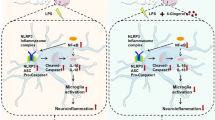
Abstract
Rationale
Microglia-mediated neuroinflammation is a vital hallmark in progression of depression, while calcitriol exerts anti-inflammatory effects in the brain. The activation of the P2X7 receptor has an important link to neuroinflammation. However, it is unclear whether calcitriol treatment exerts anti-inflammatory effects in association with P2X7R activation.
Objective
In this study, we assessed the antidepressive and neuroprotective effects of calcitriol on lipopolysaccharide (LPS)-mediated depressive-like behavior, neuroinflammation, and neuronal damage.
Methods
In in vitro experiments, the BV2 cells were exposed to LPS, and the protective effects of calcitriol were assessed. For in vivo experiment, thirty-two male C57BL/6 mice were divided into four groups of control, calcitriol, LPS and LPS + calcitriol. Calcitriol was administered at 1 µg/kg for 14 days and LPS at 1 mg/kg once every other day for 14 days. The control group mice were given equal volumes of vehicles. All treatments were delivered intraperitoneally.
Results
The in vitro experiments showed calcitriol inhibited the release of inflammatory mediators induced by LPS in BV2 cells. The in vivo experiments revealed that calcitriol alleviated LPS-induced behavioral abnormalities and spatial learning impairments. Moreover, calcitriol treatment reduced the mRNA levels of pro-inflammatory cytokines, while increasing anti-inflammatory cytokine levels in the hippocampus. Our results further revealed that calcitriol administration attenuated LPS-induced microglia activation by suppressing P2X7R/NLRP3/caspase-1 signaling. Moreover, calcitriol inhibited apoptosis of neurons in the hippocampus as evidenced by expression of apoptosis-related proteins and TUNEL assay.
Conclusions
Collectively, our findings demonstrated that calcitriol exerts antidepressive and neuroprotective effects through the suppression of the P2X7R/NLRP3/caspase-1 pathway both in LPS-induced inflammation models in vitro and in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression, a prominent psychiatric malady, stands as a profound nexus of affliction, yielding substantial rates of morbidity and mortality (Wittenborn et al. 2015). This insidious condition intimately intertwines with inflammation and the activation of microglia, as elucidated by Miller (Miller 2020). Individuals grappling with depression manifest an upsurge in proinflammatory cytokines, including IL-6, IL-1β, and TNF-α (Rizavi et al. 2016). Furthermore, the specter of depression looms prominently amongst those beset by chronic inflammatory ailments, as underscored by Han et al. (Han et al. 2019). Microglia, the cerebral realm’s indigenous immunological custodians, constitute a formidable fraction, comprising approximately 10–15% of the cerebral cellular populace. Their farreaching influence extends to cerebral maturation, neurological functionality, and active participation in the domain of injury and malaise, as expounded by Hammond et al. (Hammond et al. 2019). Under conditions of neuroinflammation, these microglial entities undergo widespread activation, precipitating neuronal detriment through a perturbed production of pro-inflammatory cytokines. (Saxena et al. 2021). The infestation of neuroinflammation within the central nervous system instigates a cascade of neurochemical and neuroendocrine alterations that significantly contribute to the shroud of depression. (Jeon et al. 2018). Consequently, a judicious modulation of neuroinflammation emerges as a viable and auspicious therapeutic avenue in the amelioration of depression.
Vitamin D (VD) is a fat-soluble vitamin and steroid hormone widely implicated in maintenance of calcium homeostasis, and is also acknowledged for its various functions in both cells and tissues (Cui et al. 2019). This essential vitamin is acquired in a biologically inert state either through dietary intake or via dermal exposure to ultraviolet light. Subsequently, it undergoes two hydroxylation steps to assume its active form, 1,25-dihydroxyvitamin D3 (calcitriol) (Jiang et al. 2014). Particularly notable is the correlation between VD deficiency and an escalated susceptibility to neurodegenerative maladies (Balion et al. 2012). In parallel, studies using murine models of Alzheimer’s disease reveal that VD insufficiency exacerbates Alzheimer’s disease-like pathologies, primarily through the promotion of inflammatory stress (Fan et al. 2020a, b). A recent publication illuminated the potential of VD in partially ameliorating neurochemical alterations linked to behavioral anomalies in Parkinson’s disease (Lima et al. 2018). This notable finding establishes a promising avenue for leveraging VD as a mitigating agent against the neurochemical aberrations. Furthermore, Calvello and co-researchers (Calvello et al. 2017) demonstrated that the administration of calcitriol, the active form of VD, exhibited discernible neuroprotective properties in animal models. This neuroprotection was chiefly achieved through the astute attenuation of pro-inflammatory responses and the facilitation of anti-inflammatory processes, presenting a hopeful prospect for future therapeutic strategies.
Purinergic signaling systems play a pivotal role in mediating depressive behaviors and regulating neurotransmission (Wang et al. 2020). Particularly, the purinergic P2X7 receptor (P2X7R), a non-selective ion channel activated by adenosine triphosphate (ATP), demonstrates permeability to calcium ions (Ca2+), sodium ions (Na+), and potassium ions (K+) P2X7R remarkably incites inflammatory responses and modulates immunity within both the central and peripheral nervous systems. (Shen et al. 2021). During instances of inflammation, extracellular ATP concentrations experience an elevation, consequently activating P2X7R and instigating the assembly of the NLRP3 inflammasome (X. Fan et al. 2020b). This inflammasome comprises diverse proteins, including NLRP3, the adaptor molecule apoptosis-related speck-like protein containing a caspase recruitment domain (ASC), and pro-caspase-1 (Swanson et al. 2019). Activation of caspase-1 through the inflammasome sets off the maturation and secretion of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and interleukin-18 (IL-18), thereby promoting an inflammatory response (Yan et al. 2012). Elevated serum levels of NLRP3 and pro-inflammatory mediators are observed in patients exhibiting neuroinflammation (Piancone et al. 2021). Furthermore, investigations have illuminated that there exists an upregulation in the levels of NLRP3 and pro-caspase-1 within models of inflammation induced by lipopolysaccharide (LPS), as expounded upon by the work of Liu et al. (LiuZhang et al. 2021b). Moreover, a correlation has been observed, wherein neuronal apoptosis intertwines with the P2X7R/NLRP3 pathway, within a murine model depicting focal cortical ischemic stroke (Ye et al. 2017).
The aim of this study was to clarify the neuroprotective function and antidepressant effect of calcitriol and to determine the molecular mechanism of calcitriol against inflammatory response, we investigated the effects of calcitriol administration on LPS-mediated depressive-like behaviors, activation of microglia, as well as neuronal damage. We further sought to determine the underlying neuroprotective mechanisms, including the possible involvement of the P2X7R/NLRP3/caspase-1 pathway in mediating calcitriol effects.
Materials and methods
Cell culture and treatment
Mice BV2 microglial cells were procured from Procell Life Sciences & Technology Co., Ltd (Wuhan, China), inoculated in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) as well as 1% antibiotics followed by incubation at 37 °C in a humid environment with 5% CO2. Then, cells were exposed to different calcitriol (Fig. 1a) doses (0.1, 1, 2.5, 5 and 10 μM) for 24 h and allocated into six groups: control group, calcitriol group, LPS group (500 ng/ml), LPS + calcitriol group, LPS + BBG (1 µM) group, and LPS + MCC950 (1 µM) group. The experimental design for the in vitro protocol is shown in Fig. 1b.
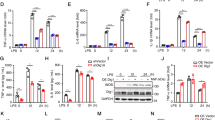
The structure of calcitriol a. The experimental design for the in vitro experimental b. Cell viability c and LDH release d after calcitriol treatment. iNOS protein expression e. mRNA levels of TNF-α f, IL-6 g, and IL-1β h. Effect of calcitriol or LPS treatment on ATP level i. ns: no significance. **p relative to control group. ##p relative to LPS group
Animals and treatment
Thirty-two male C57BL/6 mice were acquired from the Jinan Pengyue Experimental Animal Breeding Co. Ltd (Jinan, China) and maintained under standard conditions (24–25 °C temperature; 50–60% humidity; 12:12-h light/dark cycle) with water and food access. Mice were randomized into four groups (n = 8): a control, calcitriol, LPS, and LPS + calcitriol groups. The calcitriol group mice were intraperitoneally administered with calcitriol (dissolved in 5% dimethyl sulfoxide, 1 µg/kg) (for 14 days, while control group mice were given equal volumes of vehicles (5% dimethyl sulfoxide in saline). LPS (dissolved in 0.9% saline, 1 mg/kg) was administered by intraperitoneal administration every other day for a combined total of 7 injections. These assays were done as previously reported (Yang et al. 2020). The LPS + calcitriol group was subjected to daily calcitriol treatment and LPS injection. Throughout the experiment, mice were assessed daily for variations in body weights. The experimental schedule is shown in Fig. 2a. After the last behavioral test, mice were euthanized by cervical dislocation, after which brain tissues were quickly resected followed by rapid dissection of the hippocampus on ice.
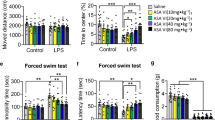
The experimental schedule of in vivo experiment a. The effects of LPS and calcitriol on body weights and behavioral tests. Body weight b. Sucrose preference c. Immobility time d. Representative heatmap of mice in EMP test e: time spent in open arms f, number of open arm entries g. ns: no significance. **p relative to control group. ##p relative to LPS group
Analysis of cell viability and cytotoxicity
This assay was done by a Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China). The BV2 cells were inoculated in a 96-well culture (1 × 104 cells/well) and treated for 24 h with various calcitriol doses. Then, 10 µl of the CCK-8 solution was added to every well, followed by 2 h of incubation at 37 °C. Absorbance was read at the wavelength of 450 nm. For cytotoxicity assay, LDH release was assessed using LDH Cytotoxicity Assay Kit (Beyotime, Shanghai, China). The BV2 cells were inoculated in a 96-well culture (1 × 104 cells/well) and treated for 24 h with various calcitriol doses, the supernatant was added to a new plate, after the incubation with working solution according to the protocol. Absorbance was read at the wavelength of 490 nm.
Sucrose preference test (SPT)
The Sucrose preference test was performed to evaluate anhedonia as described previously (Dang et al. 2017). Before the SPT, mice were separately housed and habituated to 48 h of consumption of a 1% sucrose solution from two bottles, one placed on each side of the cage. After mice adaptation, water deprivation was done for 14 h, after which they were presented with two pre-weighed bottles, one with water and the other with 1% sucrose solution, for 1 h. To avoid spatial bias, sides on which each bottle was placed was randomized. After the test, bottles were again weighed and weight differences taken as the intake from every bottle. Sucrose preferences were calculated as: sucrose intake/ (sucrose intake + water intake) × 100%.
Forced swimming test (FST)
The forced swimming test is a commonly used method for evaluating depressive-like behavior in rodents (Wang et al. 2023). Mice were independently positioned in a plastic cylinder (height: 45 cm, diameter: 25 cm) with about 35 cm of water (24 ± 1 °C). After 15 min, mice were removed from water, dried using towels, and placed in their cages. The next day, mice were again placed in the same experimental environments and subjected to 5 min of an FST. Recording of the test was done using a camera placed above the cylinder. To eliminate odors, water was renewed after each test. The observer was blinded to experimental conditions. Mice were said to be immobile when they stopped struggling in the water and floated in upright positions or only made small movements to maintain its head above water.
Elevated plus maze (EPM) test
EPM apparatus was composed of polypropylene and was placed 50 cm above the floor as previous method (Zhang et al. 2019). The apparatus was made of a cross-shaped platform comprising 2 opposing open arms (OAs, 50 × 10 cm) perpendicular to 2 opposing closed arms (CAs, 50 × 10 cm) with a small central platform (CP, 10 × 10 cm) between the arms. The walls of arms were 40 cm in height. Initially, every mouse was positioned at the maze’s center, facing an open arm, and was permitted 5 min of exploring the maze. The counts of entries into OAs as well as CAs and amounts of time spent in every arm during the assay were documented using an overhead camera.
Open field test (OFT)
The Open field test was conducted according to a classic paradigm with minor modifications (Lee et al. 2022). The OFT was performed in a square arena (90 × 90 × 45 cm) with the floor divided into 16 equal squares. The 4 squares in the center of the test arena were referred to as the central squares. Mice were placed into the center of the square and allowed to explore freely for 5 min. The total travelling distance, the duration in the central zone and rearing (standing on their hind paws) were documented using an overhead camera.
Morris water maze (MWM) test
As previous description (Cui et al. 2021), the assay was conducted in a plastic pool (diameter of 120 cm and height of 50 cm) with opaque water (24 ± 1 °C). The maze was separated into 4 quadrants with a hidden platform (diameter of 10 cm), placed in one quadrant 1 cm below the water surface. In the learning phase, at the start of every trial, mice were individually positioned in the pool facing the wall. Start location differed for every trial. Each mouse was permitted 60 s for swimming to allow the location of the hidden platform. If the platform was found by a mouse, it was permitted to stay on it for 10 s. However, if the platform was not found within the 60 s, mice were guided onto the platform and permitted to rest there for 30 s. After training, latency time for finding the hidden platform was recorded. A probe test was then done 1 day after the last orientation navigation trial. The platform was detached and the mice permitted 60 s of swimming freely. Time spent in target quadrant and the counts of times each mouse crossed the place where hidden platforms had been placed were recorded. Behavioral tests were documented using video tracking software.
Real-time reverse transcription-quantitative PCR (RT-qPCR)
Total RNA isolation from cells and hippocampal tissue was done by the Trizol reagent (Tiangen, Beijing, China), as detailed by the manufacturer. RNA integrity as well as concentration were determined by electrophoresis and spectrophotometric analysis. Reverse transcription of RNA into cDNA was dine using the FastKing gDNA Dispelling RT SuperMix (TIANGEN). qPCR was conducted using SuperReal PreMix Plus (TIANGEN) in a Cfx96 Detection System (Bio-Rad, USA). Primers for this assay are presented in Table 1. Reaction parameters comprised a preincubation step of 15 min at 95 °C, 40 denaturation cycles for 10 s at 95 °C, and an annealing, elongation step at 63 °C for 30 s. PCR reactions were run in triplicates. Normalization of mRNA levels was done to β-actin. The 2 −ΔΔCq method was used for data analyses.
Western blot analysis
Total protein extraction from cells and hippocampal tissues were done using a RIPA lysis buffer (Solarbio, Beijing, China) and their concentrations evaluated by a BCA protein assay kit (Solarbio). Then, separation of 30 µg of proteins was done by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and moved to PVDF membranes. After an hour of blocking in 5% non-fat dry milk, overnight membrane incubation at 4 °C was done with primary antibodies targeting caspase-1 (22915-1-AP, 1:1,000), iNOS (18985-1-AP, 1:1,000), cleaved caspase-3 (19677-1-AP, 1:1,000), β-actin (20536-1-AP, 1:2,000) (all from Proteintech); P2X7R (SC-514,962, 1:1,000) and ASC (SC-514,414, 1:1,000), from SCB; and NLRP3 (AF2155, 1:1,000), BAX (AF0054, 1:1,000), and Bcl-2 (AF6285, 1:1,000) (all from Beyotime). After washing, incubation with an HPR-conjugated secondary antibody (Signaling Technology, 7074 S, 7076P2, 1:2,000) was done for an hour at room temperature (RT). Signals were detected by the ECL kit (Biosharp, Beijing, China) and the intensity of the blots was analyzed by ImageJ. For quantification, signals were normalized to β-actin expressions, the internal control.
ATP contents analysis
Fresh brain tissue lysate or cell lysate adenosine triphosphate (ATP) contents were measured using colorimetric assay kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instruction. Briefly, for tissue samples, the weight was measured and added to nine times the volume of double-distilled water. The mixture was homogenized in an ice-water bath to create a 10% homogenate. It was then boiled in a water bath for 10 min, followed by centrifugation at 3500 rpm for 10 min to separate the supernatant for further analysis. For culturing cells, the cells were first centrifuged to separate them from the culture supernatant. The collected cells were then mixed with 300 to 500µL of double-distilled water and homogenized in an ice-water bath. Subsequently, the cell suspension was heated in a water bath for 10 min and is ready for analysis.
Immunohistochemical staining
Isolated hippocampi were fixed in paraformaldehyde (4%), paraffin-embedded, and prepared for histological assaying, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining, as well as immunohistochemical staining. For histology, a sledge microtome was used to prepare 5-µm-thick slices from the paraffin tissue blocks. The sections were hematoxylin-stained and counterstained with eosin.
For the TUNEL assay, paraffin-embedded tissue slices (5 μm) were dewaxed and rehydrated after which they were subjected to a TUNEL assay (Beyotime) for the assessment of apoptosis. The Olympus BX53 inverted fluorescence microscopy (Olympus, Tokyo, Japan) was performed to capture images.
Immunofluorescence
For immunofluorescence staining, dewaxing of 5-µm-thick paraffin sections was done using xylol, rehydrated, and subjected to antigen retrieval according to standard procedures. After three washes with PBS, the slices were blocked for an hour using 5% normal goat serum in PBS at RT, and incubated with anti-Iba-1 antibodies (Abcam, ab178846, 1:200) overnight at 4 °C. Then, sections were washed using PBS, incubated in the presence of Cy3-labeled goat anti-rabbit IgG (Beyotime, A0516, 1:1,000) at RT for 1 h. Immunofluorescence images were obtained by inverted fluorescence microscopy (IX53, Olympus).
Statistical analysis
The GraphPad Prism 8 software was used for data analyses, which are shown as means ± SD. Before applying parametric statistics, all variables were checked the normality of the data distribution. One-way analysis of variance (ANOVA) was used to evaluate statistical differences. When the F ratios were significant, post hoc comparisons were made using the post hoc test. p < 0.05 denoted significance.
Results
Calcitriol inhibited the LPS-induced pro-inflammatory reactions in BV2 cells
Calcitriol treatment (0.1μM, 1 μM and 2.5μM) had no effect on BV2 cell viability (Fig. 1c, F = 5.595 p*=0.0218 p**=0.0026). However, there was no difference in LDH levels between LPS group and LPS + calcitriol (0.1μM) group (Fig. 1d, F = 17.24 p**<0.0001 p#=0.025 p#=0.0462). Based on the results of the assays, calcitriol treatment with 1μM was selected for follow-up experiment to demonstrate the protective effects in vitro. LPS-stimulated activation of microglia resulted in pro-inflammatory cytokine secretion, leading to cellular damage (Lee et al. 2019). Accordingly, we evaluated pro-inflammatory cytokine levels in LPS-exposed BV2 cells. Exposure to calcitriol markedly suppressed iNOS protein levels (Fig. 1e, F = 13.00 p**=0.0003 p##=0.0048) as well as mRNA levels of TNF-α, (Fig. 1f, F = 17.23 p**0.0001 p##=0.0053), IL-1β (Fig. 1f, F = 18.42 p**<0.0001 p##=0.0063), and IL-6 (Fig. 1h, F = 18.19 p**<0.0001 p##=0.0072). Thus, calcitriol exerts anti-inflammatory outcomes in activated microglia. Additionally, the effects of calcitriol treatment on LPS-induced ATP content were investigated. The results demonstrated a significant reduction in ATP levels in cells exposed to LPS when treated with calcitriol (Fig. 1i, F = 29.70 p**<0.0001 p##<0.0001). The reduction in ATP content suggests that calcitriol may possess a potential regulatory role in mitigating the inflammatory response induced by LPS. These finding holds promise for the therapeutic application of calcitriol in conditions characterized by associated inflammatory processes, offering a potential avenue for further research into the anti-inflammatory properties of calcitriol.
Calcitriol inhibited P2X7R/NLRP3/caspase-1 signaling in BV2 cells treated with LPS
P2X7R and NLRP3 inflammasome are highly associated with activation of inflammatory responses. To investigate whether P2X7R and the NLRP3 inflammasome play roles in mediating anti-inflammatory effects of calcitriol, we assessed the effects of BBG (antagonist of P2X7R), MCC950 (NLRP3 inflammasome inhibitor), and calcitriol on LPS-treated microglia. For this, we tested the levels of related proteins (Fig. 3a). Calcitriol suppressed the LPS-mediated increase in P2X7R (Fig. 3b, F = 26.38 p**<0.0001 p##=0.0096 p##<0.0001), NLRP3 (Fig. 3c, F = 11.29 p**<0.0001 p##=0.0030 p##=0.0012 p$$=0.0002), caspase-1 P20 (Fig. 3d, F = 17.73 p**<0.0001 p##=0.0084 p##=0.0002 p##=0.0010), and ASC (Fig. 3e, F = 33.02 p**<0.0001 p##=0.0003 p##<0.0001 p##=0.0002) protein levels in BV2 cells; comparable inhibitory effects on levels of these proteins were observed following BBG or MCC950 treatmen.
Effects of calcitriol on LPS-induced body weight loss and behavioral activity
Two weeks of LPS treatment markedly reduced body weights, relative to controls, interestingly, the combination of calcitriol and LPS lost less weight than the LPS-treatment mice (Fig. 2b, F = 6.282 p** =0.0075 p#=0.0368). The LPS-treated mice showed a reduced sucrose preference in SPT (Fig. 2c, F = 15.71 p**<0.0001 p##=0.0074), and displayed a marked increase in immobility time in FST (Fig. 2d, F = 10.97 p**<0.0001 p##=0.0082). Our results showed that co-treatment with calcitriol abrogated LPS-induced behavioral abnormalities in SPT and FST. Relative to control group, calcitriol alone did not affect the body weight, sucrose preference and immobility time. Mice were further tested in the EPM for anxiety-like behavior (Fig. 2e). The results showed that, compared with control animals, LPS group mice spent less time in the open arm (Fig. 2f, F = 21.37 p** <0.0001 p##=0.0003) and had fewer open arm entries (Fig. 2g, F = 16.24 p**<0.0001 p#=0.0122). As expected, mice in the LPS + calcitriol group entered the open arm more often and spent more time in open arms, relative to those in LPS treatment group. Differences in behaviors between the control and calcitriol group were not significant. Thus, calcitriol alleviates LPS-mediated behavioral changes. Moreover, our studies have demonstrated that administering calcitriol can mitigate the depressive-like behaviors induced by LPS in mice during the open field test (Fig. 4a). In this test, mice are placed in a novel and open environment, and their exploratory and locomotor activities are observed. Calcitriol administration has been associated with increased locomotor activity, reduced anxiety-like behavior, and enhanced exploration, suggesting its potential in ameliorating the depressive effects induced by LPS (Fig. 4b, F = 15.57 p** <0.0001 p##=0.0039; Fig. 4c, F = 20.94 p** <0.0001 p##<0.0001; Fig. 4d, F = 27.95 p** <0.0001 p##=0.0002). These findings offer valuable insights into the potential therapeutic application of calcitriol in mitigating depressive symptoms associated with neuroinflammatory processes.
Representative tracks of mice in OFT a. Total travelling distance of mice in OFT b. duration of mice staying in center zone in OFT c. The number of rearing of mice in OFT d. Representative trace depicting the paths of the mice in the orientation navigation test e. The time course learning curve revealing latency for platform location from day 10 to day 14 f. Time spent in quadrant where the platform had been placed g. Counts of crossings over location where the platform had been placed h. Average speed in the test i. ns: no significance. **p relative to control group. ##p relative to LPS group
The effect of calcitriol on cognition in LPS-treated mice
The MWM test for spatial learning abilities revealed that LPS-treatment mice had a longer escape latency, relative to control group; but, co-administration with calcitriol visibly shortened escape latency and reduced the number of escape failures. Differences in escape latency between the calcitriol and control groups were insignificant (Fig. 4e, f). Upon the removal of the escape platform, LPS group mice spent less amounts of time in the target quadrant and displayed a lower frequency of crossing the platform site relative to control group mice. Moreover, calcitriol co-treatment led to a notable improvement in spatial impairment in LPS-treated mice (Fig. 4g, F = 29.59 p** <0.0001 p##=0.0002; Fig. 4h, F = 17.75 p** <0.0001 p##=0.0248). Differences in average swimming speed among all the groups were insignificant (Fig. 4i). Overall, these data indicated that calcitriol can improve the LPS-induced impairment in spatial learning ability in mice.
The effects of calcitriol on activation of microglia in brains of LPS-treated mice
To assess if calcitriol can alleviate the LPS-mediated activation of microglia in vivo, immunofluorescence staining of Iba-1, microglial activation marker was performed in hippocampal tissue of mice from the different treatment groups. Differences between the control and calcitriol groups in the number of Iba1-reactive cells were not significant, while, co-calcitriol treatment reduced the counts of LPS-induced Iba1-reactive cells (Fig. 5a, b, F = 14.42 p** =0.0049 p##=0.0063). These findings revealed that calcitriol can ameliorate LPS-induced activation of microglia in mice.
Effects of calcitriol on LPS-mediated brain inflammation
The results of the in vitro experiments revealed that calcitriol inhibited pro-inflammatory responses in LPS-activated microglia. Next, we further evaluated the anti-inflammatory effect of calcitriol in vivo by measuring the mRNA levels of TNF-α (Fig. 5c, F = 17.29 p** <0.0001 p##=0.0037), IL-1β (Fig. 5d, F = 31.50 p** <0.0001 p##=0.0011) and IL-6 (Fig. 5e, F = 30.76 p** <0.0001 p##=0.0091) in hippocampal tissue of mice from various groups. The transcript levels of pro-inflammatory markers were markedly upregulated by LPS, relative to control group, but the LPS + calcitriol group exerted obvious decrease in transcript levels, relative to LPS group. Similarly, co-treatment with calcitriol elevated the mRNA expressions of IL-10 (Fig. 5f, F = 24.40 p**=0.0028 p##=0.0036), CD206 (Fig. 5g, F = 22.43 p**<0.0001 p##=0.0016) and Ym-1 (Fig. 5h, F = 37.18 p** <0.0001 p##=0.0002), relative to LPS group. Thus, calcitriol attenuates pro-inflammatory responses in brains of LPS-treated mice.
Effects of calcitriol on P2X7R/NLRP3/caspase-1 pathway activation in brains of LPS-treated mice
Given our in vitro results, we surveyed whether calcitriol modulates the P2X7R/NLRP3/caspase-1 pathway in vivo (Fig. 6a). LPS treatment upregulated P2X7R (Fig. 6b, F = 25.73 p** <0.0001 p##=0.0031), NLRP3 (Fig. 6c, F = 30.06 p** <0.0001 p##=0.0022), caspase-1 P20 (Fig. 6d, F = 18.31 p** <0.0001 p##=0.0084) and ASC (Fig. 6e, F = 24.98 p** <0.0001 p##=0.0056) levels, relative to control group, however, these effects were offset by calcitriol supplementation in the LPS + calcitriol group. Differences in results between control group and calcitriol group were insignificant. Thus, calcitriol regulates P2X7R/NLRP3/caspase-1 signaling in brains of LPS-treatment mice.
Representative images of western blot a. The protein levels of P2X7R b, NLRP3 c, caspase-1 P20 d, and ASC e in the hippocampus were detected by Western blot. Effect of calcitriol or LPS treatment on ATP level f. Representative images g and statistics h of hematoxylin and eosin (H&E) staining in hippocampal brain slices. Bar = 50 μm. Representative images g and statistics i of TUNEL staining in hippocampus. Bar = 50 μm. Representative images of western blot j. The effects of LPS and calcitriol on protein levels of Bcl-2 k, BAX l and cleaved caspase-3 m were detected by Western blot. ns: no significance. **p relative to control group. ##p relative to LPS group
Effects of calcitriol on neuronal damage and apoptosis in LPS-treatment mice brains
Calcitriol treatment on ATP levels induced by LPS in the hippocampus of mice. The results indicated a decrease in ATP content within the hippocampal region of mice exposed to LPS when treated with calcitriol (Fig. 6f, F = 24.12 p* = 0.0114 p#=0.0009). These findings emphasize the importance of further investigating its neuroprotective and anti-inflammatory properties in the brain. Relative to control animals, hippocampal neurons of mice in LPS group showed acidophilic degeneration and nuclear condensation; however, calcitriol administration significantly reversed these effects. Differences in neuronal morphology between calcitriol and control groups were insignificant (Fig. 6g, h, F = 63.81 p** <0.0001 p##=0.0004). We further determined apoptosis levels in the hippocampus by TUNEL assay. Following LPS administration, cells in the hippocampus underwent increased cell death; however, calcitriol co-administration could reverse these effects (Fig. 6g, i, F = 14.54 p** =0.0019 p##=0.0063). To further confirm this result, we measured Bcl-2 (Fig. 6k, F = 16.88 p** <0.0001 p##=0.0095), BAX (Fig. 6l, F = 13.51 p** =0.0003 p##=0.0041) and cleaved caspase-3(Fig. 6m, F = 13.50 p** =0.0001 p#=0.0121) protein levels. After LPS treatment, proapoptotic proteins (cleaved caspase-3 and BAX) were elevated, whereas that of the antiapoptotic protein Bcl-2 was decreased; however, calcitriol co-administration greatly mitigated these effects.
Discussion
Studies have reported the existence of a strong link between changes in immune system function and depression (Dantzer et al. 2008). Pro-inflammatory cytokines are increased in depression patients, while inhibition of pro-inflammatory cytokine production can attenuate depressive symptoms (P et al. 2014). Neuroinflammation is a crucial pathological process that can result in central nervous system damage as well as injury (DiSabato et al. 2016). NLRP3 inflammasome activation enhances pro-inflammatory cytokine levels and, consequently, neuroinflammatory responses (Zhang et al. 2015). Vitamin D deficiency is highly associated with the pathogenesis of neuroinflammation and depression (Koduah et al. 2017). To investigate the antidepressive and neuroprotective effects of calcitriol as well as determine the inflammation-related mechanisms underlying these effects, we established LPS-induced inflammation models in vitro and in vivo. Calcitriol was shown to attenuate LPS-induced neuroinflammation, via calcitriol-mediated suppression of P2X7R/NLRP3/caspase-1 signaling.
The P2X7R, NLRP3 inflammasome, and caspase-1 are components of an intricate signaling pathway associated with inflammation and immune responses. The P2X7 receptor, a purinergic receptor, is a non-selective, ATP-gated ion channel that mediate the influx of calcium and other ions when activated by extracellular ATP. Moreover, it is known to activate the NLRP3 inflammasome, which in turn triggers the activation of caspase-1 (Territo et al. 2021). Caspase-1 is a protease that plays a critical role in the maturation and release of pro-inflammatory cytokines, including IL-1β. Research suggests that vitamin D, primarily through its receptor VDR, can modulate this pathway at various levels (Cui et al. 2019). The VDR is expressed in immune cells, including monocytes, macrophages, and dendritic cells, all of which are key players in the P2X7R/NLRP3/Caspase-1 pathway. Recent research underscores how vitamin D, primarily through its receptor VDR, effectively dampens NLRP3 inflammasome activation by downregulating its assembly and expression, inhibiting subsequent caspase-1 activation and IL-1β release (L et al. 2018). Moreover, the regulation of caspase-1 activity is also impacted by vitamin D/VDR signaling, potentially influencing the processing of pro-inflammatory cytokines, either directly or indirectly by modulating the expression of caspase-1 or its regulators (S et al. 2021). Interestingly, vitamin D has been suggested to modulate calcium influx by, at least in part, inhibiting the expression of P2X7Rs, thereby reducing the activation of immune cells. These actions on NLRP3 inflammasome activation, P2X7R expression, and caspase-1 activity are central to the modulation of the P2X7R/NLRP3/Caspase-1 pathway by vitamin D. Understanding the exact mechanisms by which vitamin D affects these proteins and the associated pathway is an active area of research.
Anomalous microglia activation is correlated with neuroinflammation and is considered prominent in the pathogenesis of central nervous system diseases. Previous research has shown that calcitriol exerts anti-inflammatory effects and reduces macrophage activation in the brain (de Oliveira et al. 2020). Under LPS stimulation, microglia influence neuronal function by releasing inflammatory cytokines, including IL-6, TNF-α, as well as IL-1β (LiuYang et al. 2021a). Vitamin D treatment was decreases mRNA levels of pro-inflammatory cytokines, thus exerting neuroprotective effects (Calvello et al. 2017). Consistent with previous findings (Oh et al. 2020), we observed significant elevations of inflammatory cytokines (IL-1β, TNF-α, and IL-6) in LPS-exposed BV2 microglia and the LPS-injected mouse hippocampus. As expected, calcitriol administration suppressed inflammatory cytokine production in LPS-treated microglia and downregulated the pro-inflammatory mediators in hippocampus of LPS-injected mice. Moreover, we also evaluated the anti-inflammatory properties of calcitriol, and established that mRNA levels of anti-inflammatory cytokines were elevated by calcitriol administration. These findings imply that calcitriol protects against nerve damage by suppressing microglial activation and inflammatory cytokine release.
The P2X7R/NLRP3 pathway is involved in development of neuroinflammatory and neurodegenerative diseases (Sun et al. 2021). Meanwhile, the NLRP3/caspase-1 signaling pathway promotes microglial activation and, consequently, neuroinflammation (Feng et al. 2021). Accordingly, we measured the protein levels of P2X7R, caspase-1 P20, NLRP3 as well as ASC and found that they were upregulated with LPS treatment. A lack of Vitamin D is implicated in the pathogenesis of multiple sclerosis (Lu et al. 2018). We established that calcitriol inhibited P2X7R, NLRP3, ASC, and caspase-1 P20 levels in vitro and in vivo. By administering an antagonist of P2X7R and NLRP3 inhibitor, specifically, the calcitriol treatment, similarly to BBG, inhibited P2X7R and downstream NLRP3 inflammasome under LPS stimulation, and blocking NLRP3 using inhibitor did not alter P2X7R expression. In vivo experiments, we further confirmed that the calcitriol-mediated suppression of P2X7R/NLRP3/caspase-1 signaling could inhibit inflammation. Together, our data showed that calcitriol inhibits neuroinflammatory responses by blocking P2X7R/NLRP3/caspase-1 signaling.
Neuroinflammation in the hippocampus is closely associated with behavioral and cognitive dysfunction. Numerous studies have indicated that mice experience cognitive deficits after being injected with LPS (Lan et al. 2020). In the present study, using the Morris Water Maze test, a commonly used behavioral tool for assessing hippocampal-dependent spatial learning as well as memory abilities in rodents (Zhao et al. 2019), we found that calcitriol enhanced spatial learning as well as memory capabilities in LPS-injected mice. Other behavioral tests similarly demonstrated that calcitriol ameliorates LPS-mediated depressive-like behaviors in animals. Neuronal damage and apoptosis in hippocampus have a vital role in cognitive as well as behavioral dysfunction (Neubert et al. 2011). It has been widely reported that LPS injection into the mouse brain promotes the upregulation of the levels of cleaved caspase-3, BAX and suppressing Bcl-2 (Zhu et al. 2019). Additionally, calcitriol can attenuate cell apoptosis in the brain tissue of ischemic stroke model rats, thereby mitigating brain injury (Sadeghian et al. 2019). We obtained similar results in the current study. Our data showed that treatment with LPS induced increases in cleaved caspase-3 protein levels and the BAX/Bcl-2 ratio, whereas co-administration with calcitriol diminished these effects, indicating that calcitriol exerts anti-apoptotic effects in LPS-mediated neuroinflammation. Furthermore, calcitriol also mitigated LPS-mediated neuronal damage and neuron loss in mouse hippocampus as revealed by H&E staining. Overall, these results indicated that calcitriol exhibits antidepressive and neuroprotective activity after LPS treatment.
In summary, the modulation of the P2X7R/NLRP3/Caspase-1 pathway by vitamin D is an exciting area of research with potential implications for understanding and managing inflammatory and immune-related disorders.
Data availability
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
References
Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P (2012) Vitamin D, cognition, and dementia: a systematic review and meta-analysis
Calvello R, Cianciulli A, Nicolardi G, De Nuccio F, Giannotti L, Salvatore R, Porro C, Trotta T, Panaro MA, Lofrumento DD (2017) Vitamin D treatment attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an animal model of Parkinson’s Disease, shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol 12(2):327–339. https://doi.org/10.1007/s11481-016-9720-7
Cui C, Xu P, Li G, Qiao Y, Han W, Geng C, Liao D, Yang M, Chen D, Jiang P (2019) Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol 26:101295. https://doi.org/10.1016/j.redox.2019.101295
Cui C, Wang C, Feng J, Yang M, Kong L, Han W, Jiang P (2021) Calcitriol confers neuroprotective effects in traumatic brain injury by activating Nrf2 signaling through an autophagy-mediated mechanism. Mol Med (Cambridge Mass) 27(1):118. https://doi.org/10.1186/s10020-021-00377-1
Dang R, Zhou X, Tang M, Xu P, Gong X, Liu Y, Jiao H, Jiang P (2017) Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur J Nutr 57(3):893–906. https://doi.org/10.1007/s00394-016-1373-z
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9(1):46–56. https://doi.org/10.1038/nrn2297
de Oliveira LRC, Mimura LAN, Fraga-Silva TFC, Ishikawa LLW, Fernandes AAH, Zorzella-Pezavento SFG, Sartori A (2020) Calcitriol prevents Neuroinflammation and reduces blood-brain barrier disruption and local Macrophage/Microglia activation. Front Pharmacol 11:161. https://doi.org/10.3389/fphar.2020.00161
DiSabato DJ, Quan N, Godbout JP (2016) Neuroinflammation: the devil is in the details. J Neurochem 139(Suppl 2):136–153. https://doi.org/10.1111/jnc.13607
Fan X, Ma W, Zhang Y, Zhang L (2020a) P2X7 Receptor (P2X7R) of Microglia Mediates Neuroinflammation by Regulating (NOD)-Like Receptor Protein 3 (NLRP3) Inflammasome-Dependent Inflammation After Spinal Cord Injury. Med Sci Monit 26:e925491. https://doi.org/10.12659/MSM.925491
Fan YG, Pang ZQ, Wu TY, Zhang YH, Xuan WQ, Wang Z, Yu X, Li YC, Guo C, Wang ZY (2020b) Vitamin D deficiency exacerbates Alzheimer-like pathologies by reducing antioxidant capacity. Free Radic Biol Med 161:139–149. https://doi.org/10.1016/j.freeradbiomed.2020.10.007
Feng X, Hu J, Zhan F, Luo D, Hua F, Xu G (2021) MicroRNA-138-5p regulates hippocampal neuroinflammation and cognitive impairment by NLRP3/Caspase-1 signaling pathway in rats. J Inflamm Res 14:1125–1143. https://doi.org/10.2147/JIR.S304461
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJM, Piao X, McCarroll SA, Stevens B (2019) Single-cell RNA sequencing of Microglia throughout the mouse lifespan and in the injured brain reveals Complex Cell-State changes. Immunity 50(1):253–271e256. https://doi.org/10.1016/j.immuni.2018.11.004
Han W, Zhang C, Wang H, Yang M, Guo Y, Li G, Zhang H, Wang C, Chen D, Geng C, Jiang P (2019) Alterations of irisin, adropin, preptin and BDNF concentrations in coronary heart disease patients comorbid with depression. Annals Translational Med 7(14):298–298. https://doi.org/10.21037/atm.2019.05.77
Jeon SW, Kim YK (2018) The role of neuroinflammation and neurovascular dysfunction in major depressive disorder. J Inflamm Res 11:179–192. https://doi.org/10.2147/JIR.S141033
Jiang P, Xue Y, Li HD, Liu YP, Cai HL, Tang MM, Zhang LH (2014) Dysregulation of vitamin D metabolism in the brain and myocardium of rats following prolonged exposure to dexamethasone. Psychopharmacology 231(17):3445–3451. https://doi.org/10.1007/s00213-014-3440-6
Koduah P, Paul F, Dorr JM (2017) Vitamin D in the prevention, prediction and treatment of neurodegenerative and neuroinflammatory diseases. EPMA J 8(4):313–325. https://doi.org/10.1007/s13167-017-0120-8
L. L, L. Q, C. W, L. J, L. C, Z. Z (2018) Vitamin D 3 protects against Diabetic Retinopathy by inhibiting high-glucose-Induced activation of the ROS/TXNIP/NLRP3 inflammasome pathway. J Diabetes Res 2018:8193523. https://doi.org/10.1155/2018/8193523
Lan N, Liu Y, Juan Z, Zhang R, Ma B, Xie K, Sun L, Feng H, Sun M, Liu J (2020) The TSPO-specific ligand PK11195 protects against LPS-Induced Cognitive Dysfunction by Inhibiting Cellular Autophagy. Front Pharmacol 11:615543. https://doi.org/10.3389/fphar.2020.615543
Lee E, Hwang I, Park S, Hong S, Hwang B, Cho Y, Son J, Yu JW (2019) MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ 26(2):213–228. https://doi.org/10.1038/s41418-018-0124-5
Lee HJ, Choe K, Park JS, Khan A, Kim MW, Park TJ, Kim MO (2022) O-Cyclic phytosphingosine-1-Phosphate protects against Motor dysfunctions and glial cell mediated Neuroinflammation in the Parkinson’s Disease Mouse models. Antioxidants 11(11). https://doi.org/10.3390/antiox11112107
Lima LAR, Lopes MJP, Costa RO, Lima FAV, Neves KRT, Calou IBF, Andrade GM, Viana GSB (2018) Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J Neuroinflammation 15(1):249. https://doi.org/10.1186/s12974-018-1266-6
Liu M, Yang YL, Zhang SS, Liu DN, Fang LH, Du GH, Wang YH (2021a) RKC-B1 blocks activation of NF-kappaB and NLRP3 signaling pathways to suppress neuroinflammation in LPS-Stimulated mice. Mar Drugs 19(8). https://doi.org/10.3390/md19080429
Liu M, Zhang SS, Liu DN, Yang YL, Wang YH, Du GH (2021b) Chrysomycin A attenuates Neuroinflammation by Down-regulating NLRP3/Cleaved Caspase-1 signaling pathway in LPS-Stimulated mice and BV2 cells. Int J Mol Sci 22(13). https://doi.org/10.3390/ijms22136799
Lu M, Taylor BV, Korner H (2018) Genomic effects of the vitamin D receptor: potentially the link between vitamin D, Immune cells, and multiple sclerosis. Front Immunol 9:477. https://doi.org/10.3389/fimmu.2018.00477
Miller AH (2020) Beyond depression: the expanding role of inflammation in psychiatric disorders. World Psychiatry 19(1):108–109. https://doi.org/10.1002/wps.20723
Neubert M, Ridder DA, Bargiotas P, Akira S, Schwaninger M (2011) Acute inhibition of TAK1 protects against neuronal death in cerebral ischemia. Cell Death Differ 18(9):1521–1530. https://doi.org/10.1038/cdd.2011.29
Oh YC, Jeong YH, Pak ME, Go Y (2020) Banhasasim-Tang attenuates Lipopolysaccharide-Induced Cognitive impairment by suppressing neuroinflammation in mice. Nutrients 12(7). https://doi.org/10.3390/nu12072019
P J, LH Z, HL C, HD L, MM YPL, RL T, WY D, Z., Y, X., X, H (2014) Neurochemical effects of chronic administration of calcitriol in rats. Nutrients 6(12):6048–6059. https://doi.org/10.3390/nu6126048
Piancone F, La Rosa F, Marventano I, Saresella M, Clerici M (2021) The role of the Inflammasome in neurodegenerative diseases. Molecules 26(4). https://doi.org/10.3390/molecules26040953
Rizavi HS, Ren X, Zhang H, Bhaumik R, Pandey GN (2016) Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Res 240:314–320. https://doi.org/10.1016/j.psychres.2016.04.049
S J, H, Z., X, L., B, Y., L, H., Z, H., A, L., J, D., Y, L., W, Z (2021) Vitamin D/VDR attenuate cisplatin-induced AKI by down-regulating NLRP3/Caspase-1/GSDMD pyroptosis pathway. J Steroid Biochem Mol Biol 206:105789. https://doi.org/10.1016/j.jsbmb.2020.105789
Sadeghian N, Shadman J, Moradi A, Ghasem Golmohammadi M, Panahpour H (2019) Calcitriol protects the blood-brain barrier integrity against ischemic stroke and reduces vasogenic brain edema via antioxidant and antiapoptotic actions in rats. Brain Res Bull 150:281–289. https://doi.org/10.1016/j.brainresbull.2019.06.010
Saxena S, Kruys V, Vamecq J, Maze M (2021) The role of Microglia in Perioperative Neuroinflammation and Neurocognitive disorders. Front Aging Neurosci 13:671499. https://doi.org/10.3389/fnagi.2021.671499
Shen L, Wang Z, Wang R, Chen X, Cheng S (2021) Upregulation of the P2X7 receptor promotes Ca2 + accumulation and inflammatory response in post-stroke depression
Sun K, Zhang J, Yang Q, Zhu J, Zhang X, Wu K, Li Z, Xie W, Luo X (2021) Dexmedetomidine exerts a protective effect on ischemic brain injury by inhibiting the P2X7R/NLRP3/Caspase-1 signaling pathway. Brain Res Bull 174:11–21. https://doi.org/10.1016/j.brainresbull.2021.05.006
Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19(8):477–489. https://doi.org/10.1038/s41577-019-0165-0
Territo PR, Zarrinmayeh H (2021) P2X7 Receptors in Neurodegeneration: Potential Therapeutic Applications From Basic to Clinical Approaches. Front Cell Neurosci 15:617036. https://doi.org/10.3389/fncel.2021.617036
Wang D, Wang H, Gao H, Zhang H, Zhang H, Wang Q, Sun Z (2020) P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci 10:28. https://doi.org/10.1186/s13578-020-00388-1
Wang C, Cui C, Xu P, Zhu L, Xue H, Chen B, Jiang P (2023) Targeting PDK2 rescues stress-induced impaired brain energy metabolism. Mol Psychiatry. https://doi.org/10.1038/s41380-023-02098-9
Wittenborn AK, Rahmandad H, Rick J, Hosseinichimeh N (2015) Depression as a systemic syndrome: mapping the feedback loops of major depressive disorder. Psychol Med 46(3):551–562. https://doi.org/10.1017/s0033291715002044
Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, Monga SP, Geller DA, Lotze MT, Tsung A (2012) High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology 55(6):1863–1875. https://doi.org/10.1002/hep.25572
Yang L, Zhou R, Tong Y, Chen P, Shen Y, Miao S, Liu X (2020) Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol Dis 140:104814. https://doi.org/10.1016/j.nbd.2020.104814
Ye X, Shen T, Hu J, Zhang L, Zhang Y, Bao L, Cui C, Jin G, Zan K, Zhang Z, Yang X, Shi H, Zu J, Yu M, Song C, Wang Y, Qi S, Cui G (2017) Purinergic 2X7 receptor/NLRP3 pathway triggers neuronal apoptosis after ischemic stroke in the mouse. Exp Neurol 292:46–55. https://doi.org/10.1016/j.expneurol.2017.03.002
Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T, Wang W, Wang YX, Jiang CL (2015) NLRP3 Inflammasome mediates chronic mild stress-Induced Depression in mice via Neuroinflammation. Int J Neuropsychopharmacol 18(8). https://doi.org/10.1093/ijnp/pyv006
Zhang W-y, Guo Y-j, Han W-x, Yang M-q, Wen L-p, Wang K-y, Jiang P (2019) Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int Immunopharmacol 67:138–144. https://doi.org/10.1016/j.intimp.2018.12.012
Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, Lu D, Wei W, Wang Y, Li H, Fu Y, Zhu L (2019) Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep 9(1):5790. https://doi.org/10.1038/s41598-019-42286-8
Zhu X, Liu J, Chen S, Xue J, Huang S, Wang Y, Chen O (2019) Isoliquiritigenin attenuates lipopolysaccharide-induced cognitive impairment through antioxidant and anti-inflammatory activity. BMC Neurosci 20(1):41. https://doi.org/10.1186/s12868-019-0520-x
Funding
This work was supported by Natural Science Foundation of Shandong Province (Grant no. ZR2021MH145, ZR2023MH365, ZR2023QH094 and ZR2020MH375), National Natural Science Foundation of China (Grant no. 81602846 and 82302795), Key Research and Development Projects of Jining City (Grant no. 2019MNS012 and 2021YXNS084), China International Medical Foundation (Grant no. Z-2018-35-2002), High-Level Research Project Fostering Plan of Jining Medical University (JYGC2021FKJ009), Research Fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation of Jining Medical University (JYHL2021FMS19) and Project Foundation of Affiliated Hospital of Jining Medical University (Grant no. MP-ZD-2021-005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal procedures were conducted in accordance with Guide for the Care and Use of Laboratory Animals and were approved by the Ethics Committee of Jining First People’s Hospital (JNRM-2023-DW-99).
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Cui, C., Xie, X. et al. Calcitriol attenuates lipopolysaccharide-induced neuroinflammation and depressive-like behaviors by suppressing the P2X7R/NLRP3/caspase-1 pathway. Psychopharmacology 241, 1329–1343 (2024). https://doi.org/10.1007/s00213-024-06565-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-024-06565-1