 nm).
Colloidal gold immunoassay
Nitrocellulose membranes were purchased from Pierce and nitrocellulose Hi-Flow plus membranes were purchased from Millipore. Semirigid polyethylene sheets and adhesive tape were purchased from a local market. Filter paper and analytical grade buffer chemicals were purchased from Hope Biotech (Teda, Tianjin, China). Chloroauric acid, goat anti-rabbit immunoglobulin G (IgG) and other chemicals were purchased from Sigma.
Solutions
Phosphate-buffered saline (PBS; 10 mmol L−1 sodium phosphate, 137 mmol L−1 NaCl, 2.7 mmol L−1 KCl, pH 7.5), PBS with 0.05% Tween 20 (PBST), coating buffer (50 mmol L−1 sodium carbonate buffer pH 9.6) and TMB substrate solution [prepared by adding 3.3 mg TMB in 250 μL dimethyl sulphoxide (DMSO) to 25 mL phosphate–citrate buffer (0.1 mol L−1 citric acid plus 0.2 mol L−1 Na2HPO4; pH 4.3) containing 3.25 μL of a 30% H2O2 solution] were used.
Preparation of the immunogens
The immunogen was made by coupling SMZ to KLH according to the two-step method [7]. For diazotization, 57 mg (0.2 mmol) SMZ was dissolved in 4 mL of 0.5 mol L−1 H2SO4 and the solution was cooled to 0–4 °C. One millilitre of 1 mol L−1 NaNO2 was added dropwise under stirring in an ice bath over a period of 10 min. The diazonium salt formed was kept in an ice bath until use. For azocoupling, KLH (100 mg) was dissolved in 4 mL borate–carbonate buffer (pH 9.4). While the mixture was being stirred, diazonium salt (4 mL) at 4 °C was added dropwise over the course of about 30 min. The mixture was maintained within the pH range 8–10 using 1 mol L−1 NaOH. The resultant azoprotein solutions were stirred for 1 h at room temperature before dialysis, and gel filtration on Sephadex G-25 was used to remove autocoupling products.
Preparation of two enzyme conjugates
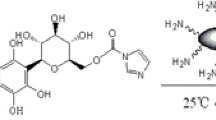
Figure 2 shows the chemical structure of the hapten for enzyme conjugate 1. The synthetic method for preparation of enzyme conjugate 1 was as follows. SMZ was first derived with succinic anhydride in accordance with the following procedure. A mixture of SMZ (0.2 mmol, 56 mg) and succinic anhydride (0.2 mmol, 20 mg) was stirred in dry pyridine (4 mL) for 22 h at room temperature. The reaction process was monitored by TLC. The mixture was then evaporated to dryness. The residue was purified by column chromatography (15:1 dichloromethane–methanol) to give the carboxylic acid SMZ derivative and it was confirmed by its mass spectrum.
The carboxylic acid SMZ derivative was coupled directly to horseradish peroxidase (HRP) as follows. Carboxylic acid of the hapten (0.025 mmol) and EDC (0.05 mmol) were dissolved in 1 mL dry dimethylformamide, then 10 mg HRP in 2 mL of 130 mmol L−1 sodium bicarbonate (pH 8.1) was added. The solution was added dropwise with mixing to the mixture. After mixing for 4–6 h at room temperature on a mixing wheel, 4.7 mg EDC was added, then the reaction solution was mixed overnight at 4 °C. The enzyme conjugate was dialysed against PBS.
Enzyme conjugate 2 was prepared by using suberic acid bis(N-hydroxysuccinimide ester) (DSS) as a linker to couple SMZ and HRP. The SMZ solution (10 mg mL−1 in dry DMSO) was added dropwise to the cross-linker solution (20 mg mL−1 in dry DMSO) such that there was a 2 mol L−1 excess of cross-linker. The mixture was stirred for 30 min at room temperature. The mixture was then added to 10 mg HRP in 2 mL of 10 mmol L−1 phosphate buffer at pH 7, with 20 mol L−1 excess of SMZ. After incubation for 2 h at room temperature, the mixture was dialysed against PBS.
Preparation of protein conjugate
The protein OVA was used for the preparation of the coating conjugate. The method was the same as the method for the conjugation of the immunogen as described earlier.
Antibody production
Antibodies were produced in rabbits as described by Wang et al. [11]. White rabbits were immunized by intradermal and intramuscular injections of the hapten conjugated to KLH. IgG from the antisera was purified by protein A–Sepharose 4B affinity chromatography.
Direct competitive ELISA
The microwell plates were coated with purified antibodies at 1 μg per well in 100 μL coating buffer, and were then incubated overnight at room temperature. The plates were then washed three times with 10 mmol L−1 PBST and unbound active sites were blocked with 200 μL of 1% BSA in PBS per well for 1 h. After the plates had been washed four times, 100 μL standards in PBS (or diluted sample solution) and 100 μL HRP–haptens in PBS were then added to each well, and the mixtures were incubated for 1 h at room temperature. After five washes, 150 μL of TMB substrate solution was added to each well. The enzymatic reaction was stopped after 30 min by adding 2.5 mol L−1 H2SO4 (50 μL per well) and the absorbance was then read in dual-wavelength mode (450 nm as the test and 650 nm as the reference).
Optimization of SMZ ELISA
In this assay, the effects of ionic strength, pH and surfactant concentration of the dilution buffer were studied to improve the performance of the selected immunoassay format [12]. Then the optimized buffer condition was used in the ELISA.
Ionic strength
Different concentrations of PBS, ranging from onefold to fivefold were tested. Using optimized PBS buffer concentration, we tested the effect of different pH ranging from 4 to 9.
Surfactant concentration
Using the optimized buffer concentration and the optimal pH, we tested the effect of different concentrations of Tween 20 ranging from 0 to 0.5%.
Colloidal gold immunoassay
Conjugation of colloidal gold solution to the anti-SMZ polyclonal antibody
Gold markers have the characteristics of binding proteins noncovalently without changing their bioactivity. This adsorption leads to the formation of a “protein–gold complex” [13]. The pH of colloidal gold solution for anti-SMZ polyclonal antibody conjugation was adjusted to pH 9.0 with 0.1 mol L−1 K2CO3 or 0.1 mol L−1 HCl. Before conjugation, the optimal concentration of the antibody for conjugation was determined. With gentle stirring, purified anti-SMZ antibody was added drop by drop to 20 mL pH-adjusted colloidal gold solution. After 10 min, the antibody–gold suspension was further stabilized by adding 1% (w/v) BSA to bring the concentration to 0.025%. BSA helps to further stabilize gold against aggregation and also blocks nonspecific binding sites [14]. After incubation overnight at 4 °C, the mixture was centrifuged twice at 10,000 rpm, 4 °C for 30 min and the pellet was resuspended in 5 mL conjugate storage buffer (2 mmol L−1 sodium borate containing 0.1% BSA and 0.1% sodium azide, pH 7.2) and diluted for use.
Procedure of flow-through colloidal gold immunoassay
The flow-through device used in this study is shown in Fig. 3. SMZ standards (120 μL) in 5% methanol (prepared in PBST buffer) were mixed with 30 μL gold–antibody conjugate. After incubation for 5 min, 100 μL mixture was added to the test strip coated with the hapten–OVA conjugate and anti-rabbit IgG. After the liquid reagent had flowed through the lines, different intensity of colour on the test lines could be observed by eye. The colour of the test line was compared with the that of the test line of a negative control strip (without SMZ). If SMZ is present in the sample, it would compete with the immobilized SMZ–OVA on the test line to bind the limited amount of colloidal gold labelled anti-SMZ antibody. The more SMZ present in the sample, the weaker appears the test line [5].
Procedure of lateral-flow dipstick colloidal gold immunoassay
The test strip was pasted onto a plastic backing with adhesive. Dried filter paper acted as an absorbent pad. SMZ standards (90 μL) in 5% methanol (prepared in PBST) were mixed with 30 μL gold–antibody conjugate in a polystyrene 96-well microwell. The strip was put into the bottom of the microwell and was taken out after 5 min. When the liquid reagent migrated towards the test line, different intensity of colour on the test line could be observed by eye. The colour of the test line was compared with that of the test line of a negative control strip (without SMZ).
Sample preparation
Six different matrices, i.e. pig muscle, chicken muscle, egg, fish (ling), milk and liver were chosen to evaluate the performance of ELISA. Samples were bought from local markets. Before the spiking and recovery studies, each test sample was verified to be SMZ-free (less than 10 μg kg−1) by HPLC.
For ELISA
For a spiking study, each sample was spiked with SMZ standard dissolved in methanol within the analytical working range, at three different concentrations (20, 50 and 100 μg kg−1).
Except for milk, the other sample tissues should be chopped before extraction. The extraction and dilution procedures for these six samples are described as follows:
-
Milk: Acetone (20 mL) was added to 20 mL milk, sedimentated for 15 min, and the liquid layer was diluted tenfold with optimized phosphate buffer for analysis.
-
Pig muscle, chicken muscle and fish (ling): The sample (5 g) and 10 mL methanol were thoroughly mixed for 2 min, and the mixture was then filtrated using filter paper. The filtrate was diluted tenfold with optimized phosphate buffer for analysis.
-
Egg: Yolk and egg white were mixed adequately, then 5 g mixture and 10 mL methanol were gently mixed on a rotary shaker (250 rpm) for 30 min to avoid the formation of foam and emulsion, and the mixture was then filtrated using filter paper. The filtrate was diluted tenfold with optimized phosphate buffer for analysis.
-
Liver: The sample (2 g) and 10 mL methanol were thoroughly mixed for 2 min, then the mixture was filtrated using filter paper. The filtrate was diluted tenfold with optimized phosphate buffer for analysis.
For colloidal gold immunoassay
Milk
Acetone (3 mL) was added to 1 mL milk. After the mixture had been standing on the bench for 10 min, the liquid layer was diluted a certain amount with PBST buffer for analysis.
Pig muscle and fish (ling)
The sample (2 g) and 4 mL methanol were thoroughly mixed for 2 min, and then the mixture was filtrated using filter paper. The filtrate was diluted a certain amount with PBST buffer for analysis.
Instrumentation for HPLC analysis
The ELISA results were verified using a Shimadzu HPLC instrument equipped with a LC-10AT vp pump with a Hamilton injector (25-μL loop), a DGU-12A online degasser and a CTO-10AS vp column oven. A C18 reversed-phase column (15 cm × 4.6-mm inner diameter, 5 μm) was used. The analysis was performed at 270 nm, and the mobile phase was methanol–water (78:22) (the pH of the mobile phase was adjusted to 3.3 before the mixing with methanol) at a flow rate of 1.0 mL min−1. The temperature of column oven was 35 °C.
Before HPLC analysis, to 4 g samples (at 0, 20, 50 and 100 ng g−1 SMZ) was added 10 g anhydrous Na2SO4. After thorough mixing, 20 mL acetonitrile was added, mixed thoroughly for 2 min, and then the mixture was centrifuged at 4,000g for 10 min. The upper liquid was evaporated under reduced pressure. The steps described above were repeated, and the double-extraction residues were resolved with 2 mL mobile phase. A sample filtered through a 0.22-μm filter was infected into the HPLC system.
Results and discussion
Optimization of SMZ ELISA
The optimization of ionic strength, pH and surfactant concentration was the preliminary study for the further determination of SMZ residues. Enzyme conjugate 1 was used for the optimization of SMZ ELISA.
The effect of ionic strength on the assay performance is shown in Fig. 4. The IC50 and absorbance (A 0) values decreased as the salt concentration increased. There were no obvious changes of IC50 and A 0 for 30, 40 and 50 mmol L−1 PBS. The increased ionic strength has a detrimental effect on interactions where ionic driving forces prevail [12]. In this assay, 30 mmol L−1 PBS was chosen (A 0 = 0.8 and IC50 = 0.43 ng mL−1) to minimize the effect of ionic strength.
The effect of the pH of the buffer is shown in Fig. 5. It was observed that immunoassay for SMZ is more sensitive at pH 8.5 than at the other pH values tested. IC50 was found to be very low at pH 4 but very high at pH 9.5; this may be because the protein was metamorphous in strong acidic and alkaline solution. It was confirmed that the stability of SMZ in slightly alkaline solution is better; therefore, pH 8.5 was selected for further study.
Different concentrations of Tween 20 had no significant effect on IC50 values. So the lowest concentration of Tween 20 (0.05%) was chosen.
Taking account of all these results, we found that the best performances were achieved with 30 mmol L−1 PBS, pH 8.5, and 0.05% Tween 20. The standard curve of SMZ is shown in Fig. 6. A ten-point standard curve was obtained for each ELISA plate, with the average result being IC50 = 0.5 ng mL−1 and IC15 = 0.05 ng mL−1, which are lower values than for congeners.
Effect of enzyme tracer on the sensitivity of the assay developed
The combination of antibody with enzyme conjugate 1 was a heterologous assay, whereas enzyme conjugate 2 coupled using DSS was determined as a homologous assay. Table 1 indicates that heterologous enzyme conjugate shows better sensitivity: the IC50 (0.5 ng mL−1) was almost twice as low as for the homologous enzyme conjugate (1.0 ng mL−1). There was no difference in specificity and precision for the analogous compound.
The stabilities of these two enzyme conjugates were tested with the antibody coated in plates. Enzyme conjugates 1 and 2 were both kept at 4 °C, room temperature and 37 °C, and tested at different times. There was no obvious difference in the IC50 and IC15 values of the two enzyme conjugates kept at 4 °C, room temperature and 37 °C between 1 and 7 days. These results indicate the two enzyme conjugates have good stability. Table 2 shows the results of the stability test for the enzyme conjugates.
Specificity assessment
The specificity of the antibody for SMZ was evaluated by testing the cross-reactivity with 14 kinds of sulphonamides. The cross-reactivities to 14 sulphonamides were all under 0.5%, with the exception of sulphamerazine, the chemical structure of which contains one methyl less than that of SMZ, showing cross-reactivity of 14%. The results indicate that the antibody has high specificity for SMZ.
Removal of matrix effects and recovery study of SMZ ELISA
Some compounds in animal tissues, such as protein and fat, might affect the binding of antibody and antigen, as well as other aspects of the assay, which is the so-called matrix effect. The matrix effect is a common problem for the immunoassay, which could reduce the sensitivity and reliability of the competitive immunoassay and cause false positives by lowering the colour development. [8, 15, 16] To remove the matrix effect, PBS, 30 mmol L−1 PBST, 1% BSA and 0.5% FG were tested as the dilution buffer. The results indicated that after the extract had been diluted tenfold with 30 mmol L−1 PBST (pH 8.5), the matrix effect of chicken muscle, pig muscle, fish, egg, milk and liver could be reduced to the minimum level, which might demonstrate that it is helpful to remove the matrix effect for surfactants at low concentration. The comparison of standard curves of SMZ in extracts of six samples is shown in Fig. 7.
Simple and efficient extraction methods with different solvents were developed. For chicken muscle, pig muscle, fish and egg, 5 g spiked samples were extracted with 10 mL methanol. For liver, 2 g spiked samples were extracted with 10 mL methanol to get good recovery. The protein and fat in milk could be sedimented by acetone. So acetone was selected as the extraction solvent for milk samples. Through these three methods, the recoveries for SMZ at 20, 50 and 100 ng mL−1 in six samples were between 74 and 117.5% (Table 3).
Correlation studies between ELISA and HPLC analysis
Although previous studies have demonstrated that ELISA is simpler and rapider than traditional instrumental methods, a confirmatory study is still required for legal and statutory purposes because of the possibility of false-positive results from ELISA. Correlation studies were performed on chicken muscle, pig muscle, fish and egg samples by HPLC. Figure 8 shows the correlations between HPLC analysis of purified extracts and ELISA results from nonpurified extracts. Good correlation (r 2 = 0.96) was obtained. The results show that the ELISA developed is reliable for the analysis of SMZ in food samples.
Optimization of colloidal gold immunoassay
Optimization experiments (three factors, three levels) were used to determine the optimal immobilization concentration of the SMZ hapten–OVA conjugate (l.0, 1.5, 2 μg), the optimal ratio of the gold–antibody conjugate and SMZ (1:3, 1:4, 1:5) and the optimal incubation time (1, 5, 10 min). Optimal immunoreagent concentrations were selected as a clear colour appearing in the negative control with the shortest time, and comparison of the intensity of the colour among samples and the control could be easily distinguished by eye. The optimal conditions for flow-through and lateral-flow dipstick colloidal gold immunoassay were as follows: SMZ hapten–OVA coated on the membrane at 1.5 μg per strip; gold–antibody conjugate and SMZ in ratios of 1:4 and 1:3, respectively; incubation time of 5 min. As shown in Figs. 9 and 10, both the flow-through colloidal gold immunoassay and the lateral-flow dipstick colloidal gold immunoassay developed in this study had a visual detection limit of 0.5 μg L−1 for SMZ.
Matrix effects and their removal
Different dilution buffers, i.e. 5 mmol L−1 borate buffer (pH 9.0), 10 mmol L−1 PBS (pH 7.2), PBST (pH 7.2) and 0.5% FG in PBS, and purified water for reducing matrix effects were tested. As a result, 100% methanol extract diluted with PBST showed a lower background colour in unspotted areas and high sensitivity. So PBST was used as the dilution buffer in all subsequent experiments. In flow-through immunogold assay, the dilution with PBST buffer was 20-fold for pork and milk, tenfold for fish; in lateral-flow dipstick immunogold assay, the dilution with PBST buffer was 20-fold for milk and fish, tenfold for pork.
Spiking and recovery studies
After the matrix effect had been removed, spiking and recovery studies were carried out by the method described earlier. The flow-through colloidal gold immunoassay results of pork, milk and fish samples are shown in Fig. 11. The lateral-flow dipstick colloidal gold immunoassay results of the three samples are shown in Fig. 12. It can be concluded that the colloidal gold immunoassay presented here successfully determined SMZ in pig muscle, in milk and fish below the MRL (20 μg kg−1). An amount of 20 μg kg−1 of SMZ caused a slight but distinguishable difference compared with the negative control. SMZ was analysed by a simple and rapid extraction and dilution procedure using methanol or acetone, and the optimal dilution buffer without any cleanup steps.
Spiked experiment of pork, milk and fish by flow-through immunogold assay. Upper line: control line (goat anti-rabbit IgG). Lower line: SMZ standard prepared in a matrix with the method described in the text. Top: matrix of pork with SMZ standard (0, 20, 50, 100 μg L−1). Middle: matrix of milk with SMZ standard (0, 20, 80, 150 μg L−1), Bottom: matrix of fish with SMZ standard (0, 10, 40, 160 μg L−1)
Spiked experiment of pork, milk and fish by lateral-flow immunogold assay. Upper line: control line( goat anti-rabbit IgG). Lower line: SMZ standard prepared in a matrix with the method described in the text. Left: matrix of pork with SMZ standard (0, 10, 40, 160 μg L−1). Middle: matrix of milk with SMZ standard (0, 20, 80, 150 μg L−1). Right: matrix of fish with SMZ standard (0, 20, 80, 150 μg L−1)
Conclusion
As expected, the assays developed in this study had high specificity and sensitivity. The heterologous assay showed higher sensitivity than the homologous assay by using two types of enzyme conjugates. The competitive direct ELISA presented in this study successfully determined SMZ in pig muscle, liver, chicken muscle, egg, milk and fish below the MRL (20 μg kg−1). The accuracy and precision of ELISA were validated by the instrumental method of HPLC. The visual detection limits of SMZ for flow-through and lateral-flow gold-based assays were both 0.5 ng mL−1 in less than 10 min. A simple and rapid extraction was developed using methanol or acetone, and matrix effects from food samples could be removed by diluting the food extracts using dilution buffer. This study demonstrated that the two immunoassays developed could be used as quantitative or qualitative tools for the rapid screening of SMZ residues in edible foods.
References
Euna KO, Hochul S, Jun HP (2000) Vet Med Sci 62(10):1121–1123
Fleeker JR, Lovet TLJ (1985) Assoc Off Anal Chem 68:172–174
EU Regulation (1999) No 508/1999, L60 (9-3-1999), pp 16–52
Cliquet P, Cox E, Haasnoot W, Schacht E, Goddeeris BM (2003) Anal Chim Acta 494:21–28
Zhang HY, Duan ZJ, Wang L, Zhang Y, Wang S (2006) Agric Food Chem 54:4499–4505
Dixon-Holland DE, Katz SE (1988) Assoc Off Anal Chem 71:1137–1140
Fránek M, Kolar V, Deng A, Crooks S (1999) Food Agric Immunol 11:339–349
Renson C, Degand G, Maghum-Roglster G (1993) Anal Chim Acta 275:323–328
Sashenbrecker PW, Fish NA (1980) Comp Med 44:338–345
Martlbauer E, Meier R, Usleber E, Terplan G (1992) Food Agric Immunol 4:219–228
Wang S, Allan RD, Skerritt JH, Kennedy IR (1998) Agric Food Chem 46:3330–3338
Nuria P, Cristina G, Angel M, Rosa P (2004) Anal Bioanal Chem 379:1095–1097
Hermanson GT (1996) Bioconjugate techniques, 1st edn. Academic, San Diego
Zhao J, He SP, Liu W, Deng AX, Nan TG, Wang BM, Zhai ZX, Li ZH (2006) Food Agric Immunol 17(3–4):173–181
Wang S, Zhang J, Yang ZY, Wang JP, Zhang Y (2005) Agric Food Chem 53:7377–7384
Wang S, Allan RD, Skerritt JH, Kennedy IR (2002) Environ Sci Health Part B 37:521–532
Acknowledgements
The authors are grateful for financial support from the Ministry of Science and Technology of the People’s Republic of China (projects no. 2006BAD05A06 and no. 2006AA10Z448) and the New Century Talent Program of Ministry of Education of the People’s Republic of China (project no. NECT-04-0243).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Wang, S., Zhang, J. et al. Enzyme-linked immunosorbent assay and colloidal gold immunoassay for sulphamethazine residues in edible animal foods: investigation of the effects of the analytical conditions and the sample matrix on assay performance. Anal Bioanal Chem 390, 1619–1627 (2008). https://doi.org/10.1007/s00216-008-1836-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-1836-z