Abstract
A novel protocol for the synthesis of dye-encapsulating liposomes tagged with DNA oligonucleotides at their outer surface was developed. These liposomes were optimized for use as signal enhancement agents in lateral-flow sandwich-hybridization assays for the detection of single-stranded RNA and DNA sequences. Liposomes were synthesized using the reverse-phase evaporation method and tagged with oligonucleotides by adding cholesteryl-modified DNA probes to the initial lipid mixture. This resulted in a greatly simplified protocol that provided excellent control of the probe coverage on the liposomes and cut the preparation time from 16 hours to just 6 hours. Liposomes were prepared using probe concentrations ranging from 0.00077 to 0.152 mol% of the total lipid, several hydrophobic and polyethylene glycol-based spacers between the cholesteryl anchor and the probe, and liposome diameters ranging from 208 nm to 365 nm. The liposomes were characterized by dynamic light scattering, visible spectroscopy, and fluorescence spectroscopy. Their signal enhancement functionality was compared by using them in lateral-flow optical biosensors for the detection of single-stranded DNA sequences. In these assays, an optimal reporter probe concentration of 0.013 mol%, liposome diameter of 315 nm, and liposome optical density of 0.4–0.6 at 532 nm were found. The spacer length between the cholesteryl anchor and the probe showed no significant effect on the signals in the lateral-flow assays. The results presented here provide important data for the general use of liposomes as labels in analytical assays, with specific emphasis on nucleic acid detection via lateral flow assays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
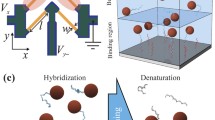
Nucleic acid-based identification is an important tool in the study of infectious diseases, genetic abnormalities [1], forensics, and biowarfare agent identification [2]. Assays based on sandwich hybridization are frequently used and rely on the target nucleic acid forming a complex between an immobilized oligonucleotide capture probe and a labeled oligonucleotide reporter probe through specific base pairing. Labels for nucleic acid diagnostics ideally yield reproducible, rapid, and sensitive analytical assays and have included radioisotopes, fluorescent and chemiluminescent markers, enzymes, and nanoparticles such as quantum dots [3, 4]. Additional considerations are introduced into the choice of label when the assays are intended for point-of-care applications, including low costs, robustness and a need for a non-time-dependent, facile mode of detection. Liposomes have been successfully used in this manner through the encapsulation of hundreds of thousands of hydrophilic dye molecules within their aqueous cores and conjugation of nucleic acid probes to their lipid bilayers [5, 6]. Unlike enzyme-based assays, the signal enhancement provided by encapsulated dye molecules is not time-dependent. The relatively large internal volume and ability to modify the surface of the bilayer with various biorecognition elements has made liposomes quite useful in optical and electrochemical biosensors, and in flow-injection analysis systems [6–10]. Their surfaces have been modified with a variety of entities, including small molecules, antibodies [11], nucleic acids [5, 6], enzymes [12, 13] and also with generic avidin [14] or streptavidin [15, 16] tags for facile conjugation to biotinylated ligands. Portable and inexpensive optical biosensors utilizing probe-tagged dye-encapsulating liposomes as a signaling mechanism for the detection of RNA from pathogens using sandwich hybridization have been successfully developed for a number of different organisms of environmental and clinical concern [7, 8, 17, 18]. These biosensors rely on the sandwich hybridization of a RNA target amplified using nucleic acid sequence-based amplification (NASBA) between a membrane-immobilized capture probe and a visible dye (sulforhodamine B (SRB))-encapsulating liposome conjugated reporter probe. The direct format of the biosensors yields a magenta-colored band in the region of the membrane where the capture probe is immobilized when the target RNA is present, and no color when the target is not present in the sample (Fig. 1).
Schematic of the lateral-flow assay. Liposomes with an immobilized reporter probe are incubated with the sample in a tube. A polyethersulfone with immobilized capture probe is inserted and the mixture is permitted to travel up the membrane by capillary action. a No binding occurs at the capture zone if the target sequence is absent from the sample. b When the target is present in the sample, the capture probe binds the target sequence/hybridized liposomes and yields a visible magenta band at the capture zone. This signal may be monitored visually or by using a handheld reflectometer
This format has been used to detect as little as one Bacillus anthracis spore [9]; 50 RNA molecules from dengue virus serotype 2 [18]; and 40 cfu/mL Escherichia coli [7]. Using synthetic DNA analogs of the amplified RNA target for characterization and optimization purposes, limits of detection ranging from 1.5 to 5.0 nM DNA [7, 9, 18] (1.5–5.0 fmol per assay) have been reported. Such lateral flow assays have been used with clinical or environmental samples and have shown excellent correlation to instrument-based methods [7, 8].
These assays require conjugation of the dye-encapsulating liposomes to DNA-oligonucleotide reporter probes. Common methods for conjugating biorecognition elements to liposomes have relied on heterobifunctional cross-linking agents, such as succinimidyl-4-(N-maleididomethyl)-cyclohexane-1-carboxylate (SMCC) [19, 20], N-succinimidyl-3-(2-pyridyldithio)propionate (SPDP) [21, 22], 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) [23, 24], and N-4-(p-maleimidophenyl)butyrate (MPB) [25], or noncovalent interactions such those as provided by the biotin–streptavidin interaction [14, 10]. Previous reports utilizing DNA-tagged liposomes relied on multistep heterobifunctional coupling procedures of maleimide and sulfhydryl groups [5, 7, 26]. These complicated reactions utilized costly coupling reagents and required additional purification steps that resulted in liposome dilution. Covalent coupling reactions have also been shown to cause leakage of entrapped contents and increases in liposome size and polydispersity. Further, high concentrations of the functionalized anchoring lipid itself can adversely influence liposome storage stability [27, 28].
Thus, an alternative was sought that was less time-consuming, cheaper, and consistently yielded higher concentrations of liposomes. A hydrophobic modification which would anchor the probes to the lipid bilayer directly was considered for this purpose. Such modified DNA probes have previously found use in microcontact printing onto hydrophobic surfaces [29], as inhibitors of normal cellular processes [30], and have been used to enhance the penetration of antisense oligonucleotides through cellular membranes [31, 32]. The incorporation of 3′-cholesteryl modified DNA reporter probes into the lipid mixture prior to commencing the reverse-phase evaporation process was thus investigated. This direct incorporation approach avoided the dilution of liposomes that occurred as a result of the purification steps required in post-formation coupling processes. Direct incorporation was also less expensive, since no costly coupling reagents and additional conjugation time were needed, and reduced the uncertainty of incomplete conjugation reactions. The use of commercially available cholesteryl-TEG modified probes allowed for the development of a modified liposome preparation procedure which can be completed within six hours. The optimization of these liposomes with respect to size, concentration, and degree of probe coverage in the lateral-flow assays is described.
Materials and methods
DPPG, DPPC, DPPE-NBD and the extrusion membranes were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Streptavidin and sulforhodamine B (SRB) were purchased from Molecular Probes, Inc. (Eugene, OR, USA). Polyethersulfone membranes were purchased from Pall Corporation (Pensacola, FL, USA). All other reagents used in these experiments were purchased from VWR (Bridgeport, NJ, USA). The DNA sequences listed in Table 1 were purchased from Operon Biotechnologies, Inc. (Alameda, CA, USA).
The liposome size distribution was determined by dynamic light scattering using a DynaPro LSR (Proterion Corporation, Piscattaway, NJ, USA). Reported diameters were obtained using the cumulant method of analysis. The liposomal phospholipid concentration was determined using the Bartlett assay [33]. SRB content was assessed using liposome lysis and detection of released fluorescence (λ excitation=565 nm and λ emission=585 nm). Optical density measurements were made at 532 nm by diluting 5 μL liposomes with 995 μL of the osmolality-adjusted HEPES buffer (described further below) in a 1.5 mL spectrometer cell. The number of dye molecules per liposome was calculated using the equation of a sphere, assuming a lipid bilayer thickness of 4 nm [34, 35] and the presence of a monodisperse, unilamellar population of liposomes which contained SRB at a concentration equal to that initially used in liposome preparation (150 mM). The theoretical number of liposomes was calculated by dividing the concentration of SRB per mL by the theoretical amount of SRB per liposome.
Liposome preparation
Direct reporter probe incorporation approach
DPPC, DPPG, and cholesterol (40.3:21:51.7 μmol, respectively) were first dissolved in a solvent mixture containing 3 mL chloroform, 0.5 mL methanol, and 3 mL isopropyl ether and sonicated in a bath sonicator to ensure homogeneous mixing. Cholesteryl-labeled reporter probe was diluted to a concentration of 300 pmol/μL in a 1:4 (v/v) mixture of methanol:formamide. Fifty microliters of this cholesteryl-labeled reporter probe (corresponding to 0.013 mol% unless otherwise specified) solution was also added to the dissolved lipids. Liposomes incorporating oligonucleotides labeled with hydrocarbon or a PEG-based spacer between the probe and cholesteryl modification were prepared using the same procedure. A 45 °C solution of dye (2 mL sulforhodamine B, 150 mM in 0.2 M HEPES) was added to the lipid mixture while sonicating for a total of four minutes. The mixture was placed onto a rotary evaporator and the solvent was removed at 45 °C. The mixture was transiently vortexed preceding and following an additional introduction of 2 mL 45 °C 150 mM SRB. The mixture was returned to the rotary evaporator before being extruded at 60 °C 19 times through 2.0 μm membranes, followed by 19 times through 0.6 μm membranes. These initial extrusions were also performed in cases where the liposomes were subsequently extruded through smaller pore size membranes. The extruded liposomes were passed through a 20×1.7 cm column packed with Sephadex G-50 at ∼4 mL/min. using 1×HEPES-saline buffer (10 mM HEPES, 200 mM sodium chloride, 0.01% sodium azide at pH 7.0), osmolality-adjusted with sucrose (∼0.22 M) to 75 mmol/kg greater than the SRB encapsulant, which was typically ∼550 mmol/kg. The liposome fractions containing high liposome densities (usually A 532>1.0) and medium liposome densities (usually A 532>0.5) were then combined separately and dialyzed overnight against the sucrose–HEPES–saline buffer. This process typically yielded 5.5±0.6 mL (n=10) of liposomes with an A 532=1.2±0.5 (n=37) (OD value obtained using 5 μL liposomes in 995 μL 1xHEPES saline; both the OD and volumes reported are for high and medium liposome concentration fractions). The phospholipid content for each liposome batch was determined using the Bartlett assay [33]. Liposome samples (3×20 μL) were dehydrated at 180 °C for ten minutes, then digested to inorganic phosphates with 0.5 mL of 3.33 N H2SO4 for two hours at the same temperature. One hundred microliters of 30% hydrogen peroxide was added and the mixture was returned to the oven for 1.5 hours. The tubes were permitted to cool to ambient temperature prior to, and vortexed vigorously following, each addition. Lastly, 4.6 mL of 0.22% ammonium molybdate and 0.2 mL of the Fiske–Subbarow reagent [36] were added. The Fiske–Subbarow reagent was prepared by mixing 40 mL 15% (w/v) sodium bisulfite, 0.2 g sodium sulfite, and 0.1 g 1-amino-4-naptholsulfonic acid at ambient temperature for 30 minutes and then filtering out undissolved solids. The tubes were then heated in a boiling water bath for seven minutes and quickly cooled in an ice water bath. The absorbance at 830 nm was recorded. Standards (composed of 0, 16, 32, 64, 128, 256 nmol phosphate) prepared from potassium phosphate dibasic in deionized water were subjected concurrently to the same procedure. Each sample was assayed for phosphorous in triplicate and the phospholipid content of the liposomes was determined from a calibration curve prepared from the standards analyzed in each run.
Multistep conjugation approach
DPPE (5 mg) was sonicated for one minute at 45 °C with 1 mL of a 0.7% (v/v) solution of triethylamine in chloroform. SATA (3.5 mg) was added to the cloudy suspension, and the mixture was sonicated at 45 °C for one minute. The mixture was then agitated at ambient temperature for 20 minutes, followed by solvent removal until dryness using additional volumes of chloroform. The residue was diluted with 1 mL of chloroform and added to the lipid/solvent mixture of the liposome preparation procedure described above, except that the cholesteryl-labeled reporter probe was not added. Once the liposomes had been dialyzed, the appropriate volume of liposomes from Eq. 1 was calculated and mixed with 0.5 M hydroxylamine hydrochloride, 25 mM EDTA, 0.4 M phosphate buffer (K2HPO4/KH2PO4) (0.1 mL/mL liposomes).
where x is the mol% tag desired (e.g., x=0.004 for a 0.4 mol% tag).
The liposome mixture was shaken at ambient temperature for two hours. While the liposome mixture was incubating, 4.3 μL of a 10 mg/mL solution of sulfo-KMUS in DMSO was added to 100 μL of a 300 pmol/μL solution of 3′-amino modified reporter probe in 50 mM phosphate buffer, 1 mM EDTA pH 7.8 and incubated on a shaker for three hours. This 30 nmol of probe was used obtain a theoretical 0.4 mol% tag concentration on the liposomes. The pH of each mixture was adjusted to a pH of 7.0 using 0.5 M phosphate buffer, and then the liposome and reporter probe solutions were combined and incubated for four hours at ambient temperature. Thirty microliters of a 100 mM ethylmaleimide in 0.05 M Tris–HCl, 0.1 M sucrose, 0.15 M NaCl, pH 7.0 solution was added and the liposome mixture was left incubating at ambient temperature for 30 minutes. The conjugated liposomes were separated from excess reagents using a Sephadex CL-4B column equilibrated with the HEPES–saline–sucrose buffer, followed by dialysis overnight against the same buffer.
Membrane preparation
Polyethersulfone membranes were prepared using a method adapted from a protocol described previously [9]. Briefly, the biotinylated capture probe and streptavidin were diluted to a concentration of 60 pmol/μL and 20 pmol/μL, respectively with 95% (v/v) 0.4 M Na2CO3/NaHCO3, 5% (v/v) methanol, pH 9.0, incubated for 15 minutes at room temperature and applied in a single line 1.5 cm from the base of the polyethersulfone membrane (8 cm length) using a Camag Linomat IV TLC applicator (Wilmington, NC, USA). The volume and rate of dispensing were adjusted such that 1 μL of the capture probe/streptavidin solution was applied per 4.5 mm width. Subsequently, the membranes were placed in a vacuum oven at 50–55 °C and 15″ Hg for 90 minutes, then blocked for 30 minutes in 0.015% (w/v) casein and 0.2% (w/v) polyvinylpyrrolidine in 1x Tris-buffered saline (20 mM Tris-HCl; 150 mM NaCl, 0.01% sodium azide, pH 7.0) at ambient temperature, then blotted dry and dried further in the vacuum oven for three hours at 15″ Hg at room temperature. The membranes were stored in vacuum-sealed bags at 4 °C and cut into 4.5 mm width strips prior to use.
Biosensor assay
The biosensor assay was performed as described previously [9]. Briefly, liposomes (2 μL, concentration varied as noted), synthetic target (1 μL, concentration varied as noted) and 4 μL of running buffer (30% formamide, 9×SSC (1.35 M sodium chloride, 0.135 M sodium citrate, 0.01% sodium azide, pH 7.0), 0.2 M sucrose, 0.2% Ficoll type 400) were mixed then incubated for ten minutes at ambient temperature in a 10×50 mm glass test tube. A polyethersulfone membrane was inserted into the test tube. The liposome mixture was permitted to be absorbed into the membrane through capillary action, and then an additional 40 μL of running buffer was added to the bottom of the test tube. Once the solution reached the top of the membrane, the intensity of the signal at the capture zone as well as the background signal below the capture zone was measured at 560 nm with a reflectometer (ESECO Speedmaster, Cushing, OK, USA).
Results and discussion
Comparison of the probe–liposome coupling protocols
The success of the direct incorporation procedure using cholesteryl-modified reporter probes was assessed, and a comparison to liposomes prepared using the multistep conjugation procedure was carried out, using the strip assays with the complementary sequence to the reporter probe immobilized on the membranes. Liposomes made through the direct incorporation protocol yielded significantly greater reflectometer readings than those made by the multistep protocol at both 100 fmol of target (126±3 vs 29±1.3, respectively) and at 10 fmol target (36±7 vs 15±3, respectively). This is a function of both the higher liposome concentration as well as an optimized coverage of DNA tag (discussed further below). Optical density may be used as a relative measure of liposome concentration assuming similar size distributions. The average optical density of liposomes resulting from the direct incorporation and the multistep approaches were 1.58±0.48 (n=18) and 0.12±0.11 (n=8), respectively. The higher liposome concentration with the direct incorporation approach resulted from the omission of coupling reagent addition and the second size exclusion chromatography purification step. When equal-diameter liposomes from both processes were normalized on the basis of optical density, the signals in the lateral flow assays were significantly higher with the cholesteryl-modified probe (Fig. 2). This result was a function of the lower reporter probe coverage (discussed further below) and the omission of reaction conditions causing leakage of entrapped dye.
Effect of coupling process on the hybridization of reporter probe-tagged liposomes to a synthetic target sequence. Reflectometer readings (y-axis) of the capture zone are reported for liposomes normalized to an OD=0.040 at 532 nm from the DPPE-ATA post-formation coupling process (diamonds) and the cholesteryl-TEG (squares) pre-formation coupling process using 1 μL of 0–100 nM synthetic DNA target per assay (x-axis). Each data point was the average of three determinations and error bars represent one standard deviation
Optimal percentage of reporter probe coverage
The optimal concentration of reporter probe on the liposome surface was determined by incorporating various amounts of 3′-cholesteryl-tagged DNA oligonucleotides (0.00077–0.152 mol% of the total lipid amount) into the liposome preparations, then comparing the reflectometer readings of these batches in subsequent lateral-flow strip assays. Given the strongly hydrophobic nature of the cholesteryl modification, it was assumed that the majority of the modified probe was retained with the liposomes during the purification process. Using 10 nM and 100 nM synthetic target in the lateral flow assays (Fig. 3), the optimal coverage of cholesteryl-TEG reporter probe was found to be approximately 0.013 mol%. The mol % tag is expressed in terms of the initial input of phospholipids and cholesterol, of which approximately 50% is expected to be on the exterior face of the lipid bilayer.
Effect of reporter probe concentration in terms of mol% of total lipid on the reflectometer readings in lateral-flow assays. Results are the average of three determinations using 0.6 μm extruded liposomes normalized to A 532=0.45 for the detection of 10 (triangles) and 100 nM (squares) of synthetic DNA target. Sixty picomoles of biotinylated capture probe and 20 pmol streptavidin were applied to the membranes used in these experiments
The optimum amount of tag on the liposome surface in sandwich hybridization assays is a delicate balance. If an insufficient amount of tag is available, then the chances of hybridization with target molecules are reduced. By contrast, multiple target molecules can bind to one liposome if an excessive amount of tag is available. Since the reporter probe-tagged liposomes were preincubated with the target prior to application on the membrane, too much reporter probe can result in a decrease in the number of liposomes that can participate in the membrane-immobilized sandwich complex. Both extremes were evidenced in this work by the observed increase in reflectometer readings up to 0.013 mol% reporter probe and the subsequent decline with higher reporter probe concentrations.
The theoretical surface tag of liposome preparations made by a multistep protocol similar to that described here was 0.4 mol%, optimized using a range between 0.05 and 1 mol% tag [7]. In addition, for a competitive assay format, Esch et al. reported better reproducibility and sensitivity when 0.1 mol% tagged liposomes were used at a target concentration of 0–0.1 pmol/assay in lieu of 0.4 mol% tagged liposomes, which were better for assays where 0.1–1.0 pmol/assay were used [37]. In contrast to this, we found the optimal reporter probe concentration with the direct incorporation procedure was 0.013 mol% and was not dependent on the synthetic DNA concentrations used (0.1–200 nM). This amount of coverage is approximately 60 times lower than achieved with the post-formation coupling approaches, since the direct incorporation approach distributes probe on both sides of the bilayer; while the post-formation approaches are believed to couple functional groups on the outer face of the liposomes. The reported degree of coupling in the multistep procedure may be lower than expected due to the uncertainty in the theoretical number of liposomes used for the calculation (Eq. 1) and a less efficient reaction under the conditions employed.
Using the direct reporter probe incorporation procedure developed in this project, 15 nmol of the reporter probe was required to produce 5–10 mL of liposomes with an optimal amount of reporter probe coverage. By comparison, the same amount of reporter probe resulted in only 0.3–0.5 mL of liposomes with the multistep protocol. In addition, the multistep approach required costly reagents such as SATA and sulfo-KMUS. The cost per mL of the tagged liposomes produced was thus $74–123 per mL, whereas the newly developed direct reporter probe incorporation procedure was only $0.85–1.70 per mL. The significantly lower concentration of coupled liposomes and the additional labor costs and equipment time required for the multistep approach are not included in these estimates. These factors become significant should the lateral-flow assays undergo commercial development.
Optimal distance between lipid bilayer and reporter probe
Increasing the length of the spacer in-between the reporter probe and liposome surface was expected to allow the immobilized probe to interact with target molecules more freely and would more closely approximate solution phase hybridizations. Thus, the effect of spacer lengths between the cholesteryl-TEG anchor and the reporter probe was investigated with the spacers composed of either straight chain hydrocarbons (C3: propanediol and C12: (CH2)12) or polyethylene glycol-based spacers (C9: triethyleneglycol (CH2O)3, C18: hexaethyleneglycol (CH2O)6) at the optimal probe concentration of 0.013 mol%. These spacers were estimated to add lengths of 7.4 Å, 18.9 Å, 13.4 Å, and 24.2 Å, respectively, to the 24.3 Å TEG spacer already present on the cholesteryl anchor [38–40].
The reflectometer readings for the detection of 10 nM and 100 nM synthetic target for the C0, C3, C9, and C18 liposomes were quite similar and within the error of the technique (Fig. 4). The signal-to-noise ratio of the lateral flow assays using the C3 spacer was slightly higher at both target concentrations than with the other spacers (1.54 and 5.78 vs 1.03–1.17 and 5.23–5.38, respectively), but this is more likely due to the larger diameter of this liposome batch (354±90 nm vs 307–319 nm). In the lateral-flow assays, the liposomes were preincubated for ten minutes with target molecules, so there was ample opportunity for hybridization regardless of spacer length provided a hydrophilic spacer was used, and therefore no significant change in signal was observed. The reflectometer readings and signal-to-noise ratios for the liposomes prepared with the C12 spacer were significantly lower than the others despite their similar diameters (320±57 nm) and optical densities. It is expected that the more hydrophobic C12 spacer did not extend into the aqueous medium as much as its PEG-based counterparts and thus may have rendered the attached reporter probe less available to target sequences.
Effect of alkyl chain length on the hybridization of reporter probe-tagged liposomes to a synthetic target sequence. Reflectometer readings of the capture zone are reported for liposomes normalized to an OD532=0.460±0.007 using 60 nM (triangles) and 10 nM (squares) synthetic DNA. Each data point was the average of 3–5 determinations, and error bars represent one standard deviation
Optimization of liposome size and concentration
The effect of liposome diameter and concentration on the lateral-flow assays was investigated using liposomes of varying size prepared by extrusion through 0.2, 0.4, 0.6, 0.8, 1.0, and 2.0 μm pore size membranes, resulting in liposomes with diameters between 208 and 365 nm. The extrusion technique typically removes liposomes with diameters larger than the pore size of the membrane used for extrusion and thus serves to downsize the upper end of the population while reducing the degree of multilamellarity. Two micrometer-extruded liposomes yielded a broad size distribution (365 nm±73 nm), whereas 0.2 μm-extruded liposomes yielded a much narrower size distribution (208 nm±42 nm). Each of the diameters reported here is the average of an assumed monomodal size distribution with its associated size distribution (Table 2).
The determination of the optimum liposome size poses a multifaceted problem. Several liposomal properties change when the liposome diameter increases as follows: (1) the number of phospholipid molecules per liposome increases and thus fewer liposomes are present for a given phospholipid concentration; (2) the internal volume increases, yielding an increased amount of encapsulant per liposome and thus fewer liposomes are present for a given dye concentration, and; (3) since the number of liposomes decreases, the amount of tag per liposome increases for a given amount of reporter probe. Thus neither phospholipid concentration nor encapsulant concentration nor optical density alone are ideal methods for normalizing liposome concentration. Since no single measurement can accurately characterize liposome concentration when different diameters are considered, the results were analyzed on the basis of a theoretical number of liposomes per mL based on the diameter obtained from dynamic light scattering measurements and the concentration of SRB as determined by fluorescence spectroscopy after liposome lysis. The estimated number of liposomes is based on the average diameter of the liposome population and does not take into account the width of the size distribution. The number of liposomes required to result in a maximum signal for a given target concentration (100 nM used here) was investigated for all of the different liposome diameters (Fig. 5).
The maximum reflectometer readings were observed when ∼2.83×109 liposomes, ∼2.04×109 liposomes, ∼1.99×109 liposomes, 2.45×109 liposomes, and ∼4.16×109 liposomes per assay were used with diameters of 208±42 nm, 312±56 nm, 318±81 nm, 348±91 nm and 365±73 nm respectively. Thus, the fewest number of liposomes per assay (∼2.0×109) were needed for populations with an average diameter of 312 nm or 318 nm, leading to an optimal average diameter of ∼315 nm. We postulate that the increased number of liposomes required at higher average diameters is a function of steric considerations. These liposome batches have a higher population of liposomes larger than the optimal diameter due to their extrusion through larger pore size membranes (1.0 and 2.0 μm). These large liposomes may have had more difficulty accessing the immobilized capture probe and may have encountered more difficulties migrating up the membrane by capillary action, resulting in a higher background signal. By contrast, the maximum signal attainable using 208±42 nm (0.2 μm-extruded) liposomes was limited by their reduced internal volume in which to encapsulate dye. These results suggest that while the 0.2 μm-extruded liposomes are of a significantly smaller diameter, the potentially greater number of liposomes, if any, capable of binding within the capture zone is insufficient to overcome their smaller encapsulant volume. Liposomes with the smallest (208±42 nm), the largest (365±73 nm) and the optimum (319±81 nm) diameters were used in order to generate dose response curves (Fig. 6). When compared to the smallest liposomes, about a tenfold lower LOD was achieved for the optimal diameter liposomes. In terms of the signal-to-noise ratio, there was no advantage to using larger rather than optimal-diameter liposomes. From these results and the above analysis on the number of liposomes required for maximum reflectometer readings, liposomes with an approximate diameter of 315 nm yield an optimal signal. This conclusion was also reached with liposomes made using the post-formation coupling process, where signals increased with increasing average liposome diameter until about 315 nm (data not shown).
Comparison of dose–response curves for liposomes extruded through 0.2 (circles), 0.6 μm (triangles), and 2.0 μm (squares). Nucleopore membranes with actual diameters of 208±42 nm, 319±81 nm, and 365±73 nm respectively. Sixty picomoles of capture probe and 20 pmol streptavidin were immobilized per lateral-flow assay. Each point is the average of three determinations, with error bars representing one standard deviation
For a given number of liposomes, larger liposomes were found to give higher signals. This is likely due to the increased volume of dye per liposome (Table 2). However, this advantage is balanced by an increased background signal. Using an optical density at 532 nm of between approximately 0.23 and 0.60, the background signal remained constant regardless of liposome diameter. At higher concentrations, the background measurement increased substantially and was directly related to the liposome size. This background measurement was taken below the capture zone; however, highly concentrated liposomes were also observed to bind nonspecifically in the capture zone. While highly concentrated liposomes adversely affected the reflectometer readings due to higher background and nonspecific signals, liposomes which were more dilute yielded reduced specific reflectometer readings. For liposomes with an optimal diameter of approximately 315 nm, the reflectometer readings were maximized at an optical density at 532 nm of approximately 0.4. Thus, to minimize nonspecific binding and background signals for the optimal diameter liposomes, an optical density at 532 nm ranging from 0.4 to 0.6 can be used to attain the optimum signal.
Conclusions
This study focused on the optimization of sulforhodamine B-encapsulating liposomes for use in sandwich hybridization lateral-flow assays for RNA detection, which considered the percentage of reporter probe coverage, the length of spacer between the liposome surface and the reporter probe, and the diameter and concentration of the liposomes. Through the incorporation of a cholesteryl-modified reporter probe in lieu of multistep coupling procedures, liposomes of higher concentration were obtained, and the need for expensive coupling reagents and time-consuming coupling was avoided.
The length of the spacer between the reporter probe and the lipid bilayer had little effect on the lateral-flow assays, presumably because the TEG spacer provided with the cholesteryl modification was sufficient. The effect of the spacer in terms of optimal reporter probe coverage was not investigated. However, it would seem reasonable that an increase in the spacer length may reduce the amount of reporter probe that is required on the surface of the liposomes due to the probe’s suspected increased conformational flexibility. Though beyond the scope of the investigation at present, further study in this regard is warranted and could be performed by a more informative technique, such as differential scanning calorimetry [41] or cyclic voltammetry [42].
Due to the modified synthesis protocol, which results in more reliable integration of the reporter probe onto the liposome surfaces, the amount of reporter probe used to generate optimized coverage on the liposomes could be reduced from 0.4 mol% to 0.013 mol% of the total lipid input. Independent from the liposome preparation method, it was found that liposome size and concentration had a significant impact on the signals obtained in the lateral-flow assay. The increased number of smaller liposomes that could potentially bind for a given concentration of capture probe/target did not result in a higher signal than that obtained from liposomes of larger diameter with a larger internal volume. The internal volume of encapsulant is therefore a key factor in maximizing the specific signals. Liposomes with an approximate diameter of 315 nm yielded the best compromise between internal volume and nonspecific binding/background effects that increased with increasing liposome diameters. With respect to the effect of liposome concentration on the hybridization strip assay, it was found that the sensitivity of the assays was diminished at low concentrations of liposomes, whereas excess concentrations led to high background signals and a nonspecific signal at the capture zone in the absence of target. The overall optimal liposome properties for this assay were an average diameter of approximately 315 nm, a concentration yielding an A 532 of between 0.4 and 0.6, and a reporter probe coverage of 0.013 mol%. Using optimally prepared liposomes and the conditions outlined for the lateral-flow assays, about 2.05 pmol of reporter probe would be expected to be present in the mixture. This corresponds to 195 molecules of tag per 319 nm liposome, though only around half of this is available on the outer surface of the liposomes. This concentration of reporter probe is well above the range of detection for the strip assays (0.5–150 fmol/assay), providing sufficient reporter molecules for an efficient detection.
Abbreviations
- Cholesteryl-TEG:
-
1-dimethoxytrityl-3-O-(N-cholesteryl-3-aminopropyl)-triethyleneglycolyl-glyceryl-2-O-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite
- DPPC:
-
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DPPG:
-
1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]
- NASBA:
-
nucleic acid sequence based amplification
- SATA:
-
N-succinimidyl S-acetylthioacetate
- SRB:
-
sulforhodamine B
- Sulfo-KMUS:
-
N-[κ-maleimidoundecanoyloxy]sulfosuccinimide ester
References
Zhou X, Huang L, Li S (2001) Biosens Bioelectron 16:85–95
Iqbal S, Mayo M, Bruno J, Bronk B, Batt C, Chambers J (2001) Biosens Bioelectron 15:549–578
Kricka L (2002) Ann Clin Biochem 39:114–129
Kricka L (1999) Clin Chem 45:453–458
Rule G, Montagna R, Durst R (1997) Anal Biochem 244:260–269
Rule G, Montagna R, Durst R (1996) Clin Chem 42:1206–1209
Baeumner A, Cohen R, Miksic V, Min J (2003) Biosens Bioelectron 18:405–413
Baeumner A, Schlesinger N, Slutzki N, Romano J, Lee EM, Montagna RA (2002) Anal Chem 74:1442–1448
Hartley H (2002) A rapid and sensitive Bacillus anthracis biosensor. MS Thesis, Cornell University, Ithaca, NY
Baeumner A, Pretz J, Fang S (2004) Anal Chem 76:888–894
Robinson A, Creeth J, Jones M (1998) Biochim Biophys Acta 1369:278–286
Jones M, Kilpatrick P, Carbonell R (1993) Biotechnol Prog 9:242–258
Jones M, Kilpatrick P, Carbonell R (1994) Biotechnol Prog 10:174–186
Plant A, Brizgys M, Locascio-Brown L, Durst R (1989) Anal Biochem 176:420–426
Rongen H, van der Horst H, Hugenholtz G, Bult A, Bennekom W, van der Meide P (1994) Anal Chim Acta 287:191–199
Rongen H, van Nierop T, van der Horst H, Rombouts R, van der Meide P, Bult A, Bennekom W (1995) Anal Chim Acta 306:333–341
Esch M, Locascio L, Tarlov M, Durst R (2001) Anal Chem 73:2952–2958
Zaytseva N, Montagna R, Lee E, Baeumner A (2005) Anal Bioanal Chem 380:46–53
Ho J, Durst R (2000) Anal Chim Acta 414:51–60
Chen C, Baeumner A, Durst R (2005) Talanta 67:205–211
Barbet J, Machet P, Leserman L (1981) J Supramol Struct Cell Biochem 16:243–258
Martin FJ, Heath TD (1992) Covalent attachment of proteins to liposomes. In: New RRC (ed) Liposomes, a practical approach. IRL Press/Oxford University Press, Oxford, pp 163–182
Zhang N, Ping Q, Huang G, Xu W (2005) Int J Pharm 294:247–259
Kung V, Redemann C (1986) Biochem Biophys Acta 862:435–439
Hansen C, Yao G, Moase E, Zalipsky S, Allen T (1995) Biochim Biophys Acta 1239:133–144
Hartley H, Baeumner A (2003) Anal Bioanal Chem 376:319–327
Wen H, DeCory T, Borejsza-Wysocki W, Durst R (2006) Talanta 68:1264–1272
Bredehorst R, Ligler F, Kusterbeck A, Chang E, Gaber B, Vogel C (1986) Biochemistry 25:5693–5698
Xu C, Taylor P, Ersoz M, Fletcher P, Paunov V (2003) J Mater Chem 13:3044–3048
Henderson GB, Stein CA (1995) Nucleic Acids Res 23:3726–3731
Letsinger RL, Zhang G, Sun DK, Ikeuchi T, Sarin PS (1989) Proc Natl Acad Sci USA 86:6553–6556
Krieg A, Tonkinson J, Matson S, Zhao Q, Saxon M, Zhang L, Bhanja U, Yakubov L, Stein C (1993) Proc Natl Acad Sci USA 90:1048–1052
Bartlett G (1959) J Biol Chem 234:466–468
Small D (1967) J Lipid Res 8:551–557
Israelachvili J, Mitchell D (1975) Biochim Biophys Acta 389:13–19
Fiske C, SubbaRow Y (1925) J Biol Chem 66:375–399
Esch M, Baeumner A, Durst R (2001) Anal Chem 73:3162–3167
Limanto J, Tallarico J, Porter J, Khuong K, Houk K, Snapper M (2002) J Am Chem Soc 124:14748–14758
Muller G, Schmidt B, Jiřiček J, Bünzli J, Schenk K (2003) Acta Crystal C 59:o353–o356
Berman H, Olson W, Beveridge D, Westbrook J, Gelbin A, Demeny T, Hsieh S, Srinivasan A, Schneider B (1992) Biophys J 63:751–759
Mrevlishvili G, Kankia B, Mdzinarashvili T, Brelidze T, Khvedelidze M, Metreveli N, Razmadze G (1998) Chem Phys Lipids 94:139–143
Huang W, Zhang Z, Han X, Tang J, Wang J, Dong S, Wang E (2003) Bioelectrochemistry 59:21–27
Acknowledgements
The authors thank Dr. John Randolph of Glen Research Corporation for calculating the lengths of the spacers used in this study. We also thank Dr. John C. March for critically reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edwards, K.A., Baeumner, A.J. Optimization of DNA-tagged dye-encapsulating liposomes for lateral-flow assays based on sandwich hybridization. Anal Bioanal Chem 386, 1335–1343 (2006). https://doi.org/10.1007/s00216-006-0705-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0705-x