Abstract
The possibility of successfully applying a common GC–MS procedure for identification in one step of all types of dyes from plants of unknown origin and from historical objects is particularly attractive due to the high separation efficiency of the capillary columns, the MS detection sensitivity and the reproducibility of results. In this work, GC–MS analysis, previously and successfully used for the characterization of anthraquinones, flavonoids and tannins from plant extracts and historical samples, has been tested on indigoid dyestuffs. An analytical procedure based on the silylating agent N,O-bis-(trimethylsilyl)trifluoroacetamide (BSTFA) with 1 % trimethylchlorosilane (TMCS) was applied to pure molecules of indigotin and indirubin and to plant extracts of Indigofera tinctoria L. and Isatis tinctoria L. Preliminary tests have been done to establish the chromatographic conditions and the derivatization amounts most suitable for the simultaneous detection of indigoid molecules and of the other natural compounds, such as fatty acids, carboxylic acids and sugars, contained within the plant extracts. In order to assess the capacity and the sensitivity of the analytical procedure in typical archaeometric applications, wool samples dyed in the laboratory with indigo were analysed by mimicking the sample amounts typically available with historical objects. The electron ionization (EI) spectra of the main silylated derivatives of indigoid molecules obtained in this way constitute the necessary data set for the characterization of natural extracts and historical works of art. Subsequently, the procedure has been applied to historical samples for the detection of indigo and of other dyestuffs eventually contained in samples. Additional information, useful for restoration and preservation of works of art, could be also obtained on the nature of stains and smudges present on the sampled textile material. The GC–MS method turns out to be an efficient and fast analytical tool also for the identification of natural indigo in plants and textile artefacts, providing results complementary to those from high-performance liquid chromatography (HPLC).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyeing and painting activities have accompanied the development of human culture since the early beginnings. Different cultures, in many aspects totally unrelated and unconnected among them, have developed similar dyeing techniques using dyes available from easily available natural sources. Indigo is one of such naturally available and frequently used dyes and has a long history [1–4]. The most important sources of indigo are Indigofera tinctoria L, Indigofera suffruticosa Mill. and Polygonum tinctorium Ait. Indigofera tinctoria and Indigofera suffruticosa are perennial herbs cultivated in Africa, Asia, East India and South America. P. tinctorium, an annual plant of the family Polygonaceae with the morphology of a marsh plant, has been the most popular indigo crop in Japan, China, Korea and Russia. This plant has been employed for large-scale indigo production and also used in herbal medicine for its therapeutic properties. Isatis tinctoria L., a biennial species of the family Cruciferae native to southeastern Russia and historically cultivated in Europe, is the source of woad [5]. Although woad gave a much smaller yield than indigo and a lighter blue colour, it was extensively cultivated in England and other European countries for a long time when the importing of it from India was uneconomical. The technology for the preparation of dye from indigoid plants is not simple because the coloured active principles mainly contained in the leaves are insoluble in water, unlike all other vegetal dyestuffs. In India, leaves, stalks, pods and twigs of Indigofera were cut, placed in vats and macerated in water for several hours to allow fermentation. The liquid was skimmed, rendered alkaline and then exposed to air oxidation, striking the surface with bamboo sticks for several days. The supernatant water was taken out and the indigo sludge transferred to a cauldron where it was fire-heated to prevent further fermentation. After cooling, the sludge was filtered and left to rest until it reached the consistency of a paste. This process allowed the trade of the product from the original lands to many parts of the world.
The preparation of woad from Isatis in Europe, as described by historical sources [6], does not differ much from the Indian method: the selected leaves were quickly crushed or ground to a pulp and left to drain until they had attained the correct consistency. The resulting product was rolled by hand into balls weighing about 1.5 lb, which were then placed in trays and left drying. The balls were dried and ground into fine powder which was sprayed with water and reduced to a paste and left to ferment for several weeks. The final mass, which becomes quite hot and with an unpleasant smell, is left to dry completely and then placed into wooden casks ready for the market [7, 8].
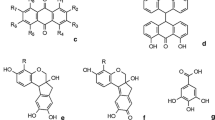
Both indigo and woad are vat dyes and contain the same active dyeing principles, shown in Fig. 1. Indigotin (trans-indigo) is the principal molecule responsible of the final blue colour. Cis-Indigo (blue), indirubin (a red isomer of indigotin) and the cis-forms of indigotin and indirubin, named respectively isoindigo (brown) and isoindirubin (red), are other minor components which can be also formed in varying proportion during the processing of indigo plants. All these active principles derive from precursors which can be extracted from the leaves of plants. Precursors are the indoxyl glycoside named indican (indoxyl-β-d-glucoside), isatan A (indoxyl-3-O-(6′-O-malonyl-beta-d-ribohexo-3-ulopyranoside)) and isatan B (indoxyl-3-O beta-d-ribohexo-3-ulopyranoside) [9, 10]. Indican and isatans present in plants are converted by enzymatic hydrolysis to nearly colourless indoxyl and sugars.
Indigotin and its isomers are formed by the coupling of two molecules of indoxyl. Indigofera spp. and P. tinctorium are characterized by the presence of indoxyl-β-d-glucoside (indican) as the only indigo precursor while Isatis contains isatan B as a major precursor and indican as a minor component. Isatin is generated from indoxyl in an oxygen-rich environment as a side reaction, and the condensation of indoxyl and isatin gives rise to indirubin. Maugard et al [11] describe another precursor of indirubin: isatan C, a dioxindole with a molecular weight of 395, although no definitive structure was proposed. Hydrolysis of isatan C produces dioxindole that may react with isatin yielding indirubin and isoindirubin. A common characteristic of vat dyes is the presence of carbonyl groups in the molecules responsible for colour; when they are treated with a reducing agent in the presence of an alkali, they form water-soluble species known as the leuco form. Idigotin, in fact, is stabilized by intramolecular hydrogen bonding between the N–H groups and the two carbonyl groups of the molecule. Intramolecular proton transfer via hydrogen bonds allows the formation of leuco indigotin, and a single bond replaces the double bond between the indene rings. Regarding the formation of leuco indirubin, it was proposed [12, 13] that intramolecular hydrogen bonding can be established initially in one indene ring followed by free rotation of the molecule around the central carbon–carbon bond. The leuco form is used in the dyeing process for obtaining initially the uncoloured textile which turns blue thanks to oxidation by air exposition. Because organic dyes are a mixture of chromophores, their identification in the field of cultural heritage is mainly based on solvent extraction of the chromophores from the yarns (or the lake matrix) followed by separation techniques. High-performance liquid chromatography with diode-array detection (HPLC–DAD) and mass spectrometer detection is by far the most commonly used technique in the analysis of natural dyes and is also used for the detection of indigoids in plant extracts [14–17]. With HPLC, however, the most appropriate separation and detection conditions may differ for the different families of dyestuffs to analyse, meaning that the analysis of complex mixtures coming from real samples can require multiple runs.
GC–MS-based methods for characterizing dyestuff from plants of unknown origin and from historical objects could be, in general, attractive due to the high separation efficiency of the capillary columns, the detection sensitivity and the reproducibility of results. A limiting step comes, eventually, from the derivatization reaction necessary for the transformation of the dye molecules into more volatile forms, but once the suitable reaction conditions are settled, the separation and identification of components can be performed routinely with results which can be complementary to HPLC.
In the analysis of organic components from artistic works, the pyrolysis–GC–MS (Py–GC–MS) technique is particularly suitable due to the very limited amount of sample which is required and the possibility of avoiding off-line sample preparation and complex derivatization [18]. However, only few examples of the use of Py–GC–MS for analysis of dyes can be found in the literature, the reason being that the fragments obtained from the dye molecules are seldom significant for discriminating among the species coming from complex matrices of origin, in particular from ancient textile samples. Fabbri et al. [19] employed Py–GC–MS with tetramethylammoniumhydroxide (TMAH) derivatization for the analysis of synthetic indigo, and the marker molecules which could be identified were 2-(dimethylamino)-benzoic acid and 2-(methylamino)-benzoic acid. Casas-Catalan et al. [20], by applying Py–GC–MS with hexamethyldisilazane on-line derivatization to the analysis of commercial indigo dyestuff, identified several indole compounds obtained as primary pyrolysis products of indigo, as well as 2-aminobenzoic acid and benzeneamine, and 2,3-dihydroindole-2-one was indicated as the principal peak of analytical interest. Similar results have been reported by Andreotti et al. by using Py-silylation–GC–MS in the presence of hexamethyldisilazane [21]. To overcome the limitations brought by the pyrolytic conditions, GC–MS should be a most suitable method, once the dye components have been properly extracted from the matrix. In respect of the indigoid molecules, the high sensitivity of GC–MS has been already demonstrated by Takao et al. [22] in the search of a practical method for the analysis of human urine; they determined a detection limit of 0.1 ppb for the analysis of silylated products of indirubin by using N,O-bis-(trimethylsilyl)-trifluoroacetamide (BSTFA) derivatization in dichloromethane/acetone (8:2) solvent, pyridine as catalyst and a reaction time of 1 h at 90 °C.
To the best of our knowledge, the application of GC–MS for the detection of indigo dye from historical textiles is reported in only one work, where dye extraction and derivatization was done with m-(trifluoromethyl) phenyltrimethylammonium hydroxide (TMTFTH); a methylated derivative of indigotin, 2-bis-(N-methylindole-3-methoxy), was identified as the principal marker molecule, together with isomers and other methylated derivatives [23]. As we have recently shown that GC–MS can be successfully used for characterization of anthraquinones, flavonoids and tannins from plant extracts [24], we extend in this contribution the application of the method, which involves extraction and derivatization with BSTFA, demonstrating positive results in the dye characterization from indigoid sources. Optimization of conditions for the derivatization procedure and the evaluation of the chromophore separation was done on standard molecules of indigotin and indirubin. The method was then validated on commercial extracts of Indigofera tinctoria L. and Isatis tinctoria L. produced for dyeing purposes. In order to assess the capacity and the sensitivity of the analytical procedure in the typical archaeometric applications, wool samples dyed in the laboratory with the same indigoid commercial extracts were analysed mimicking the sample amounts typically available with historical objects. Finally, the method was verified on historical samples from a Genovese blue bodice in silk of the eighteenth century and a Brussels tapestry of the sixteenth century. The yarns and fibres that constitute the historical samples have been preliminarily observed with an optical microscope for evaluating the nature of yarns and their general conservation conditions.
Experimental
Chemicals and materials
Indigotin, indirubin and anhydrous pyridine were purchased from Sigma-Aldrich, Italy. BSTFA with 1 % trimethylchlorosilane (TMCS) reagent was purchased from Supelco (Supelco Park, PA, USA). Plant extracts of Indigofera tinctoria L. and Isatis tinctoria L. were provided by CRITT Horticole (Rochefort/Mer, France).
Historical samples
Female silk corsage of the eighteenth century
The female corsage catalogued “T.88” in the “Civiche Collezioni Tessili of the City of Genoa” is conserved at “Museums of Strada Nuova, Palazzo Bianco”. It is a strongly altered artefact dating from the second half of the eighteenth century [25].
One sample (sample PB1) was taken from the inside of the left sleeve of the bodice, in correspondence of a flap of excess tissue due to an ancient intervention for reduction of the sleeve. The sample consists in a fragment of fabrics in blue silk, with pale pinkish–yellow wefts. The sampling area was stained and yellowish, the fibres brittle and showing characteristic transverse fractures.
Brussels tapestry of the sixteenth century
Blue wool yarns (CC22) were sampled from the back of the tapestry named “Deer hunting with mythological figures” before a restoration treatment done at the Centro Conservazione e Restauro La Venaria Reale (Turin, Italy). The tapestry is housed at Villa Necchi Campiglio in Milan (Italy), an historic building owned by FAI (Fondo Ambiente Italiano), and was realized at the Brussels manufactory in 1580, as documented by the double “B” in the selvedge on the left side.
Instrumentation
The GC–MS instrument used is a 6890 Network GC System (Agilent Technologies, USA) with a methyl-phenyl-polysiloxane cross-linked 5 % phenyl-methyl silicon capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness). The mass spectrometer coupled to the GC apparatus was a 5973 Network Mass Selective Detector (Agilent Technologies, USA). Mass spectra are recorded under electron impact at 70 eV, scan range 40–700 m/z. The interface was kept at 280 °C, ion source at 230 °C and quadrupole mass analyser at 150 °C. The gas chromatographic conditions were as follows: initial temperature 57 °C, 2 min isothermal, heating programme at 10 °C/min up to 200 °C, 3 min isothermal at 20 °C/min up to 300 °C and isothermal for 20 min. The carrier gas was helium with a constant flow rate of 1.2 mL/min. All measurements were controlled by Enhanced ChemStation software (Agilent Technologies, USA). The injection volume was 2 μL.
Sample preparation
Standard molecules
Indigotin and indirubin were analysed separately. Solutions of 30 ppm of standard molecules in pyridine were prepared. To 100 μL of each standard solution, 200 μL BSTFA with 1 % of TMCS was added and then heated at 70 °C for 75 min.
Plant extracts
Commercial plant extracts were provided as fine powders and no further extraction processing was needed. To about 0.01 mg of sample dissolved in 100 μL pyridine, 200 μL of BSTFA with 1 % TMCS was added; the derivatization reaction was performed at 70 °C for 75 min, as above described. The injection volume was 2 μL.
Reference wool samples dyed in the laboratory
-
Dyeing procedure
The dye bath was prepared by adding to 40 mL of demineralized water 13 g of sodium hydrosulphite, 2 g of sodium hydroxide and 1.5 g of indigo extract, previously dissolved in 1.5 mL of ethanol. The solution was stirred for 15 min at 50 °C. The fleece of wool was cleaned in demineralized water and Marsilla soap, then rinsed and left to dry. After drying, the wool was immersed for 15 min in a solution at 40 mg/L of sodium hydrosulphite and demineralized water, then extracted, squeezed and immersed in the dye bath at 50 °C for 10 min. The wool was then extracted from the dye bath, gently squeezed and exposed to air.
-
Extraction methods and derivatization
Different amounts of dyed wool samples with weights of ca. 4, 3, 2 and 1 mg were immersed in 500 μL of pyridine and heated for 1 h at 100 °C. After centrifugation, the supernatant liquid was transferred into a reaction vial and 300 μL of BSTFA with 1 % of TMCS was added; the vial was heated at 70 °C for 75 min. Two microlitres was injected in the apparatus.
Historical samples
The silk sample (PB1) was a fragment of blue taffeta, with pale pinkish–yellow wefts. The blue yarns were separated from the wefts and analysed separately. The blue silk fibres (ca. 0.004 g) were placed in a test tube and 500 μL of anhydrous pyridine was added. The test tube was kept at 100 °C for 60 min. The solution was then centrifuged and the supernatant liquid was collected, transferred to a vial and evaporated to dryness under a gentle flow of nitrogen. To the sample were then added 200 μL of pyridine and 500 μL of BSTFA with 1 % of TMCS. The vial was kept at 70 °C for 75 min. The injection volume was 2 μL. Extraction and derivation procedures used for the preparation of the wool samples from the Brussels tapestry were the same as those used for the blue silk sample.
Results and discussion
The efficiency of the derivatization reaction with BSTFA has been investigated by analysing the reaction products obtained from treatments of the pure standards in the time interval ranging from 60 to 120 min, in order to check the silylated derivative formation. The time tests on the derivatization of standard molecules showed that the silylated products may be clearly detected with 75 min reaction time. In the range between 75 and 120 min, no significant increase of the different silylated species was observed. Figure 2 shows a GC chromatogram of the indigotin and indirubin standard after 120 min of derivatization reaction. The main silylated product of indigotin visible in Fig. 2a is di-trimethylsilyl indigotin (peak 4). tri-Trimethylsilyl indigotin and per-trimethylsilyl indigotin (peaks 1 and 4) are also present together with a very low peak corresponding to an isomer of the di-trimethylsilyl compound (peak 2).
GC chromatograms of pure indigotin and pure indirubin after 120 min of derivatization reaction. Trimethylsilyl (TMS) derivative of indigotin (a) and indirubin (b): 1 indigotin 3TMS, 2 indigotin 2TMS isomer, 3 indigotin 4TMS, 4 indigotin 2TMS isomer, 5 indigotin, 6 indirubin 4TMS, 7 indirubin 2TMS, 8–9 indirubin isomers, 10 indirubin 1TMS
In the chromatogram of standard indirubin, Fig. 2b, the predominant silylated product is the mono-trimethylsilyl indirubin (peak 10); di-trimethylsilyl (peak 7) and per-trimethylsilyl derivatives of indirubin (peak 6) are detectable also. The electron ionization (EI) mass fragmentation spectra collected from the analysis of the derivatized standard molecules are reported in Table 1 and constitute the data set we have used for identification of the chromophores in plant extracts and in historical textiles, in addition to the matching of the GC retention times.
Analysis of plant extracts
On the plant extracts, preliminary tests have been done to establish the derivatization amounts most suitable for the simultaneous detection of chromophores and of the other natural compounds present in the extracts. The chromatographic conditions have proved to be suitable also for the separation of fatty acids, carboxylic acids and sugars typically contained within the plant extracts.
The chromatograms of Isatis tinctoria and Indigofera tinctoria extracts are shown in Fig. 3. The principal peak, recurrent in both chromatograms, corresponds to di-trimethylsilyl indigotin (peak 4). In addition to it, the per-trimethylsilyl (peak 3) and tri-trimethylsilyl (peak 1) were also present. In the chromatograms of the Isatis and of the Indigofera extracts, two mono-trimethylsilyl methyl-indigotin isomers, at 25.92 and 26.39 retention time respectively and partially co-eluting with the broad peak of indigotin, were recognized. In both extracts could be also detected two different silylated forms (peaks A and B) of the indoxyle aglycon. Several methylsilyl esters of fatty acids were present in both plant extracts as well as other carboxylic acids, while xylitol (the only polyalcohol present) and gallic acid have been detected in the Indigofera sample only (peaks C and E). Indirubin, in the per-trimethylsilylated form (peak 6), was found in the total ion current (TIC) chromatogram of Isatis only, but it was detected in both plant extracts with the selected ion monitoring (SIM) acquisition mode (data not reported). The results indicate that in the Indigofera commercial extract we have used, indirubin is present in quite low amounts; it is well known that the concentration of indirubin in plants depends not only on the species but also on the extraction processes and on the concentration of precursors which may vary according to the age of the plant, the harvest season and the part of the plant (top or bottom) from which the leaves are collected [3, 26, 27].
Chromatograms of Indigofera tinctoria (a) and Isatis tinctoria (b) extracts. A indoxyl 1TMS; B indoxyl 2TMS; C xylitol, 1,2,3,4,5-pentakis-O-(trimethylsilyl); D tetradecanoic acid, trimethylsilyl ester; E gallic acid per-trimethylsilyl; F palmitic acid, trimethylsilyl ester; G oleic acid, trimethylsilyl ester; H octadecanoic acid, trimethylsilyl ester; I 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester; L stearic acid trimethylsilyl ester; M probably 5H-imidazo(2,1-a) isoindole, 2-phenyl,P 2,2-biindolyl 2TMS; Q methyl-indigotin 1TMS isomer; R methyl-indigotin 1TMS isomer; U unknown compound; 1 indigotin 3TMS; 3 indigotin 4TMS; 4 indigotin 2TMS; 5 indigotin; 6 indirubin 4TMS; TMS trimethylsilyl
Application on model dyed samples for optimization of indigoid molecule identification in historical textiles
Wool samples dyed in the laboratory with Indigofera were analysed in order to assess the conditions and procedures suitable for archaeometric applications. Quantitative determination of the dyes is not feasible in archaeometric applications due to the many variables inherent to samples. In fact, variation of time and temperature in the dye procedure, the size of indigo particles, the nature of fibres and the exposition to light can have a strong influence on the final amount of analytes in the samples. Historical samples are also usually very small and the quantity of dye contained in them can be insufficient for GC detection in TIC mode. This fact has been verified on the wool samples dyed in the laboratory with the Indigofera extract. Four dyed wool samples having weights of ca. 4, 3, 2 and 1 mg respectively were analysed in TIC mode, and indigotin was detected only in the 4-mg sample. This empirical test shows the importance of exploiting the SIM mode for the GC analysis of historical samples which are, most often, limited at 1 or 2 mg of weight. In fact, SIM mode acquisition increases the sensitivity for the target analytes through the selected detection of one or few ions, indicative of the compound of interest. On the basis of the most abundant derivatives and on their mass spectra, the parameters to be used for SIM acquisition were selected and are reported in Table 2. SIM experiments were performed preliminarily on multiple ions for the dyed wool samples and on indigoid standard molecules in full scan acquisitions and in different runs, selecting every time the mass/charge relative to the molecules of interest for assessing the retention times of the marker compounds. Subsequently, in order to improve the acquisition of the ions in a simultaneous detection of the most important silyl derivatives of indigoids, a single run in SIM mode was performed selecting the m/z values 262, 334, 480, 408 and 552, corresponding to the main ions of the silylated derivatives of indigotin, reported in Table 2.
Silk sample from a blue bodice of the eighteenth century
In Fig. 4 are shown microscope images of the blue bodice taffeta sample and of isolated fibres; the latter appear fragile and brittle, with characteristic transverse fractures. The blue yarns from the warp (ca. 4 mg in weight) and the reddish wefts were analysed separately. In the TIC chromatogram of the blue silk warp, shown in Fig. 5, the main peaks corresponding to fatty acids and their appreciable abundance suggest that the stains which were observed on the fabric are due to fatty substances. In the chromatogram, indigotin was detected in the forms tri-trimethylsilyl (peak 1) and per-trimethylsilyl (peak 3), proving that the blue colour derives indeed from indigoid sources. Indirubin, previously recognized in the plant extracts, was not found in the silk sample, and the reason can be attributed to its much lower amount in the indigoid sources. Comparing these results with those obtained from the analysis of standard molecules and from plant extracts, it is noted that the major components from the historical samples are the 3TMS or 4TMS derivative of indigotin whereas in the reference materials and the extracts from reference materials, the major compound seen in the chromatograms is the 2TMS derivative of indigotin. The difference is attributed to the much larger excess of BSTFA in the historical samples, which substantially improved the derivatization of the dye molecules.
TIC chromatogram of blue sample PB1 from the corsage. K 1,5-cis,8-cis-undecatriene-3,7-diol bis(trimethylsilyl)ether, S 1H-indole, 1-(trimethylsilyl)-5-[(trimethylsilyl)oxy]-3-[2-[(trimethylsilyl)oxy]ethyl], T tetradecanoic acid, trimethylsilyl ester, U unknown compounds, V fatty acids, Y N-(3-methoxy-phenyl)-benzamide, Z 1-trimethylsilyloxy-n-octene, 1 indigotin 3TMS, 3 indigotin 4TMS; TMS trimethylsilyl
In order to check if other dyestuffs were present in the sample, SIM analysis was also performed by selecting mass/charge values apt to identify anthraquinones, tannins and flavonoids. The m/z values chosen were 574, 414, 662, 559, 647, 546, 371, 472, 368, 590 and 458, previously identified as characteristics in the GC–MS analysis of brazilin, genistein, haematoxylin, quercetin, rhamnetin, alizarin, purpurin, catechin, ellagic acid and gallic acid respectively [24]. The m/z value 471, characteristic of the main ion in both EI spectra of apigenin and emodin, was selected for their recognition, while m/z 559 was selected for determination of luteolin and kaempferol, inasmuch as the principal ion in both EI spectra of these compounds. The runs in SIM mode of the PB1 sample warp yarns revealed no other dye marker, and we can therefore conclude that indigo, or woad, was used alone in these yarns and no other dyestuff was employed to modify the final hue. On the other hand, alizarin, the main component of the red madder dye, was clearly detected in the SIM mode analysis of the pinkish weft (data not shown).
Wool samples from a Brussels tapestry
The optical microscope observation of the sample and its constitutive fibres (Fig. 6) has shown that the fibres have homogeneous coloration. Particles of dirt are present on the wool fibres; despite the presence of a few fractures, no signs of biological degradation were observed. The GC analysis was performed at first in TIC mode, but due to the limited quantity of material available (and the even less dye content), markers were not detected. Therefore, the SIM acquisition was performed on the m/z values 226, 408, 480 and 552, pertaining to the principal ions of indigotin and of their main silyl derivatives, as reported in Table 2. The chromatogram obtained in SIM mode, in Fig. 7, shows the presence of the tri-trimethylsilyl (peak 1) and the di-trimethylsilyl (peak 2) indigotin derivative, thereby confirming the use of an indigo source for the blue wool in the tapestry.
Conclusions
A GC–MS analytical procedure based on preliminary silylation with BSTFA was tested on indigotin and indirubin standard molecules and optimized on extracts of the indigoid plants Indigofera tinctoria and Isatis tinctoria, principal sources of indigo dyes in Europe and Asia. In the extracts from both plants, indoxyl and indigotin could be easily recognized; indirubin could be detected with TIC or SIM mode, depending on its variable amount. The EI mass fragmentation spectra collected from the derivatized standard molecules and from the plant extracts constitute the data set for the selection of the ions to monitor in the SIM analysis which is to be preferentially employed when precious artefacts are involved and adequate micro-sampling is not feasible. The analysis method based on GC–MS with silylation reaction has proven to be efficient and fast for identifying indigo dye on textiles. Indigo was detected in a wool sample coming from a Brussels tapestry of the seventeenth century and in a blue silk coming from a bodice of the seventeenth century, proving in this way the convenience of the method in archaeometric applications. Moreover, when encrusting and stains are present on the historical samples, the GC–MS analysis in TIC mode is also capable of providing information on the chemical nature of the stains which may be useful for the restoration and preservation of the textile materials. As previous studies in our laboratory have shown, a key feature to this procedure resides on its effectiveness in the identification of many different natural dyes commonly present in ancient textiles, including flavonoids, tannins and anthraquinones.
Another advantage of the GC–MS method resides in the fact that the EI mass spectra provide a higher fragmentation than APCI or ESI sources [28, 29] and consequently more significant structural information on the investigated compounds is obtained with a single run. The GC–MS method for the characterization of indigoid compounds can represent a useful and efficient tool when a multi-analytical approach is needed or for specific goals such as the recognition of degradation products formed under appropriate ageing conditions.
References
Balfour-Paul J (1998) Indigo. British Museum Press, London. ISBN 0-7141-1776-5
Melo MJ (2009) In: Bechtold T, Mussak R (eds) Handbook of natural colorants. Wiley, Chichester, UK. ISBN 978-0-470-51199-2
Cardon D (2007) Natural dyes: sources, tradition, technology and science. Archetype, London. ISBN 190498200X
Brunello F (1968) L’Arte della tintura nella storia dell’ umanita’. Neri Pozza, Vicenza
Epstein E, Nabors MW, Stowe BB (1967) Origin of indigo of woad. Nature 216:547–549
Singer C, Williams J, Illtyd T (1954) A history of technology. Oxford University Press, London ISBN 978-0-19-858155-0; ISBN: 0-19-858155-6
Legget WF (1994) Ancient and medieval dyes. Chemical Publishing CO, Brooklyn
Wisniak J (2004) Dyes from antiquity to synthesis. Indian J Hist Sci 39:75–100
Oberthur C, Schneider B, Graf H, Hamburger M (2004) The elusive indigo precursors in woad (Isatis tinctoria L.)—identification of the major indigo precursor. Isatan A, Structure Revision B, Chem Biodivers 1:174–182
Kokubun T, Edmonds J, John P (1998) Indoxyl derivatives in woad in relation to medieval indigo production. Phytochemistry 49:79–87
Maugard T, Enaud E, Choisy P, Legoy MD (2001) Identification of an indigo precursor from leaves of Isatis tinctoria (woad). Phytochemistry 58:897–904
Seixas de Melo J, Moura AP, Melo MJ (2004) Photophysical and spectroscopic studies of indigo derivatives in their keto and leuco forms. J Phys Chem A 108:6975–6981
Seixas de Melo J, Ronda R, Burrows HD, Melo MJ, Navaratnam S, Edge R, Voss G (2006) Photophysics of an indigo derivative (keto and leuco structures) with singular properties. J Phys Chem A 110:13653–13661
Hofenk-de Graaff J H (2004) The colorful past. Origins, chemistry and identification of natural dyestuffs. Archetype Publications, London ISBN: 1873132131
Surowiec I (2008) Application of high-performance separation techniques in archaeometry. Microchim Acta 162:289–302
Wouters J (1985) High performance liquid chromatography of anthraquinones: analysis of plant and insect extracts and dyed textiles. Stud Conserv 30:119–128
Degano I, Ribechini E, Modugno F, Colombini MP (2009) Analytical methods for the characterization of organic dyes in artworks and in historical textiles. Appl Spectrosc Rev 44:363–410
Colombini MP, Modugno F (2009) Organic mass spectrometry in art and archaeology. Wiley, Chichester, UK. ISBN 0470517034
Fabbri D, Chiavari G, Ling H (2000) Analysis of anthraquinoid and indigoid dyes used in ancient artistic works by thermally assisted hydrolysis and methylation in the presence of tetramethylammonium hydroxide. J Anal Appl Pyrolysis 56:167–178
Casas-Catalan MJ, Domenech-Carbo MT (2005) Identification of natural dyes used in works of art by pyrolysis–gas chromatography/mass spectrometry combined with in situ trimethylsilylation. Anal Bioanal Chem 382:259–268
Andreotti A, Bonaduce I, Colombini MP, Ribechini E (2004) Characterisation of natural indigo and shellfish purple by mass spectrometric techniques. Rapid Commun Mass Sp 18:1213–1220
Takao Y, Yamashita K, Kohra S, Inudo M, Nagae M, Tominaga N, Ishibashi Y, Sekizawa J, Miyairi S, Arizono K (2003) High sensitivity analysis of indirubin by silylation using GC-MS. J Health Sci 49:88–90
Poulin J (2007) Identification of indigo and its degradation products on a silk textile fragment using gas chromatography-mass spectrometry. J Can Assoc Conserv (JCAC) 32:48–56
Degani L, Riedo C, Gulmini M, Chiantore O (2014) From plant extracts to historical textiles: characterization of dyestuffs by GC–MS. Chromatographia 77:1683–1696
Zanus Fortes L, Fashion and color: three eighteenth century corsets. Restoration and preventive conservation, MS Thesis in Conservation Restoration of Cultural Heritage, University of Torino, 2014
Oberthur C, Graf H, Hamburger M (2004) The content of indigo precursors in Isatis tinctoria leaves—a comparative study of selected accessions and post-harvest treatments. Phytochemistry 65:3261–3268
Mohn T, Plitzko I, Hamburger M (2009) A comprehensive metabolite profiling of Isatis tinctoria leaf extracts. Phytochemistry 70:924–934
Puchalska M, Połeć- Pawlak K, Zadrożna I, Hryszko H, Jarosz M (2004) Identification of indigoid dyes in natural organic pigments used in historical art objects by high performance liquid chromatography coupled to electrospray ionization mass spectrometry. J Mass Spectrom 39:1441–1449
Papanastasiou M, Allen NS, McMahon A, Naegel LC, Edge M, Protopappas S (2012) Analysis of indigo-type compounds in natural dyes by negative ion atmospheric pressure photoionization mass spectrometry. Dyes Pigments 92:1192–1198
Acknowledgments
CRITT Horticole (Rochefort sur Mer, France) is gratefully acknowledged for supplying the plant extracts. This work has been partially supported through the PRIN 2010-2011 (MIUR, Italy) project: Sustainability in cultural heritage: from diagnosis to the development of innovative systems for consolidation, cleaning and protection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Degani, L., Riedo, C. & Chiantore, O. Identification of natural indigo in historical textiles by GC–MS. Anal Bioanal Chem 407, 1695–1704 (2015). https://doi.org/10.1007/s00216-014-8423-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8423-2