Abstract
A green, simple, non-toxic, and sensitive sample pretreatment procedure coupled with high-performance liquid chromatography (HPLC) was developed for the analysis of chloramphenicol (CAP) that exploits an aqueous two-phase system based on imidazolium ionic liquid (1-butyl-3-methylimidazolium tetrafluoroborate, [Bmim]BF4) and organic salt (Na3C6H5O7) using a liquid–liquid extraction technique. The influence factors on partition behaviors of CAP were studied, including the type and amount of salts, the pH value, the volume of [Bmim]BF4, and the extraction temperature. Extraction efficiency of the CAP was found to increase with increasing temperature and the volume of [Bmim]BF4. Thermodynamic studies indicated that hydrophobic interactions were the main driving force, although electrostatic interactions and salting-out effects were also important for the transfer of the CAP. Under the optimal conditions, 90.1% of the CAP could be extracted into the ionic liquid-rich phase in a single-step extraction. This method was practical when applied to the analysis of CAP in feed water, milk, and honey samples with a linear range of 2~1,000 ng mL−1. The method yielded a limit of detection of 0.3 ng mL−1 and a limit of quantification of 1.0 ng mL−1. The recovery of CAP was 90.4–102.7% from aqueous samples of real feed water, milk, and honey samples by the proposed method. This novel process is much simpler and more environmentally friendly and is suggested to have important applications for the separation of antibiotics.

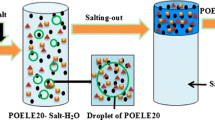
The schematic diagram of separation target molecule in ILATPS
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chloramphenicol (CAP) (2,2-dichloro-N-[(αR,βR)-β-hydroxy-α-hydroxymethyl-4-nitrophenethyl] acetamide, CAS 56-75-7) is a bacteriostatic antimicrobial originally derived from the bacterium Streptomyces venezuelae, isolated by David Gottlieb, and introduced into clinical use in 1947 [1, 2]. It exerts its action through protein inhibition and is effective in the treatment of several infectious diseases. This, together with its low cost and ready availability, has made it extensively used since the 1950s in the treatment of animals all over the world, including food-producing animals. However, it has the potential to cause serious toxic effects on humans and animals when given by mouth or injection, with non-target effects such as bone marrow depression, aplastic anemia, hypoplastic anemia, and thrombocytopenia, as well as granulocytopenia [3]. The clinical use of CAP is therefore restricted to the treatment of serious infections. In addition, China, the European Commission, the USA, and some other countries have strictly banned the use of CAP in food-producing animals and legislated maximum residue limits [4, 5]. Nevertheless, due to its low price and steady antibiosis effectiveness, illegal use of CAP in livestock and aquaculture still exists. So, it is very important to develop a sensitive, rapid, and simple method for the determination of CAP in food commodities.
The main methods for effectively monitoring and detecting CAP residues in foods include microbiological assay [6], enzymatic assay [7], immunoassay [8], chromatography [9], and sensor method [10]. However, these methods have their own disadvantages, such as complex analyzing processes, long time requirement for preparation of samples, and expensive equipment. The difficulty in determining CAP in foods is extremely low concentrations of 1–10 μg kg−1 in various samples with complex matrices. Quantitative analysis of trace level of CAP is still a significant challenge demanding a rapid and effective sample preparation procedure prior to analysis. Currently, two principal sample pretreatment methods developed for the determination of CAP are liquid–liquid extraction (LLE) [11] and solid-phase extraction (SPE) [12]. However, LLE usually requires some poisonous volatile organic solvents. SPE is a method with good purification and concentration effects, but it requires solvent desorption step with traditional volatile organic solvents, and the pretreatment processes are relatively time consuming. Sometimes, sample recovery is not always satisfactory. Therefore, novel, rapid, and accurate cleanup and enrichment methods are required for analyses involving CAP monitoring.
Recently, there are two interesting pretreatment methods falling into our concern. One is dispersive liquid–liquid microextraction (DLLME) for extraction of CAP in honey samples by Chen et al. [13]. The other is matrix solid-phase dispersion (MSPD) for extraction of CAP in fish issues by Guo et al. [14]. This two methods, coupled with high-performance liquid chromatography (HPLC), successfully determined trace CAP in real samples. However, these procedures are not yet suitable for the determination of CAP at concentrations lower than 1.5 μg kg−1, and the extraction procedures are relatively complex.
Aqueous two-phase system (ATPS) as a useful extraction technique is formed by mixing two aqueous solutions of structurally different polymers [15], a polymer and a salt [16], two surfactants [17], an ionic liquid and a salt [18], or small molecular water-miscible organic solvent and a salt [19] together when the concentrations of the components are higher than a critical value. Compared with conventional extraction methods, ATPS is more environmentally benign without using harmful volatile organic solvent in the whole process. Therefore, it has attracted much attention in several fields. As a pretreatment method, in recent years, ATPS has been successfully applied in separation, recovery, and purification of many biological materials such as proteins [20], enzymes [21], nucleic acids [22], antibodies [23], and antibiotics [24]. ATPS can also be used for extraction of metal ions [25] and dyes [17].
In recent years, the special properties of room temperature ionic liquids (ILs) have lead to their application in various fields of chemistry [26–28], due to their “green” characteristics such as negligible volatility and non-flammability under ambient conditions, large liquid range, high thermal and chemical stability, strong solvent, and a number of possible variations in cation and anion features which allow fine-tuning of the IL’s properties [29]. ATPSs based on ionic liquids and water-structuring salts have been reported by Rogers and his co-workers for the first time in 2003 [30]. These new ATPSs have been successfully used to separate drugs [18], proteins [31], and antibiotics [32]. In these studies, salting out of IL has been mainly accomplished by use of inorganic salts. However, high concentrations of these inorganic salts are not desirable in the effluent streams due to environmental problems. Recently, several authors have used a citrate or tartrate salt as a substitute for inorganic salt in polymer + salt ATPSs [33, 34] and IL + salt ATPSs [35] due to its biodegradability and non-toxicity. Such no pollution problems are known to be caused by organic salts. But there is no report on separation and extraction of antibiotics with ionic liquid + organic salt ATPSs.
In this paper, the aim of this work was to develop a novel, greener, simple and effective sample pretreatment technique, an ILATPS based on 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4) and sodium citrate, coupled with HPLC for determining the trace CAP in feed water, milk, and honey samples. The schematic diagram of separation of target molecule in ILATPS is shown in Fig. 1. The phase equilibrium of ILATPSs and the parameters, such as the types of salts, concentration of salt, pH, and the extraction temperature, were discussed in detail. Under the optimal conditions, this novel method has been successfully applied to the analysis of CAP in feed water, milk, and honey samples coupled with reversed-HPLC method.
Experimental
Chemicals and materials
The standard drug of sample of CAP was obtained from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). [Bmim]BF4 was purchased from Chengjie Chemical Co., Ltd. (Shanghai, China) with a quoted purity of greater 0.99 mass fraction and was used without further purification. Methanol of HPLC grade was from Sinopham Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were of analytical grade, and all the solutions were prepared from deionized water. The individual stock solution was prepared in methanol at a concentration of 200 μg mL−1 and stored at 4 °C in a refrigerator. Working standard solution of CAP was prepared by appropriate dilution of stock solution using deionized water.
Sample preparation
Feed water
Feed water samples were obtained from different farmsteads of standard commercial breeding. They were centrifuged at 2,000 rpm for 15 min, and the supernatant was collected. The CAP working solution was added and, finally, filtered through microfilters with a pore size of 0.45 μm to remove the denatured proteins. The extracts were stored at 4 °C until use.
Milk
Milk samples were purchased in a local supermarket. The CAP working solution was added. They were then deproteinized as follows [36]. Approximately 10 g of homogenized milk was accurately weighted in a 20-mL polypropylene tube. Then, trichloroacetic acid (1.0 mL, 15% in water) was added, and the solution was shaken and centrifuged at 2,000 rpm for 10 min and, finally, filtered through microfilters with a pore size of 0.45 μm to remove the denatured proteins. The extracts were stored at 4 °C until use.
Honey
Honey samples were purchased from local supermarkets or obtained from private beekeepers. One-gram honey was weighted into a 10-mL centrifuge tube with conical bottom. The amount of CAP working solution and 5.0 mL of water were added, and the mixture was vortexed until a homogeneous sample was obtained and, finally, filtered through microfilters with a pore size of 0.45 μm to remove the denatured proteins. The homogenous sample was stored at 4 °C until use.
Phase diagrams
The binodal curves were determined by the titration method [37]. A glass vessel, volume 50 cm3, was used to carry out the phase equilibrium determinations. The glass vessel was provided with an external jacket in which water at constant temperature was circulated using a DC-2008 water thermostat (Shanghai Hengping Instrument Factory, China). The temperature was controlled to within ±0.05 K. An [Bmim]BF4 solution of known mass fraction was taken into the vessel; then, a salt solution of known mass fraction was added dropwise to the vessel until the mixture became turbid or cloudy. The composition of this mixture was noted. Afterward, water was added dropwise to the vessel to get a clear one-phase system, and the procedure was repeated and so on. The composition of the mixture for each point on the binodal curve was calculated by mass using an analytical balance (Model BS 124S, Beijing Sartorius Instrument Co., China) with a precision of ±1.0 × 10−7 kg. The maximum uncertainty was found to be 0.001 in determining the mass fraction of both [Bmim]BF4 and salt by the titration method used.
Tie lines were determined from a series of ATPSs by mixing appropriate amounts of [Bmim]BF4, organic salt, and water in the vessel, which was placed in a thermostated bath. The system was vigorously stirred for at least 1 h; the phase separation occurred quickly after cessation of the stirring process. However, a complete phase separation was ensured by centrifuging (Model 80-2, Shanghai Surgery Instrument Factory, China) at 2,000 rpm for 10 min. Then, the systems were placed again into the thermostated bath and allowed to settle for at least 2 h to separate into two clear phases. After reaching phase equilibrium, the concentration (mass fraction) of salt in the top and bottom phases was determined by density index measurement performed at 298.15 K using a density meter (DMA-4100, Anton Paar Co., Ltd., Austria). The uncertainty in the measurement of the density index was found to be ± 0.0001 g/cm3.
Extraction process
One milliliter of [Bmim]BF4, salt, and the standard solution of CAP was added into a 10-mL centrifuge tube. And actually, both the spiked and the real samples followed the sample procedures as the standard. The mixture was diluted to the mass of 10 g with water. This mixture was gently stirred for 5 min at ambient temperature. Complete phase separation was achieved by centrifugation at 2,000 rpm for 20 min at 25°C. After centrifugation, the tubes were placed into a thermostatic bath at 25 ± 0.05 °C for 10 min to equilibrate. [Bmim]BF4 and CAP were enriched in the top phase, and salt was enriched in the bottom phase. The volume of the top phase was read accurately, and part of them was aspirated into a 50-μL microsyringe and directly injected into the HPLC system for analysis. The desired pH of the sodium citrate solution was adjusted by the addition of sodium hydroxide and citric acid. The systems with pH lower than 4.0 were not used because they cannot form two phases in the selected concentration range of the phase-forming components.
HPLC/UV analysis
CAP analyses were performed on an Agilent 1200 HPLC system including a quaternary pump and a variable wavelength UV detector (Agilent, USA). The instrument control and data processing were carried out by an Agilent ChemStation software. An Eclipse XDB-C18 (250 mm × 4.6 mm, 5 μm, serial no. G1314B) reversed-phase column was employed for chromatographic separation, with the mobile phase of water/methanol (55:45) at a flow rate of 1.0 mL. The injected volume was 20 μL, and the column effluent was monitored at a wavelength of 278 nm.
Determination of the partition parameters of CAP
The partitions of CAP among the various phases were characterized by various parameters including the partition coefficient of CAP calculated by
where C t and C b are equilibrium concentrations of the CAP in the top and bottom phases, respectively.
Phase volume ratio (R) is defined as volume ratio of the top phase to bottom phase
where V b and V t stand for the volume of the bottom phase and the top phase, respectively.
The enrichment factor (F) was calculated by
where \( {V_{{{{\rm{H}}_{{2}}}{\rm{O}}}}} \) and V t stand for the volume of water phase and the top phase, respectively.
Extraction efficiency of CAP was also calculated according to the given equation:
where m s is the amount of CAP added.
Results and discussion
Phase behaviors of the [Bmim]BF4–organic salt ILATPS
Phase diagram
Phase diagram data are required for the design of aqueous two-phase extraction process and for the development of models that predict partitioning of CAP. The phase diagrams determined at 298.15 K for the [Bmim]BF4–organic salt ILATPSs were shown in Fig. 2. These binodal curves provide information about the concentration of phase-forming components required to form two phases. It can be seen from Fig. 2 that ATPSs can be formed by adding appropriate amount of organic salt to aqueous [Bmim]BF4 solution. The increase in the salting-out strength of salt is reflected by a decrease in the amount of salt required for the formation of ILATPS under the same mass fraction of [Bmim]BF4. The ability of salts for separation is increased with increasing the salting-out strength of salt. It is clear that the ability of the organic salts for phase separation follows the order Na3C6H5O7 > (NH4)3C6H5O7 > Na2C4H4O6 > Na2C4H4O4 > NaC2H3O2. The structure-making anions, C6H5O 3−7 , C4H4O 2−6 , C4H4O 2−4 , and C2H3O −2 , which exhibit stronger interaction with water molecules, are beneficial to the formation of aqueous two phases. This implies that anions with a higher valence are better salting-out agents because they hydrate more water molecules, thus decreasing the amount of water available to hydrate the ILs. The most kosmotropic organic salt, Na3C6H5O7, results in a binodal closest to origin, indicating that less salt is needed to form an ATPS. So we choose Na3C6H5O7 as salting-out agent in this work. Another important reason was that Na3C6H5O7 can result in an appropriate pH range for the CAP extraction.
As shown in Fig. 3, it shows binodal curves and the tie lines for the [Bmim]BF4–Na3C6H5O7 ILATPS at 298.15 K and illustrates that ILATPS could be formed over a large composition range. The phase diagram for the [Bmim]BF4–Na3C6H5O7 ILATPS shows that the system is separated into two phases in the region above the binodal curve. The [Bmim]BF4–Na3C6H5O7 ILATPSs at 298.15 K requires the amounts of both [Bmim]BF4 and Na3C6H5O7 to exceed a particular threshold.
Phase volume ratio
In order to the investigate the concentration of the Na3C6H5O7 effect on the ILATPS formation and phase separation capability, various quantities of salt were added to a 10 mL 10% [Bmim]BF4 solution at 298.15 K. As illustrated in Fig. 4, the further addition of Na3C6H5O7 could improve ILATPS formation and could make phase separation easier. There would be a slight increase of the top phase volume with the addition of Na3C6H5O7, resulting in the enrichment of CAP and a minor decrease of the enrichment factor. When the concentration of Na3C6H5O7 was up to 33%, the top phase volume reached 0.7 mL and kept constant; this is because the salting-out ability of Na3C6H5O7 has reached the mixture.
Partition behaviors of CAP in [Bmim]BF4–Na3C6H5O7 ILATPS
The partitioning studies of CAP in ILATPS have great significance not only regarding its purification but also aiming at extending it to antibiotics. The success of the extractive potential of ILATPS depends on the ability to manipulate phase properties to obtain the appropriate partitioning coefficients and selectivity for the biomolecule of interest. There are several approaches to manipulate a particular solute partitioning: (1) adjust the system by applying different salts and/or ILs, thus controlling the solute’s affinity; (2) change the system composition by changing either the concentration of salt or IL; (3) introduction of additional cosolvents, antisolvents, or amphiphilic structures to the overall system.
Effect of salt species
Changing the type and concentration of salt is known to influence the partition behaviors of many biological materials. Hence, it is thought desirable to study the effects of salts on partition of CAP in [Bmim]BF4–organic salt ILATPS. The partitioning of CAP was carried out in several ILATPSs of 10% [Bmim]BF4 with different salts, including Na3C6H5O7, (NH4)3C6H5O7, Na2C4H4O6, Na2C4H4O4, and NaC2H3O2. Results show that ILATPS can be formed by adding appropriate concentration of Na3C6H5O7, (NH4)3C6H5O7, Na2C4H4O6, and Na2C4H4O4, while NaC2H3O2 cannot cause phase separation. This may due to the amount of [Bmim]BF4 being low in our ILATPS; even if NaC2H3O2 reaches the saturated concentration, phase separation could not happen. As shown in Fig. 5, the type of salt has a significant effect on the extraction efficiency and partition coefficient of CAP. The order of extraction efficiency of CAP in [Bmim]BF4–organic salt ILATPS is as follows: Na3C6H5O7 > Na2C4H4O6 > Na2C4H4O4 > (NH4)3C6H5O7; this is due to the difference of the ability of salting out of organic salt. Na3C6H5O7 is known to be a strong salting-out organic salt; it will promote CAP into an IL-rich phase more than other organic salts. From Fig. 2, we found that the ability of phase separation of (NH4)3C6H5O7 was larger than Na2C4H4O6 and Na2C4H4O4. But CAP was stable in pH = 6~10, while in acid or strong alkaline condition, CAP could not exist with the molecular solvent, and it was decomposed; so the extraction efficiency of CAP in [Bmim]BF4–(NH4)3C6H5O7 ILATPS was less than in other ILATPSs. The partition coefficient of CAP in [Bmim]BF4–(NH4)3C6H5O7 ILATPS was higher than other ILATPSs because that the phase volume ratio became less in [Bmim]BF4–(NH4)3C6H5O7 ILATPS.
Effect of the concentration of Na3C6H5O7
The effect of Na3C6H5O7 at different concentrations on the extraction efficiencies of CAP was discussed in this part. As mentioned previously, CAP was transformed into the top phase with a certain level of enrichment. We discussed the interrelationship of the extraction efficiency of CAP and amounts of Na3C6H5O7 over a range from 25.01% to 38.10%. It was clear that the extraction efficiency of CAP increased with the increasing amount of Na3C6H5O7, and when the concentration of Na3C6H5O7 reached 35.17%, the average extraction efficiency was up to 90%. This is because in aqueous solution, a certain amount of the concentration of Na3C6H5O7 employed in this system seemed to be high enough to induce CAP precipitation. So the amount of Na3C6H5O7 used in the [Bmim]BF4–Na3C6H5O7 ILATPS was 35.01%.
The measurements of water solubility of CAP in the presence of a salt by many researchers revealed only salting-out effects. Na3C6H5O7 is known to be a strong salting-out organic salt; a large amount of Na3C6H5O7 present in CAP solution would compete with CAP for water molecules, resulting in the reduction of the hydration degree of CAP. Therefore, introduction of a salt into an aqueous CAP solution promotes the transfer of the CAP from the bottom phase to the top phase. Thus, it is likely that the salting-out effect drives the CAP into the [Bmim]BF4-rich top phase.
Effect of pH
As an example, extraction efficiency of CAP and phase volume ratio were studied as a function of pH of the extraction buffers, and the results are shown in Fig. 6. [Bmim]BF4–Na3C6H5O7 ILATPS cannot form two phases at a pH below 4.0. This was because the Na3C6H5O7 is dominant at lower pH values. The CAP was stable in pH 6.0~10.0, while in acid or strong alkaline condition, CAP could not exist with the molecular solvent, and it was decomposed. So when pH < 6, the extraction efficiency of CAP was decreased. In our experiment, the pH of the [Bmim]BF4–Na3C6H5O7 ILATPS was about 8.0, so we did not adjust the pH in the whole experiment.
Effects of the mount of [Bmim]BF4 on extraction efficiency and enrichment factor
The influence of the amount of [Bmim]BF4 on the extraction efficiencies of analytes and enrichment factor was also investigated. As shown in Table 1, with the increase of the amount of [Bmim]BF4 used, the extraction efficiency of CAP obviously increased, while the enrichment factor decreased greatly. Generally, both high extraction efficiency and suitable enrichment factor are all crucial for the concentration of trace component. Considering these two respects, 1.0 mL [Bmim]BF4 was really appropriate with an enrichment factor of 9 and extraction efficiency of 90% for CAP.
Effect of extracting temperature and the main driving force for the extraction of CAP
From the viewpoint of thermodynamics, extraction of CAP can be regarded as a transfer process of the CAP from the salt-rich bottom phase to the IL-rich top phase. Analysis of the K variation with a temperature change may provide thermodynamic data as regards the partitioning process. The Gibbs energy of such a transfer process at a given temperature can be calculated from the partition data by
where K is partition coefficients of the CAP between the phases. T is the temperature (K), and R is the gas constant. The enthalpic change and entropy change associated with the CAP partition can be calculated, respectively, from the slope and intercept of the linear equation between ln K and 1/T
Figure 7 shows the temperature effect on the partition coefficient expressed as Van’t Hoff plots. A linear relation which suggests the presence of only one temperature-dependent process can be seen. The resultant \( \Delta G_T^{\theta } \), \( \Delta H_T^{\theta } \), and \( \Delta S_T^{\theta } \)values obtained by linear least-square analysis are included in Table 2 for the transfer of CAP from the Na3C6H5O7-rich phase to the [Bmim]BF4-rich phase in the [Bmim]BF4–Na3C6H5O7 ILATPS. It can be seen that the values of \( \Delta G_T^{\theta } \) are negative whereas those of \( \Delta H_T^{\theta } \) and \( \Delta S_T^{\theta } \) are positive, and \( T\Delta S_T^{\theta } \) is always greater than \( \Delta H_T^{\theta } \) in value. This indicates that the partition of the CAP is controlled by entropy changes, which is the characteristic of hydrophobic interactions. Thus, thermodynamic study revealed that hydrophobic interactions are the main driving force for the extraction of CAP in the [Bmim]BF4–Na3C6H5O7 ILATPS, although CAP molecules carry negative charge under the extraction conditions, and the electrostatic interaction between the charged CAP and [Bmim]+ was also important for the partitioning of CAP.
Method validation
Under the optimum conditions, the calibration was performed by adding standard CAP at six different concentrations (three replications for each concentration) in the range of 2~1,000 ng mL−1 to [Bmim]BF4–Na3C6H5O7 ILATPS. After phase separation, the CAP concentration in the top phase was measured by HPLC method as described in "HPLC/UV analysis". The obtained linear regression equations and correlation coefficients (r) for CAP was Area = 0.284 × c + 3.777 (r = 0.9998), where c was the concentration of CAP with the unit of nanogram per milliliter. The precision of this method was determined by successive six-time extraction and analysis of a 10 ng mL−1 standard solution of CAP; the relative standard deviations (RSDs) were 3.8%; the inter-assay precision (RSDs) was 5.1%. Considering that the European Commission Decision 2002/657/EC 2002 [38] recommends that the inter-assay precision should be lower than 23%, the values obtained by the proposed HPLC method are adequate.
The limit of detection (LOD) was obtained from the signal-to-noise ratio (S/N) and the calibration curve. In this work, the baseline noise was measured from a chromatogram of a blank sample solution. A signal value of three times the noise (S/N = 3) was used to calculate the LOD of the calibration curve. The LOD obtained was 0.3 ng mL−1, and the limit of quantification (LOQ) (S/N = 10) was 1.0 ng mL−1. The LOQ for CAP of around 1.0 ng mL−1 of IL–organic salt ILATPS reversed-phase HPLC detection system used in this study is lower than that of the DLLME–HPLC/variable wavelength detect [13] and that of the MSPD–HPLC/UV [14]. In the case of CAP residues in milk, as well as meat, eggs, aquaculture products, and honey, the European Commission established a minimum required performance limit (MRPL) of 0.3 ng g−1 to ensure the same level of consumer protection throughout the community [38]. Nevertheless, the MRPL of 0.3 ng g−1 cannot be reached by these systems. In regard to the complexity of the sample matrix, an efficient cleanup method in combination with a sensitive detection method will have to be applied. As a greener alternative method for the extraction of CAP, ILATPS shows great potential in the separation of biomolecules. Therefore, the combination of ILATPS with LC–MS/MS or other electrochemistry methods will be a task for further studies to achieve the MRPL.
Application of method in analysis of real samples
The proposed extraction technique was applied to separate and determine CAP in feed water, milk, and honey samples under the optimum conditions as described above. Before the CAP was added, no contamination of CAP residues at detectable levels was found in the real samples. The recovery tests were determined from spiked with 10–100 ng mL−1 CAP. After hydrolysis, CAP in real samples were separated to IL phase and determined with the proposed HPLC method (Fig. 8 and Table 3). Twenty microliters of the top phase was directly injected for proposed HPLC analysis. As shown in Table 3, the recovery of CAP was 90.4–102.7% with an RSD of 1.4–4.7% when spiked with 10–100 ng mL−1 CAP in the recovery tests, showing that the present method has a satisfactory reproducibility and recovery for the determination of the CAP in a wide range of concentrations. The present method can be satisfactorily applied to the quantitative determination of CAP in real food samples.
HPLC chromatograms with UV detection of a the blank sample (deionized water) added with 50 ng mL−1 CAP after ILATPS extraction; b feed water sample added with 50 ng mL−1 CAP after ILATPS extraction; c honey sample added with 50 ng mL−1 CAP after ILATPS extraction; d milk sample added with 50 ng mL−1 CAP after ILATPS extraction
Effect of interfering substances
The assay was unaffected by the presence of excipients as shown by the excellent recoveries (97.2 ± 2.7 to 101.6 ± 1.6%) obtained when analyzing the studied CAP in the presence of commonly encountered excipients under optimized conditions. Samples containing a fixed amount of the CAP (20 ng mL−1) and variable concentrations of excipients were measured. The result showed that the coexistent organic compounds, such as 1,000 times lactose, sucrose, and starch, 500 times glucose and fructose, and 200 times magnesium stearate, did not induce significant interference in the examination. This fact indicates good selectivity of method to determine the subject CAP in real samples.
Conclusions
The analytical utility of an [Bmim]BF4–Na3C6H5O7 ILATPS method was demonstrated to be an excellent strategy for determining trace CAP for the first time. Compared with traditional PEG–inorganic salt ATPS, the IL–organic salt ATPS has the advantages of lower viscosity, little emulsion formation, quick phase separation, being more environmentally friendly, and others. In this paper, thermodynamic studies indicated that hydrophobic interactions were the main driving force, although electrostatic interactions and salting-out effects were also important for the transfer of the CAP. As a viable pretreatment technique for complicated samples, this novel extraction method, combined with an HPLC method, has been successfully used to quantitatively determine CAP in feed water, milk, and honey samples. These results highlight new possibilities of IL-based ATPSs in the separation of biomolecules.
References
Gikas E, Kormali P, Tsipi D, Tsarbopoulos A (2004) J Agr Food Chem 52:1025–1030
Gottlieb D, Legator M (1953) Mycologia 45:507–515
Mottier P, Parisod V, Gremaud E, Guy PA, Stadler RH (2003) J Chromatogr A 994:75–84
Gantverg A, Shishani I, Hoffman M (2003) Anal Chim Acta 483:125–135
Gude T, Preiss A, Rubach K (1995) J Chromatogr B 673:197–204
Singer CJ, Katz SE (1985) J Assoc Off Anal Chem 68:1037–1041
Yamato S, Sugihara H, Shimada K (1990) Chem Pharm Bull (Tokyo) 38:2290–2292
Dumont V, Huet AC, Traynor I, Elliott C, Delahaut P (2006) Anal Chim Acta 567:179–183
Forti AF, Campana G, Simonella A, Multari M, Scortichini G (2005) Anal Chim Acta 529:257–263
Park IS, Kim DK, Adanyi N, Varadi M, Kim N (2004) Biosens Bioelectron 19:667–674
Akhtar MH, Danis C, Sauve A, Barray C (1995) J Chromatogr A 696:123–130
Kubala-Drincic H, Bazulic D, Sapunar-Postruznik J, Grubelic M, Stuhne G (2003) J Agric Food Chem 51:871–875
Chen HX, Chen H, Ying J, Huang JL, Liao L (2009) Anal Chim Acta 632:80–85
Guo LY, Guan M, Zhao CD, Zhang HX (2008) Anal Bioanal Chem 392:1431–1438
Shibusawa Y, Takeuchi N, Sugawara K, Yanagida A, Shindo H, Ito Y (2006) J Chromatogr B 844:217–222
Li L, Liu F, Kong X, Su S, Li KA (2002) Anal Chim Acta 452:321–328
Tong AJ, Dong JJ, Li LD (1999) Anal Chim Acta 390:125–131
Ferguson J, Baxter A, Young P, Kennedy G, Elliott C, Weigel S, Gatermann R, Ashwin H, Stead S, Sharman M (2005) Anal Chim Acta 529:109–113
Liu H, He C, Wen D, Liu H, Liu F, Li K (2006) Anal Chim Acta 557:329–336
Gu Z, Glatz CE (2007) J Chromatogr B 845:38–50
Su CK, Chiang BH (2006) Process Biochem 41:257–263
Gomes GA, Azevedo AM, Aires-Barros MR, Prazeres DMF (2009) Sep Purif Technol 65:22–30
Azevedo AM, Gomes AG, Rosa PAJ, Ferreira IF, Pisco AMMO, Aires-Barros MR (2009) Sep Purif Technol 65:14–21
Mokhtarani B, Karimzadeh R, Amini MH, Manesh SD (2008) Biochem Eng J 38:241–247
Shibukawa M, Nakayama N, Hayashi T, Shibuya D, Endo Y, Kawamura S (2001) Anal Chim Acta 427:293–300
Leone AM, Weatherly SC, Williams ME, Thorp HH, Murray RW (2001) J Am Chem Soc 123:218–222
Fukumoto K, Yoshizawa M, Ohno H (2005) J Am Chem Soc 127:2398–2399
Anderson JL, Armstrong DW, Wei GT (2006) Anal Chem 78:2892–2902
Han X, Armstrong DW (2007) Acc Chem Res 40:1079–1086
Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD (2003) J Am Chem Soc 125:6632–6633
Du Z, Yu YL, Wang JH (2007) Chem Eur J 13:2130–2137
Li CX, Han J, Wang Y, Yan YS, Xu XH, Pan JM (2009) Anal Chim Acta 653:178–183
Zafarani-Moattar MT, Tolouei S (2008) Calphad 32:655–660
Malpiedi LP, Fernández C, Picó G, Nerli B (2008) J Chem Eng Data 53:1175–1178
Zafarani-Moattar MT, Hamzehzadeh S (2008) J Chem Eng Data 54:833–841
Mena ML, Agüí L, Martinez-Ruiz P, Yáñez-Sedeño P, Reviejo AJ, Pingarrón JM (2003) Anal Bioanal Chem 376:19–25
Pei Y, Wang J, Liu L, Wu K, Zhao Y (2007) J Chem Eng Data 52:2026–2031
European Commission (2002) Implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Regulation 2002/657/EC, 12 August. Off J Eur Commun L211:8–36
Acknowledgements
This work has been supported by the National Natural Science Foundation of China (no. 20777029, no. 20976074, and no. 21076098), the Natural Science Foundation of Jiangsu Province (no. BK2010349), and the Ph.D. Programs Foundation of Ministry of Education of China (no. 200807100004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, J., Wang, Y., Yu, Cl. et al. Extraction and determination of chloramphenicol in feed water, milk, and honey samples using an ionic liquid/sodium citrate aqueous two-phase system coupled with high-performance liquid chromatography. Anal Bioanal Chem 399, 1295–1304 (2011). https://doi.org/10.1007/s00216-010-4376-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4376-2