Abstract
Rationale
Alcoholism is a serious public health problem throughout the world. Current pharmacotherapies for the treatment of this disorder are poorly effective. Preclinical and clinical findings point to nicotinic acetylcholine receptors (nAChRs) as a promising target for the development of novel and effective medications. Assuage Pharmaceuticals, in collaboration with Torrey Pines Institute for Molecular Studies, has discovered a new class of potent and selective α4β2 nAChR antagonists.
Objective
Here, it was hypothesized that α4β2 nAChR antagonism is a viable approach for treatment of alcohol use disorders.
Results
When tested in rats, one lead compound, AP-202, attenuated both operant alcohol and nicotine self-administration in a paradigm in which the two reinforcers were concurrently available. The conotoxin TP2212-59, a selective α3β4 nAChR antagonist, was only effective in reducing nicotine self-administration. AP-202 also reduced alcohol but not food responding when alcohol was presented as the only reinforcer, whereas the commercially available α4β2 nAChR antagonist dihydro-β-erythroidine failed to alter alcohol self-administration. AP-202 did not block relapse-like behavior induced by previously alcohol-associated stimuli or yohimbine stress. In a reinstatement paradigm, in which alcohol seeking was triggered by a nicotine challenge, a behavior successfully inhibited by the nonselective nAChR antagonist mecamylamine, AP-202 was not effective, while pretreatment with TP2212-59 abolished nicotine-induced reinstatement of alcohol seeking.
Conclusions
These findings suggest differential roles for α4β2 and α3β4 nAChR on alcohol taking and seeking with selective blockade of α4β2 nAChR being more implicated in modulating alcohol taking while selective blockade of α3β4 nAChR is involved in nicotine-induced alcohol seeking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As with all drug abuse, alcohol dependence is a chronic relapsing disorder that represents an enormous public health problem in the USA and worldwide. The prevalence of alcohol abuse and dependence in the USA is about 7% of the adult population (SAMHSA 2013) with an estimated annual cost of 249 billion dollars to the economy (Sacks et al. 2015). Furthermore, nearly 88,000 people die from alcohol-related causes annually (CDC 2014; Stahre et al. 2014), including more than 10,000 driving fatalities (NHTSA 2016), making it the third leading preventable cause of death in the USA (CDC 2014; Stahre et al. 2014). Current FDA-approved pharmacotherapies to reduce alcohol consumption include disulfiram, which induces nausea when taken with alcohol, acamprosate, which is thought to reduce the negative effects related to withdrawal by dampening glutamate activity, and the opiate antagonist naltrexone (Volkow and Skolnick 2012). These medications have a modicum of effectiveness when paired with behavioral therapy. Clearly new medications are a high priority.
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels made up by a pentameric arrangement of α and β subunits to create a central pore, which regulates cation flux (Albuquerque et al. 2009). nAChRs can be homomeric or heterometric proteins. The heteromeric receptors can also have a large number of different α and β subunit compositions, rendering the pharmacological actions of the receptors and drug discovery very complicated. The heteromeric α4β2* (* indicates the possibility of additional subunits) and the homomeric α7 receptors are by far the most prevalent in the CNS (Perry et al. 2002; Xiao and Kellar 2004), whereas α6-containing nAChRs or heteromeric α3β4* nAChRs are mostly found in a few brain structures (Gotti et al. 2006). However, all these subunits are well expressed in the mesocorticolimbic and the habenulo-interpeduncular circuitry and may modulate the rewarding properties of nicotine.
In addition to their effects in mediating nicotine actions, there is evidence that nAChRs are involved in alcohol addiction (Chatterjee and Bartlett 2010; Hendrickson et al. 2013; Rahman et al. 2014). Accordingly, the nonselective nAChR antagonist mecamylamine has been demonstrated to block alcohol drinking in animal models (Ericson et al. 1998; Hendrickson et al. 2009). Although there have been a considerable number of studies pertaining to the role of various nAChR subtypes in regulating alcohol-related neurochemistry and behavior, the results are confusing and inconclusive. For example, the vast majority of nAChRs in the ventral tegmental area are α4β2*, and α4 subunits are apparently crucial in mediating alcohol-rewarding properties (Hendrickson et al. 2010) whereas β2-containing receptors have been reported not to be involved in reduction of alcohol-taking behavior, since the selective α4β2* antagonist dihydro-β-erythroidine (DHβE) was not effective in reducing home cage, operant self-administration, and other alcohol-related measures (Chatterjee et al. 2011; Hendrickson et al. 2009; Le et al. 2000). However, varenicline (Chantix), the α4β2* partial agonist smoking cessation medication (Jorenby et al. 2006), blocks excessive alcohol drinking in the intermittent access two-bottle choice paradigm in rats as well as drinking in the dark in mice (Hendrickson et al. 2010; Steensland et al. 2007), and also has been demonstrated to block alcohol consumption in heavy drinkers (McKee et al. 2009). Varenicline actions might be mediated by activation of α3β4* nAChRs (Chatterjee et al. 2011), but it has been recently reported that varenicline decreases binge drinking in the drinking in the dark model through a β4 nAChR-independent mechanism (Patkar et al. 2016), and consistently, the selective α3β4* partial agonist AT-1001 was ineffective in blocking alcohol self-administration (Cippitelli et al. 2015a) in rats. Furthermore, previous evidence has shown that the α4β2* nAChR desensitizing agent sazetidine A reduced alcohol drinking using a standard two-bottle choice procedure in alcohol-preferring rats (Rezvani et al. 2010; Xiao et al. 2006). Therefore, the science remains unsettled.
Starting with combinatorial libraries followed by initial modifications using traditional medicinal chemistry, Assuage Pharmaceuticals (AP), in collaboration with Torrey Pines Institute for Molecular Studies, identified novel structures that have remarkable selectivity and high affinity for α4β2* nAChRs. In a recent publication (Wu et al. 2013), we have determined that these compounds have antagonist activity at α4β2* nAChRs but not α3β4* nAChRs. In addition, one compound, (S)-5-methyl-1-(2-(pyridin-3-yl)ethyl)imidazolidin-2-imine (AP-202) was found to attenuate nicotine self-administration in rats and to block nicotine priming—as well as cue-induced reinstatement of nicotine seeking (Wu et al. 2017). Using mixture-based combinatorial libraries, modeled after α-conotoxin, a highly selective α3β4* nAChR antagonist, TP2212-59, was also identified (Chang et al. 2014). Here, we hypothesize that α4β2* nAChR antagonism is a viable approach for treatment of alcohol use disorders. First, we examined the effects of AP-202 on an operant co-administration paradigm in which rats concurrently lever press for intravenous (iv) nicotine and oral alcohol. The same experiment was conducted with the selective α3β4* nAChR antagonist TP2212-59. Second, we investigated if alcohol-related responses induced by AP-202 were specific and consistent across a variety of behavioral paradigms. Third, we determined the effectiveness of AP-202 in modifying relapse-like behavior as assessed by extinction-based reinstatement paradigms.
Material and methods
Animals
Male Sprague Dawley rats obtained from Charles River (Portage, MI) and weighing 200–225 g at their arrival were used in this study. Rats were housed in groups of two in a room with a reverse 12-h light/12-h dark cycle (lights off at 07:30 a.m.). All experiments were conducted during the dark phase of the cycle. Animals were acclimatized for 7 days with water and chow (Teklad Diets, Madison, WI) and handled three times before the experiments were started.
Drugs
AP-202 (Fig. 1) (Wu et al. 2017) was dissolved in a vehicle of 0.9% saline and injected by subcutaneous (sc) route of administration at a volume of 1 ml/kg. The conotoxin TP2212-59 was dissolved in 10% dimethyl sulfoxide and 90% of 0.9% saline, and injected by intracerebroventricular (icv) route at the volume of 2 μl/rat. (−)-Nicotine hydrogen tartrate salt and alcohol were purchased from Sigma (St. Louis, MO). Alcohol was diluted to a concentration of 10% (v/v) in water and made available orally. Nicotine solutions for sc and iv injections were obtained by dissolving the salt in 0.9% saline and the pH adjusted to 7.0–7.4 with 3 M sodium hydroxide. Nicotine doses are reported as free base concentration. DHβE and mecamylamine were purchased from Tocris Bioscience (Bristol, UK), dissolved in 0.9% saline, and sc injected. Yohimbine hydrochloride (Sigma, St. Louis, MO) was dissolved in distilled water and administered intraperitoneally (ip). AT-1001 was suspended in a vehicle containing 2% DMSO, 1% HCl, and 97% of 0.5% aqueous hydroxypropyl-cellulose, administered in a 1 ml/kg volume injection, and given by sc route of administration.
Apparatus
The self-administration boxes consisted of operant conditioning chambers (Med Associates, Inc., St. Albans, VT) enclosed in lit, sound-attenuating, ventilated environmental cubicles. In the case of food self-administration, each chamber was equipped with two retractable levers located in the front panel, laterally to a food pellet magazine. A pellet dispenser was positioned behind the front panel of the boxes. In the case of alcohol self-administration, the food pellet magazine was substituted by a drinking receptacle. Alcohol infusions (0.1 ml) occurred by means of syringe pumps (Med Associates, Inc., St. Albans, VT) connected to the drinking receptacle through a PE-160 tube. During food and alcohol self-administration experiments, pellet dispensers as well as infusions pumps were activated by responses on the right (active) lever, while responses on the left (inactive) lever were recorded but did not result in any programmed consequences. During co-administration of iv nicotine and oral alcohol, the chambers were equipped with two active levers and two infusion pumps, one that delivered iv nicotine (0.1 ml) and one that delivered alcohol (0.1 ml) into the drinking receptacle. Thus, appropriate responding on the right lever resulted in activation of the pump containing nicotine while responding on the left lever resulted in activation of the pump that released alcohol. Nicotine infusions occurred by means of liquid swivels (Instech Solomon, Plymouth Meeting, PA), connected to plastic tubing protected by a flexible metal sheath for attachment to the external catheter terminus. Operant chambers were also equipped with auditory stimuli presented via a speaker and visual stimuli located above the levers (cue lights) and near the top of the chamber opposite the lever on the front panel (house light). A microcomputer controlled the delivery of reinforcers, presentation of auditory and visual stimuli, and recording of the behavioral data.
Iv catheter implantation
One week after arrival, rats assigned to alcohol and nicotine co-administration experiments were exposed to an intermittent (every other day) 10% (v/v) alcohol exposure in their home cages (Cippitelli et al. 2012). This procedure lasted until animals attained three alcohol exposures. Then, the same rats were subjected to iv catheter implantation under isoflurane anesthesia. Incisions were made to expose the right jugular vein, and a catheter made from silicon tubing (inner diameter = 0.020 in, outside diameter = 0.037 in) was subcutaneously positioned as previously described (Cippitelli et al. 2015b , 2016). After insertion into the vein, the proximal end of the catheter was anchored to the muscles underlying the vein with surgical silk. The distal end of the catheter was attached to a threaded cannula guide bent at a 90° angle that protruded from the rat’s back. The cannula was capped with plastic tubing and covered with threaded lightweight aluminum hood. To maintain patency for the duration of the experiment, catheters were flushed daily with 0.2 ml of heparin (1000 UPS U/ml)-containing saline solution, which also contained 0.7 mg/ml enrofloxacin.
Nicotine and alcohol co-administration
Effect of AP-202 and TP2212-59
After recovery from surgery, one group of rats (N = 6) was trained to the concurrent self-administration of iv nicotine and oral alcohol. Operant conditions used in this experiment were chosen from previous work (Cippitelli et al. 2015b; Le et al. 2010). Following each nicotine infusion (30 μg/kg/infusion), a 20-s time-out (TO) period occurred during which responses at the lever that delivered nicotine (right lever) did not lead to programmed consequences. Nicotine reinforcements were accompanied by concurrent illumination of a cue light to signal delivery of nicotine. Reinforcements of 10% (v/v) alcohol were accompanied by a flashing house light (1-s on, 1-s off) with a TO period of 20 s during which responses at the lever that delivered alcohol (left lever) did not lead to programmed consequences. An intermittent tone (7 kHz, 70 dB) was sounded throughout 60-min sessions. These co-self-administration sessions were conducted under a fixed ratio-1 (FR-1) schedule for six sessions and under a FR-3 schedule for both reinforcers for additional 6 days. Subsequently, AP-202 (0.0, 0.3, 1.0 mg/kg) was sc administered to rats 10 min before sessions using a within-subject Latin square design. For TP2212-59 testing, an additional group of rats (N = 8) was subjected to an identical procedure except that the catheter implantation was concurrent to implantation of a guide cannula (26G, 7 mm long) aimed at the IV ventricle (coordinates − 1.0 mm anteroposterior, + 1.8 mm lateral, − 2.0 mm ventral from bregma; cannula tip exposed 2.5 mm). TP2212-59 infusions (0, 15, 30 μg/2 μl per rat) occurred through a 10 μl microsyringe 15 min prior to co-administration sessions. Test sessions for both AP-202 and TP2212-59 were 4 days apart and conducted after two consecutive co-administration sessions. To control for correct cannula placement, dipsogenic response (intake of at least 6 ml of water in 5 min) was evaluated following icv administration of angiotensin II-human (Sigma, St. Louis, MO) injected at a dose of 0.1 μg/1 μl.
Alcohol self-administration
Effect of AP-202 and DHβE
Once the intermittent procedure of alcohol exposure in their home cages was terminated, rats were moved to self-administration chambers in which they were trained to self-administer 10% (v/v) alcohol in 30-min daily sessions under a FR-1 schedule over 2 weeks (10 sessions). Following a response that delivered alcohol, a 5-s TO period was in effect, during which the house light was on and responses were recorded but not reinforced. Subsequently, two groups of new animals (N = 8) and (N = 7) were used to assess the effectiveness of AP-202 (0.0, 0.1, 0.5, 1.0 mg/kg) and DHβE (0, 8 mg/kg), respectively, using a Latin square counterbalanced within-subject design. The dose of 8 mg/kg DHβE has previously been used and failed to alter voluntary alcohol consumption in a rat-limited access drinking paradigm (Le et al. 2000). Test sessions were 4 days apart. Following each test session day, animals were allowed 1 day off, and a new baseline was then established over the following 2 days. Animals received sc injections of either AP-202 or DHβE, or vehicle 10 and 15 min, respectively, prior to the self-administration sessions. Results are described as number of rewards obtained in 30 min. Responses on the inactive lever were also recorded and served as an index of unspecific motor behavior.
Food self-administration
Effect of AP-202
Two weeks following their arrival, one group of new rats (N = 8) underwent a food restriction procedure in which rats received 16–20 g of chow daily with water freely accessible. After 1 week, rats were trained to self-administer 45 mg food pellets (Test Diet, 5-TUM, Richmond, IN) in 30-min daily sessions on an FR-1 (TO = 20 s) schedule of reinforcement. The TO period was signaled by illumination of a cue light at the top of the active lever for 20 s, and lever pressing during this period did not lead to programmed consequences. Food self-administration was conducted for 10 sessions before drug testing. Rats were then treated with different doses of AP-202 (0.0, 0.1, 0.5, 1.0 mg/kg, sc) according to a Latin square counterbalanced within-subject design. Drug doses or vehicle were given 10 min before the beginning of the session. Results are described as number of rewards obtained in 30 min. Responses on the inactive lever were also recorded and served as an index of unspecific motor behavior.
Cue-induced reinstatement of alcohol seeking
Effect of AP-202
New rats (N = 8) were initially exposed to the intermittent alcohol procedure in their home cage and then trained to lever press for 10% (v/v) alcohol in daily 30-min sessions under a FR-1 schedule of reinforcement for 15 days. Sessions were performed 5 days a week. Concurrently with the lever pressing, a 5-s TO period was in effect during which the house light was on and responses were recorded but not reinforced. A stimulus predictive of alcohol (orange odor) was also presented immediately after the animals were placed in the operant chambers and immediately before the onset of every conditioning session (Cippitelli et al. 2015a). Alcohol-reinforced responding was then extinguished in daily 30-min sessions that continued for 12 consecutive days. In this phase, neither alcohol nor the house light or the orange cue were available. After the last extinction session, animals were pre-treated with AP-202 (0.0, 0.1, 0.5, and 1.0 mg/kg, sc) 10 min prior to a 30-min reinstatement session, in which odor, house light, but not alcohol were presented. Responses on the previously alcohol-associated lever were recorded. Reinstatement experiments following AP-202 testing were repeated every third day counterbalancing AP-202 doses according to a Latin square design. No extinction sessions were conducted between reinstatement sessions (Ciccocioppo et al. 2001; Liu et al. 2007). Responding on the inactive lever was recorded throughout the experiment.
Yohimbine stress-induced reinstatement of alcohol seeking
Effect of AP-202
This experiment was performed as previously described (Cippitelli et al. 2015a). A new cohort of rats (N = 7) was initially exposed to the intermittent alcohol procedure in their home cage and then trained to lever press for 10% (v/v) alcohol in daily 30-min sessions under a FR-1 5-s TO schedule of reinforcement for 15 days. Once self-administration was established, responses were extinguished over 12 consecutive daily 30-min sessions. Extinction sessions were identical to self-administration sessions, except that alcohol was no longer available. After the last extinction session, animals were pre-treated with AP-202 (0.0, 0.1, 0.5, and 1.0 mg/kg, sc) 10 min prior to ip administration of 1.25 mg/kg yohimbine dose (Ayanwuyi et al. 2013). Thirty minutes following yohimbine treatment, the reinstatement test was started under the same conditions as extinction sessions (i.e., house light was still contingently presented during both extinction and reinstatement phases). Reinstatement experiments following AP-202 testing were repeated every third day counterbalancing AP-202 doses according to a Latin square design. Two 30-min extinction sessions were carried out between testing days to avoid potential lasting or cumulative effects of the stimulus-triggering relapse. Responding on the inactive lever was recorded throughout the experiment to monitor possible nonspecific behavioral effects.
Nicotine-induced reinstatement of alcohol seeking
Effects of mecamylamine, AP-202, TP2212-59 and AT-1001
Three additional groups of new rats (N = 8 each) were exposed to the intermittent alcohol procedure in their home cage and then trained to lever press for 10% (v/v) alcohol in daily 30-min sessions under a FR-1 5-s TO schedule of reinforcement for 7 days. Sessions were conducted under identical conditions as described above and performed 5 days a week. On days 6 and 7, rats were sc injected with saline 40 min prior to self-administration sessions in order to habituate them to the injection procedure. Daily injections of nicotine [0.4 mg/kg, sc (Le et al. 2003)] were then given 40 min prior to six additional alcohol sessions. Subsequently, two alcohol self-administration sessions were conducted prior to the start of extinction without previous nicotine challenge. Responses were extinguished over 15 consecutive daily sessions. Extinction sessions were identical to self-administration sessions, except that alcohol was no longer available. After the last extinction session, one group of animals was pre-treated with mecamylamine [0, 0.5, 1, 2 mg/kg, sc (Liu et al. 2007)] 30 min prior to sc injection of 0.4 mg/kg nicotine, which in turn was administered 40 min prior to operant sessions. The second group of animals was pre-treated with AP-202 (0.0, 0.1, 0.5, and 1.0 mg/kg, sc) 10 min prior to administration of nicotine that occurred 40 min before reinstatement sessions. The third group of animals was used for TP2212-59 and AT-1001 testing. Due to poor brain penetration of TP2212-59, this group was subjected to an identical reinstatement procedure as the other groups except that a guide cannula (26G, 7 mm long) aimed at the IV ventricle (coordinates and methods as above) was implanted after eight consecutive days of extinction. Four days following surgeries, rats continued extinction until day 15. Then, TP2212-59 (0, 15, 30 μg/2 μl, icv) was infused 15 min prior to nicotine-induced reinstatement sessions. To verify that the route of administration did not affect interpretation of results, the systemically administered α3β4* nAChR ligand AT-1001 was tested in the same relapse paradigm. The effect of AT-1001 was determined in the same animals used for TP2212-59 testing. AT-1001 (1.5 mg/kg) or vehicle was sc administered 10 min prior to the nicotine-induced reinstatement sessions. Reinstatement experiments following mecamylamine, AP-202, TP2212-59, and AT-1001 were repeated every third day counterbalancing drug doses according to a Latin square design. Two 30-min extinction sessions were carried out between testing days to avoid potential lasting or cumulative effects of the stimulus-triggering relapse. Responding on the inactive lever was recorded throughout the experiment to monitor possible nonspecific behavioral effects.
Locomotor activity
Effect of AP-202
The open-field apparatus consisted of a square box with an open top, painted black, 50 cm wide × 30 cm tall (Cippitelli et al. 2016). The arena was dimly illuminated. The test consisted of an initial trial in which new rats (N = 8) were allowed to explore the open arena for 10 min. Other three 10-min trials were conducted 10 min following injection of AP-202 (0.0, 0.3, 1.0 mg/kg). Doses of AP-202 were administered (sc) in a counterbalanced order (Latin square design) every other day. Each trial was recorded by a video camera suspended above the field and interfaced with a computerized tracking system using Ethovision® XT version 5 software (Noldus Information Technology, Wageningen, The Netherlands). For each trial, the total distance traveled, immobility time, time spent in the center, and crossing into the center of the field were measured.
Elevated plus maze (EPM)
Effect of AP-202
To measure anxiety-like responses, the elevated plus maze (EPM) test was used, as previously described (Cippitelli et al. 2015a). In brief, the apparatus was made of black plastic with two open arms (50 × 10 cm) and two closed arms (50 × 10 × 45 cm) connected by a 10 × 10 cm central area. The maze was 50 cm above the floor and placed in a spacious room. Testing was performed under a source of red light. The 5-min test procedure began when the animal was placed in the center of the maze, facing a closed arm. The percent of time spent exploring the open arms and the percent of open arm entries were used as measures of anxiety-like behavior, whereas the number of entries into the closed arms was used as an indicator of general motor activity. An entry into an arm was defined as the animal placing all four paws over the line marking that area. The apparatus was cleaned with tap water between each rat performance. AP-202 (1 mg/kg) or vehicle was administered (sc) to new rats (N = 8 per group) 10 min prior to the EPM.
Data analysis
The effects of AP-202 on alcohol, food self-administration, and locomotor behavior as well as DHβE effect on alcohol lever pressing were analyzed by means of a one-way ANOVA in which “treatment” was the within-subject factor. To establish that reinstatement was successfully induced, responding during the last EXT session was compared to the respective reinstatement session of the vehicle-treated group by one-way within-subject ANOVA. The effects of AP-202, mecamylamine, TP2212-59, and AT-1001 on reinstatement experiments were analyzed using one-way repeated measures ANOVA with treatment as a within-subject factor. On co-administration experiments, the effects of AP-202 and TP2212-59 on nicotine and alcohol were analyzed separately, by means of one-way repeated measures ANOVA with treatment as a within-subject factor. EPM data were analyzed by means of one-way ANOVA that used AP-202 treatment as the only between-subject factor. The level of significance was set at p < 0.05. Where appropriate, ANOVAs were followed by Tukey’s post hoc test.
Results
Effect of AP-202 and TP2212-59 on operant co-administration of iv nicotine and oral alcohol
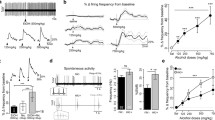
The potent α4β2* nAChR antagonist AP-202 was initially tested in a co-self-administration paradigm, in which rats learned to press one lever for nicotine and the second lever for alcohol. This allowed us to test novel compounds to examine how they affect the self-administration of each drug when they are co-administered. AP-202 attenuated self-administration of both nicotine [F(2, 10) = 10.1, p < 0.01] and alcohol [(F(2, 10) = 4.2, p < 0.05), Fig. 2a]. On post hoc analysis, 1.0 mg/kg was the effective dose for decreasing both nicotine and alcohol taking (p = 0.01 and p < 0.05, respectively). In contrast, the conotoxin TP2212-59, a selective α3β4* nAChR antagonist, only attenuated nicotine self-administration [F(2, 14) = 4.4, p < 0.05], whereas there was no effect of TP2212-59 on alcohol lever pressing ([F(2, 14) = 0.3, NS], Fig. 2b). Post hoc comparisons showed that 30 μg/rat TP2212-59 was an effective dose (p < 0.05) for decreasing responding for nicotine.
Effect of AP-202 and TP2212-59 on operant co-administration of intravenous (iv) nicotine and oral alcohol. Rats were initially trained to concurrent self-administration of nicotine (30 μg/kg/infusion) and 10% (v/v) alcohol under a fixed ratio-1 (FR-1) and then under a FR-3 reinforcement schedule in 60-min sessions. a Subcutaneous (sc) treatment with AP-202 potently decreases nicotine- as well as alcohol-reinforced lever pressing, both at the dose of 1 mg/kg (N = 6). b Intracerebroventricular (icv) administration of TP2212-59 only decreases the number of nicotine infusions at the dose of 30 μg/rat leaving alcohol self-administration unaltered (N = 8). Results are described as mean (± SEM) number of nicotine and alcohol rewards. *p < 0.05; **p < 0.01 difference from vehicle. For detailed statistics, see “Results” section
Effect of AP-202 on locomotor activity and anxiety
To determine whether AP-202 co-administration data were behaviorally specific, locomotor effects were examined in the open-field paradigm. Under familiarity conditions, rats treated with the α4β2* nAChR antagonist prior to allowing exploration of an open arena showed a small but significant decrease in total distance traveled across the 10-min test [F(2, 14) = 3.6, p = 0.05, Table 1]. Post hoc comparisons showed effect of the dose of 1.0 mg/kg (p < 0.05) but not 0.3 mg/kg dose. However, changes in distance traveled were not accompanied by changes in time of immobility [F(2, 14) = 2.1, NS]. Other variables measured in the open-field test and considered to describe anxiety-like behavior, such as transitions into the center of the field and time exploring the center of the field, were also unaltered: [F(2, 14) = 0.8, NS] and [F(2, 14) = 2.4, NS], respectively. The absence of changes in anxiety-like behavior following AP-202 treatment (1.0 mg/kg, sc) was also verified in the EPM (Table 2). AP-202 did not alter time exploring the open arms [F(1, 14) = 0.5, NS], the number of transitions onto the open arms [F(1, 14) = 0.7, NS], and transitions onto the closed arms of the maze [F(1, 14) = 0.2, NS]. These results suggest that the observed reduction of both nicotine and alcohol self-administration following AP-202 treatment at the dose of 1.0 mg/kg could be influenced by a weak reduction of the rat locomotor behavior.
Effect of AP-202 on self-administration of only alcohol
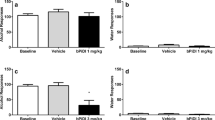
Effect of AP-202 was examined when alcohol was the only reinforcer. Although weakly, AP-202 attenuated operant responding on the alcohol-associated lever [F(3, 21) = 3.7; p < 0.05), Fig. 3a]. On post hoc comparisons, responding was significantly decreased at 1.0 mg/kg (p < 0.05) while lower doses did not significantly differ from vehicle. Responding on the inactive left lever was not significantly affected by the α4β2* nAChR antagonist [F(3, 21) = 1.5; NS]. This response confirmed the decrease in alcohol lever pressing observed in the co-administration paradigm.
Effect of AP-202 on only alcohol self-administration. a Subcutaneous (sc) administration of AP-202 (0.0, 0.1, 0.5, 1.0 mg/kg) decreased alcohol self-administration under a fixed ratio-1 (FR-1) reinforcement schedule across a 30-min session when alcohol was the only reinforcer (N = 8 rats). b AP-202 (0.0, 0.1, 0.5, 1.0 mg/kg) failed to alter food self-administration in food restricted rats (N = 8) under a FR-1 schedule. c Treatment with the α4β2* nAChR antagonist dihydro-β-erythroidine (DHβE 0, 8 mg/kg) was not sufficient to alter alcohol self-administration in rats (FR-1, N = 7). Results are described as mean (± SEM) number of alcohol or food rewards in 30 min. *p < 0.05 difference from vehicle. For detailed statistics, see “Results” section
Effect of AP-202 on food self-administration
AP-202 did not affect responding on the food-reinforced lever ([F(3, 21) = 0.9; NS], Fig. 3b), conferring specificity to the alcohol response. Responding on the inactive left lever was not significantly affected by the drug [F(3, 21) = 0.9; NS].
Effect of DHβE on alcohol self-administration
The commercially available α4β2* nAChR antagonist DHβE was tested in the alcohol self-administration paradigm under identical conditions as AP-202. This antagonist did not alter alcohol lever pressing ([F(1, 6) = 0.6; NS], Fig. 3c). Responding on the inactive left lever was not significantly affected by the drug [F(1, 6) = 0.6; NS].
Effect of AP-202 on cue-induced reinstatement of alcohol seeking
On the last day of the alcohol self-administration training, this cohort of animals reached a lever pressing response of 42.8 ± 6.2. During extinction, lever pressing progressively decreased from 34.6 ± 3.5 on the first day to 6.4 ± 1.2 on the last extinction day. In the reinstatement test, ANOVA showed that cues had a significant effect in triggering alcohol seeking [F(1, 7) = 52.3; p < 0.001]. Reinstatement of alcohol seeking was not significantly modified by pre-treatment with AP-202 ([F(3, 21) = 2.0; NS], Fig. 4a). Responses at the inactive lever did not change following exposure to cues [F(1, 7) = 0.0; NS] and were not influenced by drug treatment [F(3, 21) = 2.4; NS].
Effect of AP-202 on cue-induced and stress-induced alcohol seeking. AP-202 failed to block cue-induced reinstatement as well as yohimbine stress-induced reinstatement of alcohol seeking. a Reinstatement of lever pressing was obtained upon presentation of stimuli (cue light, orange odor) previously associated with alcohol lever pressing. Pretreatment with AP-202 (0.1, 0.5, 1.0 mg/kg) did not affect cue-induced reinstatement (N = 8). b There was significant reinstatement induction by intraperitoneal (ip) administration of yohimbine 1.25 mg/kg dose. Pretreatment with AP-202 (0.1, 0.5, and 1.0 mg/kg) did not affect stress-induced reinstatement (N = 7). Values represent the mean (± SEM) number of total responses on the alcohol-associated lever in 30 min for both reinstatement paradigms. #p < 0.05; ###p < 0.001 difference from last day extinction (EXT). For detailed statistics, see “Results” section
Effect of AP-202 on yohimbine-induced reinstatement of alcohol seeking
On the last day of the alcohol self-administration training, animals reached a lever pressing response of 49.5 ± 7.4. During extinction, lever pressing progressively decreased from 35.9 ± 6.6 on the first day to 9.3 ± 2.3 on the last extinction day. In the reinstatement test, ANOVA showed that yohimbine had a significant effect in triggering alcohol seeking [F(1, 6) = 9.7; p < 0.05]. Reinstatement of alcohol seeking was not significantly modified by pre-treatment with AP-202 ([F(3, 18) = 0.7; NS], Fig. 4b). Extinction responding between reinstatement tests always returned to similar levels as observed on the last extinction day. Responses at the inactive lever did not change following exposure to cues [F(1, 6) = 3.5; NS] and were not influenced by drug treatment [F(3, 18) = 1.2; NS].
Effects of mecamylamine, AP-202, TP2212-59 and AT-1001 on nicotine-induced reinstatement of alcohol seeking
Three groups of rats were subjected to alcohol self-administration training that encompassed repeated treatment with 0.4 mg/kg nicotine prior to sessions for 6 days. Baseline of alcohol reinforcements at the end of this training phase (average of the two sessions before extinction) were 42.4 ± 2.8, 44.9 ± 3.7, and 52.0 ± 9.9. Lever pressing was then extinguished and decreased from 35.8 ± 4.0 responses of the first extinction session to 11.5 ± 2.0 responses of the last extinction session in the group assigned to mecamylamine testing. In the groups assigned to AP-202 and TP2212-59 testing, responses decreased from 28.3 ± 3.3 to 12.8 ± 1.3 and from 46.4 ± 5.8 to 13.3 ± 2.2, respectively. In the reinstatement test of the group assigned to mecamylamine testing, nicotine (0.4 mg/kg) successfully induced alcohol-seeking behavior [F(1, 7) = 8.0; p < 0.05]. Reinstatement of alcohol seeking was significantly modified by pre-treatment with mecamylamine [F(3, 21) = 4.0; p < 0.05]. Post hoc comparisons indicated that mecamylamine, 2.0 mg/kg, was the dose that effectively blocked reinstatement (p < 0.05, Fig. 5a). Extinction responding between reinstatement tests always returned to similar levels as observed on the last extinction day. Responses at the inactive lever did not change following nicotine treatment [F(1, 7) = 0.6; NS] and were not influenced by mecamylamine [F(3, 21) = 2.6; NS]. The same dose of nicotine also induced reinstatement of alcohol seeking in the rat group assigned to AP-202 testing [F(1, 7) = 16.2; p < 0.01]. AP-202 again failed to modify relapse-like behavior ([F(3, 21) = 0.5; NS], Fig. 5b). Responses at the inactive lever did not change following nicotine treatment [F(1, 7) = 2.3; NS] and were not influenced by drug treatment [F(3, 21) = 3.0; NS]. In contrast, icv pretreatment with TP2212-59 successfully blocked nicotine-induced reinstatement of alcohol-seeking behavior (“nicotine” effect [F(1, 7) = 12.7; p < 0.01]; overall TP2212-59 effect [F(2, 14) = 11.5; p < 0.01], Fig. 5c). On post hoc analysis, both TP2212-59 doses examined were significantly different than vehicle (p < 0.05 and p < 0.01 for 15 and 30 μg/rat, respectively). Systemically administered AT-1001 also potently blocked nicotine-induced reinstatement of alcohol seeking [F(1, 7) = 184.5; p < 0.001], suggesting that the route of administration did not affect interpretation of relapse data. Responses at the inactive lever did not change following nicotine treatment [F(1, 7) = 4.2; NS] and were not influenced by the conotoxin [F(2, 14) = 1.3; NS] or AT-1001 [F(1, 7) = 0.9; NS].
Effect of AP-202 on nicotine-induced alcohol seeking. In contrast to mecamylamine TP2212-59 and AT-1001, AP-202 failed to block reinstatement of alcohol seeking induced by pretreatment of nicotine. There was significant reinstatement induction by subcutaneous (sc) administration nicotine (0.4 mg/kg). a Pretreatment with mecamylamine (0.0, 0.5, 1.0, 2.0 mg/kg, sc) significantly blocked nicotine-induced reinstatement of alcohol seeking (N = 8 rats). In contrast, b AP-202 (0.0, 0.1, 0.5, 1.0 mg/kg, sc) did not affect nicotine-induced reinstatement of alcohol seeking (N = 8). c Intracerebroventricular (icv) infusion of TP2212-59 (0, 15, 30 μg/rat) successfully blocked nicotine-induced reinstatement of alcohol seeking (N = 8). d In the same animals used for TP2212-59 testing, AT-1001 (0, 1.5 mg/kg, sc) successfully blocked reinstatement. Values represent the mean (± SEM) number of total responses on the alcohol-associated lever in 30 min. #p < 0.05; ##p < 0.01; ###p < 0.001 difference from last day extinction (EXT). *p < 0.05; **p < 0.01; ***p < 0.001 difference from vehicle. For detailed statistics, see “Results” section
Discussion
Here, we show that antagonism at α4β2* nAChRs by the potent and selective compound AP-202 attenuates responding for alcohol when alcohol is self-administered with nicotine as well as when alcohol is self-administered alone. However, AP-202 fails to decrease relapse-like behavior as assessed in a variety of extinction-based reinstatement paradigms. AP-202 was initially tested in an operant co-administration paradigm in which rats were trained to press one lever to obtain iv nicotine and a second lever to obtain alcohol orally, as previously described by Le et al. (2010). In this experiment, rats pressed approximately the same for each drug when using an FR-3 schedule. This allowed us to test novel compounds to examine how they affect the self-administration of each drug independently when nicotine and alcohol were presented at the same time.
α4β2* nAChRs account for more than 90% of the receptor subtypes in the rodent brain (Gaimarri et al. 2007). Although the role of α4β2*nAChRs in mediating addictive properties of nicotine is well established (Tapper et al. 2004), their role in mediating alcohol reward is unclear. Findings showing that the nonselective nAChR antagonist mecamylamine reduces alcohol drinking in animal models (Ericson et al. 1998; Le et al. 2000), along with evidence that varenicline decreases alcohol intake in both rats and humans (Erwin and Slaton 2014; Steensland et al. 2007) through activation of nAChRs containing the α4 subunit (Hendrickson et al. 2010), strongly indicate that α4β2*nAChRs are implicated in alcohol drinking. Furthermore, in addition to AP-202 and varenicline, other compounds with high affinity for α4β2*nAChRs including sazetidine A and cytisine as well as the nonselective nAChR antagonist lobeline have shown efficacy in regulating alcohol intake (Bell et al. 2009; Rezvani et al. 2010). However, effectiveness of mecamylamine was not confirmed in humans with alcohol use disorders (Petrakis et al. 2018), and as also demonstrated herein, the selective α4β2*nAChR antagonist, DHβE, was never found effective in altering alcohol drinking behavior in rats and mice (Chatterjee et al. 2011; Hendrickson et al. 2009; Le et al. 2000). Additionally, β2 nAChR knockout mice were shown to consume a similar amount of alcohol compared to wild type (Kamens et al. 2010), suggesting that α4β2*nAChRs may not be critical in alcohol drinking behaviors.
There is also evidence for and against other nAChR subunits being involved in alcohol self-administration. Diminished drinking in mice overexpressing α3, β4, and α5 nAChR subunit genes (Gallego et al. 2012) and anti-alcohol effects of α3β4* nAChR partial agonists (Chatterjee et al. 2011) suggest the involvement of α3β4* in alcohol self-administration. However, as we have demonstrated here with the conotoxin antagonist TP2212-59 and previously with the partial agonist AT-1001 (Cippitelli et al. 2015a, b; Toll et al. 2012), high affinity and selective α3β4* nAChR-acting compounds can potently block nicotine without affecting alcohol self-administration, while under identical experimental conditions, the α4β2*-acting compounds varenicline and AP-202 block both nicotine and alcohol self-administration. Chatterjee and collaborators used α3β4* nAChR-directed ligands with different pharmacodynamic and pharmacokinetic properties than the α3β4* ligands used here, which may account for the different behavioral observations. These results suggest that α4β2* and α3β4* nAChRs play different roles in nicotine and alcohol self-administration. The fact that alcohol and nicotine are frequently co-abused by humans highlights the translational relevance of these results.
To determine whether AP-202 co-administration data were behaviorally specific, locomotor effects were examined in the open-field paradigm. Under familiarity conditions, rats treated with the α4β2* nAChR antagonist prior to allowing exploration of an open arena showed decreased total distance traveled across the 10-min test. However, this effect was weak and was not supported by changes in other parameters that describe locomotor activity, such as immobility time in the open-field test. Furthermore, this reduced locomotor activity was not observed in the EPM test where the number of entries into the closed arms of the maze was similar between drug-treated and vehicle-treated rats, nor when responding for food was tested. The observed changes in locomotor behavior are not necessarily surprising as α4β2* nAChRs are widely expressed in the ventral tegmental area (Gotti et al. 2006) and changes in overall level of activity of dopamine neurons could lead to parallel changes in reward and locomotor activity. Therefore, the possibility that the decreased operant co-administration of nicotine and alcohol is influenced by a reduced rat’s locomotor performance cannot be ruled out.
To further investigate the function of the α4β2* nAChR in alcohol-taking behavior, AP-202 was tested in a typical operant self-administration paradigm in which alcohol was presented as the only reinforcer. Although weakly, AP-202 was able to attenuate responding for alcohol. To potentially dissociate this effect from responding for non-drug reinforcement, AP-202 was examined on self-administration of food under conditions of food restriction. We found that food pellet self-administration was not altered by the antagonist, suggesting that AP-202 decreases alcohol and nicotine due to a specific effect on drug reinforcement rather than reduction in locomotion. In addition, alcohol self-administration was examined following a challenge with DHβE. Consistent with published reports (Chatterjee et al. 2011; Hendrickson et al. 2009; Le et al. 2000), administration of DHβE failed to modify alcohol lever pressing.
Although there have been a large number of structure activity studies conducted on nAChRs, the identification of high affinity and selective α4β2* nAChR ligands has proved elusive. For example, while being very selective for the α4β2* nAChRs, DHβE has quite low potency (5–10 μM) for inhibition of receptor activity (Marks et al. 1999), which might explain why it is ineffective for inhibition of alcohol self-administration. Carroll and colleagues have developed a series of epibatidine analogs that have quite good selectivity in binding, but functional activity and selectivity do not always correlate with the binding affinities (Carroll 2004; Carroll et al. 2002, 2004). In fact, this is the case for varenicline as well, which has high selectivity in binding assays but significantly reduced selectivity, to both α3β4* and α7 nAChR, with respect to function (Chatterjee et al. 2011; Rollema et al. 2007). This is not the case for AP-202, which has high selectivity in both binding and functional studies (Wu et al. 2017).
The role of α4β2* nAChRs in alcohol relapse is poorly investigated. It is known that partial activation at this receptor by varenicline or cytisine reduces cue- as well as context-induced reinstatement of alcohol seeking and alcohol deprivation effect (Lacroix et al. 2017; Sajja and Rahman 2013; Wouda et al. 2011), rodent models of alcohol relapse. However, the effect of α4β2* nAChR antagonists on alcohol-seeking behavior has not been examined. We attempted to carry out these experiments by testing AP-202 in a variety of extinction-based reinstatement paradigms. We initially found no effect of AP-202 in modifying reinstatement induced by previously alcohol-associated cues. Then, the antagonist was examined in stress-induced relapse with AP-202 not being effective in reversing yohimbine-induced alcohol seeking. Hence, surprisingly, our potent and selective α4β2* nAChR antagonist attenuated alcohol self-administration while showing no effect in common reinstatement paradigms. These results indicate that α4β2* nAChR activation and inhibition lead to different outcomes with respect to alcohol relapse. Notably, a similar profile as AP-202 was recently observed for one selective antagonist at α6-containing nAChRs (Srisontiyakul et al. 2016), suggesting that this class of nAChR-directed compounds may influence reinforcing effects of alcohol, but not relapse into alcohol seeking. Finally, we induced alcohol-seeking behavior by administering nicotine prior to alcohol self-administration training sessions and then again immediately prior to reinstatement sessions (Le et al. 2003). Although this reinstatement behavior can be mediated by nAChRs, as demonstrated by mecamylamine inhibition, AP-202 did not show any effect, but nicotine-induced reinstatement was prevented by icv administration of conotoxin TP2212-59 and blocked by AT-1001. Hence, this behavior could be modified by a blockade of α3β4* but not α4β2* nAChRs. We recently have shown that partial activation of α3β4* nAChRs by AT-1001, which results in functional inhibition of these receptors due to desensitization (Zaveri et al. 2015), blocks stress but not cue-induced alcohol seeking (Cippitelli et al. 2015a). Thus, the successful reversal of nicotine-induced reinstatement by the conotoxin TP2212-59 and AT-1001 suggests that blockade of α3β4* nAChRs counteracts possible pharmacological stress elicited by nicotine. Consistently, nicotine has anxiogenic-like activity at doses that induce reinstatement of alcohol seeking (Ouagazzal et al. 1999), and α3β4* nAChRs are widely expressed in the medial habenula and the interpeduncular nucleus, two regions highly involved in stress mechanisms associated with nicotine dependence (Zhao-Shea et al. 2015). Furthermore, an important discovery suggests that nicotine requires stress hormones to influence neurotransmission and behavior associated with alcohol reinforcement (Doyon et al. 2013).
One limitation of the present study is that due to the low number of reinforcements earned, blood alcohol levels of rats on co-administration were probably below pharmacologically relevant levels. However, it is known that animals co-administering two substances self-administer less of each substance than when they are available alone (Le et al. 2010; Scuppa et al. 2015) and experimental conditions used here (i.e., FR-3 schedule, 1-h session length) could limit the amount of rewards earned.
In conclusion, the present results indicate different roles for α4β2* and α3β4* nAChRs in alcohol taking and seeking behaviors. Specifically, on co-administration, α4β2* nAChR-directed antagonism decreases both nicotine and alcohol taking, whereas α3β4* nAChR-directed antagonism selectively decreases nicotine taking. When alcohol is presented as the only reinforcer, α4β2* nAChR antagonism decreases alcohol self-administration but fails to block reinstatement of alcohol seeking. α3β4* nAChRs are not involved in alcohol reinforcement, and seeking unless relapse is induced by nicotine or stress. Given the elevated relapse vulnerability of alcoholic patients, it is questionable whether blockade of α4β2* nAChR can be considered a viable approach to treat alcohol use disorder. However, AP-202 may be a potential lead molecule for a therapeutic strategy aimed at lowering nicotine and alcohol consumption.
References
Albuquerque EX, Pereira EF, Alkondon M, Rogers SW (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120
Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, Cippitelli A (2013) Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Frontiers Psychiatr 4:23
Bell RL, Eiler BJ 2nd, Cook JB, Rahman S (2009) Nicotinic receptor ligands reduce ethanol intake by high alcohol-drinking HAD-2 rats. Alcohol 43:581–592
Carroll FI (2004) Epibatidine structure-activity relationships. Bioorg Med Chem Lett 14:1889–1896
Carroll FI, Lee JR, Navarro HA, Ma W, Brieaddy LE, Abraham P, Damaj MI, Martin BR (2002) Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 2-exo-2-(2′,3′-disubstituted 5′-pyridinyl)-7-azabicyclo[2.2.1]heptanes: epibatidine analogues. J Med Chem 45:4755–4761
Carroll FI, Ware R, Brieaddy LE, Navarro HA, Damaj MI, Martin BR (2004) Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 2′-fluoro-3′-(substituted phenyl)deschloroepibatidine analogues. Novel nicotinic antagonist. J Med Chem 47:4588–4594
CDC (2014) Centers for Desease Control & Prevention. Alcohol-related disease impact (ARDI) application (2006–2010)
Chang YP, Banerjee J, Dowell C, Wu J, Gyanda R, Houghten RA, Toll L, McIntosh JM, Armishaw CJ (2014) Discovery of a potent and selective alpha3beta4 nicotinic acetylcholine receptor antagonist from an alpha-conotoxin synthetic combinatorial library. J Med Chem 57:3511–3521
Chatterjee S, Bartlett SE (2010) Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets 9:60–76
Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE (2011) Partial agonists of the alpha3beta4* neuronal nicotinic acetylcholine receptor reduce ethanol consumption and seeking in rats. Neuropsychopharmacology 36:603–615
Ciccocioppo R, Angeletti S, Weiss F (2001) Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol Clin Exp Res 25:1414–1419
Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, Heilig M (2012) Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav 100:522–529
Cippitelli A, Brunori G, Gaiolini KA, Zaveri NT, Toll L (2015a) Pharmacological stress is required for the anti-alcohol effect of the alpha3beta4* nAChR partial agonist AT-1001. Neuropharmacology 93:229–236
Cippitelli A, Wu J, Gaiolini KA, Mercatelli D, Schoch J, Gorman M, Ramirez A, Ciccocioppo R, Khroyan TV, Yasuda D, Zaveri NT, Pascual C, Xie XS, Toll L (2015b) AT-1001: a high-affinity alpha3beta4 nAChR ligand with novel nicotine-suppressive pharmacology. Br J Pharmacol 172:1834–1845
Cippitelli A, Schoch J, Debevec G, Brunori G, Zaveri NT, Toll L (2016) A key role for the N/OFQ-NOP receptor system in modulating nicotine taking in a model of nicotine and alcohol co-administration. Sci Rep 6:26594
Doyon WM, Dong Y, Ostroumov A, Thomas AM, Zhang TA, Dani JA (2013) Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron 79:530–540
Ericson M, Blomqvist O, Engel JA, Soderpalm B (1998) Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol 358:189–196
Erwin BL, Slaton RM (2014) Varenicline in the treatment of alcohol use disorders. Ann Pharmacother 48:1445–1455
Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C (2007) Regulation of neuronal nicotinic receptor traffic and expression. Brain Res Rev 55:134–143
Gallego X, Ruiz-Medina J, Valverde O, Molas S, Robles N, Sabria J, Crabbe JC, Dierssen M (2012) Transgenic over expression of nicotinic receptor alpha 5, alpha 3, and beta 4 subunit genes reduces ethanol intake in mice. Alcohol 46:205–215
Gotti C, Zoli M, Clementi F (2006) Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27:482–491
Hendrickson LM, Zhao-Shea R, Tapper AR (2009) Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology 204:563–572
Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR (2010) Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci 30:10169–10176
Hendrickson LM, Guildford MJ, Tapper AR (2013) Neuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependence. Frontiers Psychiatr 4:29
Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63
Kamens HM, Andersen J, Picciotto MR (2010) Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology 208:613–626
Lacroix F, Pettorelli A, Maddux JN, Heidari-Jam A, Chaudhri N (2017) Varenicline reduces context-induced relapse to alcohol-seeking through actions in the nucleus Accumbens. Neuropsychopharmacolgy 42:1037–1048
Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK (2000) Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res 24:155–163
Le AD, Wang A, Harding S, Juzytsch W, Shaham Y (2003) Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology 168:216–221
Le AD, Lo S, Harding S, Juzytsch W, Marinelli PW, Funk D (2010) Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology 208:475–486
Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE (2007) Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology 32:710–718
Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC (1999) Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the beta2 subunit. J Pharmacol Exp Ther 289:1090–1103
McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66:185–190
NHTSA (2016) National Highway Traffic Safety Administration. 2015 motor vehicle crashes: Overview
Ouagazzal AM, Kenny PJ, File SE (1999) Modulation of behaviour on trials 1 and 2 in the elevated plus-maze test of anxiety after systemic and hippocampal administration of nicotine. Psychopharmacology 144:54–60
Patkar OL, Belmer A, Tarren JR, Holgate JY, Bartlett SE (2016) The effect of varenicline on binge-like ethanol consumption in mice is beta4 nicotinic acetylcholine receptor-independent. Neurosci Lett 633:235–239
Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ (2002) Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem 82:468–481
Petrakis IL, Ralevski E, Gueorguieva R, O’Malley SS, Arias A, Sevarino KA, Jane JS, O’Brien E, Krystal JH (2018) Mecamylamine treatment for alcohol dependence: a randomized controlled trial. Addiction 113(1):6–14
Rahman S, Engleman EA, Bell RL (2014) Nicotinic receptor modulation to treat alcohol and drug dependence. Front Neurosci 8:426
Rezvani AH, Slade S, Wells C, Petro A, Lumeng L, Li TK, Xiao Y, Brown ML, Paige MA, McDowell BE, Rose JE, Kellar KJ, Levin ED (2010) Effects of sazetidine-A, a selective alpha4beta2 nicotinic acetylcholine receptor desensitizing agent on alcohol and nicotine self-administration in selectively bred alcohol-preferring (P) rats. Psychopharmacology 211:161–174
Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD 3rd, Williams KE (2007) Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994
Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD (2015) 2010 National and state costs of excessive alcohol consumption. Am J Prev Med 49:e73–e79
Sajja RK, Rahman S (2013) Nicotinic receptor partial agonists modulate alcohol deprivation effect in C57BL/6J mice. Pharmacol Biochem Behav 110:161–167
Scuppa G, Cippitelli A, Toll L, Ciccocioppo R, Ubaldi M (2015) Varenicline decreases nicotine but not alcohol self-administration in genetically selected Marchigian Sardinian alcohol-preferring (msP) rats. Drug Alcohol Depend 156:126–132
Srisontiyakul J, Kastman HE, Krstew EV, Govitrapong P, Lawrence AJ (2016) The nicotinic alpha6-subunit selective antagonist bPiDI reduces alcohol self-administration in alcohol-preferring rats. Neurochem Res 41:3206–3214
Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X (2014) Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis 11:E109
Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A 104:12518–12523
Substance Abuse and Mental Health Services Administration (SAMHSA) (2013) National Survey on Drug Use and Health (NSDUH). Table 5.8B—Substance Dependence or Abuse in the Past Year among Persons Aged 18 or Older, by Demographic Characteristics: Percentages, 2012 and 2013. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabsPDFWHTML2013/Web/HTML/NSDUH-DetTabsSect5peTabs1to56-2013.htm#tab5.8b
Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032
Toll L, Zaveri NT, Polgar WE, Jiang F, Khroyan TV, Zhou W, Xie XS, Stauber GB, Costello MR, Leslie FM (2012) AT-1001: a high affinity and selective alpha3beta4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology 37:1367–1376
Volkow ND, Skolnick P (2012) New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology 37:290–292
Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ (2011) Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology 216:267–277
Wu J, Zhang Y, Maida LE, Santos RG, Welmaker GS, LaVoi TM, Nefzi A, Yu Y, Houghten RA, Toll L, Giulianotti MA (2013) Scaffold ranking and positional scanning utilized in the discovery of nAChR-selective compounds suitable for optimization studies. J Med Chem 56:10103–10117
Wu J, Cippitelli A, Zhang Y, Debevec G, Schoch J, Ozawa A, Yu Y, Liu H, Chen W, Houghten RA, Welmaker GS, Giulianotti MA, Toll L (2017) Highly selective and potent alpha4beta2 nAChR antagonist inhibits nicotine self-administration and reinstatement in rats. J Med Chem 60:10092–10104
Xiao Y, Kellar KJ (2004) The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther 310:98–107
Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ (2006) Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol 70:1454–1460
Zaveri NT, Bertrand S, Yasuda D, Bertrand D (2015) Functional characterization of AT-1001, an alpha3beta4 nicotinic acetylcholine receptor ligand, at human alpha3beta4 and alpha4beta2 nAChR. Nicotine Tob Res 17:361–367
Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, Gao G, Rando OJ, Martin GE, George O, Gardner PD, Tapper AR (2015) Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun 6:6770
Acknowledgements
We are thankful to Shannon Ortiz-Umpierre and Roman Ramirez for animal care and Ginamarie Debevec for support in analytical chemistry.
Funding
This work was supported by NIH grants R41 AA025298 and R44 DA036968.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Christopher J. Armishaw’s current affiliation is with Eli Lilly & Company, Indianapolis, IN 46285.
Rights and permissions
About this article
Cite this article
Cippitelli, A., Brunori, G., Schoch, J. et al. Differential regulation of alcohol taking and seeking by antagonism at α4β2 and α3β4 nAChRs. Psychopharmacology 235, 1745–1757 (2018). https://doi.org/10.1007/s00213-018-4883-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4883-y