Abstract
This paper describes the synthesis and characterization of a fluorescent ion-imprinted polymer (IIP) for selective determination of copper ions in aqueous samples. The IIP has been prepared using a novel functional monomer, 4-[(E)-2-(4′-methyl-2,2′-bipyridin-4-yl)vinyl]phenyl methacrylate (abbreviated as BSOMe) that has been spectroscopically characterized in methanolic solution, in the absence and in the presence of several metal ions, including Cd(II), Cu(II), Hg(II), Ni(II), Pb(II), and Zn(II). The stability constant (2.04 × 108 mol−2 l2) and stoichiometry (L2M) of the BSOMe complex with Cu(II) were extracted thereof. Cu(II)-IIPs were prepared by radical polymerization using stoichiometric amounts of the fluorescent monomer and the template metal ion. The resulting cross-linked network did not show any leaching of the immobilized ligand allowing determination of Cu(II) in aqueous samples by fluorescence quenching measurements. Several parameters affecting optosensor performance have been optimized, including sample pH, ionic strength, or polymer regeneration for online analysis of water samples. The synthesized Cu(II)-IIP exhibits a detection limit of 0.04 μmol l−1 for the determination of Cu(II) in water samples with a reproducibility of 3%, exhibiting an excellent selectivity towards the template ion over other metal ions with the same charge and close ionic radius. The IIP-based optosensor has been repeatedly used and regenerated for more than 50 cycles without a significant decrease in the luminescent properties and binding affinity of the sensing phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecularly imprinted polymers (MIPs) are tailor-made materials capable of recognizing a particular molecule in the presence of others as they contain selective template recognition sites (for binding or catalysis). For MIP synthesis, the selected print molecule (the analyte) or a surrogate molecule, interacts through covalent or non-covalent bonds with functional monomers that are polymerized in the presence of a cross-linker to form a three dimensional structure. The latter, upon template removal, will bear selective recognition sites with complementary size, geometry, and arrangement of functional groups to the target compound. Molecular imprinting technology has already been applied to preparation of materials selective to a wide variety of chemical and biochemical species [1–3] of interest in different areas, including solid-phase extraction, enantiomeric separations, biomimetic assays, and sensing [4–7].
In a parallel approach, ion-imprinted polymers (IIPs) can be based on the formation of a complex between a selective ligand and the target ion [8] prior to polymerization. The ligand may be trapped in the cross-linked network upon polymerization. Alternatively, the use of a monomeric ligand results in the formation of chemical bonds during polymerization that imparts higher polymer stability and reusability, avoiding the leaching problems associated to the ligand physical entrapment that may cause sample contamination and a reduction of the analytical signal over time.
In an ion-imprinting process, the selectivity of the imprinted adsorbent must satisfy the coordination geometry, oxidation state, and size of the target ion [9]. IIPs have found application to solid-phase extraction [10, 11], membrane separations [12], and in sensing [13] of different metal ions. Nowadays, one of the main challenges in the development of IIP-based sensors relies on finding selective metal chelating ligands with responsive functionalities that can be readily coupled to the transducer for signal generation [2]. In this regard, the availability of fluorescent probes selective to metal ions of environmental or biological interests has traditionally attracted much interest for chemical optosensing, due to the inherent sensitivity and selectivity of luminescent methods that can be further implemented in combination with the selectivity and cost effectiveness of ion imprinting technologies [8].
Copper is an environmentally toxic pollutant but plays an important role in several biological systems [14]. Food and water are the primary sources of copper exposure in developed countries, and the World Health Organization (WHO) recommends that the concentration of Cu(II) in drinking water should not exceed 2 mg l−1 (31 μM). In the last years, several authors have reported preparation of IIPs for preconcentration or selective separation of this metal ion [10, 11, 15–21] in aqueous samples, and IIP-based sensors using different transduction techniques such as potentiometry [22], quartz crystal microbalance [23], or optical reflectance [24], with detection limits ranging from 8 × 10−4 to 123 μM in water samples.
We have reported recently the development of an optosensor based on a novel engineered ligand containing a fluorescent bipyridine moiety, covalently immobilized in a sol–gel matrix for the simultaneous determination of Cu(II), Hg(II), and Zn(II) in mineral water samples [25].
To the best of our knowledge, there is no previous report of the synthesis of Cu(II)–IIP fluorosensors for the determination of this metal ion in water samples. This paper describes the synthesis of a fluorescent polymerizable ligand, BSOMe (4-[(E)-2-(4′-methyl-2,2′-bipyridin-4-yl)vinyl]phenyl methacrylate), and its application to the synthesis of a selective Cu(II)-IIP optosensor. The polymer has been prepared by radical polymerization of the Cu(II)–BSOMe complex to generate binding sites with the adequate coordination geometry and size in the polymeric network after removal of the metal ion. Characterization of the spectroscopic features of the novel BSOMe in the presence of Cu(II) and other heavy metal ions in solution, as well as in the sensing phase, are described and discussed.
Experimental
Reagents
Hydroxyethyl methacrylate (HEMA), acrylamide (AA), 3-(acryloyloxy)-2-hydroxypropyl methacrylate, and 2,2-dimethoxy-2-phenylacetophenone were purchased from Sigma-Aldrich, Madrid (Spain). The acrylic monomers were purified by column chromatography using an inhibitor remover (Sigma-Aldrich) immediately before use. Analytical grade reagents from Sigma-Aldrich Química (Madrid, Spain) and water purified by a Millipore Milli-Q system (Millipore, Bedford, MA, USA) were used throughout. Buffer solutions (20 mmol l−1) in the 2.0 to 9.0 pH range were prepared from dipotassium hydrogen phosphate, potassium dihydrogen phosphate, potassium phosphate, and hydrochloric acid or sodium hydroxide solutions. Anhydrous methanol (over molecular sieves) was from Fluka. Stock solutions of the different metal ions were prepared in methanol from analytical-grade mercury chloride (HgCl2), zinc chloride (ZnCl2), copper sulfate pentahydrate (CuSO4.5H2O), nickel chloride hexahydrate (NiCl2.6H2O), cadmium nitrate tetrahydrate (Cd(NO3)2.4H2O), and lead nitrate (Pb(NO3)2) obtained from Vetec (São Paulo, Brazil). Working solutions were prepared by serial dilutions of the stock solutions with phosphate buffer solution.
Apparatus
A Bruker AVANCE DPX 300 MHz-BACS60 spectrometer was employed to acquire the 1H-NMR spectra. Mass spectra were obtained with a Bruker APEX QIV FTMS high-resolution spectrometer. Surface area of the material was determined with an Autosorb I from Quantachrome Instruments (Boynton Beach, FL, USA). Scanning electron microscopy micrographs were obtained with a JEOL JSM 6360LV microscope. UV–visible absorption spectra were measured using a Varian Cary 3-Bio (Palo Alto, CA, USA) spectrophotometer. Fluorescence data were acquired with a Varian Cary Eclipse spectrofluorometer, using 10-nm slits and a 360-nm cut-off filter in the emission path. The pH of the sample and buffer solutions were adjusted with a Corning 443i pH meter (São Paulo, Brazil) equipped with a combination glass electrode.
Synthesis and characterization of the functional monomer
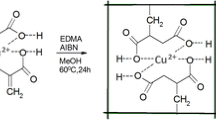
The functional monomer BSOMe (4-[(E)-2-(4′-methyl-2,2′-bipyridin-4-yl)vinyl]phenyl methacrylate) was prepared by acylation of the precursor ligand 4-[(E)-2-(4′-methyl-2,2′-bipyridin-4-yl)vinyl]phenol (BSOH) [25] with methacrylic anhydride. Briefly (scheme 1), 40 mg (0.139 mmol) of BSOH was dissolved in 2 mL of anhydrous dimethylformamide (Fluka, dried over molecular sieves) containing 57.7 μL (0.417 mmol) of triethylamine (Riedel-de Häen). After purging for 15 min with argon, 25 μL (0.17 mmol) of methacrylic anhydride (94%, Aldrich) was added, and the reaction mixture was stirred at room temperature under argon for 18 h. Then, the dimethylformamide was evaporated under vacuum (<50 °C), and the residue was purified by column chromatography (silica, CH2Cl2–MeOH, 40:1 v/v) to yield BSOMe as a yellowish solid (81% yield). 1H–NMR (300 MHz, CDCl3)—δ (ppm) = 2.08 (3H, s, –CH 3 ), 2.53 (3H, s, –CH 3 ), 5.78 (1H, s, =CH), 6.37 (1H, s, =CH), 7.10 (1H, d, J = 16.37 Hz, =CH), 7.18 (2H, d, J = 8.48 Hz, ArH), 7.30 (1H, d, J = 5.26 Hz, ArH), 7.46 (1H, d, J = 5.11 Hz, ArH), 7.57–7.64 (3H, m, ArH, =CH), 8.46 (1H, s, ArH), 8.64 (1H, d, J = 5.40 Hz, ArH), 8.68 (1H, d, J = 5.26 Hz, ArH), 8.77 (1H, s, ArH); MS—m/z calcd. for C23H21N2O +2 [M+H]+—357.1597; found—357.1589.
Synthesis of the Cu(II)-imprinted polymer and instrumental setup
For the Cu-IP preparation, 6.15 × 10−3 mmol of Cu(II) in 5 ml of methanol were mixed with 12.3 × 10−3 mmol of the BSOMe monomer (1:2 metal-to-ligand ratio) to allow coordination. The solution was mixed with HEMA (3.73 ml; 30.7 mmol), AA (1.0 g; 14.05 mmol), and 3-(acryloyloxy)-2-hydroxypropyl methacrylate (250 μL; 1.33 mmol). The mixture was homogenized and left to stand for 30 min. After adding the initiator (2,2-dimethoxy-2-phenylacetophenone, 0.39 mmol), the mixture was purged with argon for 30 min, and the vials were sealed and irradiated in a home-made photochemical carousel reactor equipped with eight “black-light” lamps (Philips F8T5/BLB, 8-W each one) with an emission maximum at 350 nm, at 4 °C for 18 h. Non-imprinted polymers (NIPs) were prepared in the same way but with omission of Cu(II) ions. The Cu-IP and NIP materials showed red and yellow colors, respectively, confirming the stability of the metal complex during the polymerization reaction. The vials were broken, and the MIP monolith was removed and broken into smaller fragments. Cu(II) ions were extracted by successive washings with 0.1 mol l−1 HCl, followed by a final washing with purified water. Thereafter, the resulting polymers were dried at 50 °C for 4 h, and then crushed and sieved using US standard 100- and 150-μm sieves (Filtra, Barcelona, Spain). The polymer particles were packed in a 100-μL, 3-mm optical path, fluorescence flow-through fused silica cell (Hellma, Germany) for the analyses as described previously [25].
The sensing flow cell was placed inside the sample holder of the fluorometer. The sample flow stream was generated by a peristaltic pump (Ismatec IPC 8, Switzerland). The carrier solution consisted of 0.05 mol l–1 pH 5.0 phosphate buffer that was pumped through the sensing phase at a constant flow rate of 0.30 ml min−1. Once a stable base line was obtained, 4.5 mL (15 min) of the heavy metal sample solution was pumped through the system and the decrease of the fluorescence signal was evaluated. Finally, the sensing phase was regenerated with 0.60 mL (2 min) of 0.05 mol l–1 HCl solution, and carrier buffer solution was pumped through the flow cell to obtain the reference signal (3.0 mL, 10 min). All determinations were carried out in duplicate.
Results and discussion
Characterization of the BSOMe monomer and its metal ion complexes
The chemical structure of the functional monomer was confirmed by 1H-NMR and high-resolution mass spectrometry (see “Experimental” section). Figure 1 depicts the absorption and emission spectra of the chelating BSOMe monomer in methanol solution. An intense absorption band is observed at 313 nm while its fluorescence peaks at 404 nm. The spectroscopic features of the precursor phenolic BSOH ligand are significantly different [25] as it shows the lowest energy absorption band at 336 nm and the fluorescence maximum at 440 nm. The blue shift observed in the emission maxima of the monomer with respect to BSOH is due to the intramolecular charge transfer (ICT) character of its lowest lying excited state. Esterification of the phenol group decreases its electron-releasing features so that formation of the ICT state is more difficult. Esterification also eliminates the capability of hydrogen bonding phenol to solvent, thus reducing the stabilization of the (polar) excited state and contributing also to the observed blue shift of the emission band. The hypsochromic shift of the absorption band has a similar origin due to the decrease in the push–pull character of the chromophoric stilbene [26].
Figure 2 shows the absorption spectra of 5.0 × 10−5 mol l−1 Cd(II), Cu(II), Hg(II), Ni(II), Pb(II), and Zn(II) solutions in the presence of 2.0 × 10−5 mol l−1 BSOMe in methanol. As it can be seen, the absorption profile for each metal ion complex is different, except for Cu(II) and Ni(II). These two ions also suppressed the emission of the fluorescent monomer, as depicted in Fig. 3.
A broad emission band (Fig. 3) was obtained in the presence of Hg(II) complex, and complexation to the Cd(II) ion causes an increase in the intensity of the BSOMe emission and a red shift to 450 nm; the Zn(II) ion strongly increases the luminescence intensity of the monomer and is included in Fig. 3 under the same experimental conditions for the sake of comparison. Similar to that of the BSOH precursor [26], the behavior of the BSOMe ligand is quite different depending on the metal ion involved in the coordination. The emission spectrum of the protonated BSOMe (Fig. 4) is similar to those observed in the presence of Zn(II), Cd(II), Hg(II), and Pb(II) ions. This fact confirms that the fluorescence red shift induced by these metal ions is due to the enhancement of the ICT as a consequence of coordination. The higher fluorescence intensity in the case of Zn(II) and Cd(II) complexes could be attributed to the increased rigidity of the ligand as observed upon complexation of many other fluorescent chelating ligands [27]. This effect is completely offset by the highly efficient photoinduced electron transfer (static) quenching observed in the case of the Cu(II) and Ni(II) complexation [25] and partially balanced by the enhanced ICT for the other investigated metal ions. However, a more detailed photochemical discussion on these phenomena is out of the scope of the present work.
The overall stability constant (2.04 × 108 mol−2 l2) and stoichiometry (L2M) of the BSOMe complex with Cu(II) was determined from the absorption spectra (Figure S2 in the Electronic supplementary material) by titration of a methanolic solution of the chelating ligand with the metal ion and using the Hyperquad 2006 computer program (Protonic Software, www.hyperquad.co.uk).
Characterization of the fluorescent ion-imprinted polymer
In this work, Cu(II) sensing is based on a fluorescence quenching mechanism. The latter determines that, when the amount of emissive ligand molecules in the polymer network which are unaffected by the Cu(II) ion (quencher) increases, the recognition material displays lower sensitivity. Therefore, the polymer was prepared with a small amount of BSOMe monomer (1:2 metal-to-ligand ratio) to prevent self-quenching and to allow noticeable deactivation by lower concentrations of the target cation. HEMA and AA were also included as monomers in polymer formulation to increase recognition in aqueous solutions, and 3-(acryloyloxy)-2-hydroxypropyl methacrylate was used as the cross-linker for enhanced water compatibility, based on previous results of our research group on the development of acrylate-based optosensing layers [28].
The resulting Cu(II)-IIPs were characterized by observation of distinct color changes in the course of the polymerization process and upon sorption and desorption of copper ion, scanning electron microscopy (SEM), N2 adsorption–desorption isotherms, and fluorescence spectroscopy. As mentioned before, the Cu(II)-IP and NIP materials displayed dramatically different red and yellow colors (Fig. S1, Electronic supplementary material), respectively, indicating the presence of the Cu(II)–BSOMe complex into the cross-linked network. Extensive washing of the materials to remove non-polymerized compounds and Cu(II) ions from the IIP did not show any leaching of the fluorescent monomer, confirming its efficient bonding to the polymer matrix through the methacrylate group that replaced the phenol group in the precursor as shown in scheme 1.
The SEM micrographs, Fig. S3 in the Electronic supplementary material, did not reveal important textural differences between the IIP and the non-imprinted material, although surface roughness seems higher for the NIP. The IIP was found to be a microporous material as assessed by nitrogen adsorption–desorption studies. The surface area for the Cu(II)-IIP, determined by applying the Brunauer–Emmett–Teller (BET) theory, was 23.0 m2 g−1, whereas the average pore radius and the pore volume, derived by the Barrett–Joyner–Halenda theory, were 59.1 Å and 0.68 μL g−1, respectively. Surprisingly enough, the experimental data obtained with the NIP material did not obey the BET isotherm, preventing textural characterization of this polymer.
IIP-based optosensor optimization
Several parameters affecting the fluorescence of the IIP as well as the complexation equilibrium of Cu(II) ions have been optimized. Firstly, the effect of pH on the sensing phase luminescence was investigated in the 1.8 and 9.2 range, using 10 mM phosphate buffer solutions. As shown in Fig. 5, similar to the behavior of the free monomer in buffer solution, maximum emission at 462 nm was obtained at pH < 3.2, while this peak shifted to 397 nm at pH > 5.2, with an isoemissive point observed at 415 nm. The significant blue shift of the emission maximum of the acidic form of the polymerized monomer with respect to that observed for the free monomer in aqueous solutions (481 nm, Fig. 4) might be related to the higher hydrophobicity imparted by the polymer network to the fluorescent probe that prevents water to fully stabilize its important ICT excited state under these conditions. The lower extent of the ICT phenomenon in the excited state of the unprotonated BSOMe determines a smaller blue shift of its emission band when going from the free to the polymerized species (402 to 397 nm, Figs. 4 and 5, respectively).
The fluorosensor response to Cu(II) ions as a function of pH was evaluated in the 5.0 to 8.0 range. Lower pH values were not tested because protonation of the BSOMe ligand impairs the complexing reaction of the metal ions. Samples containing 10 mM Cu(II) were prepared in 10 mM phosphate buffer and analyzed using the procedure described in the “Experimental” section. The sensor response was not significantly different within the 5.0–6.0 pH range; however, at higher pH values, the fluorescence signal diminished and the reproducibility of the measurements decreased due to formation of copper hydroxides. Therefore, pH 5.0 was selected for further experiments.
One of the important features of a chemosensor is its reversible response towards the target analyte. Unfortunately, most of metal ion sensors work under irreversible conditions of analysis, as the conditional formation constant between the ligand and the analyte must be high enough to drive the transfer of the later species from the aqueous solution to the solid phase. In such type of sensors, a regeneration step is necessary, which is usually performed with the aid of an acidic solution or a complexing reagent solution such as EDTA. In this way, the fluorescence of the sensing phase was recovered by flowing a 100 or 50 mM HCl solution during 2 min (0.30 ml min−1). The use of 10 mM HCl required longer regeneration times (12 min), so that a 50 mM HCl solution was selected for removing the Cu(II) retained in the sensing phase.
The effect of the ionic strength of the buffer solution on the analytical signal was also investigated measuring the optosensor response to water solutions containing 0.1 mol l−1 Cu(II) in pH 5.0 and phosphate buffer concentrations of 0.010, 0.025, and 0.050 mol l−1. The results showed that the variations in the buffer ionic strength in the assayed range did not affect the optosensor response, but the most concentrated solution, due to its higher buffer capacity, provided the most stable signals (1.5% relative standard deviation (RSD)) and was employed throughout the work.
Figure 6 shows an example of the optosensor response profile, in the optimized conditions, to injections of decreasing concentration of Cu(II) samples in the range from 61.30 to 0 μmol l−1. The corresponding calibration graphs are shown in Fig. 7. Practically no response is observed in the non-imprinted material, demonstrating the selective recognition of the template ions by the fluorescent IIP in aqueous samples and the effectiveness of the imprinting process. Fitting the response to the modified Stern–Volmer equation (I 0 − I F)/(I − I F) = 1 + K SV[Cu(II)], where I 0 corresponds to the fluorescence intensity in the absence of the analyte, I is the fluorescence intensity in the presence of Cu(II), and I F is the (unquenchable) constant scattered light, yielded a Stern–Volmer constant of K SV = 2.2 × 105 l μmol−1 for the linear range from 0.16 to 8.29 μmol l−1.
Dose–response plot for the Cu(II)-IP sensor in the presence of decreasing concentrations of Cu(II) (61.30 × 10−4–0.12 × 10−6 mol 1−l) in 10 mmol l−1 pH 5.0 phosphate buffer. Polymer regeneration with 50 mmol l−1 HCl (λ exc = 330 nm; λ em = 415 nm). Inset: NIP response in the presence of decreasing concentrations of Cu(II) (111.0 × 10−4–1.11 × 10−6 mol 1−l) in the same experimental conditions. Values shown below the peaks indicate the concentration of Cu(II) in μmol l−1 (see text)
The limit of detection (LOD), calculated according to Miller and Miller [29] as the Cu(II) concentration producing an analytical signal that is three times the standard deviation of the blank signal, was 0.04 μmol l−1, a value lower than the control level recommended by WHO for tap water (31 μmol l−1) and the LOD achieved with previous IIP-based optical sensors (123 μmol l−1) [24]. Moreover, the LOD value is about one tenth lower than that obtained with the fluorosensor manufactured with the precursor ligand (BSOH) immobilized in a silica sol–gel [25].
The total analysis time was 27 min to perform a single measurement, and the sensing phase could be regenerated and reused for more than 50 cycles without significant changes in the optosensor response. Sensor repeatability, evaluated as the relative standard deviation of triplicate measurements of water solutions containing 0.5 and 2.0 μmol l−1 Cu(II), was better than 3.0%. To evaluate the intra-laboratory reproducibility, the Cu(II) samples were analyzed in triplicate for three different days with RSDs lower than 3.0%.
The Cu(II) fluorosensor selectivity was evaluated by measuring the IIP and NIP responses to water samples containing 10 μmol l−1 each of Cu(II), Cd(II), Hg(II), Ni(II), Pb(II), and Zn(II) ions in 50 mM pH 5.0 phosphate buffer (Fig. 8). The analyte selectivity of IIPs has been shown to be determined by two main factors: (a) the affinity of the ligand for the imprinted metal ion, and (b) the size and shape of the generated cavities [17]. As discussed previously (Fig. 3), those heavy metals influence the emission characteristics of BSOMe in methanol solution but not in the ion-imprinted polymer, even though they have similar charge and, in some cases, similar ionic radius to Cu(II) demonstrating, once again, the effectiveness of the imprinting process. Only Ni(II) showed some cross-reactivity for Cu(II) determination, although much lower than in solution. This is a clear advantage over other IIP sensors for Cu(II) determination which are not based on the use of Cu(II) ion selective ligands [22, 23, 25]. The Fe(III) ion was not included in the figure, as it precipitated as hydroxide in the pH 5.0 buffer solution. Moreover, the selectivity of the optosensor prepared with the Cu(II)-IIP as recognition material turned out to be significantly better than that achieved with the precursor fluorescent ligand covalently immobilized in a sol–gel matrix [25], demonstrating the applicability of this procedure not only for molecular recognition but also for metal ion screening.
Conclusions
We have shown in this work that the application of imprinting technology may significantly enhance the selectivity and sensitivity of optosensing materials prepared with ion chelating ligands. Synthesis of such polymers requires the availability of fluorescent polymerizable ligands, such as the 2,2′-bipyridyl stilbene synthesized and characterized in this work (BSOMe), for preparation of synthetic recognition elements containing both the recognition binding site and the signalling luminescent moiety whose emission properties are modulated by the selective rebinding of the target ion. On the other hand, the use of designed polymerizable ligands, with high affinity towards the template ion, allows the preparation of polymers with highly selective binding sites and decreased non-specific binding. In comparison to other solid supports such as sol–gel matrices commonly applied to optosensor development, the IIPs display a higher selectivity and reusability for the analysis of Cu(II) in aqueous samples, where imprinted polymers usually show a poor performance. Current work is being carried out in our laboratory to extend the application of this principle to the analysis of other heavy metals for multi-analyte screening in environmental waters.
References
Sellergren B (ed) (2001) Molecularly imprinted polymers. Man made mimics of antibodies and their applications in analytical chemistry. Elsevier, Amsterdam, The Netherlands
Moreno-Bondi MC, Navarro-Villoslada F, Benito-Peña E, Urraca JL (2008) Molecularly imprinted polymers as selective recognition elements in optical sensing. Curr Anal Chem 4:316–340
Chen L, Xuab S, Lia J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942
Maier NM, Lindner W (2007) Chiral recognition applications of molecularly imprinted polymers: a critical review. Anal Bioanal Chem 389:377–397
Tse Sum Bui T, Haupt K (2010) Molecularly imprinted polymers: synthetic receptors in bioanalysis. Anal Bioanal Chem 398:2481–2492
Suriyanarayana S, Cywinski PJ, Moro AJ, Mohr GJ, Kutner W (2011) Chemosensors based on molecularly imprinted polymers. Top Curr Chem. doi:10.1007/128_2010_92
Moreno Bondi MC, Benito Peña ME, Urraca JL, Orellana G (2011) Immuno-like assays and biomimetic microchips. Top Curr Chem. doi:10.1007/128_2010_94
Prasada Rao T, Kala R, Daniel S (2006) Metal ion-imprinted polymers—novel materials for selective recognition of inorganics. Anal Chim Acta 578:105–116
Özkara S, Andaç M, Karakoç V, Say R, Denizli A (2011) Ion-imprinted PHEMA based monolith for the removal of Fe3+ ions from aqueous solutions. J Appl Polym Sci 120:1829–1836
Shamsipur M, Besharati-Seidani A (2011) Synthesis of a novel nanostructured ion-imprinted polymer for very fast and highly selective recognition of copper(II) ions in aqueous media. React Funct Polym 71:131–139
Walas S, Tobiasz A, Gawin M, Trzewik B, Strojny M, Mrowiec H (2008) Application of a metal ion-imprinted polymer based on salen–Cu complex to flow injection preconcentration and FAAS determination of copper. Talanta 76:96–101
Araki K, Marumaya T, Kamiya N, Goto M (2005) Metal ion-selective membrane prepared by surface molecular imprinting. J Chromatogr B 818:141–145
Taher A, Somaye A (2011) Preparation of nano-sized Pb2+ imprinted polymer and its application as the chemical interface of an electrochemical sensor for toxic lead determination in different real samples. J Hazard Mater 190:451–459
World Health Organization (2004) Copper in drinking-water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization, http://www.who.int/water_sanitation_health/dwq/chemicals/copper.pdf
Say R, Birlik E, Eröz A, Yilmaz F, Gedikbey T, Denizli A (2003) Preconcentration of copper on ion-selective imprinted polymer microbeads. Anal Chim Acta 480:251–258
Dakova I, Karadjova I, Ivanov I, Georgieva V, Evtimova B, Georgiev G (2007) Solid phase selective separation and preconcentration of Cu(II) by Cu(II)-imprinted polymethacrylic microbeads. Anal Chim Acta 584:196–203
Shamsipur M, Fasihi J, Khanchi A, Hassani R, Alizadeh K, Shamsipur H (2007) A stoichiometric imprinted chelating resin for selective recognition of copper(II) ions in aqueous media. Anal Chim Acta 599:294–301
Wang S, Zhang R (2006) Selective solid-phase extraction of trace copper ions in aqueous solution with a Cu(II)-imprinted interpenetrating polymer network gel prepared by ionic imprinted polymer (IIP) technique. Microchim Acta 154:73–80
Birlik E, Eröz A, Denizli A, Say R (2006) Preconcentration of copper using double-imprinted polymer via solid phase extraction. Anal Chim Acta 565:145–151
Dam HA, Kim D (2009) Selective copper(II) sorption behavior of surface-imprinted core-shell-type polymethacrylate microspheres. Ind Eng Chem Res 48:5679–5685
Jo SH, Park C, Yi SC, Kim D, Mun S (2011) Development of a four-zone carousel process packed with metal ion-imprinted polymer for continuous separation of copper ions from manganese ions, cobalt ions, and the constituent metal ions of the buffer solution used as eluent. J Chromatogr A 1218:5664–5674
Rongning L, Ruiming Z, Wenjing S, Xuefeng H, Wei Q (2011) Potentiometric sensor based on an ion-imprinted polymer for determination of copper. Sens Lett 9:557–562
Zheng-Peng Y, Chun-Jing Z (2009) Designing of MIP-based QCM sensor for the determination of Cu(II) ions in solution. Sensors Actuator B 142:210–215
Sing-Muk N, Narayanaswamy R (2010) Demonstration of a simple, economical and practical technique utilising an imprinted polymer for metal ion sensing. Microchim Acta 169:303–311
Pinheiro SCL, Raimundo IM Jr, Moreno-Bondi MC, Orellana G (2010) Simultaneous determination of copper, mercury and zinc in water with a tailored fluorescent bipyridine ligand entrapped in silica sol–gel. Anal Bioanal Chem 398:3127–3138
Likhtenshtein G (2010) Stilbenes. Applications in chemistry, life sciences and materials science. Wiley, New York
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York
Orellana G, Urraca JU, Ribeiro dos Santos A “Method for preparing thin films in optical sensors” PCT Patent Appl. WO2011/009981
Miller JC, Miller JN (2005) Statistics for analytical chemistry, 5th edn. Pearson Education, Essex
Acknowledgments
The authors thank financial support from CAPES (CAPES/DGU 125/06), the Spanish Ministry of Education and Ministry of Science and Innovation (PHB2005-0030-PC, CTQ2009-14565-C03), and Complutense University (GR58-08-910072). FAPESP (05/04258-6) and National Institute of Advanced Analytical Science and Technology-INCTAA (CNPq 573894/2008-6 and FAPESP 2008/57808-1) are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in the topical collection Biomimetic Recognition Elements for Sensing Applications with guest editor María Cruz Moreno-Bondi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 254 kb)
Rights and permissions
About this article
Cite this article
Lopes Pinheiro, S.C., Descalzo, A.B., Raimundo, I.M. et al. Fluorescent ion-imprinted polymers for selective Cu(II) optosensing. Anal Bioanal Chem 402, 3253–3260 (2012). https://doi.org/10.1007/s00216-011-5620-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5620-0