Abstract
We report a chiral high-performance liquid chromatographic enantioseparation method for free α-aminophosphonic, β-aminophosphonic, and γ-aminophosphonic acids, aminohydroxyphosphonic acids, and aromatic aminophosphinic acids with different substitution patterns. Enantioseparation of these synthons was achieved by means of high-performance liquid chromatography on CHIRALPAK ZWIX(+) and ZWIX(-) (cinchona-based chiral zwitterionic ion exchangers) under polar organic chromatographic elution conditions. Mobile phase characteristics such as acid-to-base ratio, type of counterion, and solvent composition were systematically varied in order to investigate their effect on the separation performance and to achieve optimal separation conditions for the set of analytes. Under the optimized conditions, 32 of 37 racemic aminophosphonic acids studied reached baseline separation when we employed a single generic mass-spectrometry-compatible mobile phase, with reversal of the elution order when we used (+) and (-) versions of the chiral stationary phase.

New zwitterionic ion-exchangers can separate free amino phosphonic acids and a change from Chiralpak ZWIX(+) to ZWIX(-) allows reversal of enantiomer elution order
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amino acids play a key role in nature, constituting the structural units of peptides, proteins, and enzymes. Aminophosphonic acids (APAs) can very efficiently act as biomimetics, representing phosphorus analogues of amino acids, competing with them at the active site of enzymes [1] and cell receptors [2]. Their unique characteristics render this class of compounds of considerable biological and pharmacological interest, with applications as antitumor [3–6], neuroactive [5, 7], antihypertensive [5, 8], antimicrobial [5, 9, 10], herbicidal [1, 5] and imaging [11] agents.
Biological activity is mostly observed for one enantiomer and, therefore, use strictly relies on the enantiomeric purity of these chiral compounds. Several concepts for the preparation of such chiral chemical entities with high enantiomeric excess have been reported to date [12–17]. In contrast, there are only a few enantioselective analytical methods currently available for chiral phosphonic acids, which might be useful for the study of stereochemical pathways of stereoselective reactions and for the determination of the enantiomeric purity of the final products. Most often, indirect 31P-NMR methods, based on different complexation-induced shifts of signals of diastereomeric complexes with a chiral solvating agent, are used for these purposes [13, 18]. Although several reports have been published on different enantioselective high-performance liquid chromatography (HPLC) methods for the ester analogues of phosphonic and APAs, e.g., using cellulose- or amylose-based chiral stationary phases (CSPs) [19] or “Pirkle-type” CSPs [20–22], publications dealing with the direct enantioseparation of APAs with an underivatized phosphonic acid group are scarce. In a previous article, we reported the separation of N-derivatized APAs and phosphinic acids by HPLC on quinine-derived chiral anion exchangers [23, 24]. In this contribution, we focus on direct enantioseparation of underivatized APAs and aminophosphinic acids on the recently commercialized quinine- and quinidine-based zwitterionic CSPs CHIRALPAK ZWIX(+) and ZWIX(-), respectively. The optimization of flow rate, temperature, mobile phase compositions, and buffer system is discussed, and enantioseparation of a pool of 44 amino acids including APAs, phosphinic acids, carboxylic acids, and sulfonic acids with a single mobile phase is reported.
Experimental
Materials
HPLC solvents methanol (MeOH) and acetonitrile (ACN) were of HPLC-grade quality and were purchased from Sigma-Aldrich (Vienna, Austria) and Merck (Darmstadt, Germany). Millipore-grade water was obtained from an in-house Millipore system (resistivity 18.2 MΩ cm at 25 °C). Formic acid, 7 N NH3 solution in MeOH, acetic acid (AcOH), propionic acid, and β-alanine (β-Ala) were of analytical grade from Sigma-Aldrich (Vienna, Austria).
Racemic mixtures and/or enantiomerically pure analytes employed in this study were either commercially available (Acros Organics, Belgium) or synthesized according to established procedures [16, 17]. About 1 mg of the analytes was dissolved initially in 100 μL of dimethyl sulfoxide and then diluted with 900 μL of a mobile phase. The injected sample volume was 5 μL.
Apparatus and chromatography
HPLC experiments were performed using an 1100 series liquid chromatography system from Agilent Technologies (Waldbronn, Germany) equipped with a binary gradient pump, autosampler, vacuum degasser, temperature-controlled column compartment, diode-array detector for the detection of aromatic samples and a charged aerosol detector from Thermo Fisher Scientific (Sunnyvale, CA, USA) for nonaromatic species. To reduce the baseline noise of the charged aerosol detector under reduced flow rate (0.93 and 0.66 mm/s), the gas flow was reduced from 35 to 30 psi. The data were processed with Agilent ChemStation version Rev. B.01.03.
The CSPs adopted for the study were CHIRALPAK ZWIX(+) and ZWIX(-) (150 mm × 3 mm inner diameter, 3 μm particle size) from Chiral Technologies Europe (Illkirch, France) based on trans-(1″S, 2″S)-N-{[((8S,9R)-6′-methoxycinchonan-9-yl)oxy]carbonyl}-2-2″-aminocyclohexanesulfonic acid and trans-(1″R, 2″R)-N-{[((8R,9S)-6′-methoxycinchonan-9-yl)oxy]carbonyl}-2-2″-aminocycloxexanesulfonic acid, respectively [25, 26].
In the mobile phase study the chromatographic parameters if not specified differently were as follows: flow rate 1.33 mm/s (0.55 mL/min), injection of 5 μL, detection at 258 nm. The dead time (t 0) was determined after each modification of the chromatographic parameters by three injections of methanolic acetone.
The mobile phase for the enantioseparation of the APAs reported in Table 1 consisted of MeOH with 200 mM formic acid and 50 mM NH3. Other conditions were as follows: flow rate 0.93 mm/s (0.4 mL/min on a 3 mm × 150 mm column), temperature 15 °C.
Results and discussion
This contribution describes the enantioseparation of free (nonprotected) APAs on the novel cinchona-based chiral zwitterionic ion exchangers commercialized under the names CHIRALPAK ZWIX(+) and CHIRALPAK ZWIX(-). The chemical structure of these CSPs is depicted in Fig. 1. Note that the chiral selectors in CHIRALPAK ZWIX(+) and (-) are diastereomeric, but experimentally show pseudo-enantiomeric behavior (due opposite configurations at carbon C8 and C9, but identical configurations a N1, C3 and C4), inter alia, a reversed elution order for APAs enantiomers in most cases. The selectors were immobilized onto the mercaptopropyl-modified silica gel by employing thiol–ene click chemistry, generating brush-type stationary phases [25–29].
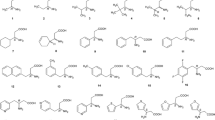
The enantiorecognition properties of the cinchona-based ZWIX CSPs towards unprotected amino carboxylic and aminosulfonic acids have been described in previous contributions [25, 27, 29–34]. However, no investigations have been performed so far with regard to the phosphonic and phosphinic acid analogues. Using these low molecular weight CSPs, we could baseline-separate (R s ≥1.5) 32 of 37 compounds (1–36 and 44) of the investigated APA analyte set with a single mass-spectrometry-compatible mobile phase.
Optimization of chromatographic parameters
To achieve the best separation performance, the separation conditions were optimized using ZWIX(-), which had been found to have lower enantiodiscrimination in our preliminary experiments. To fulfill the scope, we selected two α-APAs—([amino(phenyl)methyl]phosphonic acid 13 and [1-amino-3-(phenylthio)propyl]phosphonic acid 25—and one γ-APA—3-amino-2-(4-chlorophenyl)propyl)phosphonic acid (phaclofen) 36—(see Fig. 2a) to study the influence of different chromatographic parameters on the enantioseparation. Typically, low values of relative standard deviation were observed for replicate measurements under the same conditions (less than 0.1 % RSD for k1 and enantioselectivity; less than 2 % RSD for resolution and less than 4 % RSD for the number of theoretical plates), offering a method with good repeatability performances.
a Chemical structures of the compounds adopted for the mobile phase optimization. b Influence of buffer type of the mobile phase on enantioselectivity and resolution. FA formic acid, NH3 ammonia, AcOH acetic acid, PA propionic acid. The proportions of acid and base are given as molar ratios (150 mM/50 mM for FA–NH3, AcOH–NH3, and PA–NH3, and 100 mM/50 mM for FA–β-Ala,). Chromatographic parameters are as specified in “Experimental”
Effect of counterion type and acid-to-base ratio
Similarly to anion-exchange mode, in chiral zwitterionic ion-exchange (ZWIX) chromatography, long-range electrostatic interactions between oppositely charged atoms of the chiral selector and the zwitterionic selectand constitute the initial dominant type of driving forces for the approach of the selectand towards the chiral selector. Subsequently, directional hydrogen-bond-mediated ionic interactions can orient the zwitterionic selectand at the chiral zwitterionic selector, resulting eventually in a chiral recognition process [25, 35, 36].
In such type of chromatography, the proton activity of the mobile phase influences the ionization status of both species and therefore the strength of their interaction. As a consequence, the ion-exchange process will be affected by the strength, concentration, and ratio of the acidic and the basic additives. Therefore, all these parameters have to be considered in the phase of optimization of the buffer system.
Previously published data on chiral enantioseparation on such stationary phases revealed a weak dependency of the separation parameters by variation of the type of base [27]. Therefore, in our optimization we employed NH3 as a basic additive in the eluent and focused our study on the effect produced by the type of acidic component of the mobile phase. Furthermore, we evaluated the possibility of the use of a zwitterionic buffer (β-Ala).
As starting test conditions we adopted an acid-to-base ratio of 3:1, which was previously identified as a successful operation mode for ZWIX CSPs, using 150 mM acidic component and 50 mM NH3 in MeOH [27]. Employing such a composition, we studied the effect of the strength and different chain lengths (i.e., distinct lipophilicity and acidity) of the acidic additive (formic acid, AcOH, and propionic acid). Figure 2 summarizes the findings, comparing the effects of such variation on selectivity and resolution. The separation factors for α-APA were slightly greater with AcOH than with propionic acid, but decreased significantly with formic acid. The trend was different for compound 36, which showed the highest enantioselectivity with formic acid and the lowest with AcOH. However, the gain in enantioselectivity for α-APA with AcOH and propionic acid in comparison with formic acid was unfavorably outbalanced by the loss in efficiency. We observed, in general, a negative trend in terms of separation efficiency when formic acid was replaced by AcOH or propionic acid. The reason for such phenomena could be ascribed to the lower acidity of acids with longer aliphatic chains and therefore to their less pronounced counterion properties.
Further, we decided to test a zwitterionic additive, namely β-Ala, which, as a propionic acid derivative, should provide higher selectivity. On the other hand, it possesses a basic center as well, and therefore we assumed that this feature may compensate the negative effect of chain length observed for propionic acid. For solubility reasons, it was necessary to add an excess of formic acid (100 mM) to obtain a clear 50 mM methanolic solution of β-Ala. The resulting mobile phase had an acid-to-base ratio of 3:1 (i.e., acid consisting of two parts of formic acid and one part of carboxylic acid from β-Ala, whereas the base consisted of one part of β-Ala). Under such conditions we did not observe an improvement of the separation performances; however, the possibility of using such a zwitterionic buffer offers a further confirmation of a zwitterionic selectand–chiral selector interaction.
In addition to the variation of the type of buffer components, it was also of interest to study the effect of the ratio of formic acid and NH3 on the retention characteristics. This parameter determines the proton activity of the mobile phase and therefore the degree of ionization and interaction strength between selectands and the chiral selector. Altogether we tested five methanolic mobile phases containing 50 mM basic additive (NH3) with an acidic component (formic acid) in a molar ratio between 1:5 and 2:1 (in the case of the 2:1 ratio, 100 mM NH3 was used). The results obtained are summarized in Fig. 3. We observed a nearly constant retention factor in acidic mobile phases (ratio from 1:5 to 1:1), once more confirming the bivalent double charge interaction between the selectand and the chiral selector. The retention decreased significantly in basic medium (ratio 2:1) as a consequence of the reduced protonation of the amino group of APAs and/or the quinuclidine ring. The resolution values reached a maximum in the acidic range (at 1:5 or 1:4 ratio) but decreased significantly under neutral or basic conditions (1:1, 2:1). This finding suggests that a modification of the proton activity of the medium could influence the spatial positions of the two charged units of the CSP (intramolecular counterions). The increased closeness of these moieties in a strongly acidic medium (owing to a higher charge of the quinuclidine ring) would influence the spatial interactions between the selectand and the chiral selector, resulting in differences in chiral recognition.
Effect of the acid-to-base ratio in the mobile phase on the capacity factor and resolution. Mobile phases composed of FA–NH3. The proportions of acid and base are given as molar ratios: 250 mM/50 mM (5-1), 200 mM/50 mM (4-1), 150 mM/50 mM (3-1), 50 mM/50 mM (1-1), and 50 mM/100 mM (1-2). Chromatographic parameters are as specified in “Experimental”
In final experiments we investigated the dependence of the retention on the ionic strength at constant acid-to-base ratio. In accordance with an ion-exchange retention mechanism, higher retention factors were observed when mobile phases with lower buffer concentration were adopted. However, we did not observe significant differences in terms of selectivity and efficiency (data not shown) and therefore we selected a higher buffer concentration (200 mM formic acid–50 mM NH3) as the final optimized eluent in order to reduce the analysis time.
Generally, the trends with regard to effects of mobile phase additives on enantioseparation largely reflected those previously described for amino acids [27]. The stronger acidity of the APAs as selectands solely leads to minor shifts to higher acidity of the resulting optimized mobile phase.
Effect of solvent composition, temperature, and flow rate
As previously investigated, the cinchona zwitterionic chiral ion exchangers tested provided the best results in polar organic mode [27]. However, to further tune the performance of this chromatographic method, we investigated the effect of the bulk solvent composition. First, we tested eluent systems based on MeOH (polar protic solvent) with a formic acid to NH3 ratio of 3:1 and increasing content of ACN (polar aprotic solvent). The findings for the effect of chromatographic parameters k 1, α, and R s on the variation of the bulk solvent composition are depicted in Fig. 4. As expected, an increase in the ACN content increases the retention time because of the reduced solvation power of such a system. However, such modification does not significantly enhance the separation properties. Furthermore, we tested the effect of the addition of small percentage (1-5 %) of water to elution media of various compositions [MeOH/ACN, 50:50 (data not shown) or 75:25; v/v), but no beneficial effect was observed for such three-component mobile phase systems (Fig. 5).
Influence of acetonitrile (ACN) and methanol (MeOH) as bulk eluent solvent for CHIRALPAK ZWIX(-) a on enantioselectivity and retention and b on chromatographic profiles (λ = 254 nm) of 13. Mobile phase composed of 200 mM FA and 50 mM NH3 in ACN–MeOH mixtures (v/v). For conditions, see “Experimental”
Influence of small percentages of water in the eluent composed of 200 mM FA and 50 mM NH3 in MeOH/ACN (75:25; v/v) on retention and selectivity (a) as well as resolution and plate numbers (b). For conditions, see “Experimental”
Summarizing our findings, we can conclude that mobile phases based solely on MeOH as bulk solvent offer the best performances.
To further improve the separation efficiency, we tested the influence of the flow rate (from 0.66 to 1.33 mm/s) and temperature (from 15 to 55 °C) on the separation (the results are summarized in Fig. 6). An analysis of the data from the temperature study reveals that the retention factors and resolution decrease with increasing temperature (enthalpy-driven retention process). A low temperature of 15 °C is favorable in terms of resolution. With regard to the flow rate, increased plate heights at faster flow velocities reduce the resolution and thus low flow rates such as 0.66 mm/s are preferred. However, owing to increased analysis times at such slow flow rates, we selected a flow rate of 0.93 mm/s as the optimum.
Influence of temperature (a) and linear flow rate (b) and on retention and resolution. For conditions, see “Experimental”
To summarize, the selected chromatographic parameters for the separation of the analytes reported in Table 1 are as follows: MeOH containing 200 mM formic acid and 50 mM NH3 as the mobile phase. This generic eluent was run at a flow rate of 0.93 mm/s at a column temperature of 15 °C and provided sufficient separations for most of the target APA compounds tested.
Elements of chiral recognition and structure–enantioselectivity relationships
The chromatographic method developed allowed us to baseline-separate (R s ≥1.5) 32 APAs from the pool of 37 APAs with the generic eluent described earlier, demonstrating once more the good enantiorecognition properties of such CSPs towards free APAs and phosphinic acids besides earlier reported amino acids and sulfonic acids. The chromatographic data for all the amino acids tested are summarized in Table 1.
Recently published work investigated the enantiorecog-nition mechanism of cinchona-based zwitterionic ion exchangers by studying the influence of structural modification of the chiral selector together with the variation of the amphoteric selectand species [32]. The simultaneous ionic interactions between the protonated base and dissociated acid of the selectand towards the dissociated sulfonic group and protonated quinuclidine nitrogen of the chiral separator are the dominating forces in the retention process. Ionic forces are not spatially oriented and have to be supported by oriented intermolecular chiral selector–selectand interactions in order to establish a different affinity towards one of the selectand enantiomers. In the present case, both of the ionic interactions are, however, mediated via a hydrogen bond, which provides these interactions with directional character. Together with these elements, the cinchona-based zwitterionic CSPs present geometrically and conformationally oriented interaction sites such as the carbamate group (hydrogen-bonding) and the quinoline ring (π–π). These elements together or separately constitute the elements of chiral recognition of the CSP, which can lead to chiral discrimination between the enantiomers of the zwitterionic analytes.
CHIRALPAK ZWIX(+) and ZWIX (-) are based on quinine and quinidine, respectively, on the one hand, and are coupled with (S,S)-2-aminocyclohexylsulfonic acid and (R,R)-2-aminocyclohexylsulfonic acid, respectively, on the other hand. Although quinine and quinidine represent a diastereomeric pair with configurations 1S, 3R, 4S, 8S, and 9R in the case of quinine and 1S, 3R, 4S, 8R, and 9S for quinidine, they usually act like enantiomers (owing to opposite configurations at C8 and C9), and thus are often considered as pseudoenantiomers. The CPSs exhibit similar behavior, with reversal of the elution order of selectand enantiomers on changing from quinine- to quinidine-based CSP (Fig. 7) [26]. In the present study the reversal of the elution order of APAs on changing from ZWIX(+) and ZWIX(-) could not be extensively explored because of the lack of enantiomerically pure standards. However, with the available enantiomerically pure standards we observed reversal of elution order in analogy to what has been reported for amino acids and aminosulfonic acids [26].
Reversed elution order of compound 1 on CHIRALPAK ZWIX(+) (a) and ZWIX(-) (b). For conditions, see “Experimental”
In Fig. 8 we present a comparison of the enantiosepa-ration performances (in terms of selectivity and resolution) for the ZWIX(+) and ZWIX(-) CSPs tested. Except for some particular cases (e.g., compounds 20 and 44), we observed similar enantioselectivity properties for the two pseudoenantiomeric CSPs (Fig. 8a). However, as can be seen in Fig. 8b , ZWIX(-) generally provided lower separation efficiency and thus lower resolution. Although our analyte set was limited and it is hard to derive general conclusions, we observed better enantioresolving power of the ZWIX(+) column for α-APAs, whereas ZWIX(-) was better for γ-APAs. Such a phenomenon is most probably related to the diastereomeric nature of the selectors and their different conformations.
Comparison of enantioselectivity (a) and resolution (b) performances of CHIRALPAK ZWIX(+) versus ZWIX(-) with respect to the analyte set reported in Table 1. For conditions, see “Experimental”
The heterogeneity, in terms of structural elements, of the analyte portfolio allowed us to evaluate the influence of various structural motifs on enantioselectivity, which may provide deeper insight into the enantiorecognition process for such zwitterionic species. Generally, elongation of the alkyl side chain of the APA resulted in increased retention and enhanced enantioselectivities (compounds 1, 2, and 9–11). A similar effect was observed for branched aliphatic side chains (compounds 3 and 7). Introduction of polar thiol, sulfide, or sulfone groups in the side chain (compounds 4–6) led to an increase of retention times, with the sulfone derivative (compound 6) having the strongest influence. We assume that such behavior could be caused by additional hydrogen-bond formation, although enantioselectivity is not significantly enhanced.
From a comparison of aliphatic and aromatic α-substituents of the same carbon chain length (compounds 12 and 13), we found that the aliphatic one resulted in lower retention and enhanced enantioselectivity. This observation was further supported by better enantioselectivity and resolution achieved on enlargement of the aliphatic part of the substituent for compound 18 in comparison with compound 17. These results indicate that in this particular case repulsive interactions may occur between the selector system and the bulky groups of the respective APAs, thus playing a more significant role in chiral recognition than potentially active attractive π–π interactions.
The introduction of the second substituent in the α-position of APAs resulted in higher steric hindrance, which reduced the strength of the chiral selector–selectand interaction (lower retention factor) (compare compounds 7 and 8 for aliphatic side chains and compounds 13 and 14 as well as compounds 19 and 20 for aromatic side chains). In case of aliphatic APAs, this steric hindrance disturbed the chiral distinction and led to a worse separation (compounds 7 and 8). In contrast, for the aromatic APAs (compounds 13, 14, 19, and 20) enantioselectivity was improved. It is worth noting that increasing bulkiness of the alkyl substituent at the stereogenic center yielded increased enantioseparation factors in case of β-APAs as well (see compounds 26 and 27).
The enantioseparation of β-amino-α-hydroxyphosphonic acids was generally lower than in the case of α-APAs. No chiral recognition was observed under given conditions for β-amino-α-hydroxyphosphonic acid derivatives bearing only hydrogen or aliphatic substituents (compounds 28–30). In principle, the reason for the poor performance of the ZWIX CSPs towards these species is due to the presence of the α-hydroxyl group that apparently disturbs the chiral recognition mechanism based on hydrogen bonding, whereas other aliphatic β-APAs (e.g., compounds 26 and 27) are well separated. We found that substitution with aromatic moieties (compounds 31–34) supports chiral recognition. With increasing size of the aromatic ring, the α values increased; however, the substitution of hydrogens of the aromatic moiety with a methyl group had a disturbing influence. This indicates that π–π interactions play a more significant role, with an even stronger influence on chiral recognition of β-amino-α-hydroxyphosphonic acids, than hydrogen bonding.
Monoprotic acids such as phosphinic acids (compounds 41 and 42) and sulfonic acids (compound 43) were separated with excellent selectivity and resolution, probably because of the lack of the competing second acidic group (Fig. 9). As a special class we chose cyclic secondary α-APAs. In both cases (compounds 35 and 40) enantioseparation was not observed under the given conditions, even though the compounds were well retained. We assume that this behavior is caused by pronounced rigidity of these substrates, which led to conformationally stable species that could not specifically interact with the chiral selector. However, since the corresponding carboxylic acid analogues are well resolved, one may argue that these secondary APAs only need a specific optimization.
Effect of acidic functionality in zwitterionic analytes on enantioseparation. For conditions, see “Experimental.” A acid, AA amino acid
Conclusion
We have reported the first direct HPLC enantioseparation of nonprotected APAs on recently developed zwitterionic CSPs, CHIRALPAK ZWIX(+) and ZWIX(-), based on the cinchona alkaloid scaffold.
By variation of the chromatographic parameters, we investigated the contribution of the mobile phase to the enantiorecognition of such zwitterionic analytes, finding optimum conditions under which most of the APAs investigated were successfully enantioseparated. We found that these diprotic acidic analytes (APAs) require in comparison with amino acids or peptides [34] more acidic mobile phases; however, the character of the optimal buffer salt remained the same. In concord with this result, the use of zwitterionic buffer did not improve the general performance of the ZWIX CSPs.
Chiral recognition facilitated by cinchona-based CSPs is usually supported by strong π–π interactions of the quinoline ring of the selector and an aromatic moiety of the analyte [36]. We found that repulsive interactions of bulky aliphatic functionalities and, at the same time, their van der Waals interactions can strongly enhance chiral recognition properties of the novel zwitterionic CSPs.
We have shown that the recently commercialized CSPs CHIRALPAK ZWIX(+) and ZWIX(-) can be employed for direct enantioseparation of free APAs and aminophosphinic acids in polar organic mode.
References
Hudson HR, Kukhar VP (2000) Aminophosphonic and aminophosphinic acids: chemistry and biological activity. Wiley, New York
Mucha A, Kafarski P, Berlicki L (2011) Remarkable potential of the alpha-aminophosphonate/phosphinate structural motif in medicinal chemistry. J Med Chem 54(17):5955–5980. doi:10.1021/jm200587f
Morphy JR, Beeley NRA, Boyce BA, Leonard J, Mason B, Millican A, Millar K, Oconnell JP, Porter J (1994) Potent and selective inhibitors of gelatinase-A. 2. Carboxylic and phosphonic acid-derivatives. Bioorg Med Chem Lett 4(23):2747–2752. doi:10.1016/S0960-894x(01)80588-6
Kraicheva I, Bogomilova A, Tsacheva I, Momekov G, Troev K (2009) Synthesis, NMR characterization and in vitro antitumor evaluation of new aminophosphonic acid diesters. Eur J Med Chem 44(8):3363–3367. doi:10.1016/j.ejmech.2009.03.017
Kafarski P, Lejczak B (1991) Biological activity of aminophosphonic acids. Phosphorus Sulfur 63(1–2):193–215. doi:10.1080/10426509108029443
Grzywa R, Oleksyszyn J, Salvesen GS, Drag M (2010) Identification of very potent inhibitor of human aminopeptidase N (CD13). Bioorg Med Chem Lett 20(8):2497–2499. doi:10.1016/j.bmcl.2010.02.111
Selvam C, Goudet C, Oueslati N, Pin JP, Acher FC (2007) L-(+)-2-Amino-4-thiophosphonobutyric acid (L-thioAP4), a new potent agonist of group III metabotropic glutamate receptors: increased distal acidity affords enhanced potency. J Med Chem 50(19):4656–4664. doi:10.1021/jm070400y
Wallace EM, Moliterni JA, Moskal MA, Neubert AD, Marcopulos N, Stamford LB, Trapani AJ, Savage P, Chou M, Jeng AY (1998) Design and synthesis of potent, selective inhibitors of endothelin-converting enzyme. J Med Chem 41(9):1513–1523. doi:10.1021/jm970787c
Beck J, Gharbi S, Herteg-Fernea A, Vercheval L, Bebrone C, Lassaux P, Zervosen A, Marchand-Brynaert J (2009) Aminophosphonic acids and aminobis(phosphonic acids) as potential inhibitors of penicillin-binding proteins. Eur J Org Chem 2009(1):85–97. doi:10.1002/ejoc.200800812
Yang S, Gao X-W, Diao C-L, Song B-A, Jin L-H, Xu G-F, Zhang G-P, Wang W, Hu D-Y, Xue W, Zhou X, Lu P (2006) Synthesis and antifungal activity of novel chiral α-aminophosphonates containing fluorine moiety. Chin J Chem 24(11):1581–1588. doi:10.1002/cjoc.200690296
Manning HC, Goebel T, Marx JN, Bornhop DJ (2002) Facile, efficient conjugation of a trifunctional lanthanide chelate to a peripheral benzodiazepine receptor ligand. Org Lett 4(7):1075–1078. doi:10.1021/ol017155b
Hammerschmidt F, Hanbauer M (2000) Transformation of arylmethylamines into alpha-aminophosphonic acids via metalated phosphoramidates: rearrangement of partly configurationally stable N-phosphorylated alpha-aminocarbanions. J Org Chem 65(19):6121–6131. doi:10.1021/jo000585f
Hammerschmidt F, Li Y-F (1994) Determination of absolute configuration of α-hydroxyphosphonates by 31P NMR spectroscopy of corresponding Mosher esters. Tetrahedron 50:10253–10264. doi:10.1016/s0040-4020(01)81758-0
Hammerschmidt F, Wuggenig F (1999) Enzymes in organic chemistry. Part 9: chemo-enzymatic synthesis of phosphonic acid analogues of L-valine, L-leucine, L-isoleucine, L-methionine and L-α-aminobutyric acid of high enantiomeric excess. Tetrahedron-Asymmetry 10:1709–1721. doi:10.1016/s0957-4166(99)00152-4
Schmidt U, Krause HW, Oehme G, Michalik M, Fischer C (1998) Enantioselective synthesis of α-aminophosphonic acid derivatives by hydrogenation. Chirality 10:564–572. doi:10.1002/(sici)1520-636x(1998)10:7<564::aid-chir3>3.0.co;2-2
Woschek A, Lindner W, Hammerschmidt F (2003) Enzymes in organic chemistry, 11:[1] hydrolase-catalyzed resolution of α- and β-hydroxyphosphonates and synthesis of chiral, non-racemic β-aminophosphonic acids. Adv Synth Catal 345(12):1287–1298. doi:10.1002/adsc.200303135
Wuggenig F, Schweifer A, Mereiter K, Hammerschmidt F (2011) Chemoenzymatic synthesis of phosphonic acid analogues of L-lysine, L-proline, L-ornithine, and L-pipecolic acid of 99 % ee – assignment of absolute configuration to (–)-proline. Eur J Org Chem 2011(10):1870–1879. doi:10.1002/ejoc.201001501
Hammerschmidt F, Voellenkle H (1989) Absolute configuration of (2-amino-1-hydroxyethyl) phosphonic acid from Acanthamoeba castellanii (Neff). Preparation of phosphonic acid analogs of (+)- and (-)-serine. Liebigs Ann Chem 1989(6):577–583. doi:10.1002/jlac.1989198901101
Fischer C, Schmidt U, Dwars T, Oehme G (1999) Enantiomeric resolution of derivatives of α-aminophosphonic and α-aminophosphinic acids by high-performance liquid chromatography and capillary electrophoresis. J Chromatogr A 845(1–2):273–283. doi:10.1016/s0021-9673(99)00487-2
Pirkle WH, Burke JA (1992) Separation of the enantiomers of the 3,5-dinitrobenzamide derivatives of α-amino phosphonates on four chiral stationary phases. J Chromatogr A 598(2):159–167. doi:10.1016/0021-9673(92)85044-T
Pirkle WH, Jonathan Brice L, Caccamese S, Principato G, Failla S (1996) Facile separation of the enantiomers of diethyl N-(aryl)-1-amino-1-arylmethanephosphonates on a rationally designed chiral stationary phase. J Chromatogr A 721(2):241–246. doi:10.1016/0021-9673(95)00844-6
Pirkle WH, Jonathan Brice L, Widlanski TS, Roestamadji J (1996) Resolution and determination of the enantiomeric purity and absolute configurations of α-aryl-α-hydroxymethanephosphonates. Tetrahedron-Asymmetry 7(8):2173–2176. doi:10.1016/0957-4166(96)00263-7
Zarbl E, Lammerhofer M, Hammerschmidt F, Wuggenig F, Hanbauer M, Maier NM, Sajovic L, Lindner W (2000) Direct liquid chromatographic enantioseparation of chiral alpha- and beta-aminophosphonic acids employing quinine-derived chiral anion exchangers: determination of enantiomeric excess and verification of absolute configuration. Anal Chim Acta 404(2):169–177. doi:10.1016/s0003-2670(99)00700-x
Lämmerhofer M, Hebenstreit D, Gavioli E, Lindner W, Mucha A, Kafarski P, Wieczorek P (2003) High-performance liquid chromatographic enantiomer separation and determination of absolute configurations of phosphinic acid analogues of dipeptides and their α-aminophosphinic acid precursors. Tetrahedron-Asymmetry 14(17):2557–2565. doi:10.1016/s0957-4166(03)00537-8
Hoffmann CV, Reischl R, Maier NM, Lämmerhofer M, Lindner W (2009) Stationary phase-related investigations of quinine-based zwitterionic chiral stationary phases operated in anion-, cation-, and zwitterion-exchange modes. J Chromatogr A 1216(7):1147–1156. doi:10.1016/j.chroma.2008.12.045
Hoffmann CV, Pell R, Lämmerhofer M, Lindner W (2008) Synergistic effects on enantioselectivity of zwitterionic chiral stationary phases for separations of chiral acids, bases, and amino acids by HPLC. Anal Chem 80(22):8780–8789. doi:10.1021/ac801384f
Hoffmann CV, Reischl R, Maier NM, Lämmerhofer M, Lindner W (2009) Investigations of mobile phase contributions to enantioselective anion- and zwitterion-exchange modes on quinine-based zwitterionic chiral stationary phases. J Chromatogr A 1216(7):1157–1166. doi:10.1016/j.chroma.2008.12.044
Pell R, Sić S, Lindner W (2012) Mechanistic investigations of cinchona alkaloid-based zwitterionic chiral stationary phases. J Chromatogr A. doi:10.1016/j.chroma.2012.08.006
Wernisch S, Lindner W (2012) Versatility of cinchona-based zwitterionic chiral stationary phases: enantiomer and diastereomer separations of non-protected oligopeptides utilizing a multi-modal chiral recognition mechanism. J Chromatogr A 1269:297–307. doi:10.1016/j.chroma.2012.06.094
Hoffmann CV, Reischl R, Maier NM, Lammerhofer M, Lindner W (2009) Investigations of mobile phase contributions to enantioselective anion- and zwitterion-exchange modes on quinine-based zwitterionic chiral stationary phases. J Chromatogr A 1216(7):1157–1166. doi:10.1016/j.chroma.2008.12.044
Hoffmann CV, Reischl R, Maier NM, Lammerhofer M, Lindner W (2009) Stationary phase-related investigations of quinine-based zwitterionic chiral stationary phases operated in anion-, cation-, and zwitterion-exchange modes. J Chromatogr A 1216(7):1147–1156. doi:10.1016/j.chroma.2008.12.045
Pell R, Sic S, Lindner W (2012) Mechanistic investigations of cinchona alkaloid-based zwitterionic chiral stationary phases. J Chromatogr A 1269:287–296. doi:10.1016/j.chroma.2012.08.006
Pell R, Sić S, Lindner W (2012) Mechanistic investigations of cinchona alkaloid-based zwitterionic chiral stationary phases. J Chromatogr A 1269:287–296. doi:10.1016/j.chroma.2012.08.006
Wernisch S, Pell R, Lindner W (2012) Increments to chiral recognition facilitating enantiomer separations of chiral acids, bases, and ampholytes using Cinchona-based zwitterion exchanger chiral stationary phases. J Sep Sci 35(13):1560–1572. doi:10.1002/jssc.201200103
Lämmerhofer M, Lindner W (1996) Quinine and quinidine derivatives as chiral selectors I. Brush type chiral stationary phases for high-performance liquid chromatography based on cinchonan carbamates and their application as chiral anion exchangers. J Chromatogr A 741(1):33–48. doi:10.1016/0021-9673(96)00137-9
Maier NM, Schefzick S, Lombardo GM, Feliz M, Rissanen K, Lindner W, Lipkowitz KB (2002) Elucidation of the chiral recognition mechanism of cinchona alkaloid carbamate-type receptors for 3,5-dinitrobenzoyl amino Acids. J Am Chem Soc 124(29):8611–8629. doi:10.1021/ja020203i
Acknowledgment
This work was financially supported by the University of Vienna through the interdisciplinary doctoral program Initiativkolleg Functional Molecules (IK I041−N). We thank Jan Pícha (Institute of Organic Chemistry and Biochemistry, AS CR) and Friedrich Hammerschmidt (Institute of Organic Chemistry, University of Vienna) for providing several samples of APAs. We are further grateful to the Operational Program Research and Development for Innovations - European Regional Development Fund (project CZ.1.05/2.1.00/03.0058) and the project of Palacký University in Olomouc PRF_2012_020 for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in the topical collection Amino Acid Analysis with guest editor Toshimasa Toyo'oka.
Rights and permissions
About this article
Cite this article
Gargano, A.F.G., Kohout, M., Macíková, P. et al. Direct high-performance liquid chromatographic enantioseparation of free α-, β- and γ-aminophosphonic acids employing cinchona-based chiral zwitterionic ion exchangers. Anal Bioanal Chem 405, 8027–8038 (2013). https://doi.org/10.1007/s00216-013-6938-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6938-6