Abstract
The isoelectric points of many microbial cells lie within the pH range spanning from 1.5 to 4.5. In this work, we suggest a CIEF method for the separation of cells according to their isoelectric points in the pH range of 2–5. It includes the segmental injection of the sample pulse composed of the segment of the selected simple ampholytes, the segment of the bioanalytes and the segment of carrier ampholytes into fused silica capillaries dynamically modified by poly(ethylene glycole). This polymer dissolved in the catholyte, in the anolyte and in the injected sample pulse was used for a prevention of the bioanalyte adsorption on the capillary surface and for the reduction of the electroosmotic flow. Between each focusing run, the capillaries were washed with the mixture of acetone/ethanol to achieve the reproducible and efficient CIEF. In order to trace of pH gradients, low-molecular-mass pI markers were used. The mixed cultures of microorganisms, Escherichia coli CCM 3954, Candida albicans CCM 8180, Candida parapsilosis, Candida krusei, Candida glabrata, Candida tropicalis, CCM 8223, Proteus vulgaris, Klebsiela pneumoniae, Staphylococcus aureus CCM 3953, Streptococcus agalactiae CCM 6187, Enterococcus faecalis CCM 4224 and Staphylococcus epidermidis CCM 4418, were focused and separated by the CIEF method suggested here. This CIEF method enables the separation and detection of the microbes from the mixed cultures within several minutes. The minimum detectable number of microbial cells was less than 103.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The isoelectric point of microorganisms [1], pI, is often used for the prediction of the properties of the cell surface polymers [2] and their consequences regarding cell adhesion [3]. The acid-base titration of isolated cell walls or whole cells [4, 5] is one the applicable methods for investigation of the charge of the cell surface and/or for determination of the pH value at which the net surface charge of cells equals zero. Similarly, capillary isoelectric focusing (CIEF) was used for the successful separation of microorganisms according to their pI [6–11].
The isoelectric point, pI, of most bacterial cells lies within the pH range of 1.5–4.5 [2, 12]. The majority of cells is negatively charged within the pH range 5–9 [5]. When suitable amphoteric compounds (spacers) are added to the commercial synthetic carrier ampholytes, the local pH gradient in the pH interval of interest is less steep at the isoelectric focusing (IEF). Both “good and poor” ampholytes can be used as the spacers [13]. The spacer-modified slab-gel IEF technique was re-applied by Righetti et al. to CIEF [14, 15]. The extension of the pI separation range in CIEF, which improves the resolution of basic proteins, can also be achieved by a simple combination of ampholyte 9–11 with pharmalyte 3–10 [16]. Recently [7], 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethanesulfonic acid (HEPES) and L-aspartic acid (Asp) were used as the spacers at the segmental injection of the sample of bioanalytes in the pH gradient of 3–10. The advantage of these experiments includes the possibility of washing the capillaries between each focusing run with suitable solvents with no need of their preliminary precondition before each analysis with a mixture of carrier ampholytes [17–20]. The effect of the adsorption of spacers onto fused silica (FS) capillaries is appreciable. The linearity of the pH gradient, resolution of bioanalytes and reproducibility are maintained. For tracing of the pH gradient the low-molecular-mass pI markers 3.9–10.1 were used [7].
The capillary surfaces for CIEF should be pH stable independent of the capillary origin. With regard to a strong adherence of the microorganisms or other bioanalytes to the surfaces [21, 22], the capillaries used in CIEF are usually dynamically [23–26] or statically modified [17, 25–27]. Protein rejecting surfaces can be achieved by attaching poly(ethylene glycole) (PEG) chains to the surfaces [7, 22, 28, 29]. Similarly, Armstrong et. al. [8, 11] have used poly(ethylene oxide) (PEO) in CZE of microorganisms. In capillary electrophoresis the poly(ethylene oxide) has been used as non-bound coating for the purpose of alteration of the electroosmotic flow (EOF) [30]. The presence of carrier ampholytes in the capillary and their adsorption onto the capillary wall resulted in almost eliminating EOF in FS [24, 31, 32].
This study suggests a new protocol for CIEF of microorganisms within the pH range of 2.1–4.6 on bare FS capillaries dynamically modified by poly(ethylene glycole), PEG 4000, and with UV detection. The reproducible results and the linear pH gradient are achieved by means of a segmental injection of the sample pulse composed of the segment of spacers, solution of the selected simple ampholytic electrolytes, segment of the sample mixture of bioanalytes and the segment of the mixture of the commercial carrier ampholytes. For tracing of the pH gradients, the low-molecular pI markers [27] 2–4.9 [33] were used.
Materials and methods
Equipment and procedure
The capillary isoelectric focusing experiments were carried out using a laboratory made apparatus [17]. All measurements were made at constant voltage (-) 20 kV supplied by the high voltage unit Spellman CZE 1000 R (Plainview, NY, USA). During the experiments, the current was decreased from the beginning value of 40–60 μA to 3–6 μA at time of detection, depending on sampling time and a sample solution. The length of the fused silica capillaries—(A) Pliva-Lachema a.s, Brno, Czech Republic, and (B) Microquartz, Munich, Germany—was 250–270 mm, 180 mm to the detector. Their I.D. was 0.1 mm; their O.D. was 0.25 mm for A and 0.36 mm for B, respectively. The ends of the separation fused silica capillaries were dipped in 3-ml glass vials with the anolyte or the catholyte and electrodes.
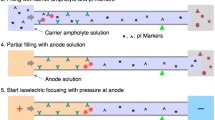
The segmental injection of the sample pulse was accomplished by the siphoning action achieved by the elevation of the inlet–anolyte reservoir relative to the outlet–catholyte reservoir [7, 17]. The height difference of the reservoirs for sample injection can be adjusted in the range of 100–180 mm for 7 to 60 s.
The on-column UV-Vis detector LCD 2082 (Ecom, Prague, Czech Republic) connected to the detection cell by optical fibers (Polymicro Technologies, Phoenix, AZ, USA) was used at a wavelength of 280 nm. The light absorption (optical density) of the microbial suspensions was measured using a DU series 520 UV/Vis spectrophotometer (Beckmann Instruments, Palo Alto, CA, USA) at 550 nm.
The aggregation among the cells was avoided by sonication of their suspension in Sonorex, Bandelin Electronic (Berlin, Germany). The frequency of 35 kHz was used for 1 min at the temperature of 30 °C. After the sonication, the microbial sample was vortexed (Vortex-Genie 2, Scientific Industries, Bohemia, USA) for 10 min and than immediately injected.
The detector signals were acquired and processed with the Chromatography station for Windows CSW v. 1.5, DataApex sro, Praha, Czech Republic; the data were processed by graphic program Sigma-Plot 3.0, Jandel Scientific Software (Point Richmond, CA, USA) including the calculation of the correlation coefficients for all the measured data in each plot.
Chemicals
The bacteria strains applied in this study, Escherichia coli CCM 3954 (E. coli), Candida albicans CCM 8180 (C. albicans), C. parapsilosis, C. krusei, C. glabrata, C. tropicalis CCM 8223, Proteus vulgaris (P. vulgaris), Klebsiela pneumoniae (K. pneumoniae), Staphylococcus aureus CCM 3953 (S. aureus), Streptococcus agalactiae CCM 6187 (S. agalactiae), Enterococcus faecalis CCM 4224 (E. faecalis) and Staphylococcus epidermidis CCM 4418 (S. epidermidis) were obtained from the Czech Collection of Microorganisms. Bovine serum Albumin (Mr 67,000, pI 4.9 [19], hydroxypropylmethylcellulose (HPMC) (H 7509), poly(ethylene glycole) (PEG 4000, Mr 4000) were from Sigma (St. Louis, MO, USA). The solution of synthetic carrier ampholytes, Biolyte, pH 3–10, was obtained from Bio-Rad laboratories (CA, USA), ampholyte high resolution, pH 2–4, and ampholyte pH 3-4.5, poly(methylhydrosiloxane), methyltrimethoxysilane, trifluoroacetic acid, methylene chloride, 2-morpholinoethanesulfonic acid monohydrate (MES), 3-morpholino-propanesulfonic acid (MOPS), N-[tris-(hydroxymethyl)-methyl]-3-amino-2-hydroxy-propansulfonic acid (TAPSO) were from Fluka Chemie GmbH (Buchs, Switzerland). Poly(dimethylsiloxane) (hydroxy terminated) was from ABCR (Germany), L-aspartic acid (Asp) from LOBA Chemie, Wien, Austria, N-(2-acetamido)-2-aminoethansulfonic acid (ACES) and 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethanesulfonic acid (HEPES) from Merck, Darmstadt, Germany. All chemicals were of analytical grade. The pI markers 2, 3.0, 3.3, 3.65, 4.25 [33], 2.7, 4.0, and 4.9 [27] and 4-morpholinyl acetic acid (MAA) [33] were synthesized at the Institute of Analytical Chemistry Academy of Sciences of the Czech Republic, Brno. MAA was prepared by the reaction of morpholin and chloracetic acid (Sigma, St. Louis, MO, USA). The specifications [34, 35] of the used spacers, simple ampholytes, are in Table 1.
Sol-gel capillary
The Sol-gel capillary was prepared according to the protocol in Ref. [6]. Newly prepared capillary was filled with the 5% (wt/vol) solution of the synthetic carrier ampholytes and 0.25% (wt/vol) HPMC for 30 min and then prior to each injection for 10 min. Then the capillary was back-flushed with catholyte. Solution of Biolyte, pH 3–10, or the mixture of the synthetic carrier ampholytes, pH 3–10, pH 2–4 and 3–4.5 (1:2:5), were used for the capillary filling before CIEF of the pI markers.
Solutions
Electrolytes
For the CIEF experiments on the sol-gel capillary, 0.001% (wt/vol) HPMC, 20 mM sodium hydroxide and 0.25% (wt/vol) HPMC, 100 mM ortho-phosphoric acid were used as the catholyte and the anolyte solutions, respectively. For the other CIEF experiments presented here, 10–100 mM sodium hydroxide and 100 mM ortho- phosphoric acid in the catholyte and the anolyte solutions were used, respectively. For the dynamic modification of the capillaries, a solution of PEG 4000 of the concentration ranging from 0 to 5% (wt/vol) was added into the catholyte and the anolyte solutions.
Microbial sample
The bacteria strains applied in this study were inoculated on blood agar (Hi-Media, Mumbai, India) and stored at −70 °C, E. coli CCM 3954, E. faecalis CCM 4224, S. epidermidis CCM 4418 and C. albicans CCM 8180 in Itest KRYOBANKA B (ITEST plus, Hradec Králové, Czech Republic). Before each experiment, the bacteria strains were thawed quickly at 37 °C and cultivated on Muller-Hinton’s agar (Bio-Rad, Marnes La Coquette, France) at 37 °C for 24 h. The microbial cultures were re-suspended in physiological saline solution (PSS). The concentrations of the re-suspended microorganisms were estimated by the optical density of the suspension. This was measured by spectrophotometer at 550 nm according to the calibration curve as defined by reference samples. These samples were prepared by re-suspension of the microbial culture in PSS. The concentrations of microorganisms in reference samples were measured by dilution and by plating 100 μl of suspension on Muller-Hinton’s agar (Bio-Rad). After cultivation at 37 °C for 24 h, the colonies were counted.
The composition of the sample pulse
The segmental injection [7] of the sample pulse into the capillary was used here. The sample pulse consisted of three segments, the first segment was composed of the solutions of the spacers and 0–5% (wt/vol) PEG 4000 both dissolved in 10–40 mM NaOH (the used spacers and their concentrations are summarized in Table 1); the second segment was composed of the 0–5% (wt/vol) solution of PEG 4000 together with the solution of bioanalytes, Albumin (50 μg ml−1) or suspension of microbes, 107–109 cells ml−1, in water or NaCl, respectively; the third segment was composed of the water solution of pI markers, 25 μg ml−1, and 5% (wt/vol) of synthetic carrier ampholytes, Biolyte, pH 3–10, ampholyte pH 3–4.5 and pH 2–4, in the ratio 1:2:5.
The amount of albumin injected into the capillary ranged from 7 to 300 ng, while the number of injected microorganisms ranged from 3×102 to 104 cells, and the pI markers ranged from 7 to 150 ng. Before each injection, the capillaries were rinsed with the mixture of organic solvent acetone/ethylalcohol, 10:1 for 10 min, and then back-flushed with the catholyte for 1 min [7]. On the sol-gel capillary, the 5% (wt/vol) solution of synthetic carrier ampholytes Biolyte, pH 3–10, or the 5% (wt/vol) solution of the mixture of carrier ampholytes was used.
Results and discussion
The optimization of the CIEF procedure
Many pIs of microorganisms lie in the acidic pH range. It is possible to expect that the difference between their isoelectric points can be very small. For better resolution of the individual microbial strains, it is necessary to flatten the acidic pH gradient. Therefore, the influence of the change of the composition of the catholyte and the anolyte solutions (Fig. 1) or the sample pulse (Fig. 2) on the pH gradient was studied.
The influence of the sample, the catholyte and the anolyte composition on CIEF of pI markers. Conditions: FS capillary A, 0.1 mm I.D., length 270 mm, 180 mm to the detection cell; applied voltage (-) 20 kV; wavelength, λ=280 nm; anolyte, 100 mM H3PO4; the composition of the stock solution of the spacers (see Table 1), DSS, 0.33, in 20 mM NaOH; Δh of the spacers segment, 180 mm; injection time, t inj, 25 s (200 nl); pI markers, pI, 2–4.9; carrier ampholytes, 5% (wt/vol) solution of Biolyte pH 3–10, ampholyte, pH 3–4.5 and pH 2–4 in the ratios 1:2:5; Δh of the segment of carrier ampholytes and pI markers, 180 mm; injection time 35 s (280 nl); rinsing procedure before each focusing run, 10 min with the solution acetone/ethanol, 10:1, 1 min with catholyte; curve 1, t 4.9 (min); curve 2, Δt (min); A, the catholyte, 40 mM NaOH; cPEG, CaAn, 0–5% (wt/vol); in the spacers segment, c PEG, inj=0% (wt/vol); B see A, c PEG, CaAn, 2% (wt/vol); c PEG, inj, 0.5% (wt/vol)
Properties of the pH gradient in the dependence on the dilution and on the injection time, t inj, of the stock solution of the spacers. Conditions and designations (see Fig. 1): the catholyte, 80 mM NaOH, c PEG, CaAn, 2% (wt/vol); spacers segment, 20 mM NaOH, c PEG, inj, 0.5% (wt/vol); A, B, curves: (1, 1a) t 4.9 (min); (2, 2a) Δt (min); A, DSS, 0–0.5; B, curves (1, 2) DSS, 0.5; curves (1a, 2a) DSS, 1
In the catholyte solution (see Fig. 1a), 40 mM NaOH and in both the catholyte and anolyte solutions, 0–5% (wt/vol) PEG 4000 was dissolved. The spacer segment (see Table 1) was injected before the segment of pI markers (2–4.9), the mixture of the synthetic carrier ampholytes, Biolyte pH 3–10, ampholyte pH 3–4.5 and pH 2–4. The ratios of the simple ampholytes in the spacer segment were kept constant. The concentration of spacers was designed as the concentration of the stock solution of the spacers. Here, the dilution of the stock solutions of the spacers, DSS, was 0.33. The spacers were dissolved in 20 mM NaOH. For the description of the properties of the pH gradients, the symbols t4.9 and Δt were used; t 4.9 stands for the migration time of the zone of pI marker 4.9 and Δt is the difference between the migration times of the zones of pI markers 2.0 and 4.9. With increasing in the concentration of PEG 4000 in the catholyte and the anolyte solutions, c PEG, CaAn, EOF decreases and therefore the value of t 4.9 increases (see Fig. 1a, curve 1). At c PEG, CaAn greater than 2% (wt/vol) the significant lengthening of the pH gradient, Δt (see Fig. 1a, curve 2) is observed. For the evaluation of the linearity of the pH gradient, we used the correlation coefficient (R 2) of the linear regression of the dependence of the migration times of the pI markers on their isoelectric points, 2, 2.7, 3.0, 3.3, 3.65, 4.0, 4.25 and 4.9. After the comparison of the values of R 2, we were able to determine that the maximum linearity of the pH gradient was reached at 2% (wt/vol) PEG 4000 in the catholyte and anolyte solution.
Unlike the concentration of NaOH in the catholyte, the addition of PEG 4000 minimally influences t 4.9 and Δt as well as the linearity of the pH gradient (see Fig. 1b, curves 1, 2). For the next experiments, the concentration 0.5% (wt/vol) of PEG 4000 in the injected sample pulse and 2% (wt/vol) of PEG 4000 in the catholyte and anolyte solutions were chosen. At the concentration of NaOH in the electrolyte higher than 60 and 70 mM, the values of the measured quantities (t 4.9 and Δt) increase; at over 80 mM NaOH in the catholyte the values of the observed quantities were changed only slightly. Therefore, for the next experiments on the capillary A, we chose the concentration of NaOH in the catholyte 80 mM. The migration time for the pI marker 4.9 is twice as long (see curve 1) and the value Δt is quadruplicate, (see curve 2) when compared with their values measured at the 20 mM NaOH in the catholyte.
The spacer segment
In these experiments (see Fig. 2a) the optimized conditions, the composition of the catholyte and the anolyte, the sample pulse, and the injection time/volume (25 s/200 nl) of the spacer segment were the same as in Fig. 1. The migration time of the pI marker 4.9 (t 4.9) did not change with increase in the concentration of the spacers in the segment at the segmental injection see Fig. 2a, curve 1). The length of the pH gradient expressed as Δt increases with increase in the concentration of spacers (see Fig. 2a, curve 2). The increase in the concentration of the simple ampholytes in the spacers segment at the segmental injection significantly influences both the steepness and the linearity of the pH gradient. It implies, from the comparison of the values of R 2, that the maximum linearity of the pH gradient was reached around DSS from 0.33 to 0.41. Without the injection of the spacers, the linearity of the pH gradient is small. The variance of the migration times for pI marker 4.0 was calculated as 1.3% from twenty runs.
In following experiments (see Fig. 2b), the injection time of the segment of spacers (t inj) was changed from 5 s (40 nl) to 40 s (320 nl). The dilution of the stock solution of the spacers was 0.5 (curves 1, 2) and 1 (curves 1a, 2a). According to the results in Fig. 2b, curve 1, 1a the value of t 4.9 is practically independent regarding the time (volume) of the injected spacers zone. The maximum difference between the migration time of the pI markers in the pH gradient (Δt) was reached when t inj of the spacer segment was about 20–25 s (160–200 nl). At that point, the maximum linearity of the pH gradient was also reached.
From this point of view, for the linearity and reproducibility of the pH gradient the sufficient concentration of the suitable ampholytic electrolytes is necessary to inject into the capillary before the segment of the analytes. Therefore, the use of the concentrated solution of the spacers in the segment, DSS=1, at the segmental injection leads to the reduction of the volume of the injected spacer segment to one-half; Δt is practically identical (see Fig. 2b; curve 2a vs. curve 2).
The steepness of the pH gradients
Recently the high separation efficiency and good reproducibility was achieved on the sol-gel capillaries [6] at CIEF of microbes in the pH gradient 3–10. Therefore, CIEF on the capillary prepared by sol-gel technique was used here “as standard” for comparison of the linearity of the pH gradient with CIEF in the pH range from 2 to 5 described in the paragraphs above (see Fig. 3). The linearity of the pH gradients was numerically evaluated with the help of the pI markers from 2 to 4.9. On the sol-gel capillary pI markers were focused via the previously described CIEF procedure [6] (see sections Sol-gel capillary and Solutions). The carrier ampholytes Biolyte, pH range 3–10 (curve 1a) or the mixture of the synthetic carrier ampholytes (curve 1b) were used. On capillary A, which was dynamically coated by 2% (wt/vol) PEG 4000, the spacer segment was injected before the segment of pI markers/mixture of the synthetic carrier ampholytes (see Fig. 3, curve 2). The spacers (DSS, 0.5) (see Table 1) were dissolved in 20 mM NaOH and 0.5% (wt/vol) PEG 4000. Between each focusing run, the untreated capillaries were rinsed with the organic solvents and the catholyte solution. The steepness of the obtained pH gradient in the CIEF procedure under the optimized conditions described above is greater in comparison with previous CIEF on the sol-gel capillary under the same composition of the carrier ampholytes (see Fig. 3, curves 2 vs. 1b).
The comparison of the slopes of the pH gradient 2–5 for different CIEF methods. Conditions and designations (see Figs. 1 and 2): curves 1a, b, sol-gel capillary, length 80 mm to the detection cell; the catholyte, 20 mM NaOH; 0.001% (wt/vol) HPMC; the anolyte, 0.25% (wt/vol) HPMC; Δh of the segment of carrier ampholytes/pI markers, 80 mm; injection time 35 s (130 nl); washing procedure before each focusing run, 10 min with 5% (wt/vol) Biolyte, pH 3–10 (curve 1a) or with 5% (wt/vol) solution of the mixture of carrier ampholytes (curve 1b) both in 0.25% (wt/vol) of HPMC; curve 2, the catholyte, 80 mM NaOH, cPEG, CaAn, 2% (wt/vol); spacer segment, DSS-0.5, 20 mM NaOH, cPEG, inj, 0.5% (wt/vol); Δh of the spacers segment, 180 mm; injection time, t inj, 25 s (200 nl); Δh of the segment of carrier ampholytes/pI markers, 180 mm; injection time 35 s (280 nl); rinsing procedure before each focusing run, 10 min with the solution acetone/ethanol (10:1) 1 min with catholyte; migration time, t (min)
CIEF of microorganisms in the pH gradient 2–5
The linearity of the pH gradient under the optimized CIEF conditions obtained in the focusing runs of eight pI markers (7 ng of each) [33] from pI 2 to 4.9 and albumin (7 ng) together with the pI markers from 2 to 4.25 was demonstrated in Fig. 4a and b, respectively. The gradient was lengthened twice in migration times in comparison with the CIEF experiments on the sol-gel capillaries (see Fig. 3 curves 1 vs. 2) the resolution was improved at the same time. The peaks of the pI markers and albumin are symmetrical. That enables the determination of their isoelectric points. The calculated isoelectric point of albumin is near the value mentioned previously (see section Chemicals). The relative standard deviation (RSD) from ten replicated measurements of the migration time of albumin was under 1.8% and that of the peak area was around 1.9%. To achieve reproducible results for the isoelectric focusing of microorganisms, both correct microbial sample and the capillary rinsing procedures (see sections Microbial sample and The composition of the sample pulse) must be used.
CIEF of the pI markers and albumin. Conditions and designations for capillary A (see Figs. 1, 2, 3); A, DSS, 0.5; Δh, 180 mm (segment of spacers, pI markers/carrier ampholytes); t inj: spacers, 25 s (200 nl), the pI markers/carrier ampholytes, 35 s (280 nl). Conditions and designations for capillary B (see A) c PEG, inj, 0.5% (wt/vol); segment of the analyte, albumin; Δh, 150 mm, t inj, 15 s (120 nl); pI markers, pI 2.0, 3.0, 4.0, 4.25
The next electropherograms demonstrate the focusing runs of microbes on FS capillaries from different producers—Pliva-Lachema, Czech Republic A (see Fig. 5) and Microquartz, Germany B (see Fig. 6). The technical parameters such as the length of the capillaries etc. were the same only the concentrations of NaOH in the spacer solution and in the catholyte were changed. The first experiments were accomplished on capillary A. The concentration of NaOH in the catholyte was 80 mM and the concentration in the injected spacer solution was 20 mM. Figure 5 represents the electropherogram of five clinically important yeasts—the strains of Candida: albicans, krusei, parapsilosis, glabrata and tropicalis. According to preliminary experiments [6, 7], the isoelectric points of these microorganisms are between pI 2–4. Therefore, the pI markers 2, 4.25 and 4.9 were chosen. The injected volume of microorganisms was around 100 nl. That represents numerically about 1,000 cells injected into the capillary. Candida zones are well focused under the protocol discussed in the previous sections. The variance of the migration times of Candidas was 1.5% from 15 measurements.
CIEF of microorganisms, capillary A. Conditions and designations (see Fig. 4); c PEG, inj, 0.5% (wt/vol); microbial sample: C. albicans, C. parapsilos, C. krusei, C. glabrata, C. tropicalis, 107 cell ml−1, resuspended in PSS; Δh of the microbial sample, 150 mm; t inj, 12 s (100 nl); pI markers pI: 2, 4.25 and 4.9
CIEF of microorganisms, capillary B. Conditions and designations (see Figs. 4 and 5); capillary 0.1 mm I.D., length 270 mm; 180 mm to the detection cell; spacers segment, DSS, 0.5, 30 mM NaOH; Δh of the microbial sample (107 cell ml−1, resuspended in PSS), 150 mm; t inj, 12 s (100 nl); c PEG, inj, 0.5% (wt/vol); A, microbial samples E. coli, C. albicans, P. vulgaris, Kl. pneumoniae, E. faecalis; B, spacers segment, DSS, 1; t inj, 12 s (120 nl); microbial samples S. aureus, S. epidermidis and S. agalactiae; pI markers 2.0, 3.0, 4.0, 4.9
The composition and/or the purity of the quartz used for the FS production influences in particular the value of the EOF and the other properties. Therefore, we found that the proper adjusting of the concentration of NaOH in the catholyte on 20 mM and in the spacer solution on 30 mM is sufficient for maintaining comparable results of both capillaries A and B (see Figs. 5 vs. 6a) - sample of pI markers 4.9, 4.25, 2 and fungi, C. albicans. The electropherogram of the pI markers and microorganisms, E. coli, C. albicans, P. vulgaris, K. pneumoniae and E. faecalis is in Fig. 6a, and S. aureus, S. epidermidis and S. agalactiae is presented in Fig. 6b. The number of injected cells into the capillary was around 1.2×103. Isoelectric points of the strains focused in Fig. 6a were in the range of 2–4.9 and 2–3 in Fig. 6b. The isoelectric point of K. pneumoniae that was about 2.5, is in good agreement with the results in Ref. [1]. The relative standard deviation from sixteen runs is similar to the one obtained for the previous FS, i.e., 1.5%.
The quantification of the microorganism, E. coli, is depicted in Fig. 7. There is good correlation between the number of the injected cells into the capillary and the detected peak areas (A) for both capillaries A and B.
Conclusions
A possible way to generate the useful acidic pH gradient is recommended via the new method of capillary isoelectric focusing in the pH range 2–5. This includes the injection of the simple ampholytic electrolytes as the spacers segment at the segmental injection of the sample pulse onto the capillary simultaneously with a suitable composition of the carrier ampholytes. The use of PEG 4000 dissolved in the catholyte and the anolyte and in the sample pulse for dynamic modification of the FS capillary minimizes both the adsorption of the bioanalytes on the capillary wall and the electroosmotic flow. Also, it is important to carefully rinse the capillary before each focusing run. All this enables high separation efficiency and good reproducibility. The relative standard deviations of retention times by CIEF of all analytes, low-molecular-mass pI markers or microorganisms and protein, were lower than 2%. The suggested technique of CIEF is a possible way to identify and quantify microorganisms.
Abbreviations
- AA:
-
L-aspartic acid
- c NaOH, Ca :
-
Concentration of NaOH in the catholyte solution (mM)
- c PEG, CaAn :
-
Concentration of PEG 4000 in the catholyte and in the anolyte solutions [% (wt/vol)]
- c PEG, inj :
-
Concentration of PEG 4000 in the spacer or sample segment [% (wt/vol)]
- DSS:
-
Dilution of the stock solution of the spacer
- Glu:
-
Glutamic acid
- Δh :
-
Height difference of the reservoirs at the siphoning injection
- MAA:
-
4-morpholinyl acetic acid
- Nic:
-
Nicotinic acid
- PSS:
-
Physiological saline solution
- t :
-
Migration time (min)
- t 4.9 :
-
Migration time of the pI marker zone 4.9 (min)
- Δt :
-
Difference between the migration time of the zones of p
- TAPSO:
-
N-[tris-(hydroxymethyl)-methyl]-3-amino-2-hydroxy-propanesulfonic acid
- t inj :
-
Injection time of the sample pulse components into the capillary (s)
References
Harden VP, Harris JO (1953) J Bacteriol 65:198–202
Jucker BA, Harms H, Zehnder AJB (1996) J Bacteriol 178:5472–5479
Rijnaarts HMH, Norde W, Lyklema J, Zehnder AJB (1995) J Colloids Surf BBiointerfaces 4:191–197
Van der Wal A, Norde W, Zehnder AJB, Lyklema (1997) J Colloids Surf B Biointerfaces 9:81–100
Ritvo G, Dassa O, Kochba MA (2003) Aquaculture 218:379–386
Horká M, Planeta J, Růžička F, Šlais K (2003) Electrophoresis 24:1383–1390
Horká M, Růžička F, Horký J, Holá V, Šlais K J (2006) Chromatogr. B (in press)
Armstrong DW, Schulte G, Schneiderheinze JM, Westenberg DJ (1999) Anal Chem 71:5465–5469
Jaspers E, Overmann J (1997) J Appl Environ Microbiol 63:3176–3181
Kenndler E, Blaas D (2001) TrAC 20:543–551
Desai MJ, Armstrong DW (2003) Microbiol Mol Biol Rew 67:38–51
Moses N, Rouxhet PG (1987) J Microbiol Methods 6:99–112
Righetti PG, Bossi A (1998) Anal Chim Acta 372:1–19
Conti M, Gelfi C, Righetti PG (1995) Electrophoresis 16:1485–1491
Conti M, Gelfi C, Bianchi-Bosisio A, Righetti PG (1996) Electrophoresis 17:1590–1596
Mohan D, Lee CS (2002) J Chromatogr A 979:271–276
Horká M, Willimann T, Blum M, Nording P, Friedl Z, Šlais K (2001) J Chromator A 916:65–71
Wehr T, Rodriguez-Diáz R, Zhu M (2001) Chromatographia 53:S45–S58
Righetti PG, Caravaggio T (1976) J Chromatogr 127:1–28
Lalljie SPD, Sandra P (1995) Chromatographia 40:519–526
Rodriguez-Diaz R, Wehr T, Zhu M (1997) Electrophoresis 18:2134–2144
Roosjen A, Kaper HJ, van der Mei HC, Norde W, Busscher J. (2003) Microbiology 149:3239–3246
Girod M, Armstrong DW (2002) Electrophoresis 23:2048–2056
Shimura K (2002) Electrophoresis 23:3847–3857
Righetti PG (2004) J Chromatogr A 1037:491–499
Kilár F (2003) Electrophoresis 24:3908–3916
Šlais K, Horká M, Nováčková J, Friedl Z (2002) Electrophoresis 23:1682–1688
Razatos A, Org YL, Boulay F, Elbert DL, Hubell JA, Sharma MM, Georgiou G (2000) Langmuir 16:9155–9158
Kaper HJ, Busscher HJ, Norde W (2003) J Biomater Sci Polymer Edn 14:313–324
Preisler J, Yeung ES (1996) Anal Chem 68:2885–2889
Kilár F, Végváry Á, Mód A (1998) J Chromatogr A 813:349–360
Zhang CX, Xiang F, Pasa-Tolic L, Anderson GA, Veenstra TD, Smith RD (2000) Anal Chem 72:1462–1468
Št’astná M, Trávníček M, Šlais K (2005) Electrophoresis 26:53–59
Hirokawa T, Nishino M, Aoki N, Sawamoto YKTY, Akiyama J-I (1983) J Chromatogr A 271:D1–D106
Acevedo F (1991) J Chromatogr A 545:391–396
Acknowledgement
This work was supported by the Grant Agency of the Academy of Sciences of the Czech republic No. A4031302.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horká, M., Růžička, F., Holá, V. et al. Capillary isoelectric focusing of microorganisms in the pH range 2–5 in a dynamically modified FS capillary with UV detection. Anal Bioanal Chem 385, 840–846 (2006). https://doi.org/10.1007/s00216-006-0508-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0508-0