Abstract
Rationale
A high number of synthetic dissociative drugs continue to be available through online stores, leading to their misuse. Recent inclusions in this category are 4-MeO-PCP and 3-MeO-PCMo, analogs of phencyclidine. Although the dissociative effects of these drugs and their recreational use have been reported, no studies have investigated their abuse potential.
Objectives
To examine their rewarding and reinforcing effects and explore the mechanistic correlations.
Methods
We used conditioned place preference (CPP), self-administration, and locomotor sensitization tests to assess the rewarding and reinforcing effects of the drugs. We explored their mechanism of action by pretreating dopamine receptor (DR) D1 antagonist SCH23390 and DRD2 antagonist haloperidol during CPP test and investigated the effects of 4-MeO-PCP and 3-MeO-PCMo on dopamine-related proteins in the ventral tegmental area and nucleus accumbens. We also measured the levels of dopamine, phosphorylated cyclic-AMP response element-binding (p-CREB) protein, deltaFosB, and brain-derived neurotrophic factor (BDNF) in the nucleus accumbens. Additionally, we examined the effects of both drugs on brain wave activity using electroencephalography.
Results
While both 4-MeO-PCP and 3-MeO-PCMo induced CPP and self-administration, only 4-MeO-PCP elicited locomotor sensitization. SCH23390 and haloperidol inhibited the acquisition of drug CPP. 4-MeO-PCP and 3-MeO-PCMo altered the levels of tyrosine hydroxylase, DRD1, DRD2, and dopamine, as well as that of p-CREB, deltaFosB, and BDNF. All drugs increased the delta and gamma wave activity, whereas pretreatment with SCH23390 and haloperidol inhibited it.
Conclusion
Our results indicate that 4-MeO-PCP and 3-MeO-PCMo induce rewarding and reinforcing effects that are probably mediated by the mesolimbic dopamine system, suggesting an abuse liability in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recreational use of new psychoactive substances (NPS) has grown rapidly over the last several years (Hill and Thomas 2011; Baumeister et al. 2015). This phenomenon has resulted, in part, from online marketing, as well as widespread availability in convenience stores and head shops (Hill and Thomas 2011). NPS are typically sold as research chemicals to circumvent governmental restrictions on the existing psychoactive drugs (Hill and Thomas 2011; Baumeister et al. 2015). One class of NPS is represented by dissociative anesthetics, which includes ketamine (KET), methoxetamine, and other analogs of phencyclidine (PCP) (Baumeister et al. 2015). These substances induce psychotomimetic effects, and based on reports, they are used recreationally or outside the medical setting, prompting authorities to impose legal restrictions on their use (Liu et al. 2016; Halberstadt et al. 2016). However, despite the ongoing legislative efforts, new synthetic dissociative anesthetics are continuously synthesized, thereby dramatically increasing their diversity by introducing chemical modifications.
Two of the recent additions to the NPS market are 1-(1-(4 methoxyphenyl)cyclohexyl)piperidine (4-MeO-PCP) and 4-[1-(3-methoxyphenyl)cyclohexyl] morpholine (3-MeO-PCMo). These substances have chemical structures similar to that of PCP, except for a few parts. As shown in Fig. 1, compared with PCP, 4-MeO-PCP has a 4-methoxy group in the phenyl ring, whereas 3-MeO-PCMo has a 3-methoxy group in the phenyl ring and a morpholine ring instead of the piperidine ring. Both drugs were shown to have a lower affinity to N-Methyl-d-aspartic acid (NMDA) receptors (4-MeO-PCP Ki = 404 nM and 3-MeO-PCMo Ki = 252.9 nM) than that of PCP (Ki = 59 nM), but higher than that of KET (Ki = 659 nM) (Roth et al. 2013; Colestock et al. 2018; Halberstadt et al. 2016). 4-MeO-PCP has been reported to induce euphoric and hallucinogenic effects, which are sometimes accompanied by adverse effects such as dizziness, confusion, psychomotor agitation, and cognitive impairment (Roth et al. 2013; Stevenson and Tuddenham 2014; McIntyre et al. 2015). Although limited information is available regarding its pharmacological profile, it has been suspected that 3-MeO-PCMo produces dissociative effects and is used recreationally (Colestock et al. 2018). Nevertheless, 4-MeO-PCP and 3-MeO-PCMo are not scheduled drugs in many countries, and to date, no studies have investigated their abuse potential. Therefore, it is important to examine their rewarding and reinforcing effects and explore the possible underlying mechanisms.
In the present study, we investigated the abuse potential of 4-MeO-PCP and 3-MeO-PCMo using the conditioned place preference (CPP) and self-administration (SA) paradigms. The CPP test examines the rewarding value of a drug, by measuring the time spent by study animals in the drug-paired compartment (Prus et al. 2009). The SA test assesses the reinforcing efficacy of the drugs, by measuring the number of lever responses that result in drug infusion (Prus et al. 2009), under the two schedules of reinforcement, fixed ratio (FR) and progressive ratio (PR). The FR schedule determines the reinforcing effects, whereas the PR schedule measures the reinforcing strength of a drug (dela Peña et al. 2012). We also examined whether these two drugs induce locomotor sensitization after 7 days of treatment and drug challenge following 7 days of abstinence. Given that the abuse potential of most drugs of abuse has been linked to the dopaminergic system (Liu et al. 2016), we pretreated mice with a dopamine D1 (DRD1) SCH23390 and D2 (DRD2) haloperidol receptor antagonists during the CPP test. We also measured the dopamine-related proteins levels in the ventral tegmental area (VTA) and nucleus accumbens (NAc), as well as the dopamine levels in the NAc of the mice treated with drugs for 7 days. Furthermore, transcription factors, cyclic-AMP response element-binding protein (CREB), and deltaFosB, and neurotrophic factor brain-derived neurotrophic factor (BDNF), proteins that are implicated in drug addiction-related neuroplasticity (Nestler 2004; McClung and Nestler 2008; Barker et al. 2015), were analyzed in the NAc. In addition, we assessed the effects of these substances on the brain wave activity using electroencephalography (EEG). Parallel experiments were also performed using KET.
Materials and methods
Animals
All animals were purchased from the Hanlim Animal Laboratory Co. (Hwasung, Korea) and were housed in a temperature- and humidity-controlled (22 ± 2 °C; 55% ± 5%) room with a 12-h light/dark (0700–1900 h light) schedule. All the experiments were performed during 0900–1700 h. Male C57BL/6 mice (6 weeks old), weighing 20–25 g, were maintained at the density of 6 individuals per cage, and Sprague Dawley rats (8 weeks old), weighing 250–300 g, were maintained at a density of 4 individuals per cage. The mice were used for the CPP, locomotor sensitization, and EEG experiments, whereas rats were used for the SA test. After arrival, the animals were acclimatized to the laboratory setting for 5 days, before they were subjected to any experiments. They had free access to food and water during the course of habituation and experiments, except for rats during lever trainings and SA sessions. Animal care and maintenance were carried out in compliance with the Principles of Laboratory Animal Care (NIH publication No. 85–23, revised 1985) and the Animal Care and Use Guidelines of Sahmyook University, Korea (SYUIACUC2018-001).
Drugs
4-MeO-PCP hydrochloride and 3-MeO-PCMo hydrochloride were synthesized (see supplementary) and generously provided by the Laboratory of Medicinal Chemistry at Kyunghee University, Seoul, South Korea. KET was purchased from BVSPharm Co., Hanam, South Korea. Haloperidol was purchased from Sigma-Aldrich, St. Louis, MO, USA. SCH23390 HCl was purchased from TOCRIS Biosciences, Bristol, UK. All the drugs were diluted with normal saline (SAL) (0.9% w/v NaCl) and administered either intraperitoneally (CPP, locomotor sensitization, and EEG) or intravenously (SA).
4-MeO-PCP
4-MeO-PCP hydrochloride was synthesized from cyclohexanone and 4-methoxymagnesium bromide (Wallach et al. 2014). Its structure was confirmed by the following spectroscopic analyses: 1H NMR (400 MHz, CD3OD) δ 7.57–7.54 (m, 2H), 7.09 (dd, J = 7.0 Hz, 2.2 Hz, 2H), 3.86 (s, 3H), 3.71 (d, J = 12.4 Hz, 2H), 3.01 (d, J = 12.4 Hz, 2H), 2.39–2.30 (m, 2H), 1.91–1.59 (m, 10H), 1.33–1.20 (m, 4H); 13C NMR (125 MHz, DMSO-d6) δ 159.97, 131.75(2C), 122.67, 114.54(2C), 70.58, 55.63, 47.03(2C), 30.46(2C), 25.10, 22.96(2C), 22.86(2C), 22.27; HR-MS calculated for C18H28NO([M + H]+): 274.2165, observed: 274.2152.
3-MeO-PCMo
3-MeO-PCMo hydrochloride was synthesized from cyclohexanone, morpholine, and 1,2,3-benzotriazole by a procedure similar to that reported previously (Prashad et al. 2005). Briefly, cyclohexanone was reacted with morpholine in the presence of 1,2,3-triazole in toluene to yield a solution of the corresponding triazolyl intermediate, which was added to a solution of phenylmagnesium bromide to yield 4-(1-(3-methoxyphenyl)cyclohexyl)morpholine. The resulting amine was treated with HCl to yield 3-MeO-PCMo as an HCl salt. Its structure was confirmed by the following spectroscopic analyses: 1H NMR (400 MHz, DMSO-d6) δ 7.47 (t, J = 8.2 Hz, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.16 (s, 1H), 7.10 (d, J = 8.2 Hz, 1.8 Hz, 1H), 3.98 (t, J = 12.1 Hz, 2H), 3.84 (dd, J = 12.1 Hz, 1.8 Hz, 2H), 3.83 (s, 3H), 3.50 (d, J = 12.1 Hz, 2H), 2.93 (d, J = 12.1 Hz, 2H), 2.43 (qd, J = 12.3 Hz, 2.7 Hz, 2H), 2.09 (t, J = 12.3 Hz, 2H), 1.73 (d, J = 12.3 Hz, 2H), 1.51 (d, J = 12.3 Hz, 1H), 1.19 (q, J = 12.3 Hz, 1H), 1.02 (q, J = 12.3 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 159.70, 131.72, 130.04, 121.86, 116.31, 114.25, 70.50, 63.05(2C), 55.28, 45.85(2C), 29.57(2C), 24.53, 22.31(2C); HR-MS calculated for C17H26NO2+ [M + H]+: 276.1958, observed: 276.1975.
Conditioned place preference test
The place preference apparatus consisted of two plexiglass compartments (17.4 cm × 12.7 cm × 12.7 cm) made of polyvinylchloride and separated by a guillotine door. To provide a visual and tactile difference between the compartments, one compartment was white in color with a 6.352-mm stainless steel mesh floor, whereas the other was black in color with a stainless-steel grid floor (3.2-mm diameter rods placed 7.9 mm apart). The compartments were covered and illuminated. Behavior was recorded and analyzed using a computer-based video tracking system (Ethovision).
The CPP test consisted of three phases: habituation and pre-conditioning, conditioning, and post-conditioning. During habituation, the mice were allowed to explore both compartments for 15 min once a day for 2 days. On the day following habituation, the time spent on each side was recorded (pre-conditioning). To eliminate the side-preference bias, the data from the pre-conditioning phase were used to group the animals that spent similar amount of time in each compartment. Mice that spent over 840 s in one compartment were excluded from the test (n = 5). During the conditioning phase, mice (n = 8/group) received 4-MeO-PCP, 3-MeO-PCMo (1, 3, or 10 mg/kg), KET (10 mg/kg), or SAL, and were confined to a randomly designated compartment for 30 min. Separate cohorts of mice (n = 10/group) were injected with SCH23390 (0.5 mg/kg), haloperidol (0.2 mg/kg), or SAL, 30 min before conditioning sessions with 4-MeO-PCP, 3-MeO-PCMo, KET (10 mg/kg), or SAL, to examine the involvement of DRD1 and DRD2 in the rewarding effects of the drugs. On alternate days, the mice received SAL and were placed in the non-drug-paired compartment. The conditioning lasted for 8 sessions (4 drug and 4 SAL conditioning sessions). The post-conditioning phase started 24 h after the final conditioning session. In this phase, the drug-free mice were allowed free access to both compartments for 15 min. The CPP score was calculated as the difference in the time spent in the drug-paired compartment between the post- and pre-conditioning phases.
Self-administration test
Rats were placed in standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA, USA), with ventilation fans to mask the external noise. Each operant chamber had a food pellet dispenser, two 4.5-cm-wide response levers (left and right), a stimulus light located 6 cm above the left lever, and a centrally positioned house light (2.5 W, 24 V) at the top of the chamber. Downward pressure (approximately equivalent to 25 g) on the levers resulted in a programmed response (described below). Beside the operant chamber, a motor-driven syringe pump was located, which delivered the solutions at 0.01 mL/s. Solutions flowed through polytetrafluoroethylene tubing connected to a swivel, which was mounted on a counterbalanced arm at the top of the chamber, allowing free movement of the animals within the chamber. The tubing was connected to an intravenous catheter implanted in the animal. The Graphic State Notation software (Coulbourn Instruments) was used to control the experimental parameters and collect data.
Initially, rats were trained to press the active (drug-paired) lever for a contingent food pellet reward, on a continuous schedule of reinforcement that lasted for 30 min/day for 3 days. During training, the rats were food-restricted such that no rat dropped below 85% of its starting body weight. Only those rats that acquired at least 80 pellets during the last session of training were selected and prepared for surgery. The surgery was performed as described in our previous study (de la Peña et al. 2012). After surgery, each rat was individually housed in a transparent plastic cage and allowed to recover for 5 days. During the first 4 days following surgery, the rats received an antibiotic treatment (gentamicin, 1 mg/kg i.p.). Thereafter, the rats (n = 10/group) were subjected to the SA experiment 2 h/day, under a FR1 schedule of reinforcement for 7 days, FR2 schedule for 3 days, and FR3 for the last 2 days. During these sessions, two levers were provided, and a press on the left lever (active lever) would result in an infusion of 0.1 mL of 4-MeO-PCP, 3-MeO-PCMo (0.03, 0.1, or 0.3 mg/kg/infusion), KET (0.3 mg/kg/infusion), or SAL. At the same time, the house light was switched off, and the stimulus light was illuminated, which remained lit for another 20 s after the end of the infusion (time-out period). Lever presses during the time-out periods were recorded but did not exert the corresponding effects. As a control for general activity, presses on the right lever (inactive lever) were recorded but not reinforced. Following the FR schedule, the rats (n = 8/group) underwent a 6-h SA session using a PR schedule of reinforcement for 4 days (note that the first 3 days served as training for rats under the PR schedule). Similar to the FR schedule, drugs were available after a lever response. The inactive lever was not provided during these sessions. The number of responses required to obtain each successive infusion of drug was determined using the following equation: responses per reinforcer delivery = [5e(injection number × 0.2)] − 5, rounded to produce the following sequence of required lever presses: 1, 2, 4, 6, 12, 15, 20, 25, 32, 40, and so on (Richardson and Roberts 1996). Breakpoint was defined as the final ratio completed within the 6-h session or until a period of 1 h with no infusion (Botly et al. 2008). Dosages used for each drug were similar under the FR schedule. Catheter patency was assessed by injecting 0.1 mL of thiopental sodium (10 mg/kg) one day before and on the last day of the SA testing period. Rats (5% of all operated rats) that did not lose muscle tone within 3–5 s were excluded from the experiments.
Locomotor sensitization test
The locomotor sensitization test was performed following our previous studies (Botanas et al. 2018). The locomotor activity was assessed in a square Plexiglas container (42 cm × 42 cm × 42 cm) with white surface, between 1000 and 1700 h under normal light condition (100 lx). The first 2 days were allowed as the apparatus acclimation period for the mice (n = 10/group) for 30 min. On the third day, their locomotor activity was recorded and used as a baseline. Thereafter, the mice (n = 10/group) were treated with 4-MeO-PCP, 3-MeO-PCMo (1, 3, 10 mg/kg), KET (10 mg/kg), or SAL for 7 days and then challenged with the same drug and dose after 7 days of abstinence. Locomotor activity was assessed for 30 min immediately after the first, third, and seventh days of drug treatment and abstinence, as well as on the challenge day. A computer-based video tracking system (Ethovision, Noldus I.T. B.V., Netherlands) was used to record the distance moved (cm) by each mouse.
Western blotting
Mice (n = 6/group) injected with 4-MeO-PCP, 3-MeO-PCMo, KET (10 mg/kg), or SAL were sacrificed by cervical dislocation and decapitation 30 min after administration for protein extraction and western blotting. Their brains were quickly and carefully removed and placed in ice-cold saline to prevent damage to the brain. The NAc and VTA were sliced into 1-mm-thick sections using a mouse brain matrix. The areas were then dissected from the appropriate slices and immediately frozen at − 70 °C until further use. The tissue samples were lysed with 300 μL homogenization buffer (RIPA buffer [Biosesang Inc., Seongnam, Korea], cOmplete™ ULTRA protease inhibitor cocktail tablets [05892791001; Sigma-Aldrich], and PhosSTOP™ phosphatase inhibitor cocktail tablets [04906845001, Sigma-Aldrich]). Tissue extract was centrifuged at 16000 rpm at 4 °C for 20 min. The samples were heated at 95 °C for 5 min. Thirty micrograms of protein lysates for each sample were loaded into 12% sodium-dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) gels, separated, and transferred to nitrocellulose membranes. The blots were blocked with 5% dry milk in Tris-buffered saline in 0.1% Tween-20 (TBST) solution for 1 h and then incubated overnight at 4 °C with specific primary antibodies (TH Rabbit Polyclonal Antibody [Merck; AB152], DAT Mouse Monoclonal Antibody [Thermo Fisher; MA5-18079], DRD1 Rabbit Polyclonal Antibody [#BS5692; Bioworld Technology, Inc.], Rabbit Polyclonal Anti-Dopamine D2 Receptor Antibody [D2R-201AP; FabGennix International, Inc.], Phosphorylated CREB (p-CREB) (Ser133) Monoclonal Antibody [#9197; Cell Signaling], CREB Monoclonal Antibody [#9197; Cell Signaling], FosB Monoclonal Antibody [ab184938; Abcam], BDNF [EPR1292] Rabbit Monoclonal Antibody [Abcam; ab108319], and Beta-Actin Mouse Monoclonal Antibody [A5441; Bio-Rad]). On the following day, the blots were washed thrice in TBST and incubated with horseradish peroxidase-conjugated anti-rabbit (1:3000) or anti-mouse secondary antibodies (1:5000) for 1 h. After three final washes with TBST, the blots were visualized using enhanced chemiluminescence (Clarity Western ECL; Bio-Rad Laboratories, Hercules, CA, USA) and the ChemiDoc Imaging System (Image Lab software, version 6.0; Bio-Rad). The values for the phosphorylation-independent levels of proteins were normalized to that of beta-actin. Levels of the phosphorylated forms of proteins were normalized to the phosphorylation-independent levels of the same proteins. Fold change was calculated by normalizing the values to that of the SAL group.
Enzyme-linked immunosorbent assay
Dopamine ELISA (#KA1887, Abnova, Taiwan) was performed to measure the total dopamine contents in the NAc in mice (n = 5/group) treated with 4-MeO-PCP, 3-MeO-PCMo, KET (10 mg/kg), or SAL, according to the manufacturer’s instructions. Standards, controls, and samples (protein lysates from NAc) were subjected to acylation assay. The acylated standards, controls, and samples were pipetted into the appropriate wells of the dopamine microtiter strips and incubated with the dopamine antiserum for 30 min at room temperature (20–25 °C) on a shaker (approx. 600 rpm). Thereafter, contents were discarded, and wells were washed thrice with a wash buffer. The conjugate was added into all wells and incubated for 15 min, and the wells were washed thrice with a wash buffer. The substrate was pipetted into all wells and incubated for 15 min. After three washes, the stop solution was added into the wells and the absorbance was measured at 450 nm (EMax Plus Microplate Reader - Molecular Devices; San Jose, CA, USA). All measurements were performed in duplicate.
Electroencephalography
Mice were anesthetized with Zoletil (50 mg/mL)/xylazine (100 mg/mL). A tethered three-channel system headmount (8200-K3-iS/iSE) was implanted and secured with two stainless screws positioned in the frontal region (A/P − 1.0 mm, M/L − 1.5 mm) and two screws over the posterior brain (A/P − 1.0 mm, M/L ± 1.5 mm) and fixed with dental cement. The mice were given 7 days to recover after the surgery. A preamplifier unit was attached to the headmount that provided the first stage amplification (100×) and initial high-pass filtering (0.5 Hz). The signals were then transmitted to a data conditioning/acquisition system through a tether and commutator. The amplified/conditioning unit provided an additional 50× signal amplification, high-pass filtering, and an eighth-order elliptic low pass filtering (50 Hz). The signals were adjusted to 400 Hz, digitized using a 14-bit A/D converter, and routed to a computer-based software package via USB (Pinnacle Technology, Inc., Lawrence, KS). The mice were habituated to the EEG apparatus for 2 h a day before the actual recording. Baseline recording was carried out for 10 min, followed by an i.p. injection of 4-MeO-PCP, 3-MeO-PCMo, KET (10 mg/kg), or SAL (n = 6/group) and a further 60 min of post-injection recordings. A separate group of mice was treated with SCH23390 (0.5 mg/kg), haloperidol (0.2 mg/kg), or SAL, 30 min before the drugs were provided.
Data analysis
All values were expressed as mean ± standard error of the mean (SEM). Results from CPP and western blotting were analyzed by one-way analysis of variance (ANOVA), followed by Dunnett’s test or Bonferroni’s multiple comparison test. A two-way ANOVA followed by Bonferroni’s test was used to analyze locomotor activity data in the CPP. Data from the active lever responses and the number of infusions during SA test under the FR schedule of reinforcement were analyzed using a two-way repeated measures ANOVA, with treatments as the between-subject factor and SA days as a within-subject factor (note that data from each FR schedule were analyzed separately). Bonferroni’s test was used for further comparison. The number of infusions and breakpoints under the PR schedule of reinforcement were analyzed using one-way ANOVA, followed by Dunnett’s test. Data from the locomotor sensitization and EEG were analyzed using a two-way repeated measures ANOVA, with treatments as the between-subject factor and treatment days/time as a within-subject factor, with Bonferroni’s test for post hoc analysis. Statistical significance was set at p < 0.05. All statistical analyses were performed using the GraphPad Prism software v. 8.01 (San Diego, CA, USA).
Results
4-MeO-PCP and 3-MeO-PCMo induce CPP
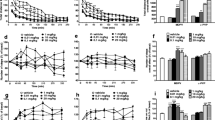
Figure 2b shows the CPP scores of mice treated with 4-MeO-PCP, 3-MeO-PCMo, KET, and SAL. A one-way ANOVA revealed a significant difference among the treatment groups [F (7,63) = 11.0, p < 0.001]. Furthermore, post hoc analyses revealed that the mice conditioned with 3 and 10 mg/kg 4-MeO-PCP, 10 mg/kg 3-MeO-PCMo, and 10 mg/kg KET displayed a significant dose-dependent increase in CPP scores compared with the SAL group.
Effects of 4-MeO-PCP, 3-MeO-PCMo, and KET on the CPP test in mice. a CPP protocol as described in the “Materials and methods” section. b Each bar represents the mean ± SEM of the CPP score(s) calculated as the difference in the time spent in the drug-paired during the post- minus pre-conditioning phases. Values are mean ± S.E.M. n = 8 animals per group. **p < 0.01, ***p < 0.001 significantly different from the SAL group (Dunnett’s post-test)
4-MeO-PCP and 3-MeO-PCMo are self-administered by rats under the FR schedule of reinforcement
Figure 3 displays the total number of active lever responses and infusions obtained by rats treated with 4-MeO-PCP, 3-MeO-PCMo, KET, and SAL (Fig. 3b). Under the FR1 schedule, a significant difference among treatments [F (4,45) = 30.2, p < 0.001] but no effect of SA days [F (6,270) = 1.20, p > 0.05], and the interaction between them [F (24,270) = 1.19, p > 0.05] was observed in active lever responses in rats self-administering 4-MeO-PCP (Fig. 3c). A significant difference in the number of infusions among treatments [F (4,45) = 86.8, p < 0.001], SA days [F (6,270) = 3.73, p < 0.01], and interaction between them [F (24,270) = 2.39, p < 0.001] was observed (Fig. 3e). SA of 3-MeO-PCMo led to a significant difference among treatments [F (4,45) = 35.5, p < 0.001], SA days [F (6,270) = 9.43, p < 0.001], and the interaction between them [F (24,270) = 1.90, p < 0.01] in active lever responses (Fig. 3f). There was also a significant difference in the number of infusions among treatments [F (4,45) = 95.1, p < 0.001], SA days [F (6,270) = 3.55, p < 0.01], and the interaction between them [F (24,270) = 1.94, p < 0.01]. Post hoc tests revealed that 4-MeO-PCP, 3-MeO-PCMo, and KET increased the number of lever presses and the number of infusions, when administered at dosages of 0.3 mg/kg/infusion, 0.1 mg/kg/infusion, and 0.03 mg/kg/infusion.
Effects of 4-MeO-PCP, 3-MeO-PCMo, and KET on the SA test in rats under the FR schedule. a SA protocol as described in the “Materials and methods” section. The data show the b, e number of active (drug-paired) lever responses, c, f number of drug infusions, and d, g inactive lever presses for the SAL, 4-MeO-PCP (0.03, 0.1, 0.3 mg/kg/inf), 3-MeO-PCMo (0.03, 0.1, 0.3 mg/kg/inf), and KET (0.3 mg/kg/inf) groups during the 12-consecutive days 2 h SA sessions under the FR1 schedule for the first 7 days, FR2 for the next 3 days, and FR3 for the last 2 days. Values are mean ± S.E.M. n = 10 animals per group. *p < 0.05, **p < 0.01, ***p < 0.001 relative to SAL group (Bonferroni’s test)
Under the FR2 schedule (Fig. 3b), there was a significant difference in the active lever response among treatments for 4-MeO-PCP [F (4,45) = 32.5, p < 0.001] and 3-MeO-PCMo [F (4,45) = 31.7, p < 0.001], but not for the SA days and interaction between them (Fig. 3c). For 4-MeO-PCP, SA led to a significant difference in the number of infusions among treatments [F (4,45) = 71.0, p < 0.001], SA days [F (2,90) = 15.9, p < 0.001], and interaction between them [F (8,90) = 5.09, p < 0.001] (Fig. 3e). Rats self-administering 3-MeO-PCMo showed a significant difference in the active lever response for treatments [F (4,45) = 31.7, p < 0.001] but not for the SA days and interaction between them (Fig. 3f). Similarly, a significant effect of treatments [F (4,45) = 37.4, p < 0.001] in the number of infusion was also observed. Post hoc analyses showed that there was an increase in lever pressing and the number of infusions of SA, upon exposure to 0.3, 0.1, and 0.03 mg/kg/infusion.
Under the FR3 schedule, two-way ANOVA revealed a significant difference in the active lever responses among treatments for 4-MeO-PCP [F (4,45) = 22.2, p < 0.001] (Fig. 3b) and 3-MeO-PCMo [F (4,45) = 22.1, p < 0.001] (Fig. 3e), but not in the SA days and interaction between them. In a similar manner, SA of 4-MeO-PCP [F (4,45) = 30.0, p < 0.001] (Fig. 3c) and 3-MeO-PCMo [F (4,45) = 24.6, p < 0.001] (Fig. 3f) showed a significant difference in the number of infusions among treatments. Post hoc test revealed an increase in lever pressing and the number of infusions in the SA test among the 0.3, 0.1, and 0.03 mg/kg/infusion groups. KET-treated rats also exhibited a significant increase in active lever responses and the number of infusions, with the post hoc tests revealing an increase in the lever pressing and number of infusions across all days of the 12-day test.
No significant difference was observed in the inactive lever response (Fig. 3d, g) among treatments across all days of the SA test.
Self-administration of 4-MeO-PCP and 3-MeO-PCMo in rats under the PR schedule of reinforcement
Figure 4 shows the breakpoints and number of infusions obtained by rats treated with 4-MeO-PCP, 3-MeO-PCMo, KET, and SAL under the PR schedule. One-way ANOVA showed a significant difference among treatments (4-MeO-PCP [F (4,35) = 13.00, p < 0.001] and 3-MeO-PCMo [F (4,35) = 9.602, p < 0.001] in breakpoints. Dunnett’s test revealed that 0.1 and 0.3 mg/kg/inf of 4-MeO-PCP and 3-MeO-PCMo induced higher breakpoint than SAL. Similarly, a significant effect of 4-MeO-PCP [F (4,35) = 21.76, p < 0.001] and 3-MeO-PCMo [F (4,35) = 17.26, p < 0.001] in the number of infusion was observed. Post hoc tests showed a significant increase in the number of infusions in the 0.1 and 0.3 mg/kg 4-MeO-PCP group and 0.03, 0.1, and 0.3 mg/kg 3-MeO-PCMo group compared with that in the SAL group. KET-treated mice also displayed a significant increase in breakpoints and the number of infusions.
Effects of 4-MeO-PCP, 3-MeO-PCMO, and KET on the SA test in rats under the PR schedule. The data show the a, c breakpoint and b, d mean number of infusions obtained during 6 h, 1 day SA for rats self-administering SAL, 4-MeO-PCP (0.03, 0.1, 0.3 mg/kg/inf), 3-MeO-PCMo (0.03, 0.1, 0.3 mg/kg/inf), and KET (0.3 mg/kg/inf). n = 8 animals per group. Values are mean ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from the SAL group (Dunnett’s post-test)
4-MeO-PCP, but not 3-MeO-PCMo, induces locomotor sensitization
Figure 5 illustrates the locomotor activity of the mice treated with 4-MeO-PCP, 3-MeO-PCMo (1, 3, and 10 mg/kg), KET (10 mg/kg), or SAL for a 7-day treatment and abstinence. Figure 5b shows that there was a significant difference among treatments [F (4,45) = 3.51, p < 0.05], treatment days [F (6,270) = 5.79, p < 0.001], and the interaction between them [F (24,270) = 3.29, p < 0.001]. Post hoc analyses showed that 10 mg/kg dose of 4-MeO-PCP increased the locomotor activity of animals on the third and seventh days of treatment (Fig. 5c). Furthermore, one-way ANOVA revealed that 4-MeO-PCP (10 mg/kg) challenge [F (9,90) = 10.22, p < 0.001] induced higher locomotor activity in mice as compared to the first treatment. In Fig. 5d, two-way ANOVA revealed a significant difference between treatments [F (4,45) = 3.62, p < 0.05] and treatment days [F (6,270) = 15.07, p < 0.001], but not for the interaction between variables [F (24,270) = 1.04, p > 0.05]. However, further analysis showed 3-MeO-PCMo did not alter the locomotor activity of the mice (Fig 5e). No significant difference was also observed between the on the first treatment of 3-MeO-PCMo and its challenge. KET-treated mice displayed a constant locomotor activity, same as SAL, during the 7-day test.
Effects of 4-MeO-PCP, 3-MeO-PCMo, and KET on the locomotor sensitization (distanced moved, cm) test in mice. a Locomotor sensitization protocol as described in the “Materials and methods” section. b, d The data show the locomotor activity before drug treatment (0), on the first, third, and seventh days of treatment and on the first, third, and seventh days of drug abstinence. c, e Comparison of locomotor activity during the first day of drug treatment versus drug challenge. Values are mean ± S.E.M. n = 10 animals per group. **p < 0.01, ***p < 0.001 significantly different from the SAL group or treatment day 1 (Bonferroni’s post-test)
SCH23390 and haloperidol inhibit the acquisition of drug CPP
Figure 6b presents the effects of SCH23390 and haloperidol pretreatment on the acquisition of 4-MeO-PCP, 3-MeO-PCMo, and KET CPP in mice. One-way ANOVA revealed a significant difference among treatment groups [F (11,119) = 17.28, p < 0.001]. Similar to the results above, 4-MeO-PCP, 3-MeO-PCMo, and KET, without SCH23390 and haloperidol pretreatment, led to increased CPP scores in mice. Pretreatment with SCH23390 and haloperidol significantly reduced the CPP scores in mice treated with compounds.
Effects of SCH23390, a DRD1 antagonist, and haloperidol, a DRD2 antagonist, on the rewarding effects of 4-MeO-PCP and 3-MeO-PCMo. a During the CPP test, SCH23390 (0.5 mg/kg) and haloperidol (0.2 mg/kg) were administered 15 min before 4-MeO-PCP, 3-MeO-PCMo, and KET (10 mg/kg) conditioning sessions. b Each bar represents the mean ± SEM of the CPP score(s) calculated as the difference in the time spent in the drug-paired during the post- minus pre-conditioning phases. c Each bar indicates the locomotor activity of mice in the SAL-paired and drug-paired compartments during the conditioning phase of the CPP. n = 10 animals per group. ***p < 0.001 significantly different from the SAL, ###p < 0.001 compared to 4-MeO-PCP, 3-MeO-PCMo, or KET pretreated with SAL (Bonferroni’s post-test)
Figure 6c displays the locomotor activity of the mice in SAL-paired and drug-paired compartment during the conditioning phase of CPP. Two-way ANOVA showed that a significant difference among treatments [F (11, 936) = 20.55, p < 0.001], compartments [F (1, 936) = 103.0, p < 0.001], interaction between treatments × compartments [F (11, 936) = 15.29, p < 0.001] was observed. Post hoc comparison revealed that no significant difference between SAL-paired and drug-paired compartment in SCH23990 + SAL and haloperidol + SAL groups was observed; however, the haloperidol + SAL group exhibited lower locomotor activity when compared to SAL + SAL group in the drug-paired compartment. SCH23390 pretreatment decreased the locomotor activity in mice treated with 4-MeO-PCP, 3-MeO-PCMo, and KET. Similarly, haloperidol reduced the locomotor activity of the 4-MeO-PCP and 3-MeO-PCMo groups but not of the KET group (although a decreasing trend was observed).
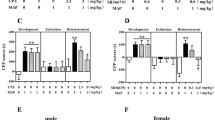
4-MeO-PCP and 3-MeO-PCMo alter DRD1, DRD2, and TH protein levels in the NAc and VTA
Figure 7 shows the effects of repeated treatment of 4-MeO-PCP, 3-MeO-PCMo, KET, or SAL on the protein expression of DRD1, DRD2, TH, and DAT in the NAc and VTA. One-way ANOVA revealed a significant difference among the treatments in DRD1 [F (3,23) = 6.697, p < 0.01] (Fig. 7b), DRD2-NAc [F (3,23) = 29.75, p < 0.001] (Fig. 7c), DRD2-VTA [F (3,23) = 8.116, p < 0.001] (Fig. 7g), and TH [F (3,23) = 9.419, p < 0.001] (Fig. 7f) protein levels. Dunnett’s post hoc test showed that TH and DRD1 protein levels in the NAc were increased, whereas DRD2 in both NAc and VTA was decreased by 4-MeO-PCP, 3-MeO-PCMo, and KET. However, neither of the drugs altered the DAT protein levels.
Effects of a 7-day treatment of 4-MeO-PCP, 3-MeO-PCMo, and KET (10 mg/kg) on the dopamine-related protein levels. a, e Representative blots of the target proteins. Protein levels of b DRD1, c DRD2, and d DAT in the NAc and of f TH, g DRD2, and h DAT in the VTA of the drug-treated mice. Values are mean ± S.E.M. n = 6 animals per group. *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from the SAL group (Dunnett’s post-test)
4-MeO-PCP and 3-MeO-PCMo increase dopamine concentrations in the NAc
Figure 8 shows the effects of repeated treatment of 4-MeO-PCP, 3-MeO-PCMo, KET, or SAL on the total dopamine concentrations in the NAc. One-way ANOVA revealed significant difference among the treatment groups for dopamine [F (3,19) = 18.98, p < 0.001], and post hoc analyses indicated that 4-MeO-PCP, 3-MeO-PCMo, and KET significantly increased the dopamine levels, compared with the SAL group.
4-MeO-PCP and 3-MeO-PCMo alter phosphorylated CREB, deltaFosB, and BDNF levels in the NAc
Figure 9 demonstrates the effects of repeated treatment of 4-MeO-PCP, 3-MeO-PCMo, KET, or SAL on the protein expression of p-CREB/CREB, deltaFosB, and BDNF in the NAc. One-way ANOVA revealed significant difference among the treatment groups in (Fig. 9b) p-CREB [F (3,23) = 134.2, p < 0.001] and post hoc analyses indicated that 4-MeO-PCP, 3-MeO-PCMo, and KET significantly decreased the p-CREB protein expression levels (Fig. 9c). In a similar manner, one-way ANOVA revealed a significant difference among the treatment groups [F (3,23) = 10.94, p < 0.001] and post hoc analyses revealed that 4-MeO-PCP, 3-MeO-PCMo, and KET significantly increased the deltaFosB protein expression levels compared with the SAL group (Fig. 9d). One-way ANOVA revealed a significant difference among the treatment groups [F (3,23) = 5.035, p < 0.01] and post hoc analyses revealed that 4-MeO-PCP, 3-MeO-PCMo, and KET significantly reduced the BDNF protein expression levels in mice, compared with the SAL group.
Effects of a 7-day treatment of SAL, 4-MeO-PCP, 3-MeO-PCMo, and KET (10 mg/kg) on CREB phosphorylation, deltaFosB, and BDNF levels in the NAc. a Representative bands of the target proteins per group. Protein levels of b p-CREB/CREB, c deltaFosB, and d BDNF in the NAc in mice. Values are mean ± S.E.M. n = 6 animals per group. *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from the SAL group (Dunnett’s post-test)
4-MeO-PCP and 3-MeO-PCMo increase the delta and gamma wave power and are inhibited by SCH23390 and haloperidol pretreatment
Figure 10 show the effects of 4-MeO-PCP, 3-MeO-PCMo, and KET on delta and gamma wave frequency, as well as the effects of SCH23390 and haloperidol on the alteration of drug-induced delta and gamma wave in mice (Fig. 10b). In delta wave, two-way repeated measures ANOVA revealed a significant effect of treatments [F (11,60) = 4.77, p < 0.001], recording time [F (7,420) = 3.19, p < 0.01], and their interaction [F (77,420) = 5.42, p < 0.001]. Dunnett’s post hoc tests revealed that there was an increase in delta waves at 10, 20, 30, 40, 50, and 60 min after administration of 3-MeO-PCMo. KET increased the delta waves only at 10 and 20 min after administration, whereas 4-MeO-PCP increased the delta waves at 50 and 60 min after administration (Fig. 10c). In a similar manner, a significant difference between treatments [F (11,60) = 15.5, p < 0.001], recording time [F (7,420) = 33.4, p < 0.001], and interaction between treatments and recording time [F (77,420) = 6.97, p < 0.001] was observed in gamma wave. Post hoc analyses showed that gamma waves were increased by 4-MeO-PCP, 3-MeO-PCMo, and KET, at 10, 20, 30, 40, 50, and 60 min after administration. In contrast, treatment with SCH23390 and haloperidol blocked the 4-MeO-PCP-, 3-MeO-PCMo-, and KET-induced delta and gamma wave power elevation. 4-MeO-PCP, 3-MeO-PCMo, and KET did not alter alpha, beta, sigma, and theta waves (data were not shown).
Effects of a single treatment of SAL, 4-MeO-PCP, 3-MeO-PCMo, and KET (10 mg/kg) on the EGG activity in mice. In addition, effects of SCH23390, a DRD1 antagonist, and haloperidol, a DRD2 antagonist, on the 4-MeO-PCP-, 3-MeO-PCMo-, and KET-induced EGG activity were examined. a EEG protocol as described in the “Materials and methods” section. The data show the b delta [0.5–4 Hz] and c gamma [35–45 Hz] band power in drug-treated mice. Data are expressed as the mean ± SEM. n = 6 animals per group. The EEG frequencies were recorded and analyzed for a total of 70 min. *p < 0.05, **p < 0.01 and ***p < 0.001 significantly different from the SAL group (Bonferroni’s post-test)
Discussion
Our results show that 4-MeO-PCP and 3-MeO-PCMo induced CPP, indicating that these substances may induce rewarding effects. 4-MeO-PCP and 3-MeO-PCMo also supported SA under the FR schedule of reinforcement, which suggests their reinforcing effects. These results are consistent with previous findings showing that other dissociatives elicit place preference and induce SA in animals (Marglin et al. 1989; Trujillo et al. 2011; de la Peña et al. 2012; Botanas et al. 2015). The findings for the PR schedule with 4-MeO-PCP and 3-MeO-PCMo indicate a positive relationship between the dose of each drug and its reinforcing efficacy. However, it is noteworthy that KET appears to induce higher breakpoints and number of infusions than 4-MeO-PCP and 3-MeO-PCMo at the same dose. As the PR schedule is used to assess the reinforcing strength of drugs with abuse liability (dela Peña et al. 2012), our data suggest that KET likely has a greater reinforcing efficacy than the two drugs. This is an interesting result, given that 4-MeO-PCP and 3-MeO-PCMo have a greater binding affinity to NMDA receptors and induce higher dopamine levels (Fig. 8) than KET. A plausible explanation for this is the high affinity of 4-MeO-PCP and 3-MeO-PCMo to serotonin transporters (Roth et al. 2013; Colestock et al. 2018). Previous studies suggest that the serotonergic system can inversely affect the reinforcing effects of some drugs (Rickli et al. 2015; Loh and Roberts 1990; Mueller and Homberg 2015). Additional studies are warranted to verify this hypothesis. Nevertheless, we suggest that 4-MeO-PCP and 3-MeO-PCMo can induce rewarding and reinforcing effects.
We also found that repeated 4-MeO-PCP and 3-MeO-PCMo injection differentially affected the locomotor activity in mice. 4-MeO-PCP at 10 mg/kg increased the locomotor activity during the third and seventh drug treatments as well as on the challenge day, whereas 3-MeO-PCMo did not, suggesting that 4-MeO-PCP can induce locomotor sensitization. This is in line with previous PCP studies that reported an increased locomotor activity in rodents after repetitive drug administrations and further enhance locomotor activation upon drug challenge (Phillips et al. 2001; Halberstadt et al. 2016). Locomotor sensitization is a common effect of abused drugs or psychostimulants during repetitive drug administrations and/or to subsequent drug challenges (Valjent et al. 2010). It is attributed to the pharmacological action of drugs and learned associations with the drug experience and is believed to be a consequence of a sensitized reward system in the brain that plays a role in certain components of drug addiction (Steketee and Kalivas 2011). Therefore, our results for 4-MeO-PCP may correspond with the observed rewarding and reinforcing effects of the drug. The lack of locomotor effects for 3-MeO-PCMo and KET are in line with previous studies showing that KET does not alter locomotor activity in rodents (Trujillo et al. 2011; Halberstadt et al. 2016). It indicates that the rewarding effects of 3-MeO-PCMo are independent of increases in locomotor activity. Nevertheless, the differential locomotor effects of 4-MeO-PCP and 3-MeO-PCMo highlight the pharmacological variation between the two substances, which could be a worthwhile focus for future studies.
Pretreatment with SCH23390 and haloperidol blocked the acquisition of 4-MeO-PCP and 3-MeO-PCMo CPP, suggesting that DRD1 and DRD2 are associated with the rewarding effects of these substances. Similar results were also observed for KET. Previous study reported that acquisition of ketamine-CPP was blocked by l-THP, a DRD1, and DRD2 antagonist, suggesting that the dopamine receptors are involved in ketamine reward (Du et al. 2017). However, SCH23390 and haloperidol decreased the locomotor activity of the mice treated with 4-MeO-PCP and 3-MeO-PCMo during the conditioning phase of CPP, in addition to the locomotor-reducing effect induced by haloperidol itself. Therefore, caution may be exercised when interpreting the CPP result as the decrease in CPP might be a nonspecific effect. Alternatively, some studies showed that despite the locomotor suppression induced by pharmacological blockings (e.g., haloperidol, pregabide, cysteamine) during conditioning, CPP was not affected (Di Scala et al. 1985; Mithani et al. 1986; Martin-Iverson et al. 1986). Nevertheless, SCH23390 and haloperidol even at the doses lower than the dose used in the present study have been shown to block or reduce the locomotor effects of some drugs in rodents (Mithani et al. 1986; Lapin and Rogawski 1995; Lisek et al. 2012; Pina and Cunningham 2014; Oh et al. 2018). Additional studies are needed to clarify the present CPP findings.
Repeated KET, 4-MeO-PCP, and 3-MeO-PCMo treatments increased DRD1 levels in the NAc, even though these drugs decreased the DRD2 levels. Repeated KET administration has been shown to decrease DRD2 mRNA expression in the brain (Li et al. 2015). Likewise, repeated exposure to different drugs of abuse is shown to downregulate DRD2 in the striatum, including the NAc, which has been associated with animals that show a propensity to self-administer drugs (Ikemoto et al. 1997; Krasnova et al. 2013, 2016). KET, 4-MeO-PCP, and 3-MeO-PCMo increased the TH levels in the VTA, similar to previous studies that reported an approximately twofold increase in TH mRNA levels and TH immunoreactivity in the midbrain of mice chronically treated with KET (Tan et al. 2012; Xu and Lipsky 2015). As TH is the rate-limiting enzyme that controls dopamine synthesis, changes in its synthesis likely affect the production of dopamine; therefore, increases in TH would result in increased dopamine production (Lu et al. 2003; Nestler 2005). Indeed, KET, 4-MeO-PCP, and 3-MeO-PCMo elevated the dopamine levels in the NAc, corresponding to the increased TH levels. The reduction of DRD2 levels in the VTA could also have contributed to the increased dopamine levels, given that DRD2 in the VTA acts as an auto-inhibitory receptor and regulator of the production and release of dopamine (De Jong et al. 2015). KET can increase dopamine levels in the mesolimbic area by blocking the NMDA receptors in gamma-aminobutyric acid (GABA) neurons inside the thalamic reticular nucleus, which decreases GABA release and disinhibits dopaminergic neurons, leading to excessive dopamine production (Tan et al. 2012; Liu et al. 2016). Based on this, we speculate that 4-MeO-PCP and 3-MeO-PCMo activate the mesolimbic dopamine system in a manner similar to KET. The lack of effect on DAT is consistent with previous finding showing that KET does not induce a significant change in DAT availability within the brain (Roth et al. 2013). Overall, we suggest that 4-MeO-PCP and 3-MeO-PCMo activate the mesolimbic dopamine system, which likely mediates the rewarding and reinforcing effects of these substances.
Studies have shown that the neuroadaptive changes that develop during repeated exposure to drugs of abuse lead to drug addiction, likely through regulation of gene expression via transcription regulation, among others (Chao and Nestler 2004; Nestler 2008). The two transcription factors implicated in these neuroadaptations are CREB and deltaFosB (Nestler 2004; McClung and Nestler 2008). CREB is widely expressed in the brain and can be activated through the cyclic adenosine 3,5-monophosphate (cAMP) pathway and other second messenger systems (Nestler 2004). Its activity, particularly in the NAc, can be altered by repeated exposure to several drugs of abuse (Nestler 2004, 2005, 2012). DeltaFosB is a member of the Fos family of transcription factors that accumulate in the NAc and dorsal striatum after chronic exposure to abused drugs (Nestler et al. 2001; McClung et al. 2004). These changes in CREB and deltaFosB have been linked to drug reward and addiction (Nestler 2004, 2005, 2012). We found that repeated 4-MeO-PCP, 3-MeO-PCMo, and KET treatments decreased the p-CREB levels in the NAc, similar to previous findings that showed decreased levels of CREB phosphorylation after repeated cocaine administration in animals. Reduced CREB function in the striatum, particularly in the NAc, is reported to potentiate behavioral responses to drugs of abuse (e.g., cocaine and morphine) (Barrot et al. 2002; Carlezon et al. 1998; McClung and Nestler 2003). Thus, the drug-induced reduction of p-CREB in this study might have contributed to the rewarding effects of 4-MeO-PCP, 3-MeO-PCMo, and KET. Furthermore, all drugs enhanced the deltaFosB levels, indicating the involvement of deltaFosB, in addition to that of CREB, in the rewarding effects of the drugs.
As a transcription factor, CREB produces its behavioral effects via regulation of target genes, including BDNF (Wang et al. 2018). BDNF is a neurotrophic factor that supports the survival of existing neurons and promotes the growth and differentiation of new neurons and synapses (Park and Poo 2013). Increased BDNF expression has been associated with drug self-administration and drug craving and seeking behaviors (Barker et al. 2015). Conversely, 4-MeO-PCP, 3-MeO-PCMo, and KET reduced the BDNF levels in the NAc, which may be attributed to the reduced p-CREB given that BDNF expression can be influenced by CREB activity. Alternatively, it may indicate that the BDNF and neuroprotective functions were impaired after repeated drug treatments. Previous studies had found that serum BDNF levels are reduced in methamphetamine abusers during the first 3 weeks of withdrawal (Chen et al. 2014), although a similar study also reported increased BDNF levels after 1 month of withdrawal (Ren et al. 2016). These data indicate that BDNF levels are initially decreased and then increased after drug withdrawal, suggesting that repeated drug exposures first impair neuroprotective function, followed by a neuroadaptive process. The inconsistent results between the previous studies and the current one may be attributed to methodological differences (i.e., time points when brain tissue was collected after the last drug treatment and duration of treatment). Altogether, the alterations in CREB, deltaFosB, and BDNF activity in the NAc may reflect adaptive changes in the brain caused by repeated 4-MeO-PCP and 3-MeO-PCMo treatments, which may play an important role in the rewarding and reinforcing effects of the drugs.
Lastly, 4-MeO-PCP, 3-MeO-PCMo, and KET increased the delta and gamma wave activity in the brain. Delta wave is associated with many basic biological processes and higher-order functions, including motivation and cognition (Knyazev 2012; Hunt and Kasicki 2013). Methamphetamine users or those recently withdrawn from its use, and MDMA abusers have been reported to have increased delta wave activity (Newton et al. 2003). In line with this, the increased delta wave activity in our present study may correlate with the rewarding and reinforcing effects of the drugs. Besides, KET has been shown to increase the delta wave activity in monkeys (Keavy et al. 2016). Gamma wave is associated with information processing, consciousness, and attention (Lazarewicz et al. 2010). NMDA receptor antagonism has often been linked to increases in gamma wave activity, which is suggested to represent aberrant diffuse network noise and a potential electrophysiological correlate of a psychotic-like state (Lee et al. 2003; Jones et al. 2012). Based on this information, the increased gamma wave activity may correlate with the reported dissociative effects of 4-MeO-PCP, 3-MeO-PCMo, and KET. Conversely, SCH23390 and haloperidol inhibited the effects of 4-MeO-PCP, 3-MeO-PCMo, and KET on the delta and gamma wave activity, indicating that the dopaminergic system may be associated with the effects of these substances in delta and gamma wave activity. Overall, we suggest that 4-MeO-PCP and 3-MeO-PCMo can alter the brain wave activity, which may contribute to their rewarding and pharmacological effects.
In summary, the present study showed that 4-MeO-PCP and 3-MeO-PCMo induce rewarding and reinforcing effects, which are likely mediated via the mesolimbic dopamine system. These drugs can also induce neuroplastic changes and affect brain wave activity, which likely contribute to their rewarding and reinforcing effects. Taken together, our results suggest that 4-MeO-PCP and 3-MeO-PCMo have a high abuse liability in humans. More importantly, this study provides significant insights that can be useful for the development of appropriate legislative measures on these substances and for predicting the abuse potential of future synthetic dissociative drugs.
References
Barker JM, Taylor JR, De Vries TJ, Peters J (2015) Brain-derived neurotrophic factor and addiction: pathological versus therapeutic effects on drug seeking. Brain Res 1628:68–81
Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Zachariou V (2002) CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci 99(17):11435–11440
Baumeister D, Tojo LM, Tracy DK (2015) Legal highs: staying on top of the flood of novel psychoactive substances. Ther Adv Psychopharmacol 5(2):97–132
Botanas CJ, de la Peña JB, Dela Pena IJ, Tampus R, Yoon R, Kim HJ, Lee YS, Jang CG, Cheong JH (2015) Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: evidence of its abuse potential. Pharmacol Biochem Behav 133:31–36
Botanas CJ, Yoon SS, de la Peña JB, dela Peña IJ, Kim M, Custodio RJ, Woo T, Seo JW, Jang CG, Yang JS, Yoon YM, Lee YS, Kim HJ, Cheong JH (2018) A new synthetic drug 5-(2-aminopropyl) indole (5-IT) induces rewarding effects and increases dopamine D1 receptor and dopamine transporter mRNA levels. Behav Brain Res 341:122–128
Botly LC, Burton CL, Rizos Z, Fletcher PJ (2008) Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology 199(1):55–66
Carlezon WA, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ (1998) Regulation of cocaine reward by CREB. Science 282(5397):2272–2275
Chao J, Nestler EJ (2004) Molecular neurobiology of drug addiction. Annu Rev Med 55:113–132
Chen PH, Huang MC, Lai YC, Chen PY, Liu HC (2014) Serum brain-derived neurotrophic factor levels were reduced during methamphetamine early withdrawal. Addict Biol 19(3):482–485
Colestock T, Wallach J, Mansi M, Filemban N, Morris H, Elliott SP, Westphal F, Brandt SD, Adejare A (2018) Syntheses, analytical and pharmacological characterizations of the ‘legal high’4-[1-(3-methoxyphenyl) cyclohexyl] morpholine (3-MeO-PCMo) and analogues. Drug Test Anal 10(2):272–283
De Jong JW, Roelofs TJ, Mol FM, Hillen AE, Meijboom KE, Luijendijk MC, van der Eerden HA, Garner KM, Vanderschuren LJ, Adan RA (2015) Reducing ventral tegmental dopamine D2 receptor expression selectively boosts incentive motivation. Neuropsychopharmacology 40(9):2085–2095
de la Peña JBI, Lee HC, Ike C, Woo TS, Yoon SY, Lee HL, Han JS, Lee JI, Cho YJ, Shin CH, Cheong JH (2012) Rewarding and reinforcing effects of the NMDA receptor antagonist–benzodiazepine combination, Zoletil®: difference between acute and repeated exposure. Behav Brain Res 233(2):434–442
dela Peña I, Yoon SY, Lee JC, dela Peña JB, Sohn AR, Ryu JH, Shin CY, Cheong JH (2012) Methylphenidate treatment in the spontaneously hypertensive rat: influence on methylphenidate self-administration and reinstatement in comparison with Wistar rats. Psychopharmacology 221(2):217–226
Di Scala G, Martin-Iverson MT, Phillips AG, Fibiger HC (1985) The effects of progabide (SL 76002) on locomotor activity and conditioned place preference induced by d-amphetamine. Eur J Pharmacol 107(2):271–274
Du Y, Du L, Cao J, Hölscher C, Feng Y, Su H, Wang Y, Yun KM (2017) Levo-tetrahydropalmatine inhibits the acquisition of ketamine-induced conditioned place preference by regulating the expression of ERK and CREB phosphorylation in rats. Behav Brain Res 317:367–373
Halberstadt AL, Slepak N, Hyun J, Buell MR, Powell SB (2016) The novel ketamine analog methoxetamine produces dissociative-like behavioral effects in rodents. Psychopharmacology 233(7):1215–1225
Hill SL, Thomas SH (2011) Clinical toxicology of newer recreational drugs. Clin Toxicol 49(8):705–719
Hunt MJ, Kasicki S (2013) A systematic review of the effects of NMDA receptor antagonists on oscillatory activity recorded in vivo. J Psychopharmacol 27(11):972–986
Ikemoto S, Glazier BS, Murphy JM, McBride WJ (1997) Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci 17(21):8580–8587
Jones NC, Reddy M, Anderson P, Salzberg MR, O’Brien TJ, Pinault D (2012) Acute administration of typical and atypical antipsychotics reduces EEG gamma power, but only the preclinical compound LY379268 reduces the ketamine-induced rise in gamma power. Int J Neuropsychopharmacol 15(5):657–668
Keavy D, Bristow LJ, Sivarao DV, Batchelder M, King D, Thangathirupathy S, Macor JE, Weed MR (2016) The qEEG signature of selective NMDA NR2B negative allosteric modulators; a potential translational biomarker for drug development. PLoS One 11(4):e0152729
Knyazev GG (2012) EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev 36(1):677–695
Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes C, Adair JE, Lehrmann E, Kobeissy FH, Gold MS, Becker KG, Goldberg SR, Cadet JL (2013) CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis 58:132–143
Krasnova IN, Justinova Z, Cadet JL (2016) Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways. Psychopharmacology 233(10):1945–1962
Lapin IP, Rogawski MA (1995) Effects of D1 and D2 dopamine receptor antagonists and catecholamine depleting agents on the locomotor stimulation induced by dizocilpine in mice. Behav Brain Res 70(2):145–151
Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ (2010) Ketamine modulates theta and gamma oscillations. J Cogn Neurosci 22(7):1452–1464
Lee KH, Williams LM, Breakspear M, Gordon E (2003) Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Rev 41(1):57–78
Li B, Liu ML, Wu XP, Jia J, Cao J, Wei ZW, Wang YJ (2015) Effects of ketamine exposure on dopamine concentrations and dopamine type 2 receptor mRNA expression in rat brain tissue. Int J Clin Exp Med 8(7):11181–11187
Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM (2012) Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend 126(1-2):257–262
Liu Y, Lin D, Wu B, Zhou W (2016) Ketamine abuse potential and use disorder. Brain Res Bull 126:68–73
Loh EA, Roberts DC (1990) Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology 101(2):262–266
Lu L, Grimm JW, Shaham Y, Hope BT (2003) Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem 85(6):1604–1613
Marglin SH, Milano WC, Mattie ME, Reid LD (1989) PCP and conditioned place preferences. Pharmacol Biochem Behav 33(2):281–283
Martin-Iverson MT, Radke JM, Vincent SR (1986) The effects of cysteamine on dopamine-mediated behaviors: evidence for dopamine-somatostatin interactions in the striatum. Pharmacol Biochem Behav 24(6):1707–1714
McClung CA, Nestler EJ (2003) Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nat Neurosci 6(11):1208–1215
McClung CA, Nestler EJ (2008) Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology 33(1):3–17
McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ (2004) ΔFosB: a molecular switch for long-term adaptation in the brain. Mol Brain Res 132(2):146–154
McIntyre IM, Trochta A, Gary RD, Storey A, Corneal J, Schaber B (2015) A fatality related to two novel hallucinogenic compounds: 4-methoxyphencyclidine and 4-hydroxy-N-methyl-N-ethyltryptamine. J Anal Toxicol 39(9):751–755
Mithani S, Martin-Iverson MT, Phillips AG, Fibiger HC (1986) The effects of haloperidol on amphetamine-and methylphenidate-induced conditioned place preferences and locomotor activity. Psychopharmacology 90(2):247–252
Mueller CP, Homberg JR (2015) The role of serotonin in drug use and addiction. Behav Brain Res 277:146–192
Nestler EJ (2004) Molecular mechanisms of drug addiction. Neuropharmacology 47:24–32
Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8(11):1445–1449
Nestler EJ (2008) Transcriptional mechanisms of addiction: role of ΔFosB. Philosophical Transactions of the Royal Society B: Biol Sci 363(1507):3245–3255
Nestler EJ (2012) Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci 10(3):136–143
Nestler EJ, Barrot M, Self DW (2001) ΔFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci 98(20):11042–11046
Newton TF, Cook IA, Kalechstein AD, Duran S, Monroy F, Ling W, Leuchter AF (2003) Quantitative EEG abnormalities in recently abstinent methamphetamine dependent individuals. Clin Neurophysiol 114(3):410–415
Oh JH, Hwang JY, Hong SI, Ma SX, Seo JY, Lee SY, Kim HC, Jang CG (2018) The new designer drug buphedrone produces rewarding properties via dopamine D1 receptor activation. Addict Biol 23(1):69–79
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23
Phillips M, Wang C, Johnson KM (2001) Pharmacological characterization of locomotor sensitization induced by chronic phencyclidine administration. J Pharmacol Exp Ther 296(3):905–913
Pina MM, Cunningham CL (2014) Effects of dopamine receptor antagonists on the acquisition of ethanol-induced conditioned place preference in mice. Psychopharmacology 231(3):459–468
Prashad M, Liu Y, Har D, Repič O, Blacklock TJ (2005) 1, 2, 3-Triazole as a safer and practical substitute for cyanide in the Bruylants reaction for the synthesis of tertiary amines containing tertiary alkyl or aryl groups. Tetrahedron Lett 46(33):5455–5458
Prus AJ, James JR, Rosecrans JA (2009) Conditioned place preference, in Methods of Behavior Analysis in Neuroscience, Chapter 4, 2nd edn. (Buccafusco JJ ed.), pp 59–76. CRC Press, Boca Raton, FL
Ren W, Tao J, Wei Y, Su H, Zhang J, Xie Y, Guo J, Zhang X, Zhang H, He J (2016) Time-dependent serum brain-derived neurotrophic factor decline during methamphetamine withdrawal. Medicine 95(5)
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66(1):1–11
Rickli A, Kopf S, Hoener MC, Liechti ME (2015) Pharmacological profile of novel psychoactive benzofurans. Br J Pharmacol 172(13):3412–3425
Roth BL, Gibbons S, Arunotayanun W, Huang XP, Setola V, Treble R, Iversen L (2013) The ketamine analogue methoxetamine and 3-and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate NMDA receptor. PLoS One 8(3):e59334
Steketee JD, Kalivas PW (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63(2):348–365
Stevenson R, Tuddenham L (2014) Novel psychoactive substance intoxication resulting in attempted murder. J Forensic Legal Med 25:60–61
Tan S, Lam WP, Wai MS, Yu WHA, Yew DT (2012) Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS One 7(8):e43947
Trujillo KA, Smith ML, Sullivan B, Heller CY, Garcia C, Bates M (2011) The neurobehavioral pharmacology of ketamine: implications for drug abuse, addiction, and psychiatric disorders. ILAR J 52(3):366–378
Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault JA (2010) Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology 35(2):401–415
Wallach J, Paoli GD, Adejare A, Brandt SD (2014) Preparation and analytical characterization of 1-(1-phenylcyclohexyl) piperidine (PCP) and 1-(1-phenylcyclohexyl) pyrrolidine (PCPy) analogues. Drug Test Anal 6(7-8):633–650
Wang H, Xu J, Lazarovici P, Quirion R, Zheng W (2018) cAMP response element-binding protein (CREB): a possible signaling molecule link in the pathophysiology of Schizophrenia. Front Mol Neurosci 11
Xu K, Lipsky RH (2015) Repeated ketamine administration alters N-methyl-D-aspartic acid receptor subunit gene expression: implication of genetic vulnerability for ketamine abuse and ketamine psychosis in humans. Exp Biol Med 240(2):145–155
Funding
This study was supported by the Ministry of Food and Drug Safety (MFDS) of Korea (19182MFDS410).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abiero, A., Botanas, C.J., Custodio, R.J. et al. 4-MeO-PCP and 3-MeO-PCMo, new dissociative drugs, produce rewarding and reinforcing effects through activation of mesolimbic dopamine pathway and alteration of accumbal CREB, deltaFosB, and BDNF levels. Psychopharmacology 237, 757–772 (2020). https://doi.org/10.1007/s00213-019-05412-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05412-y