Abstract
Dragon's blood is a common but non-specific name for red-coloured resins that are produced by various plants, particularly exudations from plant species belonging to the genera Dracaena and Daemonorops. Although dragon's blood is mentioned in historic sources as a colourant, it has hardly ever been identified in real artworks. This paper reports the identification and discrimination of dragon's blood produced by Dracaena cinnabari, Dracaena draco as well as Daemonorops draco and Daemonorops micracantha by means of gas chromatography/mass spectrometry (GC/MS) within the context of a routine analysis of binding media used in works of art. The detection of specific flavonoid marker compounds in both underivatised and methylated methanol extracts provided the first evidence for the use of dragon's blood from all four species in various works of art from the fifteenth to nineteenth centuries. Dragon's blood was mainly used as a red colourant in gold lacquers as well as translucent glazes and paints, e.g. in reverse-glass paintings (Hinterglasmalerei).

Splendid and colourful Hinterglasmalerei in the Amelierung technique with gold foils on two panels of the Corning House Altar, ca. 1560–1580, before restoration. The red paints contain dragon’s blood from Dae. draco. The width of the altar with closed wings is 19.5 cm. Photo: Simone Bretz, Oberau, © The Corning Museum of Glass, Corning (NY)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dragon's blood is a non-specific name for red resinous exudations from quite different plant species endemic to various regions around the globe that belong to the genera Dracaena (Africa) and Daemonorops (South-East Asia), more rarely also to the genera Pterocarpus and Croton (both South America) [1]. The name traces back to the myth of a struggle between a dragon and an elephant that, at its climax, led to the mixing of blood from the two creatures, resulting in a magical substance: dragon's blood [2]. Dragon's blood is used for medicinal purposes where it is endemic [1], and has also been traded as a colourant for use in works of art for centuries [3, 4]. The earliest historical source referring to dragon's blood as a red colour to paint blood is given by Pliny (∼23–79 AD) [5]. Before the seventeenth century, only a few sources mention the use of dragon's blood, e.g. Heraclius (Chapters 52, 56 and 58 [6]), Cennini (Chapter 43 [7]) and Boltz von Ruffach ([8], p. 68). In later centuries, the deep red resin is mentioned more often as a colourant as well as a lacquer component [3, 9]. It has always been highly prized and expensive. Nowadays, dragon's blood is still used as a varnish component in red lacquers, e.g. for violins; however, due to its tendency to fade, more stable (synthetic) dyestuffs are used instead.
To date, dragon's blood has very rarely been identified in art objects [10]. Previous work focused on spectroscopic methods, such as infrared and Raman spectroscopy [11, 12] or nuclear magnetic resonance spectroscopy [13]. However, because dragon's blood is usually mixed with a broad range of other materials for use in works of art and has undergone considerable ageing and degradation, chromatographic methods are necessary for its unequivocal identification. This can be achieved by liquid chromatography with UV–VIS diode array detection (HPLC-DAD), which even allows discrimination of Dracaena and Daemonorops resins [3, 14]. Unfortunately, this method is limited to chromophoric compounds which are strongly affected by ageing and fading [9, 15]. This paper describes the identification of dragon's blood by means of very specific and mainly non-chromophoric flavonoids that are detectable by GC/MS and which are preserved even in considerably aged and degraded artwork samples. This method can be used to discriminate dragon's blood from individual species, Dracaena cinnabari, Dracaena draco as well as Daemonorops draco and Daemonorops micracantha.
It goes without saying that sampling of a precious work of art has to be kept to a minimum and as much information as possible has to be obtained from a sample once it has been taken. For this purpose, a special procedure for the analysis of complex and aged binding media mixtures in artworks has been developed at our institute [10, 16, 17]. This analysis procedure includes stepwise extraction of the samples with isooctane, methanol, chloroform and methanol with anhydrous oxalic acid. One advantage of this successive extraction is the separation of chemically different binding media, such as oils, waxes, resins, proteins and polysaccharides. As a consequence, the complexity of the gas chromatograms is reduced, and minor components can be detected more sensitively and reliably. Resins such as dragon's blood appear in the methanol extract, which is analysed twice with GC/MS, firstly without any derivatisation, and secondly, after simultaneous hydrolysis and methylation with trimethyl sulfonium hydroxide (TMSH) [18]. The identification and differentiation of dragon's blood is thus achieved by recognising marker substances in both the underivatised and the methylated methanol extracts. The aim is not a complete chemical characterisation of the resins but their identification within the context of a routine binding medium analysis. The parallel analysis of derivatised and underivatised extracts is advantageous because specific markers can sometimes only be detected in either one or the other chromatogram [10].
Experimental
Reference materials
Reference materials were purchased from Kremer Farbmühle (88317 Aichstetten, Germany) and GED Gerhard Eggebrecht (25361 Süderau, Germany). Currently available commercial plant material has only limited botanic variety because mainly Dae. draco resin is still being traded. In addition, the relatively expensive dragon's blood may be adulterated with cheaper red colourants or natural resins. It is thus important to obtain reference materials with a verified botanical origin. Such samples were obtained from Katja Lewerentz during her diploma thesis on dragon's blood at the Cologne University of Applied Sciences [9], and included plant material from Tenerife, Socotra (Dr. Krekel, Stuttgart State Academy of Art and Design and Cologne University of Applied Sciences, Germany), Malaysia (Forest Research Institute Malaysia FRIM, 52109 Kepong, Malaysia), and Sumatra (Dr. Psota, Historisches Museum Bern, Switzerland). Additional resins were obtained from botanic collections in Germany (Dr. Esser, Botanischer Garten München and Dr. Vogt, Botanisches Museum Berlin–Dahlem) and from Kew Gardens (Dr. Nesbitt, Economic Botany Collection, United Kingdom) [19]. The Doerner Institut also houses a large collection of natural resins and colourants from the Martius Pharmacognosy Collection (dating around 1825–63) [20, 21], which was included in the study. A complete list of samples is given in the Electronic Supplementary Material (Table S1).
Analytical procedure
Samples (0.1–0.2 mg) of the dragon's blood reference materials were dissolved in methanol. In contrast, approx. 0.2–0.5 mg of historic samples from artworks (paintings, frames, reverse-glass paintings, lacquer cabinets) were pre-treated by stepwise extraction with different solvents of increasing polarity (isooctane, methanol, chloroform/methanol [7:3 v/v], anhydrous oxalic acid in methanol [10% w/v]), all chromatographic grade (Merck Chemicals, 64293 Darmstadt). Reference materials as well as sample extracts were first injected into the GC/MS system without derivatisation. For the methylation/transesterification step, a solution of TMSH in methanol (0.2 M, Macherey-Nagel, 52355 Düren) was added to the dried extracts, and the mixture was heated for approximately 30 min at 50 °C in a closed sample vial [18].
Instrumentation
This paper presents analysis results collected over a period of 10 years. During that time, two GC/MS systems were employed that were equipped with comparable GC columns from several manufacturers. Hence, retention times of specific compounds may differ. These two GC/MS systems were, firstly, an HP 5890 series II gas chromatograph (Hewlett-Packard/Agilent) coupled to a Hewlett-Packard quadrupole mass spectrometer type 5989B (MS Engine) and secondly, a GC 6890 N coupled to a MSD 5975 (Agilent). Detection of mass spectra: EI mode, 70 eV, scan range m/z 40–500. The chromatographs were usually equipped with DB-5ht columns (J&W), 30 m, 0.25 mm ID, 0.1 µm film thickness. Measurement conditions: carrier gas helium 5.0 (purified), constant-flow mode, 1.7 ml/min; split/splitless injector: injection temp. = 250 °C, splitless mode, 1–2 µl injection volume, 0.5 min splitless; purge flow 36 ml/min; oven programme: T1 = 55 °C, t1 = 1 min, R1 = 15 °C/min, T2 = 150 °C, R2 = 10 °C/min, T3 = 360 °C. The identification of small peaks was sometimes enhanced with selected ion extraction in the Agilent data analysis software (MSD ChemStation, Rev. D.02).
Analysis of reference materials
Dragon's blood is a phenolic resin that is mainly produced by monocots of the genera Dracaena and Daemonorops [22]. A clear botanical differentiation between several subspecies of Dracaena was first made by Balfour [23]. Nowadays the Dracaena genus comprises approximately 60 species that are mainly found in tropical and subtropical Africa. The Daemonorops genus comprises about 115 known species, most of which are native to India, South China and the Malay Archipelago. However, only a few species deliver the red resin that is traded as dragon's blood [1, 19, 24–26], and only some of these are considered to be the main sources of dragon's blood traded in recent centuries: Dae. draco (Willd.) Blume, as well as Dr. draco (L.) L. and Dr. cinnabari Balf. fil. [9, 26, 27]. It is unclear to what extent other species constitute underestimated sources for dragon's blood. In addition to these three species, dragon's blood from Dae. micracantha (Griff.) Becc., a species not normally associated with being a commercial source, was analysed.
Several other plants deliver red resins or red wood that are sometimes called dragon's blood and which have been traded for centuries. It is not entirely clear whether these materials were not distinguished or maybe even confused with dragon's blood from Dracaena and Daemonorops sources. Such exudates or wood extracts from Pterocarpus or Croton genera [1] will only be briefly discussed here.
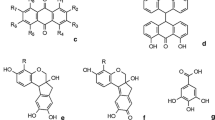
The chemical composition of the different dragon's blood resins has been studied by various authors in the past. Despite significant differences, they were all found to consist of mainly flavonoids. An overview of their basic structures is given in Table 1. Representative gas chromatograms and mass spectrometric data for the different resins are given in the following sections and the Electronic Supplementary Material (Tables S3, S4, S5 and S6). The relative amounts of individual compounds vary to some extent. A tentative assignment of the peaks to compounds identified in dragon's blood is given on the basis of mass spectrometric reference values in the respective cited literature as well as general mass spectrometric data of flavonoids [28–31]. Flavans, flavanones and flavones generally fragment by means of retro-Diels–Alder processes, thus giving rise to characteristic fragments. In the case of homoisoflavones, the benzyl side-group is usually a dominant fragment. Chalcones can be cleaved on either side of the carbonyl group, but isomerisation to flavanones or aurones in the spectrometer with subsequent fragmentation clearly complicates the situation. It has to be stressed that many compounds identified in dragon's blood have no correlation to peaks in the chromatograms reported here and vice versa. An unambiguous assignment of individual compounds is beyond the scope of this work because there are too many possible isomers and fragmentation in the mass spectrometer is greatly influenced by the substitution pattern of the flavonoids [29]. Co-elution of different compounds further confuses the situation. As already stated, it is not the aim of this study to achieve a complete analysis of dragon's blood, but rather its identification and differentiation by marker compounds within a more comprehensive analysis routine for binding media.
It is important to note that flavonoids are ubiquitous in plants. In addition to the dragon's blood of the sources discussed here, other plant exudates or parts of plants used in works of art also contain flavonoids. This is especially true for many yellow dyes. Therefore, the identification of flavonoids in an artwork sample does not necessary mean that dragon's blood is present. To interpret such a finding, the context of the sample has to be taken into account. This paper deals with plant sources for Western European art (see the “Dragon’s blood in works of art” section). Other sources of flavonoids might be relevant for Asian or ethnographic art.
Production of dragon's blood, historic products and adulterations
Dragon's blood from Daemonorops spp. is obtained from the immature fruits. The dried fruits are shaken in sacks or baskets to detach the resinous layer on the outside of the fruits. The resulting powder is softened by heat and moulded into cakes or sticks, sometimes with the aid of water or steam [9, 24, 26]. In contrast, the Dracaena resins are exuded from the wounded trunk or branches of the tree [19, 22]. They are sold as small pieces or cakes and sticks after melting and purification [9].
The reference materials in historic collections are often inscribed with Latin names, such as resina sangui[ni]s draconis. In addition, descriptions of the form are given, e.g. in granis, in lacrimis, in massis (Electronic Supplementary Material, Table S2). These names correspond to the form and size of the pieces of commercial resin; however, they provide no information on the origin of the dragon's blood. This is confirmed by the literature [32–34] as well as by our analyses; indeed, the dragon's blood in the Martius collection was obtained from diverse plant sources.
Adulterations of valuable dragon's blood with cheaper red colourants or natural resins are regularly detected. In our studies, one specimen was mixed with shellac (Electronic Supplementary Material, Table S1). Diterpenoids with the typical pattern of colophony are sometimes detected in commercial resins [35], but not in genuine reference materials. The large amounts of abietic and pimaric acids and their isomers certainly derive from adulterations with colophony, as previously suspected [36]. However, the situation is more complex with dragon's blood from Dae. draco, which fairly often contains considerable amounts of triterpenoids, even in reliable reference samples [37, 38]. It is not entirely clear whether these derive from adulterations with other resins, such as dammar, upon collection of the dragon's blood or during processing for trade. Another possibility might be that these compounds are common components in other parts of the plant and may constitute minor components of the resins [39]. The role of triterpenoids will be discussed in more detail in the “Dragon’s blood from Dae. draco (Willd.) Blume” section.
Dragon's blood from Dracaena cinnabari Balf. fil.
In contrast to other Dracaena species growing in Macaronesia, East Africa and Arabia, Dr. cinnabari is endemic to the island of Socotra (Yemen). It is a distinct species and thus has several unique morphological as well as chemical features [40]. Dragon's blood from the botanical collection in Berlin–Dahlem as well as plant material collected in Socotra was used for reference analyses.
According to the literature, dragon's blood of Dr. cinnabari mainly consists of homoisoflavans, along with flavans, flavanones, flavones, chalcones, dihydrochalcones, di- and triflavonoids and other compounds [41–45]. This is in agreement with our analyses. Representative gas chromatograms of underivatised and methylated methanol extracts are given in Figs. 1 and 2. Peak labels correspond to those in Tables 2 and 3. The dominant compound in the extracts of fresh material was 7-hydroxy-3-(4-hydroxybenzyl)-8-methoxy-chroman (DrC11), and many other compounds are detectable as minor compounds in varying amounts. After derivatisation, some of the resulting peaks were assigned to permethylated compounds from the methanol extract, but no assignments could be made for the other compounds even though they deliver quite characteristic peaks (Fig. 2). The reason for this is unclear, but as previously mentioned, the aim of this work is to characterise marker compounds and not to unambiguously identify all components, which would require much more in-depth mass spectral analysis. For the same reason, it is not always clear whether some unassigned compounds from different species with similar mass spectra are identical or not.
Representative gas chromatograms of underivatised methanol extracts of resins from a Dracaena cinnabari (DrC), b Dracaena draco (DrD), c Daemonorops draco (DaeD) and d Daemonorops micracantha (DaeM). Peak labels correspond to those given in Table 2
Representative gas chromatograms of methylated methanol extracts of resins from a Dracaena cinnabari (DrC), b Dracaena draco (DrD), c Daemonorops draco (DaeD) and d Daemonorops micracantha (DaeM). Peak labels correspond to those given in Table 3
Dragon's blood from Dracaena draco
Among the Dracaena species growing in West Africa, mainly Dr. draco is an important source of dragon's blood. There are two varieties: subspecies draco in the Madeira, Canary and Cape Verde archipelagos and subspecies ajgal in SW Morocco [40]. The latter was probably not used for harvesting resin [9]. Reference materials from the herbarium of the Botanic Garden Munich and fresh material from Gran Canaria provided reliable sources for the analyses presented here (Electronic Supplementary Material, Table S1).
The resin mainly consists of flavans and methylflavans, along with flavanones, homoisoflavans, homoisoflavones, chalcones, dihydrochalcones and others (e.g. dracaenone) [13, 46, 47]. Representative gas chromatograms of underivatised and methylated methanol extracts are given in Figs. 1 and 2, peak labels correspond to those given in Tables 2 and 3. In contrast to dragon's blood from Dr. cinnabari, the resin of Dr. draco shows about ten major peaks, and the dominant flavonoid DrC11 is missing. Therefore, a clear differentiation can be made between these two Dracaena species. No significant difference was detected between resins of subspecies Dr. draco draco and Dr. draco ajgal.
Dragon's blood from Daemonorops draco (Willd.) Blume
Nowadays, Dae. draco is grown commercially for the production of dragon's blood. As early as 1950, the main colourants were demonstrated to be dracorubin and dracorhodin [48], and these compounds are suitable for detecting the resin in artworks [27] and discriminating Dae. draco by means of HPLC [3, 14]. Dracorhodin (anhydro-7-hydroxy-5-methoxy-6-methyl-2-phenyl-benzopyranol, DaeD10) can also be identified by GC/MS [49], but the gas chromatogram of the methanol extract is dominated by two flavonoids, a flavan and a methylflavan (Figs. 1 and 2, Tables 2 and 3). The resin also contains chalcones, dihydrochalcones, bi- and triflavonoids, and other compounds, according to the literature [13, 38, 50–53].
Some reference samples of Dae. draco were shown to contain additional triterpenes [37, 38] that reveal similarities with dammar, a well-known pale yellow resin produced in South-East Asia, mainly by trees of the Dipterocarpaceae family [54, 55]. Furthermore, greatly varying amounts of triterpenoids were identified in several Dae. draco resins studied here. In agreement with the literature, many of the identified compounds are typical of dammar, but some are not (Electronic Supplementary Material, Table S7). Triterpenoids with mass fragments of m/z 218 are usually typical for amyrins, which are very common in many plants, but not in dammar resin. Analysis of the Dae. draco reference material revealed that the resin adhering directly to the fruit contained no triterpenoids. Therefore, we checked whether these compounds might be extracted from parts of the fruit during the processing of dragon's blood (cf. “Production of dragon’s blood, historic products and adulterations” section). Although analyses of the inner fruit parts obtained from the Economic Botany Collection in Kew did indeed reveal extractable triterpenoids, these were not identical to the compounds detected in the resins. Hence, it seems more likely that other resins from plants growing in the same areas as Dae. draco might be added during harvesting or incorporated during the production of the dragon's blood cakes. This might indeed be true for dammar resin [25]. Another explanation would be that resins with triterpenoids are derived from yet another Daemonorops species, although this seems rather unlikely.
Dragon's blood from Daemonorops micracantha (Griff.) Becc.
In contrast to the other types of dragon's blood, little is known about the composition of the Dae. micracantha resin. The precisely characterised botanical reference material from Malaysia reveals two main flavonoid components in the methanol extracts, similar to Dae. draco resin, but they clearly differ from the latter (Figs. 1 and 2, Tables 2 and 3). The molecular weights could not be unambiguously assigned because the chemical structures of the compounds are unknown. However, judging by the very similar infrared spectra and mass spectral patterns in both Dae. draco and Dae. micracantha resins, the main compounds DaeM4 and DaeM5 are probably flavonoids, but with a higher degree of methylation of the phenolic groups.
One sample of dragon's blood in the Martius collection revealed the same pattern as Dae. micracantha. We therefore conclude that resins from Dae. micracantha species — due to their similar appearance (Ref. [22], plate 47) to other Daemonorops resins of the Malay region — have been traded under the name of dragon's blood since historic times. This is further supported by nineteenth century samples of resinous fruit from the Economic Botany Collection Kew that are inscribed as Dae. draco Blume, but were found to originate from Dae. micracantha (Electronic Supplementary Material, Table S1).
Other sources of dragon's blood—Croton and Pterocarpus
Several Latin American species of the Croton genus (C. draco, C. draconoides, C. lechleri, C. palanostigma, C. urucurana and C. xalopensis) yield red exudates commonly called dragon's blood [3, 22, 56, 57]. Similar to the Dracaena species, the dragon's blood is collected from incisions made in the stem. Four reference samples from Croton species were analysed (Electronic Supplementary Material, Table S1). In all cases, no flavonoids comparable to the Dracaena and Daemonorops resins were identified. This is in agreement with the literature, which reports the main components of these resins as being oligomeric proanthocyanidins [56]; these are too large and polar to be detected by GC/MS. Even if monomeric catechin, epicatechin, gallocatechin and epigallocatechin [58] were more abundant in degraded material, the pattern should be clearly distinguishable from the resins discussed above. However, more work is needed here.
The main dyes in red sandalwood (from Pterocarpus santalinus L.) [27], native to East India, Sri Lanka and the Philippines, are santalin A, B and C [59]. In addition, characteristic colourless isoflavonoids are detectable by GC/MS: pterocarpin C17H14O5 (MW 298) and homopterocarpin C17H16O4 (MW 284) [27, 60]. These marker compounds were found in a sample of dragon's blood from the Collection Martius and Pterocarpus wood samples from Kew (Electronic Supplementary Material, Table S1). This demonstrates that wood extracts of sandalwood have been traded together with dragon's blood from more common sources in past centuries, and sometimes they were confused or at least not distinguished from each other.
Dragon's blood in works of art
In recent years, dragon's blood from all the species discussed here was detected in a broad variety of art objects dating from the fifteenth to the nineteenth centuries (Table 4). According to these analyses, dragon's blood was predominantly used in gold lacquers and Hinterglasmalerei (reverse-glass paintings), and relatively rarely in glazes on conventional paintings or lacquers on furniture. In all these cases, the artists took advantage of the special properties of dragon's blood: a film-forming resin with a natural red colour that is nevertheless translucent. In lacquers, dragon's blood was mixed with other natural resins such as sandarac [61], larch turpentine [62] and mastic [63], and also with other red resins such as shellac or gum benzoin, which is a dark red balsamic resin obtained from several species of the genus Styrax [64].
Dragon's blood in gold lacquers
So-called gold lacquers were used to imitate gold on altars, statues and frames: a foil of less expensive silvery metal, mostly tin or even silver, was applied to the object and covered with a yellowish to reddish translucent lacquer to achieve the illusion of gold.
Dragon's blood was detected in two gold lacquers on silver-coated frames within the collection of the Alte Pinakothek in Munich: on a neoclassical frame dating around 1767–1800 (Fig. 3) and on a frame created in 1730 by Johann Effner. The binding media in both cases were based on shellac, sandarac and mastic, but the golden colour was further intensified by the addition of dragon's blood. The markers of Dae. micracantha were detected in both samples (Fig. 4). The retention times of the DaeM4 and DaeM5 markers differ slightly from Fig. 1 because the chromatograms were recorded with different analytical systems (cf. “Instrumentation” section).
Gas chromatograms of the methanol extracts of two artwork samples containing dragon's blood. Above gold lacquer from the neoclassical frame (Fig. 3). The main binding media are shellac (Sh), sandarac (S) and mastic (M). The reddish translucent colour was achieved by the addition of dragon's blood from Dae. micracantha and probably by the natural dyes of shellac. Below red lacquer from the 16th century reverse-glass painting known as the Spanische Landkarte. The binding medium is larch turpentine (L) with very little mastic (not visible in the gas chromatogram). Dragon's blood from Dracaena draco as well as gum benzoin (B) were used as red colourants
Dragon's blood was also identified on the altar figure of St. Alto in the Rococo church of the Birgitten cloister Altomünster, Bavaria, created around 1770. The methanol extract of two gold lacquer samples on metal leaf from the Saint's coat and shoe were shown to contain the flavans DaeD1 and DaeD2, typical of Dae. draco, as well as binding media based on larch turpentine, sandarac and colophony (Table 4). Additional typical components, such as dracorhodin or triterpenes, were missing in the St. Alto samples. The colour of the now faded, but originally reddish lacquer, was further intensified by the addition of gum benzoin. This resin was identified by small amounts of benzoyl and cinnamyl esters.
Dragon's blood in Hinterglasmalerei
Objects of hinterglasmalerei are painted on the backside of glass panels. Obviously, the paint layers have to be applied in reverse order, starting with the uppermost layer. The finished hinterglas painting is seen through the glass and thus reveals an impressive gloss and depth of colour. To intensify this impression, translucent paints were often applied in the sixteenth and seventeenth centuries. The Amelierung technique involves covering the reverse side of the glass with gold leaf, which is then etched and painted with colourful translucent lacquers. Metal foils were used as a third layer to reflect the light and intensify the luminosity of the paints [65]. As a consequence, the binding media of such reverse-glass paints are often resins, as prior analyses have shown [10]. Resins dry quickly, adhere well to the glass and can form translucent coloured lacquers with many dyes or pigments. In a research project studying Hinterglasmalerei, several of the investigated red glazes were found to contain dragon's blood, sometimes in combination with another red component, mainly gum benzoin.
Six of the Dr. draco markers were identified in a small flake of red lacquer on a splendid sixteenth century rock crystal painted in Hinterglasmalerei, known as the Spanische Landkarte (Rijksmuseum Amsterdam). Larch turpentine and small amounts of mastic form the translucent binding media of the paint layer that was coloured with dragon's blood (Fig. 4, Table 4). The concentration of the aged Dr. draco is still high enough to allow detection of the markers in the methanol solution. The lacquer's colour was further intensified by the addition of gum benzoin.
In two samples of red lacquer from a South-German house altar (The Corning Museum of Glass, New York, cf. Fig. 5), dating probably around 1560–1580, dragon's blood was identified in addition to a cochineal dye [66]. The binding medium is based on larch turpentine, colophony and mastic. The methanol extract exhibited the typical mass spectrum of dracorhodin; the dragon's blood thus originates from Dae. draco. Additional triterpenoids with dammarane structures seem to be derived from triterpenoid portions within the dragon's blood, as discussed above (Electronic Supplementary Material, Table S7).
Splendid and colourful Hinterglasmalerei in the Amelierung technique with gold foils on two panels of the Corning House Altar, ca. 1560–1580, before restoration. The red paints contain dragon's blood from Dae. draco. The width of the altar with closed wings is 19.5 cm. Photo: Simone Bretz, Oberau, © The Corning Museum of Glass, Corning (NY)
A small pilgrim bottle with a medallion painted in the reverse-glass technique (late sixteenth century, Rüstkammer Dresden) was also analysed. The transparent red lacquer was completely soluble in methanol and the binding medium was based on mastic resin. Due to the very small sample size, the red colourant of the investigated paint flake was not distinguishable at first; however, the typical markers of Dr. cinnabari were identified after methylation.
As a last example, an amber game box dating around 1680 (Grünes Gewölbe Dresden) should be mentioned. The reverse side of the amber pieces decorating a folding box were ornamented by the Amelierung technique. During its conservation, it was possible to analyse two untouched samples of red lacquer [67]. The GC/MS results of the underivatised methanol extracts of both samples show larch turpentine as the main binder, and the marker compounds of Dae. micracantha were also identified (Table 4).
Dragon's blood in paintings and lacquers on furniture or wood surfaces
The use of dragon's blood on paintings, furniture or wood surfaces could only be demonstrated in isolated cases. More objects have to be studied to verify how often dragon's blood has been used in these kinds of applications.
The only painting on which we were able to detect dragon's blood is a votive panel dating from 1457 (Table 4). In a glaze on a red paint imitating brocade, it was possible to identify dracorhodin (DaeD10). This represents the oldest verified use of dragon's blood in this study, and also demonstrates a special application regarding the used binding media: the Dae. draco resin was applied with a drying oil (linseed oil). Another red lake pigment was present, but could not be identified by HPLC due to detection limits.
Similar to the use of gold lacquers or resin-based paints in Hinterglasmalerei, dragon's blood was also used in resinous lacquers on furniture. However, its use has only been demonstrated in one case so far: a cabinet inscribed Gérard and Jacques Dagly (1660–1728) from the castle of Weilburg (Germany). Parts of this cabinet are decorated with a so-called aventurine lacquer: copper filings bound in several layers of transparent lacquer placed on top of a black ground [68]. The appearance of the metal powder was modulated by a translucent red finishing lacquer composed of shellac, larch turpentine and dragon's blood of the species Dae. draco (Table 4).
Dragon's blood was presumably also used in a nineteenth century translucent lacquer on the wood panelling and furniture of the lecture hall in the Munich gynaecological hospital (Table 4). Again it was used to enhance the red colour of the coating based on shellac and thus produce a dark-red impression of the wood surface. Although the identified flavonoid markers were similar, they did not exactly match the pattern reported above. It is not clear at the moment whether this resin represents an additional, hitherto unspecified Dracaena species.
Another example of a dark-red wood polish based on shellac was found on a Saxonian table with a cherry wood veneer that dates around 1810/15 (Table 4). In this case, homopterocarpin was detected (“Other sources of dragon’s blood—Croton and Pterocarpus” section), thus sandalwood was used as an additional colourant. Whether this red wood extract was consciously selected by the artist or accidentally used instead of true dragon's blood is not known.
Conclusions
Certainly, a wide variety of plant materials was used as red colourants in works of art. After the characterisation of carefully selected reference resins, the thus identified specific marker compounds allow differentiation between dragon's blood from the genera Daemonorops and Dracaena and even their species Dae. draco, Dae. micracantha, Dr. draco and Dr. cinnabari, in addition to dragon's blood derived from sandalwood. The red resins of all these species were indeed used in a broad range of artworks dating from the fifteenth to the nineteenth centuries.
This paper summarises the detection of dragon's blood in artworks investigated over the last 10 years in our institute. Although we were able to study a very broad variety of objects, the number of positive identifications is still rather small, and it is clear that it is difficult to draw certain conclusions at the moment. Nevertheless, it is quite obvious that dragon's blood has mainly been used in translucent red lacquers and glazes, predominantly in resinous binding media systems. This allows subtle colouring of artworks with lustrous, luminous paints to provide an impressive depth of colour. Accordingly, dragon's blood was often used as a red colourant for Hinterglasmalerei and in gold lacquers on metal foils to imitate gold on picture frames or altars in churches. Other applications such as glazes on paintings, lacquers on furniture or for the tinting of wood surfaces are more rare, but occasionally identified. The colour was often further intensified and modified with other red resins such as shellac or gum benzoin.
The analyses performed so far demonstrate that Daemonorops resins from South-East Asia have been used in European Art at least since the fifteenth century, and it seems they were used more frequently than dragon's blood from African Dracaena species. This is surprising because the respective resins from Socotra or the Canaries, which have been known in Europe much longer, are always mentioned in historic sources and have definitely been traded during the last centuries because specimens are also included in botanic and pharmacognostic collections. However, the number of positive identifications in artworks is still rather small at the moment. The characterisation of marker compounds presented in this paper will hopefully result in further identifications of dragon's blood in the future. This will enable a more detailed and accurate perception of the use of different sorts of dragon's blood in art, leading to a clearer picture and a deeper understanding of ancient trading routes. Most of all, however, it demonstrates that the colourfulness of works of art that include organic colourants susceptible to fading were originally more vivid and impressive than can be seen today.
References
Gupta D, Bleakley B, Gupta RK (2008) J Ethnopharmacol 115:361–380
Thomson DV (1956) The materials and techniques of medieval painting. Dover, New York
Wallert A, van Bommel MR (2008) Dyes in History and Archaeology 21:75–88
Harley RD (1982) Artists' pigments c. 1600–1835. Butterworth, London
Plinius Secundus G (1984) Book XXXIII-Metallurgy. In: König R, Winkler G (eds) Naturalis Historia - Naturkunde, München, pp 82–85
Heraclius (1873) De coloribus et artibus romanorum. In: Ilg A (ed) Quellenschriften für Kunstgeschichte und Kunsttechnik des Mittelalters und der Renaissance, Wilhelm Braumüller, Wien
Cennini C (1871) Il libro dell'arte o trattato della pittura. In: Ilg A (ed) Quellenschriften für Kunstgeschichte und Kunsttechnik des Mittelalters und der Renaissance. Wilhelm Braumüller, Wien
Boltz von Ruffach V (1913) Illuminierbuch - Wie man allerlei Farben bereiten, mischen und auftragen soll. Benzinger CJ (ed) after the first edition of 1549, Georg Callwey, München
Lewerentz K (2000) Drachenblut. Materialkunde, quellenkundliche Studien zur maltechnischen Verwendung und Analysemöglichkeiten der verschiedenen Farbharze dieses Namens. Diploma thesis, Fachhochschule Köln
Baumer U, Dietemann P, Koller J (2009) Int J Mass Spectrom 284:131–141
Edwards HGM, de Oliveira LFC, Quye A (2001) Spectrochim Acta, Part A 57:2831–2842
Edwards HGM, LFCd O, Prendergast HDV (2004) Analyst 129:134–138
Camarda L, Merlini L, Nasini G (1983) Heterocycles 20:39–43
Sousa MM, Melo MJ, Parola AJ, de Melo JSS, Catarino F, Pina F, Cook FEM, Simmonds MSJ, Lopes JA (2008) J Chromatogr A 1209:153–161
Whitmore PM, Cass GR, Druzik JR (1987) J Am Inst Conserv 26:45–58
Koller J, Irene F, Baumer U (1998) Die Bindemittel auf Dürers Tafelgemälden. In: Goldberg G, Heimberg B, Schawe M (eds) Albrecht Dürer-Die Gemälde der Alten Pinakothek. Bayerische Staatsgemäldesammlungen, München, pp 102–119
Koller J, Baumer U (1997) Baroque and Rococo Transparent Gloss Lacquers-II. Scientific study of lacquer systems. In: Walch K, Koller J (eds) Baroque and Rococo Lacquers, Arbeitshefte des Bayerischen Landesamtes für Denkmalpflege. Lipp, München, pp 52–84
Butte W (1983) J Chromatogr 261:142–145
Pearson J, Prendergast HDV (2001) Econ Bot 55:474–477
Schneider W (1959) Pharm Ztg 104:865–879
Thoma M, Dressendörfer W (2007) Martius-Pharmakognosie-Sammlung. In: Andraschke U, Ruisinger MM (eds) Die Sammlungen der Universität Erlangen-Nürnberg. Tümmels, Nürnberg, pp 135–144
Langenheim JH (2003) Plant resins - chemistry, evolution, ecology, and ethnobotany. Timber, Portland, Cambridge
Balfour IB (1888) Trans R Soc Edinb 31:292–294
Pearson J (2002) Horticulturist 11:10–12
Dransfield J, Kramadibrata P, Madulid DA, Manokaran N, Mogea JP, Tipot L (1993) Minor Rattans. In: Dransfield J, Manokaran N (eds) Rattans. Pudoc, Wageningen
Rustiami H, Setyowati FM, Kartawinata K (2004) J Trop Ethnobiol 1–2:65–75
Schweppe H (1993) Handbuch der Naturfarbstoffe. Nikol Verlagsgesellschaft, Hamburg
Mabry TJ, Ulubelen A (1980) Flavonoids and related plant phenolics. In: Waller GR, Dermer OC (eds) Biochemical applications of mass spectrometry. Wiley, New York, Chichester, Brisbane, Toronto, pp 1131–1158
Audier H (1966) Bull Soc Chim France 9:2892–2899
Hedin PA, Phillips VA (1992) J Agric Food Chem 40:607–611
Berahia T, Gaydou EM, Cerrati C, Wallet JC (1994) Gas chromatography-mass spectrometry of polymethoxylated flavones. In: Brouillard R, Jay M, Scalbert A (eds) Polyphenols 94. Institut national de la recherche agronomique, Paris, Palma de Mallorca, pp 441–442
Pomet P (1987) Der aufrichtige Materialist und Specerey-Händler. Reprint of the Leipzig, Gleditsch & Weidmann Edition (1717), VCH Verlagsgesellschaft, Weinheim
Zedler JH (1734) Großes vollständiges Universallexicon aller Wissenschaften und Künste. Johann Heinrich Zedler, Halle und Leipzig
Lojander H (1887) Beiträge zur Kenntnis des Drachenblutes. Buchhandlung von Karl J. Trübner, Straßburg
Piozzi F, Passannanti S, Paternostro MP (1974) Phytochem 13:2231–2233
Mills JS, White R (1977) Stud Conserv 22:12–31
Nasini G, Piozzi F (1981) Phytochem 20:514–516
Shen C-C, Tsai S-Y, Wei S-L, Wang S-T, Shieh B-J, Chen C-C (2007) Nat Prod Res 21:377–380
Masaoud M, Schmidt J, Adam G (1995) Phytochem 38:795–796
Marrero A, Almeida RS, Gonzáles-Martín M (1998) Bot J Linn Soc 128:291–314
Suchý V, Bobovnický B, Trojánek J, Budésínský M, Ubik K (1991) Homoisoflavans and other constitutents of Dragon's blood form Dracaena cinnabari. In: Pezzuto JM, Kinghorn AD, Fong HHS, Cordell GA (eds) Progress on terrestrial and marine natural products of medicinal and biological interest. American Botanical Council, Illinois, pp 110–118
Masaoud M, Ripperger H, Porzel A, Adam G (1995) Phytochem 38:745–749
Machala M, Kubinova R, Horavova P, Suchy V (2001) Phytother Res 15:114–118
Masaoud M, Himmelreich U, Ripperger H, Adam G (1995) Planta Med 61:341–344
Himmelreich U, Masaoud M, Adam G, Ripperger H (1995) Phytochem 39:949–951
Gonzáles AG, León F, Sánchez-Pinto L, Padrón JI, Bermejo J (2000) J Nat Prod 63:1297–1299
Gonzáles AG, León F, Hernández JC, Padrón JI, Sánchez-Pinto L, Bermejo Barrera J (2004) Biochem Syst Ecol 32:179–184
Robertson A, Whalley WB, Yates J (1950) J Chem Soc (Resumed):3117-3123
Baumer U, Koller J (2001) Drachenblut - Herkunft, Analyse und Verwendung. In: Archäometrie und Denkmalpflege–Kurzberichte 2001, Jahrestagung an der Fachhochschule Köln, pp 151-153
Cardillo G, Merlini L, Nasini G (1971) J Chem Soc (C):3967-3970
Nakashima K-I, Abe N, Kamiya F, Ito T, Oyama M, Iinuma M (2009) Helv Chim Acta 92:1999–2008
Arnone A, Nasini G, Merlini L (1990) J Chem Soc Perkin Trans 1:2637–2640
Arnone A, Nasini G, Vajna de Pava O, Merlini L (1997) J Nat Prod 60:971–975
Wenders E (1998) Dammar als Gemäldefirnis - Untersuchung zu Löslichkeit, Glanz und Oberflächenrauhheit. Diploma thesis, Staatliche Akademie der Bildenden Künste, Stuttgart
Van der Doelen GA (1999) Molecular studies of fresh and aged triterpenoid varnishes. PhD thesis, University of Amsterdam
Salatino A, Faria Salatino ML, Negri G (2007) J Braz Chem Soc 18:11–33
Murillo RM, Jakupovic J, Rivera J, Castro VH (2001) Rev Biol Trop 49:259–264
Cai Y, Evans FJ, Roberts MF, Phillipson JD, Zenk MH, Gleba YY (1991) Phytochem 30:2033–2040
Kinjo J, Uemura H, Nohara T (1995) Tetrahedron Lett 36:5599–5602
Tyman JHP (1998) The chemistry of some natural colourants. In: Atta-ur-Rahman (ed) Studies in natural products chemistry—structure and chemistry (Part F). Elsevier, Amsterdam, pp 730, 774–776
Koller J, Baumer U, Schmid E, Grosser D (1997) Sandarac. In: Walch K, Koller J (eds) Baroque and Rococo Lacquers, Arbeitshefte des Bayerischen Landesamtes für Denkmalpflege. Lipp, München, pp 379–394
Koller J, Baumer U, Grosser D, Walch K (1997) Larch turpentine and Venetian Turpentine. In: Walch K, Koller J (eds) Baroque and Rococo Lacquers, Arbeitshefte des Bayerischen Landesamtes für Denkmalpflege. Lipp, München, pp 359–378
Koller J, Baumer U, Grosser D, Schmid E (1997) Mastic. In: Walch K, Koller J (eds) Baroque and Rococo Lacquers, Arbeitshefte des Bayerischen Landesamtes für Denkmalpflege. Lipp, München, pp 347–358
Pastorova I, de Koster CG, Boon JJ (1997) Phytochem Anal 8:63–73
Bretz S (1999) Maltechnik und Glastechnik in der Hinterglasmalerei 1600 bis 1650. In: Lanz H, Seelig L (eds) Farbige Kostbarkeiten aus Glas. Bayerisches Nationalmuseum, München/Zürich, pp 181–219
Bretz S, Baumer U, Stege H, von Miller J, von Kerssenbrock-Krosigk D (2008) Stud Conserv 53:209–224
Bertogg A (2001) Z Kunsttechnol Konserv 15:215–235
Koller J, Walch K, Baumer U (2000) Lacquerwork from the Workshop of the Dagly Brothers. In: Kühlenthal M (ed) Japanese and European Lacquerware, Arbeitshefte des Bayerischen Landesamtes für Denkmalpflege. Lipp, München, pp 381–405
Acknowledgements
We are especially thankful to Katja Lewerentz (1969–2009), who provided the first impetus to investigate dragon's blood with adequate botanical references in 2000, during her diploma thesis. Sadly, she is not able to experience the completion of this research in a bigger context and in connection with samples of works of art.
We are deeply grateful that several collections granted generous access to their precious botanical material; first of all, our thanks go to Dr Marc Nesbitt and Dr Monique Simmonds from the Royal Botanic Gardens Kew (UK). Also, we would like to thank Dr Vogl from the Botanische Sammlung Berlin–Dahlem (Germany), Dr Esser from the Botanical Garden Munich (Germany) and Dr Thomas Psota, Historisches Museum Bern (Switzerland).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 85.2 kb)
Rights and permissions
About this article
Cite this article
Baumer, U., Dietemann, P. Identification and differentiation of dragon's blood in works of art using gas chromatography/mass spectrometry. Anal Bioanal Chem 397, 1363–1376 (2010). https://doi.org/10.1007/s00216-010-3620-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3620-0