Abstract
Rationale
Recent reports describe a restricted access ethanol consumption paradigm where C57Bl/6J mice drink until intoxicated. Termed “drinking in the dark” (DID), this paradigm has been used as a model of binge drinking. Although neuronal nicotinic acetylcholine receptors (nAChRs) have been implicated in alcohol drinking in rats pre-trained to self-administer ethanol, their role in binge-like ethanol consumption is unknown.
Objectives
To determine if nAChRs are involved in binge drinking as measured by the DID assay in C57Bl/6J mice.
Materials and methods
Adult male C57Bl/6J mice were injected i.p. with nicotinic receptor antagonists including mecamylamine, hexamethonium, dihydro-β-erythroidine, and methyllycaconitine. Immediately following injection, mice were presented with 20% ethanol for 2 h in the DID assay to measure ethanol consumption. Nicotinic agonists including cytisine and nicotine were also evaluated. The effects of mecamylamine and nicotine on ethanol-induced dopaminergic neuronal activation in the VTA were evaluated via immunohistochemistry.
Results
Mecamylamine dose dependently reduced ethanol consumption; whereas, the peripheral antagonist hexamethonium had no significant effect. Nicotinic agonists, cytisine and nicotine, reduced ethanol consumption. None of the effective nicotinic receptor drugs reduced sucrose drinking. Mecamylamine blocked ethanol activation of dopaminergic neurons while nicotine alone activated them without additional activation by ethanol.
Conclusions
Neuronal nAChRs are involved in ethanol consumption in the DID paradigm. The effects of mecamylamine, nicotine, and cytisine on ethanol intake appear to be specific because they do not reduce sucrose drinking. Mecamylamine reduces alcohol consumption by blocking activation of dopaminergic neurons; whereas, nicotinic agonists may activate the same reward pathway as alcohol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcoholism is the third preventable cause of mortality in the world and few therapeutic treatments are available highlighting the importance of understanding the underlying molecular mechanisms of ethanol’s reinforcing properties (Centers for Disease Control and Prevention 2004). Animal models of voluntary alcohol drinking provide a unique tool to study potential pharmacological means to reduce ethanol intake, but few of these models yield intoxicating blood alcohol levels. Recently, a straightforward voluntary drinking paradigm whereby high-alcohol-preferring C57Bl/6J mice are exposed to 20% ethanol for 2 or 4 h during the dark cycle has been established. Termed “drinking in the dark” (DID), this novel assay reliably produces pharmacologically relevant blood ethanol concentrations even upon first exposure and has been utilized as a mouse model of “binge drinking” (Rhodes et al. 2005, 2007).
A major goal of alcohol addiction research is to identify molecules that may play a significant role in ethanol’s euphoric effects that could promote persistent voluntary drinking and acute intoxication. Achieving this goal has proven problematic due to ethanol’s properties to interact with multiple proteins expressed in the CNS (Harris 1999). Neuronal nicotinic acetylcholine receptors (nAChRs) have emerged as candidate molecules in at least partially mediating the reinforcing properties of alcohol (Soderpalm et al. 2000). Neuronal nAChRs are ligand-gated cation channels that are activated by the endogenous neurotransmitter, acetylcholine, as well as the addictive component of tobacco smoke, nicotine. Currently, 12 mammalian neuronal nicotinic acetylcholine receptor subunits have been identified (α2-10 and β2-4). Most high affinity nAChRs are heteromeric pentamers consisting of α and β subunits. Thus, multiple receptor subtypes with varying subunit compositions and electrophysiological properties exist (Jones et al. 1999; Laviolette and van der Kooy 2004; Lindstrom et al. 1996). Indeed, many neuronal nAChR subtypes are expressed throughout the mesocorticolimbic reward pathways especially in the ventral tegmental area (VTA) in both dopaminergic neurons projecting to nucleus accumbens and in local GABAergic interneurons (Klink et al. 2001; Wooltorton et al. 2003). Systemic ethanol has been shown to increase acetylcholine concentrations in the VTA, presumably, activating nAChRs in this area (Ericson et al. 2003). Ethanol can also potentiate nAChR activity depending on the subtype of nicotinic receptor expressed (Forman and Zhou 2000; Zhou et al. 2000; Zuo et al. 2002). Because a variety of subtypes exist in these nuclei, identification of the specific nicotinic receptor subtype(s) that may underlie ethanol reward is paramount.
The nonspecific antagonist, mecamylamine, when injected systemically or locally within the VTA, blocks ethanol self-administration in high-ethanol-preferring rats that have acquired robust ethanol drinking through increasing concentration of ethanol exposure over a 2-week period (Blomqvist et al. 1996; Ericson et al. 1998). Using a similar paradigm in rats, studies have shown that dihdro-β-erythroidine (DHβE) and methyllycaconitine (MLA), antagonists selective for α4β2 and homomeric α7 nAChRs, respectively, fail to block ethanol consumption (Le et al. 2000) and dopamine (DA) overflow in nucleus accumbens (Ericson et al. 2003; Larsson et al. 2002). On the other hand, it has been shown that the α3β2*, β3*, and α6* subunit specific antagonist, α-conotoxin MII, does inhibit ethanol consumption, activity, and DA release in nucleus accumbens (Jerlhag et al. 2006; Larsson et al. 2004). More recently, varenicline, an α4β2 partial agonist clinically approved as a smoking cessation therapeutic (Coe et al. 2005; Gonzales et al. 2006; Steensland et al. 2007; Tonstad et al. 2006), was found to reduce both ethanol intake and seeking in rats (Steensland et al. 2007). To our knowledge, the role of nAChRs in acute ethanol intake in mice has not been examined.
The goal of the current study was to test the hypothesis that nAChR signaling is involved in acute alcohol intake (i.e., “binge drinking”) as measured using the DID assay in C57Bl/6J mice. Toward this end, we exposed mice to a panel of nAChR antagonists and agonists prior to the presentation of ethanol and measured alcohol intake.
Materials and methods
Animals
Male C57BL/6J mice (Jackson Laboratory) used in the experiments were between 8 and 14 weeks of age and were housed three to four animals per cage up until the start of each experiment. During acclimation, animals were kept on a standard 12-h light/dark cycle with lights on at 7:00 AM and off at 7:00 PM. The animals were given food and water ad libitum, except when ethanol was substituted for water for 2 h at night as described below. All experiments were conducted in accordance with the guidelines for care and use of laboratory animals provided by the National Research Council (National Research Council 1996), as well as with an approved animal protocol from the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Drugs and drinking solutions
Ethanol solutions were prepared from 190 proof absolute anhydrous ethanol (Pharmco-Aaper brand, Brookfield, CT, USA) diluted to 20% ethanol (v/v) using tap water. Sucrose (EMD) was dissolved in tap water to make a 10% (w/v) concentration. Mecamylamine hydrochloride, hexamethonium hydrochloride, MLA, DHβE, nicotine hydrogen bitartrate, and cytisine (all purchashed from Sigma-Aldrich, St. Louis, MO, USA) were dissolved in 0.9% saline and were administered via intraperitoneal (i.p.) injections at the indicated doses. Nicotine concentrations are reported as nicotine base.
Drinking in the dark procedure
Animals were singly housed in experimental chambers for 1 week prior to the beginning of the DID sessions. The mice received a 15-ml graduated cylinder water bottle fitted with a one-hole rubber stopper with a stainless steel double-ball-bearing sipper tube which was sealed with parafilm to prevent leakage. Our drinking assay is a modified 2-day version of a limited access drinking procedure first described in Rhodes et al. (2005). On the first night, 2 h after the lights were turned off, half of the mice were given an i.p. injection of saline and the other half were i.p. injected with drug. Immediately after the injections, the water bottle was removed and replaced with a single bottle of 20% ethanol and left in place for 2 h. On the second night, the injection groups were switched (i.e., mice that received saline on the first night received drug on the second; whereas, mice that received drug on the first night received saline on the second) and again given a single 20% ethanol bottle for 2 h. The amount of ethanol consumed was recorded immediately after each 2-h session and converted to grams per kilogram per each animal’s ethanol consumption and body weight. For control experiments, mice received 10% sucrose for 2 h instead of ethanol.
Experimental design
Table 1 lists the experiment number, type, drug injected, dose, and number of animals used. For DID experiments, each mouse received two DID sessions with a low and high dose of the same drug, except in experiments 1, 3, 4, 11, and 12 where the mice only received saline and one dose of drug. Mice that received two doses of drug were given 7 days of rest between 2-day DID experiments, and tested again in the same 2-day DID procedure. Lower doses were used in the initial 2-day DID round followed by higher doses in the second DID round (see Table 1). In experiments 14 and 15, DID procedure was exactly the same as described above except that ethanol measurements were taken in 15-min intervals throughout the 2-h drinking session.
Blood ethanol concentration
For experiment 5 (blood ethanol concentration measurements), prior to ethanol drinking, one group of mice was injected i.p. with saline and a separate group was i.p. injected with 1 mg/kg mecamylamine. Trunk blood was obtained from the mice after completion of the 2-h ethanol drinking assay. Blood was collected in heparinized capillary tubes, centrifuged at 1,500×g for 5 min and blood analyzed using an alcohol oxidase-based assay. Blood ethanol concentrations were measured on a GM7 Micro-Stat Analyzer (Analox Instruments Ltd.).
Immunohistochemistry
Mice were i.p. injected with saline for 3 days prior to the start of the experiment to habituate them to handling and to reduce c-Fos activation due to stress. Two groups of nine mice were used. Mice from the first group received two injections: An i.p. injection of 3.0 mg/kg mecamylamine followed by a 2.0 g/kg ethanol injection, or a saline injection followed by a 2.0 g/kg ethanol injection, or a saline injection followed by a second saline injection. The time between the first and second injection was 45 min and was estimated based on the delayed effect that mecamylamine had on drinking pattern (Fig. 4). The second group of mice received an i.p. injection of 0.5 mg/kg nicotine followed by a saline injection, or a 0.5 mg/kg nicotine injection followed by a 2.0 g/kg ethanol injection, or a saline injection followed by a second saline injection. The time between injections was 15 min based on nicotine’s more rapid effect on drinking pattern.
Ninety minutes after the second injection, all mice were deeply anesthetized with sodium pentobarbital (200 mg/kg, i.p.) and perfused transcardially with 10 ml of 0.1 M phosphate-buffered saline (PBS) followed by 10 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4). Brains were removed and post-fixed for 2 h with the same fixative and cryoprotected in sodium phosphate buffer containing 30% sucrose until brains sank. VTA serial coronal sections (20 μm) were cut on a microtome (Leica SM 2000 R, Leica Microsystems Inc., Bannockburn, IL, USA) and collected into a 24-well tissue culture plate containing 1× PBS. Slices containing VTA were collected between −2.92 and −4.04 mm from bregma. After rinsing sections in PBS twice for 5 min, they were treated with 0.4% Trition X-100 PBS (PBST) twice for 2 min followed by incubation in 2% BSA/PBS for 30 min. Sections were washed with PBS once and then incubated in a cocktail of primary antibodies for Tyrosine Hydroxylase (TH, monoclonal, 1:250, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and c-Fos (polyclonal, 1:400, Santa Cruz) in 2% BSA/PBS overnight at 4°C. The sections were then washed with PBS three times for 5 min followed by incubation in secondary fluorescent-labeled antibodies (goat anti-rabbit Alexa Fluor® 488 and goat anti-mouse Alexa Fluor ®594, 1:300, Molecular Probes, Inc., Eugene, USA) at room temperature in dark for 30 min. After washing with PBS five times for 5 min/wash, sections were mounted on slide by using VECTASHIELD® Mounting Medium (Vector laboratories, Inc., Burlingame, CA, USA). The number of positive neurons was counted under a fluorescence microscope (Zeiss, Carl Zeiss MicroImmaging Inc., NY, USA) at a magnification of 400×. The intensity of fluorescence was quantified by using a computer-associated image analyzer (Axiovision Rel. 4.6). Neurons were counted as signal positive if intensities were at least two times higher than that of the average value of background (sections staining without secondary antibodies).
Data analysis
The effect of preinjections of nicotinic agonists and antagonists on ethanol intake was compared to saline preinjections using one of two statistical tests. In experiments where one group of mice received one dose of drug, one-way ANOVAs followed by Tukey post hoc tests were used. In experiments where one group of mice received two doses of drug, a repeated measure ANOVA followed by Tukey post hoc test was used. Data were analyzed using Graphpad software (Graphpad software, Inc.). Student t tests were used to analyze immunohistochemistry data. Results were considered significant at p < 0.05. All data are expressed as means ± standard errors of means (SEM).
Results
Effects of mecamylamine on alcohol consumption in the DID assay
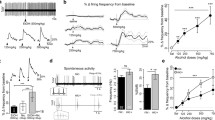
To determine if mecamylamine can inhibit ethanol drinking in the DID paradigm, mice were pre-injected, i.p. with 0.5, 1, or 3 mg/kg mecamylamine immediately prior to 20% ethanol exposure. Mecamylamine dose dependently reduced the volume of ethanol drinking (Fig. 1a). Mice receiving a preinjection of 1 or 3 mg/kg mecamylamine consumed significantly less ethanol compared to saline-injected mice (Fig. 1b, 1.30+/−0.44 and 1.53+/−0.24 compared to 2.62+/−0.28 and 3.05+/−0.28 g/kg ethanol, respectively). Repeated measure ANOVA indicated an overall significant difference between saline and mecamylamine preinjection on ethanol intake (F 3,18 = 9.33, p < 0.001). Tukey post hoc analysis indicated a significant effect of mecamylamine with a preinjection dose of 1 or 3 mg/kg compared to respective saline values. The antagonist did not affect sucrose intake in mice at the tested doses of 1 or even as high as 6 mg/kg (Fig. 1c, for 1 mg/kg F 1,12 = 0.54, p > 0.05, for 6 mg/kg F 1,10 = 3.23, p > 0.05). Preinjection of the peripheral nAChR antagonist, hexamethonium, at a dose of either 1 or 3 mg/kg, also did not significantly reduce ethanol intake (F 3,21 = 0.20, p > 0.05, data not shown).
Mecamylamine dose dependently reduces ethanol DID. a Total ethanol drinking volume (milliliters ethanol solution) over the course of 2 h starting 2 h after lights off. Immediately prior to introduction of the ethanol solution into each individual cage, mice were injected i.p. with either 0 (saline), 0.5, 1, or 3, mg/kg mecamylamine. One group of animals was used for the 0.5 mg/kg dose; whereas, a second group of animals was used for the 1 and 3 mg/kg doses (see “Materials and methods”). b Bar graph representation of total ethanol intake over the 2-h DID assay (gram per kilogram) for the three mecamylamine doses. c Total 10% sucrose volume intake (milliliter) after an i.p. injection of 0 (saline), 1, or 6 mg/kg mecamylamine. Mice had access to 10% sucrose for 2 h during the dark cycle starting 2 h after lights out. d Blood ethanol concentration (millimolar) in mice given an i.p. preinjection of saline (n = 5) or 1 mg/kg mecamylamine (n = 6) immediately prior to an alcohol bottle. Blood was isolated immediately after the 2-h drinking session. Data presented as mean+/−SEM. *p < 0.05, **p < 0.01 compared to same group saline controls, one-way or repeated measure ANOVA, Tukey post hoc test (see “Materials and methods” and “Results” sections for details)
To determine if the effect of mecamylamine ultimately resulted in a lower blood ethanol concentration, we acquired blood samples immediately following the 2-h DID assay in mice that received either a saline or 1 mg/kg mecamylamine preinjection (Fig. 1d). Mice that received mecamylamine prior to their ethanol bottle exhibited significantly lower blood ethanol levels compared to mice that received a preinjection of saline (Fig. 1d, 13.5+/−3.9 mM compared to 25.8+/−2.8 mM ethanol, F 1,9 = 6.2, p < 0.05).
Effects of selective nAChR antagonists on ethanol consumption
Preinjection of a low (1 mg/kg) or high (3 mg/kg) dose of the nAChR competitive antagonist, DHβE, did not significantly affect ethanol intake in C57Bl/6J mice compared to a preinjection of saline. Repeated measure ANOVA yielded a non-significant effect of pretreatment: F 3,21 = 0.67, p > 0.05 (data not shown). Similarly, preinjection of a low (5 mg/kg) or high (10 mg/kg) dose of the α7-selective antagonist, MLA, did not significantly reduce ethanol intake (F 3,18 = 0.56, p > 0.05, data not shown).
Effects of nAChR agonists on ethanol consumption in the DID assay
To evaluate the effects of nAChR agonists on ethanol intake in the DID assay, we pre-injected C57Bl/6J mice with nicotine immediately prior to presentation of the 20% ethanol bottle. Compared to a saline injection, both 0.25 and 0.5 mg/kg nicotine decreased the volume of ethanol drinking (Fig. 2a). Repeated-measure ANOVA indicated an overall effect of pretreatment on intake (F 3,18 = 6.33, p < 0.01, Fig. 2b). Post hoc comparisons indicated that 0.5 mg/kg nicotine significantly reduced ethanol intake compared to saline (p < 0.01+/−0.32 compared to 3.76+/−0.36 g/kg ethanol). Preinjection of either dose did not significantly reduce consumption of sucrose solution (Fig. 2c, F 3,21 = 0.24, p > 0.05). The β4* nAChR full agonist, and α4β2-selective partial agonist, cytisine also dose dependently reduced the volume of ethanol drinking compared to saline (Fig. 3a). There was a significant effect of 3 mg/kg cytisine on ethanol intake (F 1,6 = 29.8, p < 0.05, Fig. 3b, 1.37+/−0.39 compared to 4.01+/−0.39 g/kg ethanol) but not with 1 mg/kg (F 1,16 = 0.15, p > 0.05). Repeated measure ANOVA on the effect of preinjection on sucrose drinking revealed a significant interaction (F 3,21 = 9.63, p < 0.01). However, post hoc analysis revealed no significant difference between mice given 1 or 3 mg/kg cytisine compared to saline-injected controls (Fig. 3c, p > 0.05, NS).
Nicotine reduces ethanol DID. a The effect of a preinjection of nicotine on ethanol drinking volume is shown. One group of mice were used for both drug concentrations (n = 7) b Ethanol intake (gram per kilogram) from a. c Average effect of a preinjection of nicotine on sucrose intake. Data are presented as mean+/−SEM. **p < 0.01 compared to same group saline controls, repeated measures ANOVA, Tukey post hoc test
Cytisine reduces ethanol DID. a Total volume of ethanol intake after saline, 1, or 3 mg/kg cytisine preinjection. Separate groups of animals were used for each dose. b Ethanol intake (gram per kilogram) from a. Asterisk indicates significance compared to within group intake after a saline preinjection. c Effect of saline, 1, or 3 mg/kg cytisine on sucrose intake. *p < 0.05 compared to same group saline controls, one-way ANOVA, Tukey post hoc test
Effects of mecamylamine and nicotine on ethanol drinking patterns
To determine if mecamylamine, a nicotinic receptor antagonist, and nicotine, an agonist could affect ethanol intake differently, we measured the pattern of alcohol drinking in mice pre-injected with each drug. Ethanol intake was measured in 15-min intervals over the course of 2 h. Figure 4a illustrates ethanol intake in two separate groups of mice that received either saline/nicotine or saline/mecamylamine preinjections. Data from each group were normalized to their average saline values per 15-min interval so comparisons could be made between groups. Preinjection of 0.5 mg/kg nicotine decreased ethanol intake during the first hour of drinking (Fig. 4a). Conversely, 3 mg/kg mecamylamine reduced ethanol intake predominantly during the second hour of the DID assay. Figure 4b illustrates average interval intake in the first hour compared to the second hour of the DID assay. One-way ANOVA indicated a significant effect of nicotine on average interval intake in the first hour compared to saline (Fig. 4b). Actual values from the first hour are 0.502 g/kg/interval after saline injection compared to 0.159 g/kg/interval after nicotine (F 1,46 = 14.5, p < 0.001). Mecamylamine significantly inhibited ethanol intake in the second hour of the assay (0.688 g/kg/interval after saline compared to 0.33 mg/kg/interval, F 1,54 = 11.0, p < 0.01).
Mecamylamine and nicotine differentially affect DID ethanol drinking pattern. a Normalized drinking bouts in two separate groups of mice that received saline/3 mg/kg mecamylamine or saline/0.5 mg/kg nicotine. Dotted line represents the normalized saline value for each group. b Average 15-min bout during the first and second hour of the DID assay in the two groups of animals. **p < 0.01, ***p < 0.001 compared to same group saline controls, one-way ANOVA, Tukey post hoc test
Effects of mecamylamine and nicotine on ethanol-induced VTA DAergic neuron c-Fos expression
To gain mechanistic insight into how nicotinic antagonists and agonists may influence ethanol intake, we analyzed expression of the immediate early gene, c-Fos, as a measure of neuronal activation (Cole et al. 1989) in tyrosine hydroxylase (TH) positive neurons of the VTA via immunohistochemistry. The number of c-Fos, TH double positive cells in VTA, was counted in mice that received mecamylamine or nicotine prior to an i.p. injection of 2.0 g/kg ethanol (Fig. 5). A single ethanol exposure significantly increased the number of double positive cells in VTA compared to saline injection (Fig. 5a, c, p < 0.01, independent two-sample student t test). Preinjection of 3 mg/kg mecamylamine 45 min prior to ethanol injection significantly reduced the number of c-Fos/TH positive cells compared to a saline preinjection (Fig. 5c, p < 0.05).
Mecamylamine and nicotine exhibit distinct effects on ethanol-induced VTA DAergic neuron activation. a Representative images depicting VTA slices from mice receiving two saline injections (left), saline followed by a 2.0 g/kg ethanol injection (middle), or 3.0 mg/kg mecamylamine followed by a 2.0 g/kg ethanol injection (right). Slices are fluorescently double-labeled with anti-tyrosine hydroxylase (red) and anti-c-Fos (green). b Representative images depicting VTA slices from mice receiving saline injections (left), 0.5 mg/kg nicotine followed by saline (middle), or 0.5 mg/kg nicotine followed by 2.0 g/kg ethanol (right). c Average number of c-Fos positive, TH positive cells per slice from mice treated as in a. d Average number of c-Fos positive, TH positive cells per slice from mice treated as in b. Baseline c-Fos positive, TH positive cells from saline-injected control mice were subtracted from each value. Cells were counted from 23 to 33 VTA slices per mouse. Three mice per treatment were used for analysis. Asterisks directly above each bar indicate significance from saline-treated control mice. *p < 0.05, **p < 0.01
To determine how nicotine may affect ethanol-induced c-Fos expression, we injected mice with 0.5 mg/kg nicotine, followed by either a saline or 2.0 g/kg ethanol injection. In the absence of ethanol, nicotine significantly increased the number of VTA c-Fos/TH double positive neurons compared to saline-injected animals (Fig. 5b, d, p < 0.01). Ethanol exposure after the initial nicotine injection did not significantly increase or decrease the number of double positive neurons compared to nicotine alone (Fig. 5d, p > 0.05).
Discussion
Previously, the nonspecific nicotinic receptor antagonist, mecamylamine, has been shown to reduce ethanol intake in rats that have learned to drink ethanol through at least 2-week training with increasing concentration of free or limited access ethanol (Blomqvist et al. 1996; Le et al. 2000)). In addition, mecamylamine has been reported to reduce the subjective euphoria of ethanol in humans (Blomqvist et al. 1996; Chi and de Wit 2003; Le et al. 2000). To our knowledge, this is the first report that nAChR blockade reduces ethanol consumption in mice during the DID paradigm, a model of binge drinking where C57BL/6J mice consume alcohol until intoxicated. Mecamylamine dose dependently reduced alcohol intake and this also leads to a significant reduction in blood–ethanol concentration suggesting that mecamylamine was not inhibiting the metabolism of ethanol. Sucrose intake, however, was not reduced indicating specificity for alcohol consumption and not a general effect on reward signaling. Reduction of ethanol intake by mecamylamine was mediated by blockade of neuronal nAChRs expressed in the CNS because the non-specific nAChR antagonist, hexamethonium, did not significantly alter alcohol consumption. Prior studies indicate that mecamylamine delivered systemically or directly into the VTA blocks elevation of ethanol-mediated dopamine release in the nucleus accumbens (Blomqvist et al. 1993, 1997). Thus, it is likely that mecamylamine is reducing ethanol intake via a similar mechanism in the DID assay. Although there have been reports that high doses of mecamylamine can non-competitively inhibit NMDA receptors (Fu et al. 2008; O’Dell and Christensen 1988), we observe a decrease in the volume of ethanol consumption at doses as low as 0.5 mg/kg suggesting that mecamylamine is acting via blockade of neuronal nAChRs.
Because of the vast array of nAChR subtypes expressed in the CNS, identifying the specific composition of receptors involved in ethanol reinforcement is a difficult, but important question. High affinity α4β2 and low affinity α7 nAChRs are two of the most abundant nicotinic receptors in the CNS and could represent potential candidates for at least partially mediating ethanol reward, α4β2 in particular since these receptors have been clearly implicated in nicotine dependence (Picciotto et al. 1998; Tapper et al. 2004). However, the α4β2-selective (DHβE) and α7-selective (MLA) antagonists, both of which readily cross the blood-brain barrier, failed to significantly reduce ethanol intake. These data support prior studies that have shown little effect of these compounds on both operant responding, ethanol-mediated dopamine release in nucleus accumbens, and ethanol self-administration in rats (Le et al. 2000; Soderpalm et al. 2000). The straightforward interpretation of these data would be that α4β2 and α7 nAChRs are not involved in alcohol self-administration. However, caution in this interpretation is warranted especially in regard to higher affinity heteromeric nicotinic receptors that could contain α4β2 in addition to a third or even fourth subunit that may render them relatively insensitive to DHβE (Salminen et al. 2004).
Interestingly, acute exposure to nicotine dose dependently reduced alcohol intake in the DID paradigm. This is in opposition to at least one previous study that indicates that nicotine can enhance ethanol intake in rats in a restricted access drinking assay (Smith et al. 1999). The most likely difference between studies is that our DID assay utilized mice from the C57BL/6J strain which are high-alcohol-preferring animals; whereas the study of Smith et al. utilized rats that needed to be given low doses of ethanol for weeks before voluntary drinking was established. Throughout the adaptation period, where rats learned to drink increasing alcohol doses that produced robust blood ethanol concentrations, they were exposed to nicotine daily. Thus, chronic nicotine enhanced ethanol consumption, while our study illustrates that acute nicotine in naïve mice reduces ethanol intake. It will be interesting to determine the effect of chronic nicotine exposure on consumption in the DID assay.
Our results indicate that cytisine can also reduce ethanol drinking. While nicotine is a full agonist, cytisine is known to be a full agonist for β4* nAChRs and a partial α4β2 agonist (Mineur et al. 2007; Picciotto et al. 1995). The α4β2-selective partial agonist, varenicline is a derivative of cytisine and recently has been shown to inhibit alcohol intake and seeking in rats (Coe et al. 2005; Steensland et al. 2007). Based on these observations, cytisine may also be a candidate compound for alcohol cessation.
Mecamylamine and nicotine differentially modulate alcohol drinking patterns. Mecamylamine reduced ethanol intake predominantly in the second hour of the DID assay; whereas nicotine reduced intake during the first hour, perhaps indicating independent mechanisms of action for each compound. Drinking patterns may be explained by differences in the pharmacokinetics of each drug and how readily they cross the blood brain barrier. For example, nicotine is known to permeate the brain on the order of seconds (Lockman et al. 2005), while mecamylamine likely has a longer latency to reach effective concentrations in the CNS (Young et al. 2001).
Because of the complexity of nAChR subunit composition, as well as the robust expression patterns of nAChRs throughout the CNS, it is not so surprising that blocking nAChRs (i.e., with mecamylamine) and activating them with agonist can both reduce ethanol intake. However, could both classes of compounds impact the same ethanol reward circuit to modulate voluntary ethanol intake? Based on multiple studies indicating that nAChRs rapidly desensitize after a single nicotine exposure, often for prolonged periods of time (Mansvelder et al. 2002; Pidoplichko et al. 1997), it is possible that an acute injection of nicotine or cytisine prior to ethanol exposure desensitizes the relevant nAChR subtype precluding activation of circuits involved in voluntary drinking. Thus, blocking nAChRs with an antagonist or desensitizing nAChRs with pre-exposure to agonists would both reduce alcohol consumption. Our c-Fos/TH double labeling experiments support this idea. Preinjection of mecamylamine significantly reduced the number of DAergic neurons in the VTA that were activated by a subsequent exposure to ethanol suggesting that mecamylamine may block ethanol reward.
Alternatively, ethanol intake may be reduced by the nAChR agonists because the agonists themselves elevate nucleus accumbens DA release, thereby increasing DA signaling prior to ethanol drinking (Marubio et al. 2003; Picciotto et al. 1998). Indeed, preinjection of nicotine increased c-Fos induction in DAergic neurons and a subsequent exposure to ethanol did not further increase c-Fos compared to nicotine alone, suggesting that nicotine and alcohol may activate similar reward pathways. The DA reuptake blocker GBR 12909 has been shown to also reduce ethanol intake in the DID paradigm, presumably via a similar mechanism (Kamdar et al. 2007) but this compound was also shown to decrease sugar water intake. Our results argue against a common reward pathway because nicotine and cytisine reduced ethanol intake without reducing sucrose drinking suggesting that nicotinic receptor activation is involved in alcohol/nicotine reward specifically.
In summary, our data indicate that nAChRs are involved in acute ethanol drinking until intoxication. Identification of the specific nAChR subtypes involved in this behavior should lead to novel therapeutic targets that could be used to prevent binge drinking.
References
Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B (1993) The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol 249:207–213
Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B (1996) Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol 314:257–267
Blomqvist O, Ericson M, Engel JA, Soderpalm B (1997) Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol 334:149–156
Centers for Disease Control and Prevention (2004) Alcohol-attributable deaths and years of potential life lost—United States, 2001. MMWR Morb Mortal Wkly Rep 53:866–870
Chi H, de Wit H (2003) Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res 27:780–786
Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD 3rd, O’Neill BT (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477
Cole AJ, Saffen DW, Baraban JM, Worley PF (1989) Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature 340:474–476
Ericson M, Blomqvist O, Engel JA, Soderpalm B (1998) Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol 358:189–196
Ericson M, Molander A, Lof E, Engel JA, Soderpalm B (2003) Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol 467:85–93
Forman SA, Zhou Q (2000) Nicotinic receptor pore mutations create a sensitive inhibitory site for ethanol. Alcohol Clin Exp Res 24:1363–1368
Fu H, Dou J, Li W, Luo J, Li KC, Lam CS, Lee NT, Li M, Han Y (2008) Mecamylamine prevents neuronal apoptosis induced by glutamate and low potassium via differential anticholinergic-independent mechanisms. Neuropharmacology 54:755–765
Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296:47–55
Harris RA (1999) Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exp Res 23:1563–1570
Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA (2006) Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol Alcohol 41:486–493
Jones S, Sudweeks S, Yakel JL (1999) Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci 22:555–561
Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS (2007) Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 192:207–217
Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21:1452–1463
Larsson A, Svensson L, Soderpalm B, Engel JA (2002) Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol 28:157–167
Larsson A, Jerlhag E, Svensson L, Soderpalm B, Engel JA (2004) Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol 34:239–250
Laviolette SR, van der Kooy D (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5:55–65
Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK (2000) Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res 24:155–163
Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G (1996) Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res 109:125–137
Lockman PR, McAfee G, Geldenhuys WJ, Van der Schyf CJ, Abbruscato TJ, Allen DD (2005) Brain uptake kinetics of nicotine and cotinine after chronic nicotine exposure. J Pharmacol Exp Ther 314:636–642
Mansvelder HD, Keath JR, McGehee DS (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33:905–919
Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP (2003) Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci 17:1329–1337
Mineur YS, Somenzi O, Picciotto MR (2007) Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology 52:1256–1262
National Research Council (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C
O’Dell TJ, Christensen BN (1988) Mecamylamine is a selective non-competitive antagonist of N-methyl-D-aspartate- and aspartate-induced currents in horizontal cells dissociated from the catfish retina. Neurosci Lett 94:93–98
Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, Vincent P, Pich EM, Brulet P, Changeux JP (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374:65–67
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Pidoplichko VI, DeBiasi M, Williams JT, Dani JA (1997) Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390:401–404
Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63
Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr, Crabbe JC (2007) Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav 6:1–18
Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65:1526–1535
Smith BR, Horan JT, Gaskin S, Amit Z (1999) Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl) 142:408–412
Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA (2000) Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res 113:85–96
Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A 104:12518–12523
Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032
Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR (2006) Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 296:64–71
Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA (2003) Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci 23:3176–3185
Young JM, Shytle RD, Sanberg PR, George TP (2001) Mecamylamine: new therapeutic uses and toxicity/risk profile. Clin Ther 23:532–565
Zhou QL, Zhou Q, Forman SA (2000) The n-alcohol site in the nicotinic receptor pore is a hydrophobic patch. Biochemistry 39:14920–14926
Zuo Y, Kuryatov A, Lindstrom JM, Yeh JZ, Narahashi T (2002) Alcohol modulation of neuronal nicotinic acetylcholine receptors is alpha subunit dependent. Alcohol Clin Exp Res 26:779–784
Acknowledgments
This study was supported in part by National Institutes on Alcohol Abuse and Alcoholism grant R01AA017656 (A.R.T.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hendrickson, L.M., Zhao-Shea, R. & Tapper, A.R. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology 204, 563–572 (2009). https://doi.org/10.1007/s00213-009-1488-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1488-5