Abstract
Matrix solid-phase extraction has been successfully applied for the determination of multi-class preservatives in a wide variety of cosmetic samples including rinse-off and leave-on products. After extraction, derivatization with acetic anhydride, and gas chromatography–mass spectrometry analysis were performed. Optimization studies were done on real non-spiked and spiked leave-on and rinse-off cosmetic samples. The selection of the most suitable extraction conditions was made using statistical tools such as ANOVA, as well as factorial experimental designs. The final optimized conditions were common for both groups of cosmetics and included the dispersion of the sample with Florisil (1:4), and the elution of the MSPD column with 5 mL of hexane/acetone (1:1). After derivatization, the extract was analyzed without any further clean-up or concentration step. Accuracy, precision, linearity and detection limits were evaluated to assess the performance of the proposed method. The recovery studies on leave-on and rinse-off cosmetics gave satisfactory values (>78% for all analytes in all the samples) with an average relative standard deviation value of 4.2%. The quantification limits were well below those set by the international cosmetic regulations, making this multi-component analytical method suitable for routine control. The analysis of a broad range of cosmetics including body milk, moisturizing creams, anti-stretch marks creams, hand creams, deodorant, shampoos, liquid soaps, makeup, sun milk, hand soaps, among others, demonstrated the high use of most of the target preservatives, especially butylated hydroxytoluene, methylparaben, propylparaben, and butylparaben.

MSPD GC-MS analysis of preservatives in cosmetics
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cosmetic products are complex formulations containing many additives and ingredients. To ensure protection of human health, cosmetic products are regulated and controlled worldwide. The European Union (EU) Cosmetic Products Regulation [1] constitutes the main regulatory system on cosmetic products in Europe. Among the different groups of cosmetic additives, preservatives (added to maintain the integrity of the product), especially p-hydroxybenzoic acid (parabens) and potentially nitrosating compounds, are a matter of scientific and social concern due to the negative side effects on human health.
The esters of parabens, iodopropynyl butylcarbamate (IPBC), 2,4,4′-trichloro-2′-hydroxydiphenyl ether (triclosan (TCS)), and bromine-containing preservatives as 5-bromo-5-nitro-1,3-dioxane (bronidox) and 2-bromo-2-nitropropane-1,3-diol (bronopol), are included in a wide variety of cosmetics and personal-care products to prevent or retard bacterial growth. Parabens are the most widely used antimicrobial preservatives in cosmetic products. Their antimicrobial activity is generally selective, so their mixtures or mixtures with other classes of preservatives offer powerful antimicrobial activity against an extremely broad spectrum of microorganisms [2]. 2-tert-Butyl-4-methoxyphenol (butylated hydroxyanisole (BHA)) and 2,6-bis(1,1-dimethylethyl)-4-methylphenol (butylated hydroxytoluene (BHT)) are antioxidant preservatives frequently used to prevent oxidation in foods and cosmetics. The use of mixtures of both of them is very common since there is a synergic increase of their antioxidant power [3].
The preservatives allowed in the EU context are listed in the Annex V of the EU Cosmetics Regulation [1] where limitations, requirements, label warnings, and the maxima permissible concentrations are indicated (see Table 1 last column for the target preservatives).
To guarantee consumer health and ensure compliance to existing government regulations, there is a need for the development of effective and convenient methodologies to identify and determine preservatives in cosmetics. A great part of the analytical effort has been focused on parabens determination [4–7] while methods for the determination of other preservatives in cosmetic formulation are very limited or inexistent. Simultaneous analysis of more than one class of preservatives is scarce and mainly based on liquid chromatography [8–10] and capillary electrophoresis [11, 12]. Flow injection analysis has also been employed enhancing sample throughput [13]. For most cosmetic samples, it is not possible to simply dilute the sample in an adequate solvent prior analysis since several sample components would not be solubilized and we would not obtain homogeneous extracts. In addition, the complexity of the obtained solutions would cause chromatographic contamination after few analyses, and coelution of matrix components, making really hard to obtain satisfactory analytical results for the target compounds. In most procedures, sample preparation is usually tedious and time consuming, and the use of hazardous solvents is frequently required. In addition, the possible presence of interferences that could distort the results is not rejectable. To overcome some of these drawbacks, supercritical fluid extraction [8, 14] or solid-phase extraction [4] have been recently applied for the determination of different additives in cosmetics. Recently, the authors have developed a pressurized solvent extraction procedure followed by gas chromatography–mass spectrometry (GC-MS) analysis for the determination of various groups of preservatives in leave-on cosmetics [15].
Matrix solid-phase dispersion (MSPD) was introduced by Barker et al. [16]. The possibility of performing extraction and clean-up at the same time is one of the main advantages of this technique, which reduces sample contamination during the procedure and decreases the amount of solvent required [17, 18]. MSPD developments and applications are compiled in several reviews [17–20]. This technique has been applied for the isolation of a wide variety of analytes, such as drugs, pesticides [21], polychlorinated biphenyls, antibiotics, surfactants, and naturally occurring compounds, in different matrices. It has also been applied to the analysis of some cosmetic ingredients including preservatives [21, 22] in environmental samples, but to our knowledge, this technique has not been applied to cosmetic samples.
The aim of this work is to develop a simple, efficient, and inexpensive MSPD method followed by derivatization GC-MS analysis to simultaneously determine different classes of preservatives such as bromine-containing preservatives, parabens, IPBC, TCS, and the antioxidants BHA and BHT, in a broad spectrum of cosmetic samples, including both rinse-off and leave-on products.
Experimental
Chemicals
Bronidox (≥99.0%) was acquired from Fluka (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland). Bronopol (98%), methylparaben (MeP; 99%), ethylparaben (EtP; 99%), propylparaben (PrP; 99%), butylparaben (BuP; 99%), benzylparaben (BzP; 99%), butylated hydroxyanisole (BHA; ≥98.5%), butylated hydroxytoluene (BHT; 99%), IPBC (97%), and triclosan (TCS; ≥97.0%) were purchased from Aldrich (Milwaukee, WI, USA). Isopropylparaben (iPrP; ≥99%) and isobutylparaben (iBuP; ≥97%) were purchased from TCI Europe (Belgium). Table 1 shows the IUPAC names and CAS numbers of the studied compounds.
Deuterated methyl-4-hydroxybenzoate-2,3,5,6-d4 (MePd4; 98 atom % D), used as surrogate standard, was obtained from C/D/N Isotopes (Quebec, Canada). The internal standard (IS) PCB-30 (2,4,6-trichlorobiphenyl) was purchased from Dr. Ehrenstorfer (Augsburg, Germany). Acetone, ethyl acetate, n-hexane, pyridine, and acetic anhydride (Ac2O) were provided by Merck (Darmstadt, Germany). Florisil (60–100 mesh) was purchased from Supelco Analytical (Bellefonte, PA, USA). Neutral alumina, C18, and sand (50–70 mesh) were achieved from Sigma-Aldrich (St. Louis, MO, USA). Silica gel 60 (230–240 mesh) was obtained from Merck KGaA (Darmstadt, Germany). Before being used, Florisil, alumina, and silica were activated at 130 °C for 12 h and then allowed to cool down in a desiccator. Sodium sulfate anhydrous (99%) was purchased by Panreac (Barcelona, Spain).
Individual stock solutions of each compound were prepared in acetone. Further dilutions and mixtures were prepared in acetone, hexane, hexane/acetone (1:1, v/v), and ethyl acetate. All solutions were stored in amber glass vials at −20 °C. All solvents and reagents were of analytical grade. Calibration standards were prepared covering a concentration range from 0.02 to 10 μg mL−1 in hexane/acetone. Surrogate and the internal standards were added. The solutions were derivatized by adding 100 μL of acetic anhydride containing 2.5% of pyridine to 1 mL of the standard. The mixture was then maintained at 80 °C for 10 min, and then allowed to cool down before GC analysis.
Cosmetic samples
Different cosmetics from national and international brands were purchased from local sources. They included leave-on and rinse-off products such as body milk, moisturizing creams, anti-stretch marks creams, hand creams, makeup, sun milk, deodorant, shampoos and liquid soaps, hand soaps, among others. Samples were kept in their original containers at room temperature until their analysis.
MSPD and derivatization procedures
A 0.5 g of cosmetic sample was exactly weighted into a 10-mL glass vial and spiked with 20 μL of MePd4 surrogate solution (1,000 μg mL−1). When it was necessary, the sample was spiked with 50 μL of the corresponding acetone solution of the target compounds to get the desired final concentration. After homogenization, the sample was gently blended with 1 g of a drying agent (anhydrous Na2SO4) and 2 g of dispersing sorbent into a glass mortar using a glass pestle until a homogeneous mixture was obtained (ca. 5 min). Then, the mixture was transferred into a column with a polypropylene frit at the bottom containing 0.5 g of Florisil (to obtain a further degree of fractionation and sample clean-up). A second frit was placed on top of the sample before compression with a syringe plunger. Elution was made by gravity flow with hexane/acetone (1:1, v/v), collecting 5 mL of extract into a graduated conical tube; 100 μL of PCB 30 solution (10 μg mL−1) were finally added.
Acetylation was carried out by adding 100 μL of acetic anhydride containing 2.5% of pyridine to 1 mL of the standard or extract solutions. The mixture was then maintained at 80 °C for 10 min, and then allowed to cool down to room temperature. The derivatized MSPD extracts, diluted when necessary, were directly analyzed by GC-MS.
GC-MS analysis
The GC-MS analysis was performed using an Agilent 7890A (GC)-Agilent 5975C inert mass spectra detector (MSD) with triple axis detector and an Agilent 7693 autosampler from Agilent Technologies (Palo Alto, CA, USA). The temperatures of the transfer line, the quadrupole, and the ion source were set at 290, 150, and 230 °C, respectively. The system was operated by Agilent MSD ChemStation E.02.00.493 software.
Separation was carried out on a HP5-MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness). Helium (purity, 99.999%) was employed as carrier gas at a constant column flow of 1.0 mL min−1. The GC oven temperature was programmed from 80 °C (held 2 min) to 100 °C at 8 °C min−1, to 150 °C at 20 °C min−1, to 200 °C at 25 °C min−1 (held 5 min), to 220 °C at 8 °C min−1, and a final ramp to 280 °C at 30 °C min−1. Pulsed splitless mode was used for injection (25 psi, held 1.2 min). After 1 min, the split was opened at a flow of 75 mL min−1 and the injector temperature was kept at 220 °C. The injection volume was 1 μL. The MSD operated in selected ion monitoring mode, monitoring at least two ions per compound (Table 1). The electron multiplier was set at a nominal value of 1,200 V.
Statistical analysis
Basic and descriptive statistics, as well as experimental design analysis were performed using Statgraphics-Plus v5.1 (Manugistics, Rockville, MD, USA) as software package. The experimental design was applied in the optimization of the extraction method, to analyze the simultaneous effect of the main parameters affecting MSPD.
Results and discussion
Derivatization and GC-MS analysis
Optimization of the chromatographic conditions including a previous derivatization step to improve the chromatographic analysis was optimized elsewhere [15].
Different families of preservatives are studied in this work (see Table 1). Acetylation with acetic anhydride is one of the most simple and cheap derivatization procedures for phenolic compounds. Three of the compounds (bronidox, IPBC, and BHT) did not undergo derivatization. Bronidox and IPBC do not undergo derivatization since these compounds do not have chemical groups susceptible to acetylation. In the case of BHT, the acetylation could not be demonstrated since the retention time, peak shape, chromatographic response, and mass spectra were equivalent before and after the addition of the acetylation reagents. The highly hindered hydroxyl group with poor nucleophilicity may prevent the acetylation under the studied conditions. For the other compounds, reaction yield was quantitative, and satisfactory, improving significantly the chromatographic analysis of the target compounds. No trace of the un-derivatized analytes was detected. The reaction was carried out with standard solutions in ethyl acetate and hexane/acetone (1:1, v/v) demonstrating the suitability of these solvents to accomplish derivatization. Derivatization time was studied in order to improve the throughput of the method; identical results were obtained when it was reduced from 30 [15] to 10 min and, therefore, this time was selected for the rest of the experiments. Figure 1 shows the extracted ion chromatograms of a derivatized standard solution obtained from the direct injection of an hexane/acetone solution containing 2 μg mL−1 of each preservative.
MSPD optimization
Efficiency of MSPD extraction depends on various experimental factors, being one of the most important the nature of the dispersing phase. Preliminary experiments were conducted to study the influence of this parameter. Five different solid phases were tested: Florisil, neutral alumina, C18, silica gel, and sand. The sample used consisted of a leave-on cosmetic (body milk) containing several of the target analytes. In these experiments we decided to work with the sample as it, without compound addition, to really evaluate the capability of MSPD to break analyte-matrix interactions in this kind of complex sample. In all cases, the MSPD was conducted applying the most usual sample/solid support material ratio (1 to 4), blending 2 g of solid support with 0.5 g of sample [19]. Since drying of the sample is essential for an efficient extraction, in all experiments 1 g of anhydrous sodium sulfate was added. In these initial trials, the MSPD column was eluted with two fractions of 5 mL of ethyl acetate, which were analyzed separately. All experiments were performed twice. The results obtained for the first fraction were analyzed by ANOVA, and they are given in Fig. 2. As can be seen, the responses obtained were statistically equivalent for the different phases.
Regarding the second fraction, the percentage of compound was in all cases lower than 0.5% compared with the first fraction, excluding alumina (1–2%), and so, only one fraction of 5 mL was considered for further experiments.
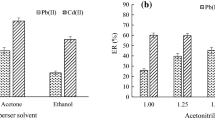
To investigate the simultaneous effect of the elution solvent and the dispersing phase, an experimental design was conducted. Considering the previous ANOVA study, only two sorbents (factor A) were selected: Florisil and sand. Sand was chosen due to its low cost, and Florisil was selected taken into account its dispersive and clean-up properties. For an efficient extraction, the solvent must solubilize the target compounds while leaving the sample matrix as intact as possible [19]. Considering the polar analyte nature, the derivatization and chromatographic performance, and our own experience [15], two solvents (factor B) were investigated: hexane/acetone (1:1, v/v) and ethyl acetate. Since we intended to develop a general method applicable to leave-on and rinse-off cosmetic samples, two different samples (factor C) were included in this study: a moisturizing lotion (leave-on) and a liquid hand soap (rinse-off). For this study, and with the aim of extending the research to all target compounds, the samples were fortified with all analytes (100 μg g−1). The factorial experimental design 23 was carried out in duplicate, so the total number of experiments was sixteen. The extracts were acetylated at 80 °C for 10 min before GC-MS analysis (see experimental section). Data analysis was made with the statistical software package Statgraphics-Plus v5.1. The global analysis of the results showed that the type of sample was statistically significant for most compounds. Therefore, we decided to analyze the design results in two blocks, the first one corresponding to the leave-on experiments and the second one to the rinse-off ones. Numerical analysis of the results obtained leads to the ANOVA table shown in Table 2. Regarding the rinse-off sample, main factors, dispersant and solvent, were not significant in all cases (excluding the dispersant for benzylparaben). However, the interaction factor was statistically significant for all compounds. The design results can be represented using several graphic tools. In Fig. 3 some representative graphics, showing the general behavior, are included. Figure 3a illustrates the pareto charts. In these graphs, the length of each bar is proportional to the absolute value of the standardized factor effect (vertical line represents the significance bound at the 95% confidence level). Figure 3b shows the main effects diagrams. The length of the lines is proportional to the effect magnitude of each factor in the extraction process, and the sign of the slope indicates the level of the factor that produces the highest response. Finally, Fig. 3c shows the interaction plots, where the importance of the second order factor is clearly represented. Best extractions were obtained using Florisil as dispersing phase and hexane/acetone as eluting solvent.
Regarding the leave-on sample (see Table 2), the solvent employed was significant for most compounds, and the extraction was most efficient using hexane/acetone. The dispersant was non-significant with the exception of BHT (Florisil). The interaction between both factors was also non-significant. Some illustrative graphics are included in Fig. 4, showing the most favorable conditions.
In view of the results, the selected general conditions for the simultaneous extraction of the target preservatives and antioxidants both in leave-on and rinse-off cosmetics comprise the use of Florisil (2 g), as dispersant and the elution with hexane/acetone (5 mL).
Method performance. Application to real samples
Method quality parameters were evaluated (Table 3). The instrumental linearity was proved at a concentration range between 0.02 and 10 μg mL−1 (including six concentration levels) using derivatized standard solutions prepared in hexane/acetone (see experimental section). Each concentration level was injected in duplicate or triplicate and the relative area (A/AIS) of each analyte was plotted versus its corresponding concentration. The response function was found to be linear with correlation coefficients (R) higher than 0.9961.
Instrumental detection limits were calculated as the concentration giving a signal-to-noise ratio of three (S/N = 3). Values ranged from 0.15 to 11 ng mL−1, as can be seen in Table 3. Other validation parameters were calculated using real cosmetic samples. In this way, recovery studies were carried out by applying the optimized method to the extraction of three real samples spiked at 100 μg g−1: a liquid soap (LS3), a body milk (BM3), and a hand soap (HS2). This last sample was also spiked at 20 μg g−1. Previous analyses of the samples showed the presence of some of the target compounds (see Tables 4 and 5), and these initial concentrations were taken into account to calculate the recoveries. As can be seen in Table 3, recoveries were between 88% and 110% in all cases. Precision was also evaluated and relative standard deviation (RSD) values were lower than 10%, with an average value of 4.2%, excluding bronopol (11%).
The limits of detection and quantification of the overall method were calculated as the compound concentration giving a signal-to-noise ratio of three (S/N = 3) and ten (S/N = 10), respectively. These values are shown in Table 3, expressed as percentage (%, w/w) in order to be consequent with the units used in the European Cosmetics Regulation [1]. The obtained limits are much lower than the established restrictions, and it is important to emphasize that, if necessary, these limits could be reduced by concentrating the extract (5 mL).
Finally, the method was applied to the analysis of real cosmetic samples including rinse-off (hand soaps, hair conditioner, shampoo, and liquid soaps) as well as leave-on (lipstick and makeup, hand cream, sun milk, body milks, moisturizing creams, anti-stretch marks creams, deodorant, and face cream) products. Results are shown in Tables 4 and 5 for rinse-off and leave-on cosmetics, respectively. For all the samples, the recoveries of MePd4 (surrogate standard) were satisfactory, with values ranging from 78.7 to 102 in rinse-off samples and ranged from 75.3 to 107% in leave-on samples (see last row, Tables 4 and 5). The maximum allowed concentration of parabens in ready for use preparations is 0.4% for single ester and 0.8% for mixtures of esters, expressed as acid [1]. For this reason, the total content of parabens in the samples was determined and expressed as % (w/w) as acid, being included in both Tables.
In the case of rinse-off cosmetics (Table 4), BHT was the most frequently found target preservative, appearing in four out of seven samples. BHA was found in two samples, in one of them associated to BHT, which increases the antioxidant power due to the synergism. Although there is some concern about the safety of both compounds, there are no restrictions about their use in cosmetic formulations. The amount and concentration of parabens in the samples were quite low with an average value of less than two parabens per sample and an average total paraben concentration lower than 0.1% (w/w) expressed as acid. Triclosan was found in three samples. Bronidox was not detected in any sample while bronopol and IPBC were detected in just one sample each.
Regarding the leave-on samples, the general preservative content was clearly higher. Most samples presented five to six of the target analytes. BHT, and the parabens MeP, PrP, and BuP were the most abundant preservatives found in more than ten out of 16 leave-on samples (Table 5). Triclosan was found in five samples, BHA in two, and Bronopol in one sample. Although in most of the samples the total paraben content can be considered quite high (>0.1% expressed as acid), the legal restrictions were fulfilled in all cases. The selected ion monitoring chromatogram corresponding to the sample BM2 is shown in Fig. 5.
Chromatogram of a real cosmetic sample (BM2, see concentrations in Table 5)
According to the European Regulation [1], a list of ingredients should be included on the label of the cosmetic product. The most of the samples were properly labeled with some exceptions. Bronopol was included as ingredient of all the samples containing this compound. Parabens were, in general, included as ingredients on the cosmetic labels. Nevertheless, BHA was just included on the label of one of the samples (MU2), BHT was only included in the 25% of the labels of the positive samples, TCS was listed in three out of eight positive samples, and IPBC was not included as an ingredient of sample Sh.
Conclusions
MSPD followed by derivatization and GC-MS analysis has been successfully applied to the determination of multi-class preservatives, including two bromine-containing preservatives, seven parabens, IPBC, TCS, and the antioxidant preservatives BHA and BHT, in leave-on and rinse-off cosmetics. To our knowledge, this study constitutes the first application of MSPD to cosmetics analysis. Optimization was carried out using real cosmetic samples and several statistical tools. Recovery studies were performed on leave-on and rinse-off samples, verifying the reliability of the optimized procedure.
The method was applied to a broad range of cosmetics demonstrating the high use of most of the target preservatives, especially BHT, and MeP, PrP, and BuP. Regarding paraben content, the concentrations found were below the legal limits in all cases.
With the developed method, some of the most relevant criteria required for an extraction procedure, such as low solvent consumption, and short process times are fulfilled. In addition, MSPD presents other clear advantages: it does not require special extraction equipment, it is cheap, and it can be easily implemented in any laboratory.
References
Regulation (EC) no. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast). Official Journal of European Union 59: L342
Ingredients Prohibited and Restricted by US Food and Drug Administration (FDA) Regulations (2007). Available at: http://www.fda.gov/Cosmetics/ProductandIngredientSafety/SelectedCosmeticIngredients/ucm127406.htm. Accessed April 2011
Polati S, Gosetti F, Gennaro MC (2007) Preservatives in cosmetics. Regulatory aspects and analytical methods (p. 211). In: Salvador A, Chisvert A (eds) Analysis of cosmetic products. Elsevier, Amsterdam, p 211
Márquez-Sillero I, Aguilera-Herrador E, Cárdenas S, Valcárcel M (2010) J Chromatogr A 1217:1–6
Wang SP, Chang CL (1998) Anal Chim Acta 377:85–93
Saraji M, Mirmahdieh S (2009) J Sep Sci 32:988–995
Melo LP, Queiroz EMC (2010) J Sep Sci 33:1849–1855
Lee MR, Lin CY, Li ZG, Tsai TF (2006) J Chromatogr A 1120:244–251
Wu T, Wang C, Wang X, Ma Q (2008) Int J Cosmetic Sci 30:367–372
Gagliardi L, Cavazzutti G, Turchetto L, Manna F, Tonelli D (1990) J Chromatogr A 508:252–258
Wang J, Zhang D, Chu Q, Ye J (2010) Chinese J Chem 28:313–319
Huang HY, Lai YC, Chiu CW, Yeh JM (2003) J Chromatogr A 993:153–164
García-Jiménez JF, Valencia MC, Capitán-Vallvey LF (2007) Anal Chim Acta 594:226–233
Yang TJ, Tsai FJ, Chen CY, Yang TCC, Lee MR (2010) Anal Chim Acta 668:188–194
Sanchez-Prado L, Lamas JP, Lores M, Garcia-Jares C, Llompart M (2010) Anal Chem 82:9384–9392
Barker SA, Long AR, Short CR (1989) J Chromatogr A 475:353–361
Barker SA (2000) J Chromatogr A 885:115–127
Kristenson EM, Brinkman UAT, Ramos L (2006) TrAC Trend Anal Chem 25:96–111
Barker SA (2007) J Biochem Biophys Meth 70:151–162
Capriotti AL, Cavaliere C, Giansanti P, Gubbiotti R, Samperi R, Laganà A (2010) J Chromatogr A 1217:2521–2532
Sanchez-Brunete C, Miguel E, Albero B, Tadeo JL (2010) J Sep Sci 33:2768–2775
Calderon-Preciado D, Jimenez-Cartagena C, Penuela G, Bayona JM (2009) Anal Bioanal Chem 394:1319–1327
Acknowledgments
This research was supported by FEDER funds and project CTQ2010-19831 (Ministerio de Ciencia e Innovacion, Spain). L.S.-P. and J.P.L. acknowledge Xunta de Galicia for a postdoctoral Angeles Alvariño and Isabel Barreto contracts, respectively. G.A.-R. acknowledges Ministerio de Ciencia e Innovación for a FPI grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanchez-Prado, L., Alvarez-Rivera, G., Lamas, J.P. et al. Analysis of multi-class preservatives in leave-on and rinse-off cosmetics by matrix solid-phase dispersion. Anal Bioanal Chem 401, 3293–3304 (2011). https://doi.org/10.1007/s00216-011-5412-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5412-6