Abstract
Neuroinflammation has emerged as a crucial factor in the development of depression. Despite the well-known anti-inflammatory properties of 6-gingerol, its potential impact on depression remains poorly understood. This study aimed to investigate the antidepressant effects of 6-gingerol by suppressing microglial activation. In vivo experiments were conducted to evaluate the effect of 6-gingerol on lipopolysaccharide (LPS)-induced behavioral changes and neuroinflammation in rat models. In vitro studies were performed to examine the neuroprotective properties of 6-gingerol against LPS-induced microglial activation. Furthermore, a co-culture system of microglia and neurons was established to assess the influence of 6-gingerol on the expression of synaptic-related proteins, namely synaptophysin (SYP) and postsynaptic density protein 95 (PSD95), which are influenced by microglial activation. In the in vivo experiments, administration of 6-gingerol effectively alleviated LPS-induced depressive behavior in rats. Moreover, it markedly suppressed the activation of rat prefrontal cortex (PFC) microglia induced by LPS and the activation of the NF-κB/NLRP3 inflammatory pathway, while also reducing the levels of inflammatory cytokines IL-1β and IL-18. In the in vitro experiments, 6-gingerol mitigated nuclear translocation of NF-κB p65, NLRP3 activation, and maturation of IL-1β and IL-18, all of which were induced by LPS. Furthermore, in the co-culture system of microglia and neurons, 6-gingerol effectively restored the decreased expression of SYP and PSD95. The findings of this study demonstrate the neuroprotective effects of 6-gingerol in the context of LPS-induced depression-like behavior. These effects are attributed to the inhibition of microglial hyperactivation through the suppression of the NF-κB/NLRP3 inflammatory pathway.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mental health is a global concern that can significantly impede the normal daily functions of an individual (Wainberg et al. 2017). Depression, which is the most prevalent incapacitating mental disorder, is marked by a lack of interest in activities, low energy and motivation, disrupted sleep and eating habits, low self-worth, and even suicidal tendencies, resulting in a considerable global health and economic burden (König et al. 2019; Konnopka and König 2020). The World Health Organization reports that depression has a global impact on more than 350 million individuals, with only half of cases achieving full remission through antidepressant therapy and a lifetime prevalence of 10.8% (Lim et al. 2022; Johnston et al. 2019). Consequently, it is crucial to acquire a comprehensive comprehension of depression’s pathophysiology and to develop efficacious treatments. Nevertheless, effective solutions remain elusive.
Research suggests that the prefrontal cortex (PFC) may play a pivotal role in depression (Pan et al. 2014). Individuals with depression exhibit a decrease in PFC volume compared to those who are healthy (Drevets et al. 2008). Microglia, which are the resident macrophages of the central nervous system, serve as the primary immune defense in the brain. Evidence suggests that depression may be a disease associated with microglial cells, wherein microglia in the PFC undergo a transformation from a quiescent to an activated state in response to chronic stress stimulation (Smith et al. 2016). The activation of microglia and subsequent production and secretion of cytokines and other inflammatory mediators, particularly pro-inflammatory cytokines, play a crucial role in neurological inflammation and may be implicated in the pathophysiology of depression (Bachiller et al. 2018; Maes et al. 2011; Yirmiya et al. 2015). The escalation of pro-inflammatory cytokines in both plasma and cerebrospinal fluid can exacerbate the progression and severity of depression (Young et al. 2014). The escalation of pro-inflammatory factors derived from microglia may incite neuroinflammation, which in turn may intensify neuronal harm, thereby augmenting the likelihood of developing depression.
The activation of microglia and the occurrence of neuroinflammation are closely linked to the development of depression, whereby interleukin 1β (IL-1β) is a significant proinflammatory cytokine responsible for microglia activation and neuroinflammation (Ge et al. 2020). Patients undergoing antidepressant treatment have been observed to exhibit a reduction in depressive symptoms, which is associated with a decrease in cortical IL-1β levels (Zhang et al. 2016). Therefore, IL-1β is considered to be a crucial factor in the pathogenesis of depression. The Nucleotide-binding oligomerization structural domain-like receptor protein 3 (NLRP3) inflammatory vesicles are fundamental proteins within the NLRs family (Choi and Ryter 2014). These vesicles can be stimulated by diverse inflammatory signals, resulting in the maturation of Caspase-1 and the processing of its substrates, namely IL-1β and IL-18, ultimately initiating an inflammatory response (Lemprière 2020; Nazarian-Samani et al. 2020). According to recent research, the presence of NLRP3 inflammatory vesicles has been linked to depression caused by stress (Iwata et al. 2013). Therefore, targeting NLRP3 may hold promise as a therapeutic approach for depression.
The phenols of 6-Gingerol (6-Gin) exhibit robust anti-inflammatory and antioxidant characteristics, as well as demonstrate antitumor, anti-biofilm, and antiviral activities. The primary bioactive component of phenolic compounds in ginger is 6-Gin, as evidenced by research (Gravina et al. 2020; Han et al. 2019, 2020). Furthermore, studies have shown that 6-Gin can mitigate neonatal hypoxic-ischemic brain injury, restore brain function by reducing neuronal apoptosis and release of pro-inflammatory factors (Zhao et al. 2021), and inhibit scopolamine-induced behavioral changes and memory impairment in mice, with neuroprotective effects (Kim et al. 2018). However, the efficacy of 6-Gin in mitigating depression and its underlying mechanism of action remain ambiguous.
The objective of the current study was to assess the effects of 6-Gin on changes in inflammatory markers in the PFC in a depression model through in vivo experiments. Additionally, we investigated the impact of 6-Gin on factors linked to microglial pathways, neuronal viability, and synaptic alterations in vitro. Ultimately, this investigation aimed to provide empirical evidence supporting the use of 6-Gin as a treatment and intervention for depression.
Materials and Methods
Animal and Treatment
The animal experiments conducted in this study were executed in accordance with the general regulations established by the National Institutes of Health (NIH) and were sanctioned by the Animal Experimental Ethics Committee of Shantou University Medical College (approval number: 2013-010). Epidemiological surveys have indicated a greater incidence of depression in females relative to males (Thériault and Perreault 2019), thereby prompting the investigation of the pathogenesis of depression in female rats as the primary objective of this study. Female Wistar rats weighing 220 ± 20 g, bred under specific pathogen-free (SPF) conditions, were procured from Jinan Peng Yue Animal Experimental Center (scxk (Lu) 20,140,007, Jinan, Shandong, China). The rats were housed in a controlled environment with a 12-hour day/night light cycle at 25 °C and provided with ad libitum access to food and water prior to the commencement of the experiments. The rats were allocated randomly into four groups (n = 10 per group): control, 6-Gin, LPS, and LPS + 6-Gin groups. The rats in the 6-Gin and LPS + 6-Gin groups were administered 6-Gin (Sigma, USA) orally at a dose of 50 mg/kg once daily for 14 consecutive days (Li et al. 2017), while the rats in the LPS and LPS + 6-Gin groups were given LPS (Sigma, USA) via intraperitoneal injection at a dose of 500 µg/kg every 2 days for a total of seven injections (Jiang et al. 2017; Dang et al. 2018). The control group received the same dosage of PBS. The experimental design is illustrated in Fig. 1.
Behavioral Assays
The behavioral experiments were conducted in a randomized double-blind manner, with the rats being allowed to acclimate to the experimental area for a minimum of 30 min prior to testing.
Open Field Test
During exploration tests, rats were introduced into a 100 × 100 cm2 testing arena for a duration of 5 min. To document and evaluate the exploratory trajectories, distances covered during movement, and duration spent in the intermediate region, a SMART video tracking system (SMART v3.0, Panlab, Spain) was utilized. Ethanol was employed to meticulously clean the facility between experiments involving two rats.
Novelty-suppressed Feeding Test
The current investigation employed the Novelty-Suppressed Feeding Test (NSFT) to assess the behavioral reactions of rats in a state of food deprivation. A test chamber of standardized dimensions measuring 50 × 50 cm2, containing a 2-cm-thick layer of wood chips on the floor, was utilized. The center of the chamber was positioned on a white paper base. Prior to the test, the animals underwent a 24-hour fast. Following this, the rats were introduced into the test box through multiple entry points and their behavior was observed for a duration of 5 min using the SMART video tracking system. The behavioral parameters, including activity trajectory, frequency, and duration of food area entries, were meticulously documented.
Forced Swimming Test
The rats were situated within a Plexiglas cylinder with dimensions of 45 cm in height and 25 cm in diameter, filled with approximately 35 cm of water (24 ± 1 °C), for 5 min. Their behavior was recorded using a video camera, and the duration of immobility was subsequently analyzed and quantified.
Cell Culture and Processing
BV2 microglial cell line, obtained from the Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China), was cultured in DMEM high glucose medium (10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin) at 5% CO2 and 37 °C. Following a pretreatment period of 15 min with 100 µM 6-Gin, a co-culture of neurons and microglia was established. Subsequently, microglia were subjected to a 24-hour treatment with LPS at a concentration of 1 µg/mL.
Cortical tissues from neonatal mice within 24 h of birth was subjected to isolation and enzymatic digestion using 0.125% EDTA-trypsin (Gibco, USA) for 10 min at 37 °C. The resulting cell suspension was subjected to filtration and centrifugation at 1000 rpm for 5 min, and the supernatant was discarded while the pellet was resuspended. The plates were then seeded with the obtained cell suspension at 1 × 106 cells/ml density for 6 hours in serum-free Neurobasal plus system medium(A3653401, Gibco, USA) containing 2% B27. After 7 days of incubation, neurons were co-cultured with microglia, which were added to the upper chamber with a 0.4 μm Transwell chamber (3414, COSTAR, USA).
Cell Viability Test
Cell viability was quantified using the CCK-8 kit (Beijing Solarbio Science&Technology Inc, China). Briefly, 100 µL of the cell suspension was seeded onto a 96-well plate at a concentration of 5000 BV2 cells per well, was incubated at 37 °C, and treated with 0, 4, 20, 100, 500 or 2500 µM 6-Gin for 24 h or with 100 µM 6-Gin for 24 h, 36 h, 48 h, 60 h, or 72 h. The optical density was measured at 450 nm using a microtiter plate reader.
Enzyme-linked Immunosorbent Assay (ELISA) Analysis
The protein levels of IL-1β and IL-18 in the PFC of rats and BV2 cells were detected using relevant ELISA kits (Shanghai Westang Biotech Inc, China) based on the manufacturer’s instructions. The concentrations were calculated based on OD values at 450 nm and their corresponding standard curves.
Western Blotting Analysis
Protein samples extracted from rat PFC, BV2 cells and neurons were separated by gel electrophoresis and transferred onto a PVDF membrane(FFP39, Beyotime, China). The membrane was subsequently blocked with 5% skimmed milk for 2 h at room temperature and incubated overnight at 4 °C with specific primary antibodies, including rabbit anti-NLRP3 (1:200, Abcam, USA), rabbit anti-Caspase1 (1:200, Abcam, USA), rabbit anti-NF-κb (1:500, Cell Signaling Technology, USA), rabbit anti-Synaptophysin (1:20000, Abcam, USA), rabbit anti-PSD95 (1:1000, Cell Signaling Technology, USA), and mouse anti-GAPDH (1:2000, Proteintech, USA) antibodies. After three rinses with TBST, the membrane was incubated with HRP-conjugated goat-anti-mouse IgG or goat-anti-rabbit IgG (1:2000, Millipore, China) at ambient temperature for 2 h. The protein bands were visualized by ECL (BIO-RAD, USA) chemiluminescence and quantified using a chemiluminescence gel imaging system (12,003,153, BIO-RAD, USA) and Image-Pro Plus software.
Real-time Quantitative PCR (RT-qPCR) Analysis
Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) was used to extract the total RNA of the Rat PFC and BV2 cells. The 1st strand cDNA was synthesized using a commercial kit (Sangon, Shanghai, China) following the supplier’s instructions. RT-qPCR analyses were conducted based on SYBR Green dye in a 20 µL reaction system containing 10 µL premix (Ssofast, Bio-rad, CA, USA), 10 µM primers for each, deionized water, and approximately 50 ng cDNA. The RT-qPCR amplification protocol involves an initial denaturation step at 95 ℃ for 30 s, followed by 40 cycles of denaturation at 95 ℃ for 5 s and annealing/extension at 60℃ for 30 s, concluding with a melting curve analysis. The relative mRNA levels of Caspase-1, NLRP3, Nf-κb, Asc were calculated by the 2−ΔΔCT method and normalized by β-actin. The primer sequences used in the present work were presented in Table 1.
Immunohistochemistry
Rats were anesthetized and were transcardially perfused with 50 mL 0.9% saline and 50 mL 4% paraformaldehyde (0.1 M PBS as a solvent, pH = 7.4) successively. After perfusion, the brain tissues were collected, serially immersed in 20, 25 and 30% sucrose solution, and preserved at 4 °C. The brain tissue was then embedded in the Tissue Tek O.C.T. compound (Sakura Finetek, USA), and the continuous coronal slices (15 μm) were prepared for immunofluorescence.
The slices were immersed in a 0.01 M of citrate buffer solution, with a volume of 200 mL (pH = 6.0) at 95 °C for a duration of 1 h for antigen retrieval after being washed three times using PBS. After blocking using 10% goat serum, slices were incubated overnight at 4 °C with mouse anti-Iba1 antibody (1:100, Abcam, Boston, USA). Then, the slices were incubated with Alexa488-conjugated anti-mouse antibody (1:200, Invitrogen, Grand Island, NY, USA). Afterward, the slices were stained with a Hoechst mixture (Beijing Solarbio Science). Images were captured utilizing laser confocal microscopy (SP8, Leica, Mannheim, Germany) and analyzed by Image-Pro Plus software (version 6.0, Media Cybernetics Inc, Rockville, MD). Five images with high magnification were taken for the quantification of each sample.
Immunocytochemistry
Terminal Deoxynucleotidyl Transferase-mediated dUTP Nick-end Labeling (TUNEL)
BV2 cells were subjected to individual cultivation, followed by treatment with cold phosphate-buffered saline (PBS) and fixation with paraformaldehyde. After being blocked in 10% goat serum for 1 h, cells were incubated with TUNEL (Roche, DE) reaction solution according to the manufacturer’s instructions. Then Hoechst staining and anti-quenching mounting were performed successively. The slices were investigated by laser confocal microscopy and analyzed by Image-Pro Plus software. Five representative images of each sample were selected for analysis.
Immunocytochemical Staining
BV2 cells were treated with 4% paraformaldehyde for 15 min. After washing three times with PBS, the cells were blocked with sheep serum and incubated overnight at 4 °C with rabbit anti-mouse NF-κb p65 antibody (1:100, Cell Signaling Technology, USA). Secondary antibodies were then added and incubated for 1 h. Color development was undertaken using DAB. Microscopic observation was performed after sealing of cells with neutral gum. Five representative images of each sample were selected for analysis.
BV2 or neuronal cells were collected, washed with cold PBS, and fixed in paraformaldehyde for immunofluorescence staining. The cells were then blocked with 10% goat serum and incubated with primary antibodies. BV2 cells were incubated with mouse anti-Iba1 antibody (1:100, Boston, USA). Neuronal cells were incubated with a mixture of mouse anti-MAP2 (1:150, Abcam, USA) and either rabbit anti-synaptophysin (1:200, Abcam, USA) or rabbit anti-PSD95 (1:200, Abcam, USA) antibodies overnight. BV2 cells were incubated with Alexa488-conjugated anti-mouse antibody (1:200, Invitrogen, Grand Island, NY, USA), while neurons were incubated with a mixture of Alexa488-conjugated anti-mouse antibody (1:200, Invitrogen, Grand Island, NY, USA) and Alexa594-conjugated anti-rabbit antibodies (1:200, Invitrogen, Grand Island, NY, USA). Afterward, cells were stained with a Hoechst mixture (Beijing Solarbio Science). Images were captured utilizing laser confocal microscopy (SP8, Leica, Mannheim, Germany) and analyzed by Image-Pro Plus software (version 6.0, Media Cybernetics Inc, Rockville, MD). Five images with high magnification were taken for the quantification of each sample.
Statistical Analysis
Statistical analyses were conducted using SPSS software (version 22.0, SPSS Inc., USA) and GraphPad Prism 8.0 (Cabit Information Technology Co., Ltd., China). All data were exhibited as mean ± SEM. The results of Cell viability were analyzed by one-way ANOVA, and Sidak post-hoc tests was used for pairwise comparison between groups. All the other data were analyzed by two-way ANOVA. Two-way ANOVA results were reported as the F-statistics and P values, and a Sidak post-hoc test was used for within-group comparisons. Boxes represent interquartile ranges, with middle lines representing the medians; whiskers (error bars) above and below the box indicate the 90th and 10th percentiles, respectively; P values less than 0.05 were considered statistically significant.
Results
Gingerol Alleviated LPS-induced Depression-like Behavior in rats
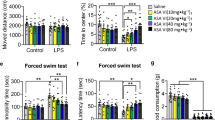
Initially, the open field test was executed to document and monitor the rats’ spontaneous motor activity. The outcomes revealed that the LPS treatment considerably diminished the overall distance of activity, as well as the distance and duration of activity in the central region of the rats, signifying a decline in active exploratory behavior. However, this behavior was effectively reinstated through the co-administration of 6-Gin, as illustrated in Fig. 2A-B.
Subsequently, the novelty-suppressed feeding test was executed to assess depression-like conduct, revealing a noteworthy decrease in the capacity to consume food, as well as the quantity and duration of entry to the food zone following LPS administration (Fig. 2C-D). Conversely, the intervention of 6-Gin counteracted these outcomes to a degree equivalent to that of the control group.
The forced swimming test was conducted to evaluate depression-like behavior, revealing a noteworthy increase in resting duration and a significant manifestation of depression following LPS administration. However, the intervention of 6-Gin resulted in a significant decrease in resting duration and an improvement in the LPS-induced depressive state, as depicted in Fig. 2E. In summary, the findings indicate that administering 6-Gin may have a mitigating effect on depressive behavior induced by LPS in rats.
(A) This study provides an illustrative example of the travel pathways exhibited by rats during the open field test. (B) The variables measured in this study include the distance traveled in the center, the duration spent in the center square, and the total distance traveled by the rats. (C) The travel pathways of rats during the novelty-suppressed feeding test are depicted through an illustrative example in this study. (D) The variables of interest in this study include the number of entries made by the rats in the food area and the duration of time spent in the food area. (E) The results of the forced swimming test were analyzed using a two-way ANOVA with Sidak post-hoc tests, with statistical significance indicated by *p < 0.05 and **p < 0.01. The mean ± SEM (n = 8 per group) are presented in the accompanying graphs
Gingerol Alleviates LPS-induced Neuroinflammation in the rat PFC
Microglia are known to play a crucial role in neuroinflammation. In this study, we investigated the activation of microglia in the PFC of rats. Our findings indicate that the administration of LPS resulted in abnormal microglia activation, as evidenced by an increase in microglia and a decrease in the length of protrusions and the number of terminal branches. However, the administration of 6-Gin intervention effectively reversed the LPS-induced abnormal microglia activation in the rat PFC (Fig. 3A and B).
The present study demonstrates that the abnormal activation of microglia induced by LPS in the PFC of rats can be alleviated through intervention with 6-Gin. This effect is evidenced by the reduced expression of Iba1, as indicated by green staining, and nuclear staining (Hoechst, blue) in the PFC. The quantity of Iba1+ cells exhibited a decrease, while the process length and endpoints demonstrated a significant increase subsequent to 6-Gin intervention, as confirmed through two-way ANOVA with Sidak post-hoc analyses (*p < 0.05 and **p < 0.01). The graphs presented in this study indicate the mean ± SEM (n = 5 per group). Original Magnification, 200×, scale bar = 100 μm for the original region, and scale bar = 20 μm for the enlarged boxed region
The present study investigated the impact of administering LPS on the expression of pro-inflammatory cytokines IL-1β and IL-18 in the PFC of rats, given their crucial role in regulating microglia activation and neuroinflammation (Ge et al. 2020; Ślusarczyk et al. 2018). The results revealed a significant increase in the expression of IL-1β and IL-18 in the PFC of rats following LPS administration. However, co-administration of 6-Gin effectively reversed the LPS-induced increase in IL-1β and IL-18 levels in the PFC of rats, as evidenced by statistically significant differences (Fig. 4A, B). We then investigated the expression of pro-inflammatory mediators, including NF-κB p65, NLRP3, and Caspase1, in the upstream pathways of IL-1β and IL-18. Our findings revealed a significant increase in the protein expression of NF-κB p65, NLRP3, and Cleaved Caspase1, accompanied by a decrease in Pro-Caspase1 expression in the PFC of rats following LPS administration. Furthermore, the co-administration of 6-Gin effectively rectified the aberrations induced by LPS in the expression of NF-κB p65, NLRP3, and Caspase1 in the PFC of rats (Fig. 4C, D). The alterations observed at the protein level were in line with the changes noted at the mRNA level (Fig. 4E). These collective findings indicate that Gingerol can mitigate the activation of the microglial NLRP3 inflammatory axis in the PFC of rats induced by LPS.
Neuroinflammation induced by LPS is alleviated by 6-Gin intervention in the PFC of rats. The alterations in protein levels of IL-1β and IL-18 (A, B) and NF-κB p65, NLRP3, Pro-Caspase1, and Cleaved-Caspase1 (C, D) were assessed. The normalization of all protein levels was conducted with respect to GAPDH. Additionally, the changes in mRNA levels of Nf-κb, NLRP3, and Caspase1 (E) were evaluated, with normalization of all mRNA levels being performed with respect to Gapdh. Statistical significance at *p < 0.05 and **p < 0.01 were determined through the utilization of a two-way ANOVA with Sidak post-hoc tests. The graphical representation of the data displays the mean ± SEM, with a sample size of n = 5 per group for protein levels and n = 8 per group for mRNA levels (n = 5–8 per group)
Gin Alleviates LPS-induced Microglia Apoptosis, Increased Activation and NF-κB p65 Nucleoplasmic Translocation
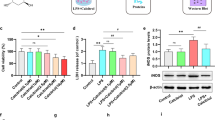
We then studied whether 6-Gin can ameliorate LPS-induced abnormal microglia activation. First, we determined that 100 µM 6-Gin treatment of BV2 cells for 24 h was the optimal treatment based on CCK8 assay (Fig. 5A, B) and western blot analysis of NF-κB, NLRP3, and cleaved Caspase1 protein expression levels (supplemental Fig. 1). Next, apoptosis was observed by TUNEL staining. Finally, a significant increase in apoptosis was observed in microglia solely treated with LPS, which could be reversed in LPS-treated BV2 cells pretreated with 6-Gin (Fig. 5C, D). The findings of this study suggest that 6-Gin has the potential to mitigate LPS-induced microglial cell apoptosis. Additionally, the microglial apoptosis levels in the 6-Gin groups did not exhibit significant changes when compared to the control group (Fig. 5C, D), indicating that the administration of 100 µM 6-Gin did not result in cytotoxicity.
Apoptosis induced by LPS in BV2 cells is alleviated by 6-Gin intervention. (A) Cell viability of BV2 cells after treatment with different concentrations of 6-Gin (0, 4, 20, 100, 500 or 2500 µM) for 24 h. (B) Time-dependent effect of 100 µM 6-Gin on cell viability at different time points (24 h, 36 h, 48 h and 72 h). (C) The apoptotic ratio of BV2 cells in each group. (D) TUNEL-positive cells in BV2 cells. TUNEL staining (green) and Hoechst nuclear staining (blue). *p < 0.05 (cell viability data were analyzed by one-way ANOVA; the other data were analyzed by two-way ANOVA with Sidak post-hoc test). Graphs indicate mean ± SEM (n = 5 per group). Original Magnification, 200×, scale bar = 100 μm
Subsequently, an assessment was conducted to determine the impact of 6-Gin on the activation of BV2 cells. The findings indicated that the cells in the LPS group underwent a transition from a quiescent state to the pro-inflammatory M1 phenotype, characterized by an escalation in Iba1-positive cell protrusions and a gradual augmentation in the cytosol with an amoeboid morphology (Fig. 6A). Conversely, treatment with 6-Gin resulted in a transformation of the cells into the M2 phenotype, which exhibited anti-inflammatory properties, primarily featuring rounded, retracted protrusions and a limited number of short pseudopods, akin to the control group (Fig. 6A). The findings suggest that 6-Gin possesses the ability to convert M1-type pro-inflammatory microglia into M2-type anti-inflammatory microglia.
Given the observed changes in NF-κB p65 expression in the PFC of LPS-induced rats, we proceeded to investigate whether activation could lead to the translocation of NF-κB p65 into the nucleus in BV2 cells. The findings of this study demonstrate that the translocation of NF-κB p65 from the cytoplasm to the nucleus in BV2 cells was observed in the LPS group (Fig. 6B), indicating the activation of the NF-κB pathway in BV2 cells. Conversely, pretreatment with 6-Gin resulted in a significant decrease in the nuclear translocation of NF-κB p65 from the cytoplasm to the nucleus (Fig. 6B). Further analysis was conducted on the protein expression of NF-κB p65, revealing a significant increase in NF-κB p65 protein expression following LPS treatment. This increase was notably suppressed upon administration of 6-Gin, as depicted in Fig. 6C. These findings indicate that 6-Gin effectively hinders the activation of the NF-κB pathway in LPS-treated microglia.
Abnormal activation of microglia and NF-κB p65 induced by LPS is alleviated by 6-Gin intervention. (A) Immunofluorescence staining of Iba1 in BV2 cells. Iba1 staining (green) and Hoechst nuclear staining (blue). Magnification is 200×, scale bar = 100 μm. (B) NF-κB p65 staining in BV2 cells. (C) Protein level changes of NF-κB p65. **p < 0.01 (analyzed by two-way ANOVA with Sidak post-hoc tests). Graphs indicate mean ± SEM (n = 5 per group)
Gin Alleviates LPS-induced Activation of the NLRP3 Inflammasome Pathway in Microglia
We conducted an investigation in BV2 cells to ascertain the conformity of the NLRP3 pathway with the alterations observed in the in vivo experiments. The findings revealed a rise in the levels of NLRP3 protein subsequent to LPS exposure in BV2 cells. Furthermore, the observed escalation in the expression of ASC mRNA strongly indicates the formation of CARD (ASC) specks. The augmented level of cleaved Caspase1 and the reduced pro-Caspase1 (Fig. 7A-C) further corroborate these results. We also observed a significant increase in the release of inflammatory factors IL-18 and IL-1β in response to LPS, as evidenced by a statistically significant difference (Fig. 7A-E). Treatment with 6-Gin resulted in a decrease in the expression of NLRP3, ASC, Cleaved-Caspase1, IL-18 and IL-1β, thereby mitigating the inflammatory response (Fig. 7A-E). These findings suggest that 6-Gin intervention may alleviate the inflammatory response of microglia by inhibiting the activation of the NLRP3 pathway.
Abnormal activation of the NLRP3 inflammasome pathway induced by LPS is alleviated by 6-Gin intervention in BV2 cells. (A, B and C) Protein level changes of NLRP3, pro-Caspase1 and cleaved-Caspase1, respectively. All protein levels were normalized to GAPDH, respectively. (D) mRNA level changes of Asc. (E) Protein level changes of IL-1β and IL-18, respectively (*p < 0.05, **p < 0.01; two-way ANOVA with Sidak post-hoc tests). Graphs indicate mean ± SEM (n = 5 per group)
Gin Alleviates Neuronal Damage Caused by LPS-induced Microglia Activation
An in vitro co-culture system of microglia and neurons was established to investigate neuronal synaptic changes. The results of fluorescence analysis revealed that the LPS intervention alone led to a reduction in neuronal dendritic complexity, and a significant decrease in the number of SYP/MAP2 and PSD95/MAP2 double-labeled cells compared to the control group. Conversely, the number of MAP2 and PSD95/MAP2 double-labeled cells in the LPS + 6-Gin group was significantly restored to normal levels (Fig. 8A-B). Subsequently, the aforementioned outcomes were corroborated through western blot analysis, which revealed a significant reduction in the expression of synaptic-related proteins SYP and PSD95 in the LPS group as compared to the control group. However, upon administering 6-Gin intervention, a marked improvement was observed in the LPS-induced decrease of neuronal PSD95 and SYP expression (p < 0.05, p < 0.01; Fig. 8C).
Effects of 6-Gin intervention on neuronal synapse in co-culture of microglia and neurons in vitro. (A, B) SYP/MAP2 and PSD95/MAP2 labeled neurons. SYP staining (green), PSD95 staining (green), MAP2 staining (red) and nuclear staining (Hoechst, blue). Original magnification, 200×; scale bar = 100 μm. (C) Protein level changes of SYP and PSD95, respectively. All protein levels were normalized to GAPDH, respectively. *p < 0.05, **p < 0.01 (analyzed by two-way ANOVA with Sidak post-hoc tests). Graphs indicate mean ± SEM (n = 5 per group)
Discussion
Depression is a multifaceted disorder, and despite extensive research, there remains a dearth of efficacious pharmacological interventions to mitigate its symptoms. However, 6-Gin, the principal bioactive constituent of phenolic compounds found in ginger, exhibits potent anti-inflammatory and antioxidant properties. Empirical evidence suggests that 6-Gin may facilitate the functional recuperation of neonatal hypoxic-ischemic brain injury and confer a protective effect against peripheral mononeuropathy via its anti-inflammatory attributes (Zhao et al. 2021; Borgonetti et al. 2020). Moreover, in the context of isoproterenol (ISO)-induced myocardial fibrosis, the administration of 6-Gin has been shown to reduce inflammation and apoptosis, thereby exerting a protective effect on the myocardial fibrosis (Han et al. 2020). Based on this premise, we postulate that 6-Gin may mitigate the progression of depression by ameliorating neuroinflammation. To test this hypothesis, we induced a classical rat depression model using LPS and concurrently administered 6-Gin treatment to evaluate the resultant behavioral changes in the rats. The present study reveals that treatment with 6-Gin resulted in an improvement in depression-like behavior in rats induced with LPS. Furthermore, the enhanced active exploratory behavior was significantly restored. These findings suggest that 6-Gin may hold promise as a potential therapeutic candidate for the treatment of depression.
The role of neuroinflammation in the pathogenesis of depression is significant. Of particular interest are microglia, specific immune cells within the central nervous system, which have been implicated in the process of neuroinflammation. Recent research suggests that depression may be attributed to a disease of microglial cells [8]. The current investigation confirmed that LPS administration resulted in the activation of microglia in the PFC of a rat model of depression, as evidenced by an augmented microglial population and a reduction in the length of protrusions and the number of terminal branches. The in vitro experiments also demonstrated microglial activation following LPS exposure, with a trend that was consistent with the in vivo observations. Moreover, the induction of microglia apoptosis was observed following LPS stimulation. Previous research has indicated that microglia apoptosis is frequently observed in cases of depression (Kreisel et al. 2014). Additionally, intermittent alcohol exposure during adolescence in rodents has been shown to result in hippocampal microglia apoptosis, which can lead to depression-like behavior (Hu et al. 2020). As such, it is postulated that the acceleration of depression onset may be attributed to the occurrence of microglia apoptosis.
Subsequently, an examination was conducted to determine the impact of 6-Gin on microglia. The results indicated that 6-Gin mitigated the hyperactivation of microglia induced by LPS in the PFC of rats. In vitro experiments further demonstrated that 6-Gin reduced LPS-induced microglia activation and improved the status of microglia apoptosis. These findings suggest that 6-Gin may possess anti-inflammatory properties and alleviate the incidence of abnormal apoptosis by inhibiting microglia activation.
The pleiotropic transcription factor, NF-κB, is known to activate numerous cellular signaling pathways and regulate other factors that contribute to the inflammatory response, ultimately leading to an increase in the production of inflammatory cytokines. Studies have shown that NF-κB activation in glial cells induced by LPS results in the upregulation of inflammatory mediators (Zusso et al. 2019). Consequently, the inhibition of NF-κB-mediated cellular and molecular processes may serve as a crucial approach to mitigate inflammatory responses. We first validated that inhibitory effect of 6-Gin supplementation treatment on LPS-induced upregulation of NF-κB expression in the rat PFC. In vitro, the activation of NF-κB was confirmed in microglia through the observation of nuclear translocation, which can be impeded through pre-treatment with 6-Gin.
The NLRP3 is a group of regulators involved in the neuroinflammatory response. The activation of NLRP3 is considered a potential link between immune activation and stress, and has therefore become a subject of research in the field of neuroinflammation and depression (Haneklaus et al. 2013). The activation of NLRP3 inflammatory vesicles entails the involvement of two distinct signaling pathways. One pathway necessitates the activation of NF-κB, which directly stimulates the production of IL-1β and IL-18. The other pathway is responsible for the activation and assembly of NLRP3 inflammatory vesicles, which involves the recruitment of Pro-Caspase1 and the subsequent generation of mature IL-1β and IL-18 (Iwata et al. 2016). The results of our study utilizing animal and cellular models indicate that LPS stimulation induces activation of both signaling pathways associated with NLRP3 inflammatory vesicles in microglial cells, leading to an increase in the expression of NF-κB, NLRP3, ASC, Cleaved-Caspase1, IL-1β, and IL-18. Moreover, the activation of the NF-κB/NLRP3 inflammasome pathway in microglia induced by LPS was notably mitigated following the administration of 6-Gin intervention. This implies that 6-Gin may alleviate neuroinflammation by inhibiting the activation of the microglia NF-κB/NLRP3 inflammasome pathway, thereby reducing the incidence of depression.
Increasing evidence suggests a nuanced interplay between microglia and neurons, with microglia serving as a crucial cellular component in the preservation of synaptic density in brain neurons (Wright-Jin and Gutmann 2019). In the event of inflammation, microglia release immune-related signaling molecules that negatively impact synaptic connectivity and plasticity, both of which are essential for learning and memory (Wu et al. 2015).
Depression is characterized by pathological changes such as neuronal atrophy and synaptic inhibition in the PFC, as evidenced by studies (Abdallah et al. 2015; Duman et al. 2016; Duman and Aghajanian 2012). Post-mortem examinations of individuals with depression have revealed a decrease in the number of synapses in the PFC (Kang et al. 2012).Synapses serve as crucial locations for the transmission of neural information between neurons, and the communication between neurons is fundamental to the genesis of all brain activity and intricate behaviors. The integral membrane glycoprotein, synaptophysin (SYP), is situated within the synaptic vesicle membrane, while the postsynaptic density (PSD) is an electron-dense structure located beneath the postsynaptic membrane of excitatory synapses, typically positioned at the apex of dendritic spines. And both SYP and PSD95 can be used as specific markers of synapses(Sun et al. 2021). The significance of synaptic vesicle formation and function, as indicated by SYP during intrauterine development, for the proper development of the brain has been established (Fang et al. 2021). Consequently, any disruption to PSD95 may result in a disturbance of synaptic plasticity at dendritic spines, which may lead to the emergence of synaptic malformations that are linked to neurological disorders (Coley and Gao 2018).
The present study employed co-culture systems of microglia and neurons to investigate the impact of LPS-induced microglia activation on neuronal function. Our findings indicate that LPS stimulation of microglia resulted in a reduction in neuronal dendritic complexity and a decrease in the expression of synapse-associated proteins SYP and PSD95. These results suggest that neuroinflammation plays a significant role in the pathogenesis of neuronal injury, with pro-inflammatory cytokines, chemokines, and other inflammatory mediators produced by neuroinflammation contributing to this process (Tang et al. 2019; Wang et al. 2019). Our findings indicate that 6-Gin has the potential to reverse the reduction in the expression of synaptic proteins SYP and PSD95, which is a result of microglia activation. This suggests that 6-Gin may have a therapeutic effect in ameliorating neuronal damage by increasing the expression of both SYP and PSD95 in the LPS-induced rat depression model. In summary, our study demonstrates that 6-Gin has ability to improve neuronal damage caused by microglia activation and restore neuronal synaptic plasticity.
In conclusion, the neuroprotective properties of 6-Gin were observed in the context of LPS-induced depression-like behavior. This effect was attributed to the inhibition of microglial hyperactivation through the suppression of the NF-κB/NLRP3 inflammatory pathway.
Data Availability
No datasets were generated or analysed during the current study.
References
Abdallah CG, Sanacora G, Duman RS, Krystal JH (2015) Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66:509–523. https://doi.org/10.1146/annurev-med-053013-062946
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A (2018) Microglia in Neurological diseases: a Road Map to Brain-Disease Dependent-Inflammatory response. Front Cell Neurosci 12:488. https://doi.org/10.3389/fncel.2018.00488
Borgonetti V, Governa P, Biagi M, Pellati F, Galeotti N (2020) Zingiber officinale Roscoe Rhizome extract alleviates neuropathic pain by inhibiting neuroinflammation in mice. Phytomedicine 78:153307. https://doi.org/10.1016/j.phymed.2020.153307
Choi AJ, Ryter SW (2014) Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases. Mol Cells 37:441–448. https://doi.org/10.14348/molcells.2014.0104
Coley AA, Gao WJ (2018) PSD95: a synaptic protein implicated in schizophrenia or autism? Prog Neuropsychopharmacol Biol Psychiatry 82:187–194. https://doi.org/10.1016/j.pnpbp.2017.11.016
Dang R, Zhou X, Tang M, Xu P, Gong X, Liu Y, Jiao H, Jiang P (2018) Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur J Nutr 57:893–906. https://doi.org/10.1007/s00394-016-1373-z
Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118. https://doi.org/10.1007/s00429-008-0189-x
Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. https://doi.org/10.1126/science.1222939
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. https://doi.org/10.1038/nm.4050
Fang Y, Wan C, Wen Y, Wu Z, Pan J, Zhong M, Zhong N (2021) Autism-associated synaptic vesicle transcripts are differentially expressed in maternal plasma exosomes of physiopathologic pregnancies. J Transl Med 19:154. https://doi.org/10.1186/s12967-021-02821-6
Ge F, Yang H, Lu W, Shi H, Chen Q, Luo Y, Liu L, Yan J (2020) Ovariectomy induces microglial cell activation and inflammatory response in Rat Prefrontal cortices to accelerate the chronic unpredictable stress-mediated anxiety and depression. Biomed Res Int 2020(3609758). https://doi.org/10.1155/2020/3609758
Gravina G, Svedin P, Ardalan M, Levy O, Ek CJ, Mallard C, Lai JCY (2020) Staphylococcus epidermidis sensitizes Perinatal Hypoxic-Ischemic Brain Injury in male but not female mice. Front Immunol 11:516. https://doi.org/10.3389/fimmu.2020.00516
Han JJ, Li X, Ye ZQ, Lu XY, Yang T, Tian J, Wang YQ, Zhu L, Wang ZZ, Zhang Y (2019) Treatment with 6-Gingerol regulates dendritic cell activity and ameliorates the severity of experimental autoimmune encephalomyelitis. Mol Nutr Food Res 63:e1801356. https://doi.org/10.1002/mnfr.201801356
Han X, Liu P, Liu M, Wei Z, Fan S, Wang X, Sun S, Chu L (2020) [6]-Gingerol ameliorates ISO-Induced Myocardial Fibrosis by reducing oxidative stress, inflammation, and apoptosis through inhibition of TLR4/MAPKs/NF-κB pathway. Mol Nutr Food Res 64:e2000003. https://doi.org/10.1002/mnfr.202000003
Haneklaus M, O’Neill LA, Coll RC (2013) Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: recent developments. Curr Opin Immunol 25:40–45. https://doi.org/10.1016/j.coi.2012.12.004
Hu P, Wang D, Zhang Y, Cai Z, Ye T, Tong L, Xu X, Lu J, Liu F, Lu X, Huang C (2020) Apoptosis-triggered decline in hippocampal microglia mediates adolescent intermittent alcohol exposure-induced depression-like behaviors in mice. Neuropharmacology 170:108054. https://doi.org/10.1016/j.neuropharm.2020.108054
Iwata M, Ota KT, Duman RS (2013) The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 31:105–114. https://doi.org/10.1016/j.bbi.2012.12.008
Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, Banasr M, Duric V, Yamanashi T, Kaneko K, Rasmussen K, Glasebrook A, Koester A, Song D, Jones KA, Zorn S, Smagin G, Duman RS (2016) Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol Psychiatry 80:12–22. https://doi.org/10.1016/j.biopsych.2015.11.026
Jiang P, Guo Y, Dang R, Yang M, Liao D, Li H, Sun Z, Feng Q, Xu P (2017) Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: involvement of autophagy and NLRP3 inflammasome. J Neuroinflammation 14:239. https://doi.org/10.1186/s12974-017-1013-4
Johnston KM, Powell LC, Anderson IM, Szabo S, Cline S (2019) The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord 242:195–210. https://doi.org/10.1016/j.jad.2018.06.045
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS (2012) Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18:1413–1417. https://doi.org/10.1038/nm.2886
Kim CY, Seo Y, Lee C, Park GH, Jang JH (2018) Neuroprotective Effect and Molecular Mechanism of [6]-Gingerol against Scopolamine-Induced Amnesia in C57BL/6 Mice. Evid Based Complement Alternat Med, 2018, 8941564. https://doi.org/10.1155/2018/8941564
König H, König HH, Konnopka A (2019) The excess costs of depression: a systematic review and meta-analysis. Epidemiol Psychiatr Sci 29:e30. https://doi.org/10.1017/s2045796019000180
Konnopka A, König H (2020) Economic burden of anxiety disorders: a systematic review and Meta-analysis. PharmacoEconomics 38:25–37. https://doi.org/10.1007/s40273-019-00849-7
Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R (2014) Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 19:699–709. https://doi.org/10.1038/mp.2013.155
Lemprière S (2020) NLRP3 inflammasome activity as biomarker for primary progressive multiple sclerosis. Nat Rev Neurol 16:350. https://doi.org/10.1038/s41582-020-0366-y
Li Y, Xu B, Xu M, Chen D, Xiong Y, Lian M, Sun Y, Tang Z, Wang L, Jiang C, Lin Y (2017) 6-Gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-κB signalling. Pharmacol Res 119:137–148. https://doi.org/10.1016/j.phrs.2017.01.026
Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC (2022) Author correction: prevalence of Depression in the community from 30 countries between 1994 and 2014. Sci Rep 12:14856. https://doi.org/10.1038/s41598-022-19021-x
Maes M, Leonard B, Fernandez A, Kubera M, Nowak G, Veerhuis R, Gardner A, Ruckoanich P, Geffard M, Altamura C, Galecki P, Berk M (2011) (Neuro)inflammation and neuroprogression as new pathways and drug targets in depression: from antioxidants to kinase inhibitors. Prog Neuropsychopharmacol Biol Psychiatry 35:659–663. https://doi.org/10.1016/j.pnpbp.2011.02.019
Nazarian-Samani Z, Sewell RDE, Rafieian-Kopaei M (2020) Inflammasome Signaling and other factors implicated in Atherosclerosis Development and Progression. Curr Pharm Des 26:2583–2590. https://doi.org/10.2174/1381612826666200504115045
Pan Y, Chen XY, Zhang QY, Kong LD (2014) Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun 41:90–100. https://doi.org/10.1016/j.bbi.2014.04.007
Ślusarczyk J, Trojan E, Głombik K, Piotrowska A, Budziszewska B, Kubera M, Popiołek-Barczyk K, Lasoń W, Mika J, Basta-Kaim A (2018) Targeting the NLRP3 inflammasome-related pathways via Tianeptine Treatment-suppressed Microglia polarization to the M1 phenotype in Lipopolysaccharide-Stimulated cultures. Int J Mol Sci 19. https://doi.org/10.3390/ijms19071965
Smith BL, Schmeltzer SN, Packard BA, Sah R, Herman JP (2016) Divergent effects of repeated restraint versus chronic variable stress on prefrontal cortical immune status after LPS injection. Brain Behav Immun 57:263–270. https://doi.org/10.1016/j.bbi.2016.05.004
Sun XY, Li LJ, Dong QX, Zhu J, Huang YR, Hou SJ, Yu XL, Liu RT (2021) Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J Neuroinflammation 18:131. https://doi.org/10.1186/s12974-021-02182-3
Tang Q, Huang K, Liu J, Wu S, Shen D, Dai P, Li C (2019) Fine particulate matter from pig house induced immune response by activating TLR4/MAPK/NF-κB pathway and NLRP3 inflammasome in alveolar macrophages. Chemosphere 236:124373. https://doi.org/10.1016/j.chemosphere.2019.124373
Thériault RK, Perreault ML (2019) Hormonal regulation of circuit function: sex, systems and depression. Biol Sex Differ 10:12. https://doi.org/10.1186/s13293-019-0226-x
Wainberg ML, Scorza P, Shultz JM, Helpman L, Mootz JJ, Johnson KA, Neria Y, Bradford JE, Oquendo MA, Arbuckle MR (2017) Challenges and opportunities in Global Mental Health: a research-to-practice perspective. Curr Psychiatry Rep 19:28. https://doi.org/10.1007/s11920-017-0780-z
Wang S, Yuan YH, Chen NH, Wang HB (2019) The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol 67:458–464. https://doi.org/10.1016/j.intimp.2018.12.019
Wright-Jin EC, Gutmann DH (2019) Microglia as Dynamic Cellular mediators of Brain function. Trends Mol Med 25:967–979. https://doi.org/10.1016/j.molmed.2019.08.013
Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B (2015) Microglia: dynamic mediators of Synapse Development and Plasticity. Trends Immunol 36:605–613. https://doi.org/10.1016/j.it.2015.08.008
Yirmiya R, Rimmerman N, Reshef R (2015) Depression as a microglial disease. Trends Neurosci 38:637–658. https://doi.org/10.1016/j.tins.2015.08.001
Young JJ, Bruno D, Pomara N (2014) A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord 169:15–20. https://doi.org/10.1016/j.jad.2014.07.032
Zhang JQ, Wu XH, Feng Y, Xie XF, Fan YH, Yan S, Zhao QY, Peng C, You ZL (2016) Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: involvement of the neuroinflammatory pathway. Acta Pharmacol Sin 37:1141–1153. https://doi.org/10.1038/aps.2016.63
Zhao M, Yao Y, Du J, Kong L, Zhao T, Wu D, Man L, Zhou W (2021) 6-Gingerol alleviates neonatal hypoxic-ischemic cerebral and White Matter Injury and contributes to functional recovery. Front Pharmacol 12:707772. https://doi.org/10.3389/fphar.2021.707772
Zusso M, Lunardi V, Franceschini D, Pagetta A, Lo R, Stifani S, Frigo AC, Giusti P, Moro S (2019) Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflammation 16:148. https://doi.org/10.1186/s12974-019-1538-9
Acknowledgements
We thank anonymous reviewers whose constructive comments and suggestions would help improve the quality of our manuscript.
Funding
The authors acknowledge funding sources from the National Natural Science Foundation of China (Grant No. 81171138), and Jiangsu Province Shuangchuang Talent Plan (Grant No. JSSCRC 2021533).
Author information
Authors and Affiliations
Contributions
C. L.: Methodology, Writing - original draft. Y. Z.: Methodology, Writing – review & editing. W. Z.: Writing – review & editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All procedures were conducted according to the Ethics Committee of Shantou University Medical College.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Zhao, Y. & Zhao, Wj. Positive Effect of 6-Gingerol on Functional Plasticity of Microglia in a rat Model of LPS-induced Depression. J Neuroimmune Pharmacol 19, 20 (2024). https://doi.org/10.1007/s11481-024-10123-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11481-024-10123-z