Abstract
The hydration number of \(\hbox {Sc}^{3+}\) aquaion is still an ongoing debate from both experimental and theoretical perspectives, in fact values between 6 and 9 have been proposed. This theoretical study presents the development of \(\hbox {Sc}^{3+}\)–\(\hbox {H}_{2}\hbox {O}\) intermolecular potentials based on ab initio potential energy surfaces of two scandium hydrates: \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_6]^{3+}\) and \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_7]^{3+}\). A flexible and polarizable cation and water model has been employed based on the mobile charge density harmonic oscillator (MCDHO) potential. Two classical molecular dynamics simulations of \(\hbox {Sc}^{3+}\) in water were carried out with these two new potentials. Data analysis shows very similar results from both simulations: The hydration number obtained is 6 and the average Sc–O distance for the first hydration shell is \(2.15\pm 0.01\) Å. Estimated hydration enthalpy is very close to the experimental one. The simulated Sc K-edge EXAFS spectrum obtained from the structural information provided by the MD simulation agrees fairly well with the experimental spectrum, as well as the main bands of the vibrational power spectra with the IR and Raman spectra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The scandium(III) ion in aqueous solution has long been studied without reaching a consensus about its hydration structure at acidic and dilute conditions. Only under these conditions, the aquaion is observed in solution [1]. The more common coordination numbers proposed in the literature are in the range 6–9 [2, 3]. Some evidence proposes the hexacoordination as the most favorable for scandium hydrates. Other studies suggest higher hydration numbers, from 7 to 9, supported by the lability of its hydration shell and its atomic radius, in between \(\hbox {Al}^{3+}\) hexacoordinated aquaion, and the closest heavy trivalent cation of its group, \(\hbox {Y}^{3+}\), whose aquaion is an octahydrate [4].
Pioneering Raman studies by Kanno et al. [5] discarded the octahedral symmetry comparing the absorption bands with the well-established case of the hydrated aluminum. A combined XRD and EXAFS study by Yamaguchi et al. [6] found a Sc–O distance of 2.18 Å and a coordination number of 7. However, a more recent Raman and quantum mechanics (QM) study [2] carried out by Rudolph and Pye, supported the octahedral geometry of \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_6]^{3+}\) in \(\hbox {Sc}(\hbox {ClO}_4)_3\) solution where it has been proved that the counterion does not penetrate the first shell. Smirnov et al. [7, 8] observed by means of X-ray diffraction a decreasing coordination with temperature, with a seven coordination at \(25\,^{\circ }\hbox {C}\) for a 1 M \(\hbox {Sc}(\hbox {ClO}_4)_3\) solution and an aquocomplex with six water molecules and one chloride for a 1 M \(\hbox {ScCl}_3\) solution.
Rode et al. [9] studied the Sc(III) hydration using molecular dynamics (MD) simulations at different levels. Classical MD simulations with or without 3-body corrections led to a coordination 7.8–9.0 and Sc–O distance 2.27–2.10 Å. When they used a hybrid QM/MM method including one or two shells in the QM part of the system a heptacoordination with Sc–O distance of 2.14–2.15 Å was obtained.
A QM study [10] about the water exchange on the aquaions of the d–series concluded that the water exchange proceeds via an associative mechanism for the scandium aquaion and sets the hexahydrate more stable than the heptahydrate, but both coordinations could coexist in solution.
The groups of Persson and Sandström [11–13] used crystal compounds including hydrates with different coordination numbers as references to compare with aqueous solutions containing \(\hbox {Sc}^{3+}\). The samples were studied by EXAFS and LAXS techniques, proposing an aquaion bearing six water molecules at 2.17 Å and one or two additional water molecules in apical positions, 2.32–2.5 Å, although experimental resolution precludes to assign the number of them unambiguously. The lack of a splitting in the pre-edge on the Sc K-edge XANES in the aqueous solution sample which is observed in the solid sample of the octahedral hydrate scandium salts is invoked by these authors to support a coordination higher than six [14].
Migliorati and D’Angelo have very recently carried out a MD-EXAFS study [3] finding an octahydrate with a broad Sc–O peak formed by six water molecules at 2.16 Å, two additional water molecules at 2.26 and 2.31 Å, and one apical molecule in a meso-shell [15] placed between 2.9 and 3.7 Å. This result is supported by the EXAFS analysis, where a good agreement for an octa-coordination with an average distance of 2.19 Å was obtained.
The aim of this work is to get insight into the ongoing debate on \(\hbox {Sc}^{3+}\) hydration number. To fulfill this aim we have built an ab initio intermolecular potential based on the hydrated ion approach developed by our group [16–18], but improved by the use of a polarizable and flexible water model. Two hydrates of coordination six and seven are chosen as reference to build the QM potential energy surface to be fitted. The available experimental EXAFS spectrum [11] will be compared with the simulated one derived from the MD simulation structural information.
2 Methodology
2.1 Intermolecular potential
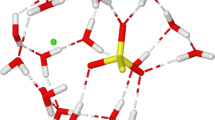
The basic idea of the hydrated ion (HI) model is the recognition that the species interacting in aqueous solution is the metal cation surrounded by a given number of water molecules [4]. The statistical implementation of the (HI) model was proposed to deal with stable aquaions, then \([\hbox {M}(\hbox {H}_{2}\hbox {O})_{\mathrm{n}}]^{\mathrm{m+}}{-}\hbox {H}_{2}\hbox {O}\), hydrated ion-water (HIW) interaction potential was built [16, 18]. A second potential \([\hbox {M}{-}(\hbox {H}_{2}\hbox {O})_{\mathrm{n}}]^{\mathrm{m+}}\) was needed to describe the intrinsic aquaion dynamics, the IW1 (ion-water \(1^{\text {st}}\) shell) potential [17, 18]. This methodology was applied to a wide set of stable aquaions, e.g., \(\hbox {M} = \hbox {Cr}^{3+}\), \(\hbox {Ir}^{3+}\), \(\hbox {Rh}^{3+}\), \(\hbox {Al}^{3+}\), \(\hbox {Mg}^{2+}\), \(\hbox {Be}^{2+}\), \(\hbox {Pd}^{2+}\), \(\hbox {Pt}^{2+}\) [15, 18, 19]. The limitation of this approach is that first-shell water molecules are different from those of bulk. That is, water exchange is not allowed during the simulation. To overcome this shortcoming of our original HI model, we developed a methodology allowing the exchange of water molecules from the first shell to the bulk [20, 21]. The ion and the water molecule were defined by means of a polarizable and flexible model, the mobile charge density harmonic oscillator (MCDHO) potential developed by Ortega-Blake et al. [22] on the basis of a mobile charge density joined to the nuclei by a harmonic oscillator. The basic idea is to develop the IW1 potential in the framework of a polarizable and flexible water model, allowing deformation and elongation of the aquaion geometry larger than those considered in the original IW1 methodology [17]. This exchangeable HI potential has been already applied to the description of trivalent lanthanoid and actinoid cations aqueous solutions [20, 21, 23], as well as to the monovalent cations of the alkaline group [24]. In this work, we have applied the exchangeable HI potential to a stable aquaion, \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_{\mathrm{n}}]^{3+}\), using a reduced prospection of the potential energy surface. Figure 1 presents the type of structures chosen and the corresponding deformation followed. The use of \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_{\mathrm{n}}]^{3+}\) aggregate as the reference structure to extract the ion-water interaction potential implies that in the QM interaction energy many-body terms up to \((n-1)\)th order are implicit. In this study we will focus our development on two aquaions which have been taken as alternative references to build two different site–site \(\hbox {Sc}^{3+}\)–\(\hbox {H}_{2}\hbox {O}\) potentials, the hexa- and the hepta-hydrated aquaion, hereafter called POT6 and POT7, respectively. Nevertheless, once provided that n is greater than a given value which allows the inclusion of the main many-body contributions, the potential behavior must be quite similar regardless the n value taken to build the potential. To check this point, we have developed two additional potentials based on \(n=4\) (POT4) and \(n=8\) (POT8). Results are given in Supplementary Material (SM), Figure S4, and both of them lead to results very similar to those obtained with POT6 and POT7. A modified version of MCDHO water model recently proposed, MCDHO2 [25], was chosen as it gives a better description of liquid water mobility.
The general expression of the exchangeable HI potential is given in the SM, together with the set of coefficients obtained for POT6 and POT7. A plot showing the goodness of fit is also included in SM. The typical deviations of these fits are 0.77 and 1.29 kcal/mol for POT6 and POT7, respectively. It is worth pointing out that for previous fitting of the (HIW+IW1) potentials of stable aquaions, which used non-polarizable models, the number of structures included in the fitting was close to 1000. In this case, only 40 structures are needed to get reliable potentials. Such a low number of structures for sampling should not be surprising because in each distorted \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_{\mathrm{n}}]^{3+}\) cluster considered there are implicitly n Sc–H2O interactions corrected from (\(n-1\))-body contributions. Therefore, 280 Sc–H2O interactions, including many-body corrections up to sixth-order were considered in the POT7 development.
Representative set of structures chosen from \(\hbox {Sc}^{3+}\) hexahydrate and heptahydrate used to build the intermolecular potentials POT6 (left) and POT7 (right). Arrows indicates the type of distortion (normal mode) considered in the hydrates. From top to bottom shortening and lengthening of only one H2O to the central cation, bending mode and asymmetric stretching mode
2.2 Computational details
QM calculations at the second order Moller-Plesset level with the aug-cc-pVTZ basis set for oxygen and hydrogen and the Stuttgart pseudopotential with the recommended basis set for the Scandium [26] were carried out to prospect the potential energy surface of the hexahydrate and the heptahydrate. Computations were carried out with the Gaussian 09 package [27].
Two molecular dynamics (MD) simulations were run for a system formed by 1 \(\hbox {Sc}^{3+}\) and 1000 MCDHO2 water molecules at 300 K in the canonical ensemble. Boxlength (31.1 Å) was set up to reproduce the liquid water density at this temperature. In one of them, POT6 was used to describe \(\hbox {Sc}^{3+}\)–H2O interactions, whereas in the second one POT7 was employed. The Noose-Hoover thermostat with a relaxation time of 0.5 ps and the Ewald sum for electrostatics were employed. The polarizable character of the particle was described by an adiabatic shell model. A timestep of 0.1 ps was needed to describe the high-frequency moves. A modified version of DLPOLY code [28] incorporating the force field used (see SM) was employed. An equilibration period of 150 ps was run before the production time. 1 ns simulation time was analyzed in each simulation. Longer simulation runs up to 5 ns were carried out to check the Sc(III) hexahydrate stability and the convergence of the present results for the case of POT6.
2.3 Simulated EXAFS spectrum
K-edge Sc experimental EXAFS spectra from \(\hbox {Sc}(\hbox {ClO}_{\text {4}})_{\text {3}}\) solution corresponds to the work of Lindqvist-Reis et al. [11]. Simulated K-edge EXAFS function has been built averaging the signal of 500 evenly distributed snapshots. Scandium, oxygen and hydrogen atoms were considered in the calculation of the backscattering potential and considering only the ion and oxygen atoms in the calculation of the scattering paths using the FEFF 9.6 code [29]. The Hedin-Lundqvist exchange-correlation potential was used to compute the electron density distribution within the SCF approach. FEFF input files for the EXAFS calculations are given in SM. The cutoff radius used to select the extension of the hydration water shell around the Sc absorber atom was large enough to include well beyond the second hydration shell (\(R_{\text {cut}} = 8\) Å, see RDF in Fig. 2). \(\Delta E_{\text {o}}\) applied for the simulated EXAFS spectrum was chosen such as it matches the first EXAFS oscillation (3–5 Å\(^{-1}\)). Previous examples of the methodology applied to others highly charged metal cations in water can be found elsewhere [20, 21, 30, 31].
3 Results
3.1 Clusters
Table 1 checks the behavior of the developed potentials to predict the interaction energies (\(E_{\text {int}}\)) of several scandium hydrates at different geometries in the gas phase. The upper part of the table compares the QM and the POT6 \(E_{\text {int}}\) for several optimized geometries of \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_{\mathrm{n}}]^{3+}\). The lower part of the table corresponds to the QM and POT7 comparison. The column headers A//B denotes A for the method used to compute \(E_{\text {int}}\) and B for the geometry optimization method. To facilitate the comparison of the MP2 results (MP2//MP2) which are the ab initio energy references for the fitting potentials, this column is repeated for POT6 and POT7 results. The agreement of \(E_{\text {int}}\) clusters provided by the two potentials at the MP2 geometries is satisfactory, the mean error being less than 2% (see POT6//MP2 and POT7//MP2 columns). Both potentials show the trend to favor the clusters where a seventh or eighth water molecule is placed in the second shell instead of remaining in the first shell. This is also the MP2 behavior and was already found by Rudoplh and Pye [2] for a set of \(\hbox {Sc}^{\text {3+}}\) hydrates at the MP2 level. When the examined structures are those optimized with POT6 and POT7 the preferred cluster with eight solvent molecules is a hexahydrate with two water molecules in the second shell (see POT6//POT6 and POT7//POT7 columns). The comparison between the clusters optimized by the two potentials as well as using the MP2 geometries shows that POT7 is a more attracting potential than POT6 by \({\sim }\)15 kcal/mol. However, the optimized geometries of the different clusters are very similar as can be verified by \(E_{\text {int}}\) values provided by the MP2 single points on the geometries optimized by POT6 or POT7. (cf. MP2//POT6 vs. MP2//POT7).
Table 2 collects the average Sc–O distance of the hexa- and hepta-hydrates obtained when the QM MP2 level is used under two conditions, isolated or in a cavity immersed in a continuum dielectric, which is described by the PCM model [32]. The table also collects the distances supplied by the optimized structure with POT6 and POT7. The hexahydrate under solvation effect introduced by the PCM approach has a distance of 2.14 Å, close to the experimental range of the aqueous scandium studies 2.15–2.18 Å, meanwhile the heptahydrate has a longer average interatomic distance 2.21 Å. As noted with \(E_{\text {int}}\) values, POT6 and POT7 give distances very close to their QM references and the difference with the distance of the hydrate for which it has not been fitted is only \({\sim }\)0.01–0.02 Å.
3.2 MD simulations
Figure 2 shows the Sc–O (red lines) and Sc–H (black lines) radial distribution functions (RDF) obtained from the simulation using POT6. Running coordination numbers are also included in the figure. The corresponding RDFs obtained from the simulation using POT7 are very similar to the function plotted in the figure, then it has not been included, but the most representative structural parameters have been incorporated into Table 3. The first conclusion is that \(\hbox {Sc}^{3+}\) aquaion is a very stable hexahydrate, predicted by both POT6 and POT7: a well-defined and narrow Sc–O peak centered at 2.15 Å is observed in the RDF which integrates to 6. It is worth pointing out that the hexacoordination is also predicted by the MD simulation where the Sc–H2O intermolecular potential was built from the hepta-hydrated structures. There is a depletion zone in the intermediate region, 2.5–3.7 Å, between the first and the second hydration shells, which indicates that no exchange between these shells took place during the 1 ns simulation time. In Table 3, the main structural and dynamical results for the POT6 and POT7 MD simulations are collected. The Sc–H first peak is very well-defined and centered at 2.85 Å. The gap of 0.7 Å with respect to the corresponding Sc–O first shell and the tilt angle of the water molecules in this shell, \({\sim }20^{\circ }\), indicate that these water molecules are arranged following an ion–dipole orientation. The depletion zone after the first peak in the Sc–H RDF confirms the stable character of \(\hbox {Sc}^{3+}\) aquaion, a hexacoordinated hydrate. The consistency of this aquaion leads to promote a very well-defined second hydration shell, where the Sc–O peak is centered at 4.3 Å and the Sc–H one at 4.9 Å, with 13–14 water molecules. Differing from the first shell, beyond the second peak the RDF minimum does not go down to zero but to 0.6, implying a significant second shell-bulk water exchange. First-shell Debye-Waller factor, DW, computed as the average of the mean-square displacements of R(Sc–O)\(_{{\text {I}}}\), \([\sigma ^2_I = \langle(R_{{\text {I}}} - \overline{R_{{\text {I}}}})^2\rangle]\), provides a value within the range of the experimental DW values obtained in the literature. The water molecule polarization in the two hydration shells is quantified by the dipole moment: A strong polarization in the first-shell, 4.6 D, with respect to the water molecule bulk value, 2.9 D is observed. Such a high dipole moment has a double origin. First of all the short ion-water distance for this trivalent cation, 2.15 Å, compared to the trivalent lanthanoid ions, where the distances are in the 2.35–2.55 Å range, and the first-shell water molecule dipole moment are in between 3.3 and 3.95 D [25, 33]. Second the metal-first-shell water molecule charge transfer is significant for this cation, but it is not included explicitly in the interaction potential. The charge transfer is then compensated by a strong interaction leading to a higher polarization of the first-shell water molecules. Second hydration shell still exhibits polarization effects as its value is 3.2D.
Regarding water molecule residence times (MRT), the only available experimental datum was given by Merbach and Helm [34], 10 ns. Since our simulation time was 1 ns and no exchange was observed, only we can state that MRT value is greater than 1 ns. Translational diffusion coefficient of \(\hbox {Sc}^{3+}\) is underestimated with respect to the experimental one (see Table 3). This difference is partly due to the fact that the MCDHO2 water model underestimates \(D_{\mathrm{H}_2\mathrm{O}}\) with respect to the bulk water experimental one, \(1.8\,vs\,2.3 \cdot\) \(10^{-5}\,\hbox {cm}^2\,\hbox {s}^{-1}\) [25], then \(\hbox {Sc}^{3+}\) aquaion is partially restrained by the low bulk water mobility.
Hydration enthalpy (\(\Delta H_{\text {hyd}}\)) is fairly well estimated by the model, an underestimation smaller than 4% is observed in the worst case. It is interesting to note that the main difference between the two developed intermolecular potentials is found in the energy, POT7 is 16 kcal/mol more stabilizing than POT6. Reexamining POT6//POT6 versus POT7//POT7 interaction energies in Table 1, one can verify that \(E_{\text {int}}\) gap is \({\sim }\)15 kcal/mol, roughly the same value found for \(\Delta H_{\text {hyd}}\) gap. The information provided by Tables 1 and 3 allows us to make an energy decomposition of the hydration energy into two contributions. The first one corresponds to \(E_{\text {int}}\), associated to \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_6]^{3+}\) aquaion formation, \(-543\) and \(-558\) kcal/mol for POT6 and POT7, respectively. The second contribution corresponds to the hydration energy of the hexahydrated aquaion, which is computed as the difference between, \(\Delta H_{\text {hyd}}\) and \(E_{\text {int}}\) of \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_6]^{3+}\) plus the corresponding thermal corrections to deal with energies at the enthalpy level. Thus, the hydration enthalpy of \(\hbox {Sc}^{3+}\) hexahydrate is \(-368\) and \(-370\) kcal/mol, for POT6 and POT7, respectively. This energy decomposition tells us that more than 60% of the aquaion stability is due to the specific interactions inside the aquaion. Bulk contribution is greater than that corresponding to the classical Born term, for a triply charged sphere of 4 Å radius (\({\sim }-190\) kcal/mol), what indicates that important first–second hydration shell interactions still contribute to the whole hydration energy.
The information provided by Tables 1, 2 and 3 indicates that POT6 and POT7 are giving almost the same values for the physicochemical properties of the ion in solution except for the interaction energy, where a threshold of \({\sim }\)15 kcal/mol shifts one energy reference from the other. This POT6–POT7 shifting represents less than 2% of the mean ion-water interaction energy operating in the system.
Figure 3 displays the power spectra of the Sc (black line) and the first-shell oxygen (red line) velocity autocorrelation functions provided by the MD simulation. We will analyze the low-frequency part of the power spectrum, which is the region where the developed Sc–H2O intermolecular potential is revealed. The results correspond to the simulation using POT6, similar spectra are obtained when using POT7 instead. The top of the figure presents spectra derived from a simulation of \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_6]^{3+}\) cluster isolated at 300K. The blue lines denote the position of the quantum mechanical MP2 frequencies of the optimized \(\hbox {Sc}^{3+}\) hexahydrate corresponding to the more representative bending and stretching intermolecular modes. The hexahydrate power spectra present two peaks at low frequency, 115 and \(180\,\hbox {cm}^{-1}\) that can be associated to bending modes, whereas at higher frequencies three bands appear at 350–385, 460 and \(510\, \hbox {cm}^{-1}\), which can be associated to the ScO stretching mode region. There is a parallelism with the QM frequencies with a blue shifting of \({\sim }50\)–70 \(\hbox {cm}^{-1}\). This is due to the difficulty of the intermolecular potential to reproduce the potential energy surface at the second derivative level. The shape of the potential energy surface in the region of one ScO stretching and OScO bending normal modes given by QM and POT6 is compared in the SM (Figures S7 and S8). It is shown that the POT6 curves do have curves leading to larger force constants and their associated frequencies. An analysis of correlation functions associated to dynamic variables of the hydrate internal geometrical parameters allows the assignment of the peak observed in the power spectra to several normal modes [18, 37]. Thus, \(370\,\hbox {cm}^{-1}\) broad band can be associated to \(E_g\) stretching mode, whereas \(460\,\hbox {cm}^{-1}\) peak is an \(A_g\) symmetric stretching mode. A final stretching mode, mixed with some bending moves, is centered at \(510\,\hbox {cm}^{-1}\). When the aquaion is immersed in the solution, the power spectra change (bottom of Fig. 3): (i) A general broadening of the narrow bands observed in gas phase; (ii) the appearance of new bands due to the intermolecular bulk water interactions incorporated, this new band appearing in the 30–100 \(\hbox {cm}^{-1}\) region. The other two bands come from the convolution of the stretching and bending modes, whose peak center is \({\sim }50\, \hbox {cm}^{-1}\) blue-shifted with respect to gas phase. Rudolph and Pye [2] studied the vibrational spectra of a 1.65 M aqueous solution of \(\hbox {Sc}(\hbox {ClO}_4)_3\) containing a perchloric acid excess to prevent hydrolysis of \(\hbox {Sc}^{3+}\) aquaion. Raman spectrum presents two bands, one of them depolarized (\(E_g\)) at \(410\,\hbox {cm}^{-1}\), and the other one, polarized (\(A_{1g}\)) at 442 cm \(^{-1}\), which corresponds to the stretching modes. A third vibrational IR active band (\(F_{1u}\)) is centered at 460 cm\(^{-1}\), which may also by assigned to a ScO asymmetric stretching mode. Bearing in mind the 50–60 \(\hbox {cm}^{-1}\) blue shifting of our power spectra, these three bands are well recognized in the gas-phase spectra. Solution of the aquaion leads to convolution of the three bands in only one broad band containing all of them. It must be pointed out that the computed power spectrum does not include the IR and Raman intensity, this being the reason precluding the implicit deconvolution of the total vibrational power spectrum provided by the experimental use of IR and Raman spectroscopies.
3.3 EXAFS spectroscopy
Figure 4 shows the comparison between experimental \(k^2\)-weighted EXAFS [11] and the simulated ones obtained using the snapshots of the simulations carried out with POT6(blue line) or POT7(red line). The agreement is remarkable, particularly in the main range [3–10.5 Å\(^{-1}\)]. At higher k values, there is experimental noise, and the signal is distorted, although the main oscillation is roughly followed by the simulated ones. Regarding the POT6–POT7 comparison, it is clear again that the structural description provided by the two developed potentials is almost the same. The fact that both spectra almost match is a sound test of the convergence of the structural result given the high sensitivity of EXAFS to the structural ensemble of Sc–O distances, coordination numbers and structural and dynamical disorder. The fairly good agreement strongly supports one option for the open question on \(\hbox {Sc}^{3+}\) hydration, i.e., \(\hbox {Sc}^{3+}\) aquaion is an hexahydrate. The angle distribution function (ADF) OScO derived from the MD trajectory is shown in SM (Figure S9). The distribution shows two well-defined peak centered at 90\(^\circ\) and 180\(^\circ\) with a ratio 4:1, respectively. This is just the expected distribution for an octahedron. Migliorati and D’Angelo [3] have recently published an study where a Sc–H\(_2\)O intermolecular potential was built to carry out classical MD simulations which resulted in an octa-coordination for \(\hbox {Sc}^{3+}\). They simulated the EXAFS spectrum using a strategy similar to that here presented. They display in Figure 3 of their work [3] a good agreement with the experimental EXAFS spectrum, but in a k-range [5–10 Å\(^{-1}\)] shorter than the range shown in Fig. 4. The exclusion of this important low-k part of the spectrum explains the different final conclusion reached by these authors with respect to that here proposed.
4 Concluding remarks
This work has developed a modified version of the hydrated ion model to build highly charged metal cation–water intermolecular potentials and shown its good performance. The key-point of the new procedure is the combination of a polarizable and flexible water model (MCDHO2) and the explicit consideration of the ensemble of metal cation–water interactions present in a given aquaion, \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_{\mathrm{n}}]^{3+}\). Two intermolecular potentials have been built by fitting the MP2 interaction energies of \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_6]^{3+}\) or \([\hbox {Sc}(\hbox {H}_{2}\hbox {O})_7]^{3+}\) clusters. Only 40 structures have been needed to obtain reliable intermolecular potentials. This number could seem low for the usual sampling, but it must be pointing out that each structure considered in the sampling contains 6 or 7 Sc–H2O interactions. Likewise, the polarizable character of the particles adds intrinsic ability to be well-adapted to non-explored arrangements. Both MD simulations supply the same structural and dynamical description of \(\hbox {Sc}^{3+}\) aqueous solution. The average Sc–O first-shell distance is \(2.15 \pm 0.01\) Å and the hydration number is 6. The good agreement of the simulated vibrational power spectra and the EXAFS spectrum with the corresponding experimental information, together with the fair agreement of the computed hydration enthalpy, strongly support the developed potential, the new methodology, and the results derived from the MD simulation. The ion radius size invoked to expect a higher hydration number does not seem to be confirmed. Differing from some previous works [3, 5, 9] where hepta- or octa-coordinations were proposed for \(\hbox {Sc}^{3+}\), we found a metal cation behavior close to that of other small and hard trivalent cations, such as \(\hbox {Cr}^{3+}\) or \(\hbox {Al}^{3+}\), in agreement with Rudolph and Pye [2] and the theoretical proposal of Rotzinger [10].
References
Cotton S (1999) Polyhedron 18(12)
Rudolph WW, Pye CC (2000) J Phys Chem A 104:1627
Migliorati V, D’Angelo P (2016) Inorg Chem 55:6703
Richens DT (1997) The chemistry of aqua ions. Wiley, Chichester
Kanno H, Yamaguchi T, Ohtaki H (1989) J Phys Chem 93:1695
Yamaguchi T, Niihara M, Takamuku T, Wakita H, Kanno H (1997) Chem Phys Lett 274:485
Smirnov P, Wakita H, Yamaguchi T (1998) J Phys Chem B 102:4802
Smirnov P, Trostin T (2011) Russ J Gen Chem 83(1):18
Vchirawongkwin V, Kritayakornupong C, Tongraar A, Rode BM (2012) Dalton Trans 41:11889
Rotzinger FP (1997) J Am Chem Soc 119:5230
Lindqvist-Reis P, Persson I, Sandström M (2006) Dalton Trans 28(32):3868
Sandström M, Persson I, Jalilehvand F, Lindqvist-Reis P, Spanberg D, Hermansson K (2001) J Synchrotron Rad 8:657
Abbasi A, Lindqvist-Reis P, Eriksson L, Sandström D, Lidin S, Persson I, Sandström M (2005) Chem Eur J 11:4065
Yamamoto T (2008) X-Ray Spectrom 37:572
Martínez JM, Torrico F, Pappalardo RR, Sánchez Marcos E (2004) J Phys Chem B 108:15851
Pappalardo RR, Sánchez Marcos E (1993) J Phys Chem 97:4500
Martínez JM, Pappalardo RR, Sánchez Marcos E (1998) J Chem Phys 109:1445
Martínez JM, Pappalardo RR, Sánchez Marcos E (1999). J Am Chem Soc 121:3175
Torrico F, Pappalardo RR, Sánchez Marcos E, Martínez JM (2006) Theor Chem Acc 115:196
Galbis E, Hernández-Cobos J, den Auwer C, Naour CL, Guillaumont D, Simoni E, Pappalardo RR, Sánchez Marcos E (2010) Angew Chem Int Ed 22:3811
Galbis E, Hernández-Cobos J, Pappalardo RR, Sánchez Marcos E (2014) J Chem Phys 140:214104
Saint-Martin H, Hernández-Cobos J, Bernal-Uruchurtu MI, Ortega-Blake I, Berendsen HJC (2000) J Chem Phys 113:10899
Morales N, Galbis E, Martínez JM, Pappalardo RR, Sánchez Marcos E (2016) J Phys Chem Lett 7:4275
Caralampio DZ, Martínez JM, Pappalardo RR, Sánchez Marcos E (2017) Submitted for publication
Villa A, Hess B, Saint-Martin H (2010) J Chem Phys 133:044509
Dolg M, Weding U, Stoll H, Preuss H (1987) J Chem Phys 86:866
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 Revision D.01. Gaussian Inc. Wallingford CT
Smith W, Forester T, Todorov IT (2012) The dl_poly classic. STFC Daresbury Laboratory, Daresbury (UK)
Rehr JJ, Kas JJ, Vila FD, Prange MP, Jorissen K (2010) Phys Chem Chem Phys 12:5503
Merkling PJ, Muñoz-Páez A, Sánchez Marcos E (2002) J Am Chem Soc 124:10911
Carrera F, Torrico F, Richens DT, Muñoz-Paez A, Martínez JM, Pappalardo RR, Sánchez Marcos E (2007) J Phys Chem B 111:8223
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999
Terrier C, Vitorge P, Gaigeot MP, Spezia R, Vuilleumier R (2009) J Phys Chem B 113:7270
Helm L, Merbach A (2005) Chem Rev 105:1923
Marcus Y (2015) Ions in solution and their solvation. Wiley, New York
Helm L, Merbach A (1999) Coord Chem Rev 187:151
Irish DE, Brooker MH (1976) Advances in infrared and Raman spectoscopies. Heyden, vol 2, chap 6
Acknowledgements
We thank Prof. Persson for providing us with the original Sc K-edge EXAFS spectrum of Scandium aqueous solution published in Ref. [11]. We acknowledge the Spanish Junta de Andalucía for financial support (Proyecto de Excelencia, P11-FQM7607) and DZC for a predoctoral grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles derived from the 10th Congress on Electronic Structure: Principles and Applications (ESPA-2016).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Caralampio, D.Z., Martínez, J.M., Pappalardo, R.R. et al. Development of a polarizable and flexible model of the hydrated ion potential to study the intriguing case of Sc(III) hydration. Theor Chem Acc 136, 47 (2017). https://doi.org/10.1007/s00214-017-2075-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-017-2075-1