Abstract
Objective
The aim of our study was to test whether ketamine produces an antidepressant effect in animal model of olfactory bulbectomy and assess the role of mammalian target of rapamycin (mTOR) pathway in ketamine’s antidepressant effect.
Methods
Bulbectomized (OBX) rats and sham controls were assigned to four subgroups according to the treatment they received (ketamine, saline, ketamine + rapamycin, and saline + rapamycin). The animals were subjected to open field (OF), elevated plus maze (EPM), passive avoidance (PA), Morris water maze (MWM), and Carousel maze (CM) tests. Blood samples were collected before and after drug administration for analysis of phosphorylated mTOR level. After behavioral testing, brains were removed for evaluation of brain-derived neurotrophic factor (BDNF) in prefrontal cortex (PFC) and hippocampus.
Results
Ketamine normalized hyperactivity of OBX animals in EPM and increased the time spent in open arms. Rapamycin pretreatment resulted in elimination of ketamine effect in EPM test. In CM test, ketamine + rapamycin administration led to cognitive impairment not observed in saline-, ketamine-, or saline + rapamycin-treated OBX rats. Prefrontal BDNF content was significantly decreased, and level of mTOR was significantly elevated in OBX groups.
Conclusions
OBX animals significantly differed from sham controls in most of the tests used. Treatment had more profound effect on OBX phenotype than controls. Pretreatment with rapamycin eliminated the anxiolytic and antidepressant effects of ketamine in task-dependent manner. The results indicate that ketamine + rapamycin application resulted in impaired stress responses manifested by cognitive deficits in active place avoidance (CM) test. Intensity of stressor (mild vs. severe) used in the behavioral tests had opposite effect on controls and on OBX animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first report of ketamine’s antidepressant effect after intravenous infusion comes from Berman and his colleagues (Berman et al. 2000). The single administration of ketamine has therapeutic effect and improves depressive symptoms lasting up to 7 days (Zarate et al. 2006, 2012; Diazgranados et al. 2010; Murrough et al. 2013a), possibly up to 4 weeks (Ibrahim et al. 2012). The significant improvement was also reported with repeated infusions in treatment-resistant depressive patients (Murrough et al. 2013b; Shiroma et al. 2014). These findings have triggered the immense interest in research of ketamine’s antidepressant effect and its mechanism of action.
Ketamine is a non-competitive NMDA receptor antagonist (Muir and Lees 1995). However, its antidepressant effect cannot be explained solely by inhibition of NMDA receptors. Ketamine administration sets off signaling cascades resulting in rapid antidepressant effect persisting long after the drug had been eliminated from the body (Hijazi et al. 2003). One of the proposed mechanisms of action responsible for ketamine’s antidepressant effect is activation of mammalian target of rapamycin (mTOR) signaling pathway (Abdallah et al. 2015).
mTOR is serine/threonine protein kinase regulating cell growth, proliferation, transcription, and proteosynthesis, thus directly participating in synaptic plasticity (Swiech et al. 2008). mTOR signaling pathway is activated as early as 30 min after ketamine administration via elevated level of Akt and ERK protein kinases. Two hours later, the level of phosphorylated proteins returns to normal. This effect is dose-dependent; lower doses of ketamine (5–10 mg/kg) have stimulating effect while high anesthetic doses (80 mg/kg) have no effect on mTOR signaling pathway. When rapamycin (inhibitor of mTORC1) is administered prior to ketamine administration, ketamine’s antidepressant effect is eliminated. It indicates that ketamine-induced synaptogenesis is dependent on mTORC1 complex activation (Li et al. 2010; Duman et al. 2012).
Two hours after ketamine administration, the levels of synaptic proteins such as synapsin I, PSD95, and GluR1 increase. This effect is sustained for 7 days. Twenty-four hours after ketamine application, the number of mushroom spines increases (Li et al. 2010; Duman et al. 2012), which indicates the maturing and strengthening of synaptic connections (Yoshihara et al. 2009). The increased affectivity of synaptic transmission is apparent from the increased amplitude of EPSCs facilitated by insertion of AMPA receptors into postsynaptic membrane (Li et al. 2010). Ketamine stimulates signaling pathways involved in protein synthesis and formation of dendritic spines. Recent studies confirmed the negative effect of stress upon number, density, and function of dendritic spines and decreased synaptic plasticity in depressive patients (Marsden 2013). Single ketamine application reverses the deficit in number and function of dendritic spines caused by exposure to chronic stress (Liu and Aghajanian 2008; Li et al. 2011). In addition, stress and depression decrease the expression and release of brain-derived neurotrophic factor (BDNF), resulting in disruption of neurotrophic support leading to atrophy and loss of neurons (Duman 2014). Autry and colleagues showed that ketamine administration increases BDNF translation that mediates rapid behavioral antidepressant responses (Autry et al. 2011).
Understanding of ketamine’s mechanism of action can help us to target mTOR signaling pathway more effectively and come up with strategies leading to the discovery of novel, fast-acting antidepressants with fewer adverse effects. The goal of present study is to examine antidepressant effect of ketamine in the animal model of depression. The bilateral olfactory bulbectomy is an animal model of depression that disrupts the integrity of limbic system known to be affected in patients suffering from major depressive disorder. Bilateral olfactory bulbectomy (OBX) leads to cellular, structural, biochemical, and behavioral changes that can be reversed by chronic treatment with antidepressants (Song and Leonard 2005).
The behavioral tests we chose served to assess locomotor activity (open field), anxiety (elevated plus maze), and cognitive functions (passive avoidance, Morris water maze, and Carousel maze), because hyperactivity, decreased anxiety, and deficits in learning and memory are some of the behavioral changes detected after olfactory bulbectomy. In addition, hippocampi and prefrontal cortex were removed for BDNF analysis and blood samples were collected for analysis of phosphorylated mTOR level. The antidepressant effect of ketamine was assessed 24–144 h after application in order to inspect its prolonged effect on behavior. Since the antidepressant effect of ketamine was reported to last 7 days, all the tests were terminated within 1 week postinjection. Rapamycin was administered in order to investigate if the ketamine’s antidepressant effect was mediated by mTOR signaling pathway.
Materials and methods
Animals
Male Wistar rats (300–350 g) used in the experiments were obtained from the Institute of Physiology; accredited breeding colony originated from Charles River Laboratories, Inc. Animals were housed in transparent plastic cages (30 × 30 × 40 cm) in an air-conditioned animal facility with constant temperature, humidity, and 12/12 light/dark cycle. Water and food were available ad libitum. Experiments were carried on during daylight hours. All procedures were in accordance with Czech and European legislation regarding treatment of laboratory animals (Directive 86/609/EEC).
Experimental design
Figure 1 depicts the time scheme of experiments and the sample collections. After the surgery, the animals had 3 weeks for recovery before they were administered with drugs. Blood samples were collected before and 30 min after drug application, and the experiments started 24 h later. In the first day of behavioral procedures, the animals were tested in open field (OF) and elevated plus maze (EPM). The cognitive tests were pursued on subsequent days. The animals were tested either in passive avoidance (PA) and Morris water maze (MWM) or in active place avoidance on a rotating arena (Carousel maze (CM)). Animals were not tested in all cognitive tests in order to complete the planned experiments within 1 week. Numbers of animals in behavioral tests were as follows: in OF, EPM, and CM (OBX saline n = 14, OBX ketamine n = 14, OBX saline + rapamycine n = 12, OBX ketamine + rapamycine n = 12, sham saline n = 9, sham ketamine n = 11, sham saline + rapamycine n = 11, and sham ketamine + rapamycin n = 15); in PA (OBX saline n = 6, OBX ketamine n = 7, OBX saline + rapamycine n = 8, OBX ketamine + rapamycine n = 8, sham saline n = 7, sham ketamine n = 8, sham saline + rapamycine n = 8, and sham ketamine + rapamycin n = 9); and in MWM (OBX saline n = 8, OBX ketamine n = 9, OBX saline + rapamycine n = 8, OBX ketamine + rapamycine n = 8, sham saline n = 9, sham ketamine n = 11, sham saline + rapamycine n = 9, and sham ketamine + rapamycin n = 9). For mTOR analysis, the group sizes were as follows: OBX saline n = 10, OBX ketamine n = 10, OBX saline + rapamycin n = 10, OBX ketamine + rapamycin n = 8, sham saline n = 12, sham ketamine n = 8, sham saline + rapamycin n = 8, and sham ketamine + rapamycin n = 14 for 30-min analysis and OBX n = 12 and sham n = 10 for 0-min analysis. On the seventh day after drug application, the brains were removed for analysis of BDNF. The numbers of animals for BDNF analysis were OBX saline n = 7, OBX ketamine n = 8, OBX saline + rapamycin n = 11, OBX ketamine + rapamycine n = 8, sham saline n = 9, sham ketamine n = 9, sham saline + rapamycine n = 7, and sham ketamine + rapamycin n = 9.
Surgery
The rats were anesthetized with isoflurane (no. B306, Abbot Laboratories, Queen-Borought, UK) in a preparation chamber (3 % of isoflurane) and then placed in a stereotactic apparatus (no. 430005-GR-GP/K, TSE systems, Germany). Level of isoflurane anesthesia during the operation was held on 2 %. For local anesthesia, 0.5 ml of mesocaine 1 % (Zentiva) was applied before an incision. The eyes were treated with Vidisic ocular gel (Bausch + Lomb). The incision was made in the scalp above the olfactory bulbs. Two 2-mm burr holes were made by a microdrill (Dremel), −8 mm AP and ±2 mm ML from bregma. The olfactory bulbs were removed by a blunt hypodermic needle connected to a water pump (performed according to van der Stelt et al. 2005). The hemorrhage was stopped and the incision was sutured using absorbable material. The sutures were treated with local antibiotic Framykoin (Zentiva). Sham-operated rats underwent the same procedure without removal of the olfactory bulbs. After surgery, 2.5 ml of sterile water was administered intraperitoneally (i.p.) and analgesic Nurofen was added into drinking water. The experiments were performed after 3 weeks of postoperative period. After the termination of behavioral procedures, the brains were extracted and the lesions were inspected. Animals with remains of olfactory tissue were excluded from the analyses.
Drug administration
Within OBX or sham-operated group, animals received either a single i.p. injection of ketamine (10 mg/kg) or saline or were i.p. co-injected with ketamine (10 mg/kg) and rapamycin (1 mg/kg) or saline and rapamycin (1 mg/kg). Rapamycin (Sirolimus) was purchased from ApexBio and dissolved in a vehicle solution containing 20 % DMSO and saline. An injection of rapamycin was applied 30 min prior to ketamine or saline administration. Ketamine was obtained as a ketamine hydrochloride from Vétoquinol Ltd. All animals received the same volume of liquid per 1 kg of body weight (1 ml/kg of b.w.). Blood samples from the rat tail vein were collected at 0 and 30 min after the drug application for later analysis of phosphorylated mTOR level. Rats were subjected to behavioral testing 24 h after drug administration. After behavioral tests were completed, rats were decapitated and hippocampi and prefrontal cortex were removed and stored in −80 °C for later BDNF analysis.
Immunoassay
ELISA for mTOR analysis
Phosphorylated mTor activity was detected in cell lysates by K-LISA™ mTor Activity Kit (Merck KGaA, Darmstadt, Germany). All procedures were done according to the manufacturer’s instructions. Briefly, cell’s pellet was washed twice with Tris-buffered saline (TBS). Lysate was prepared by adding 1 ml of lysis buffer and incubated on ice for 20 min. Cell’s lysate was precleared by adding protein A/protein G-plus agarose beads (Merck KGaA, Darmstadt, Germany) according to the manufacturer’s instructions twice. Anti-mTOR/FRAP mouse monoclonal antibody (Merck KGaA, Darmstadt, Germany) needed to be added to precleared lysates to efficiently immunoprecipitate mTOR from crude biological samples. Protein agarose beads (now bound to immunoprecipitated mTOR protein) were added 2× Kinase Assay Buffer wash buffer and mTor substrate wash buffer to prepare reaction mixture for the assay. Before use, the mixture was centrifuged to pellet agarose beads. Supernatant was transferred to clear tube and stored at −70 °C before use. Standards as well as samples were pipetted to designated wells on a glutathione-coated plate. The contents of the wells were aspirated and washed three times by adding 200 μl of TBS. Particular substances were added to the assay in following order: 100 μl of the p70S6K–GST fusion protein, 100 μl of HRP conjugate, and 100 μl of tetramethylbenzidine (TMB) substrate. Each substance was incubated for 1 h on the plate. After incubation, all substances were washed out. The TMB reaction was stopped by adding 100 μl of stop solution. The plate was read at a wavelength of 450 nm with a reference wavelength of 595 nm using a 96-well plate spectrophotometer (Victor, Work Out version 2.5, Perkin Elmer, USA).
ELISA for BDNF analysis
Excised brain parts (prefrontal cortexes and hippocampi) were extracted according to “modified extraction procedure” of Szapacs et al. (2004), in “lysis buffer” (with slightly modified concentrations of antiproteases, 100 mM PIPES pH 7.0, 500 mM NaCl, 0.2 % Triton X-100, 0.1 % NaN3, 2 % BSA, 2 mM EDTA, 1 mM PMSF, 2.5 μM leupeptin, 0.5 μM pepstatin, and 0.75 μM aprotinin). Of the homogenates, 10 % w/v was prepared by sonication (Bioblock Scientific 72442, France) with microtip (2 mm) on ice by four pulses (setting 60) for 5 s. The homogenates were acidified (25 μl 1 N HCl/0.2 ml homogenate in Eppendorf microtube) and left overnight at refrigerator (ca. 4 °C). The next day, extracts were centrifuged (12 600 rpm/15 min/4 °C in Eppendorf 5415R centrifuge) and to 0.15 ml of supernatants, 1 μl of Neutral Red (10 mg/ml 50 % ethanol) were added, and the samples were neutralized by 1 N NaOH under visual control (careful titration until the color started to change from red to yellow). Neutralized samples were frozen and kept at −40 °C until assayed by ELISA. Content of BDNF was assayed with R&D Systems DuoSet ELISA BDNF Kit DY248 (USA) according to the manufacturer’s instructions.
Behavioral procedures
OF
Rat’s spontaneous activity in new environment was observed in the open field test for 10 min. OF (50 × 50 cm) was dimly illuminated by red light considered to be less distressing for rodents. Activity was detected by evenly spaced infrared light beams. Beam interruptions caused by movements of the animal were registered by the software (Multi Conditioning System, TSE Systems, Germany). Software analyzed the number of rearings and total distance travelled. In addition, the hyperactivity (defined as movement exceeding 20 cm/s) was examined as well. The analysis was carried out twice, the first 2 min and the last 2 min of OF activity separately, since the activity is supposed to be the most intense shortly after exposure to the new environment.
EPM
The apparatus consisted of two open arms (45 × 10 cm) crossed at right angles with two opposed arms of the same length. Two of the opposed arms were enclosed by walls 40 cm high, except for the central platform where the arms crossed. The whole apparatus was elevated 50 cm above the floor. At the beginning of each experiment, the rat was placed on the central platform facing the closed arm. Time spent in open arms, closed arms, and central platform and distance moved were recorded during a 10-min test session by a camera positioned above the maze and analyzed by the software (Tracker, Biosignal Group, USA).
Step-through PA
Arena (50 × 50 cm) consisted of two equally sized compartments divided by sliding doors (Multi Conditioning System, TSE Systems, Germany). Animal was always placed into intensively illuminated compartment. During habituation session, rats moved freely between two compartments for 5 min; no shock was delivered. Thirty minutes after habituation, rats were placed into arena again for training session. After the animal crossed to the dark compartment, the sliding door dropped down. The rat received the mild electric shock (2.5 mA, 8 s) through the stainless steel grid floor. The actual testing followed 1 and 24 h after training session. During the 5-min test session, the shocks were not delivered. The latency to cross to the dark chamber was evaluated by the software.
MWM
MWM is used to study spatial learning and memory (Morris 1984; Stuchlik et al. 2007). Rat was placed into circular pool (180 cm in diameter) filled with water to a height of 40 cm. A transparent Plexiglas platform was submerged 1 cm under the water surface. The animal navigated using distal cues as points of reference. Escape latency was assessed as a measure of learning. The rat was detected by infrared camera attached to the ceiling, and trajectory was digitalized by the software (Tracker, Biosignal Group, USA and DT-3155 Card, Data Translation, USA). Escape latencies were measured by a stopwatch. Each rat underwent eight trials per day for four subsequent days. At the beginning of every trial, the rats were released from the same location positioned in the “east” of the pool. The platform was located in the middle of “southwest” quadrant.
Active place avoidance on CM
CM is an apparatus for assessment of active avoidance behavior consisting of rotating circular arena. CM can be used to test cognitive coordination and spatial avoidance navigation. It consisted of a metallic circular arena (82 cm in diameter) placed 1 m above the floor with transparent Plexiglas wall (40 cm high). The rat was tracked by an infrared camera located on the ceiling directly above the arena, and trajectory was digitized by the software (Tracker, Biosignal Group, USA). On the arena surface, a stable unmarked 60° to-be-avoided sector was defined by the room-frame coordinates. The arena rotated with the constant speed of one rotation per minute. Upon the entrance to the sector, animal received a mild electric footshock. The animal had to distinguish between two conflicting spatial representations (arena vs. room) and navigate according to relevant stimuli. The apparatus is described in detail elsewhere (Stuchlik et al. 2013).
Behavioral training consisted of four sessions lasting 20 min. The animals underwent two sessions per day. The performance was evaluated using cognitive parameters—the number of entrances into the to-be-avoided sector and the maximum time avoided within a session. These parameters assess the overall cumulative performance and an ability to maintain avoidance throughout a session, reflecting both between-session and within-session learning. The escape response was measured by the number of shocks rats received. The locomotor activity expressed as the total distance actively walked by the animal (after filtering out passive movement of the arena) was evaluated as well. For statistical analysis of cognitive performance, data from the last session only were taken into account. Since hyperactivity of OBX animals is the most apparent in a novel environment, the distance walked and shocks received were evaluated during the first 20-min session.
Statistical analysis
Statistical analyses were performed by the program GraphPad Prism 5.0 (San Diego, CA, USA) and IBM SPSS Statistics 20 (MWM). The statistical significance for OF test, EPM, and PA tests was detected by a two-way ANOVA with treatment effect (four-factor lever) and the bulbectomy effect (two-factor level) serving as independent variables. In MWM, the effect of treatment and effect of bulbectomy on the performance of animals were assessed by two-way repeated measure ANOVA. Bonferroni’s post hoc test was used when appropriate. Outliers identified by Grubbs test were removed from the analysis. If normality was not assumed, data were transformed. Data are presented as the group means ± standard error of mean (SEM). The significant level was set at <0.05.
Results
OF
Statistical analysis of spontaneous activity during the first 2 min revealed significant effect of the factor bulbectomy, F (1, 90) = 14.61, p = 0.0002, on the locomotion. No significance was detected for the effect of treatment and interaction. Post hoc test did not detect any differences (Fig. 2a). Analysis of the last 2 min did not show any effect of either variable or their interaction (Fig. 3a).
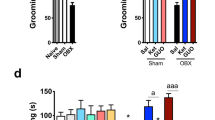
The first 2 min in the open field. The graphs illustrate spontaneous activity in open field during the first 2 min of 10-min session. In all parameters assessed, OBX animals significantly differed from sham controls. a Distance walked by OBX rats was significantly increased when compared to controls. b OBX rats displayed significantly elevated hyperactivity as well. c The most profound difference between OBX and controls was seen in vertical activity. Number of rearings was significantly greater in OBX animals. All values represent group means ± SEM
The last 2 min in the open field. The graphs illustrate activity during last 9 and 10 min of a 10-min session. The increased ambulation expressed in terms of distance walked (a) and time spent hyperactive (b) normalized as rats became habituated to the new environment. The number of rearing remained significantly increased in OBX rats. c The habituation rate of vertical activity was slower indicating increased emotionality of OBX rats. All values represent group means ± SEM. *p < 0.05
Similarly; the effect of bulbectomy on cumulative time spent by hyperactivity (˃20 cm/s) came out as significant, F (1, 90) = 11.66, p = 0.0010, for first 2-min analysis. No effect of treatment and interaction on behavior was detected (Fig. 2b). Analysis of last 2 min found no significant difference between groups (Fig. 3b).
The effect of bulbectomy on number of rearings during first 2 min was considerable, F (1, 81) = 35.86, p < 0.0001.The effect of treatment and interaction between two effects was not significant (Fig. 2c). The analysis of the first 2 min copied the results from last 2-min analysis, F (1, 81) = 8.709, p = 0.0041, for the effect of bulbectomy. No difference in the effect of interaction and treatment was revealed. Bonferroni’s post hoc test revealed significant difference between OBX rats treated with ketamine vs. OBX rats treated with saline + rapamycin (p < 0.05; Fig. 3c).
EPM
Two-way ANOVA detected significant effect of the treatment F (3, 90) = 3.044, p = 0.0329 and bulbectomy F (1, 90) = 7.877, p = 0.0061 on the distance travelled in EPM. The interaction between two effects did not come out as significant. The OBX animals treated with saline significantly differed from OBX animals treated with ketamine + rapamycin (p < 0.001) or ketamine alone (p < 0.01; as post hoc test showed) with saline-treated animals being more active (Fig. 4a).
Elevated plus maze. In both parameters tested, OBX animals exhibited significantly altered behavior when compared to sham controls. The effect of treatment was significant as well. a Locomotor activity was significantly increased in OBX rats as a response to novelty exposure. Ketamine treatment resulted in suppression of hyperlocomotion exhibited by saline-treated OBX rats. However, co-application of ketamine + rapamycin had similar effect on locomotion as ketamine administration alone. b Open arm avoidance was significantly reduced in OBX rats compared to controls. Time spent in open arms was increased in OBX rats treated with ketamine. Rapamycin eliminated the anxiolytic effect of ketamine in ketamine + rapamycin-treated OBX animals. All values represent group means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001
Two-way ANOVA revealed significant effect of treatment F (3, 90) = 4.327, p = 0.0042 and bulbectomy F (1, 90) = 8.609, p = 0.0068 on time spent in open arms. No effect of the interaction between two factors was recognized. The significant difference between OBX animals treated with ketamine and OBX animals treated with ketamine + rapamycin (p < 0.001) or saline + rapamycin (p < 0.05) was detected by post hoc test. Ketamine-treated animals exhibited lowered anxiety (Fig. 4b). As the data did not pass normality test, the square root transformation was conducted to attain Gaussian distribution. However, for better illustration, original units and scales were used in the graph.
Step-through PA
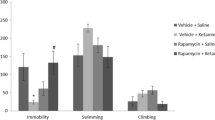
Two-way ANOVA detected significant effect of treatment F (3, 53) = 3.089, p = 0.0348 on memory and learning in PA test while interaction and bulbectomy had no effect. Within a group of OBX rats, the post hoc test revealed significant difference between saline- vs. ketamine-treated animals (p < 0.05), saline- vs. saline +rapamycin-treated animals (p < 0.01), and saline vs. ketamine + rapamycin animals (p < 0.05; Fig. 5). Data were transformed using common logarithm in order to pass normality test. The graph is portrayed with original units (seconds). The treatment that was applied has positively influenced performance in the test and enhanced the latency to cross into dark compartment.
Step-through passive avoidance. No effect of bulbectomy was revealed, but the effect of treatment came out significant. Ketamine, ketamine + rapamycin, and saline + rapamycin treatments prolonged the latency to enter dark compartment after previous shock exposure. All values represent group means ± SEM. *p < 0.05, **p < 0.01 compared to the saline-treated OBX group
MWM
For evaluation of spatial memory and learning in MWM, two-way repeated measure ANOVA was carried out. The performance during 4 days (escape latencies, first to fourth day) was set as within-subject effect and the bulbectomy and treatment used as between-subject effects. The within-subject effect came out significant, F (3, 177) = 117.023, p < 0.0001, showing that animals from all groups improved with each subsequent day. The interaction between the day and bulbectomy was significant as well, F (3, 177) = 5.521, p = 0.004. It indicates that the rate of learning was influenced by bulbectomy regardless of the treatment applied. The only significant between-subject effect was bulbectomy F (1, 59) = 84.827, p < 0.0001 (Fig. 6).
Morris water maze. OBX group significantly differed from controls in spatial navigation evaluated during 4 days when animals were released from the same starting position in the pool. Each rat was subjected to eight trials a day. Escape latencies of OBX rats were substantially increased indicating cognitive deficit caused by bulbectomy. Treatment used had no effect. Both groups improved with each subsequent day; however, the rate of learning in controls was faster. All values represent group means. For better clarity, error bars are not present
CM
Numbers of shocks received during the first and the last sessions were assessed by two-way ANOVA. The effect of bulbectomy was significant even during the first session, F (1, 90) = 13.55, p = 0.0004. No effect of treatment and interaction was detected. Post hoc test revealed significant difference between saline-treated and ketamine-treated OBX animals (Fig. 7a). Ketamine application resulted in reduced number of shocks delivered, indicating enhanced escape response. In the last session, the effect of bulbectomy on shock number was more prominent, F (1, 90) = 37.32, p < 0.0001. The treatment effect reached the level of significance, as well F (3, 90) = 2.83, p = 0.0428. Ketamine +rapamycin-treated OBX rats significantly differed from all other OBX groups (post hoc analysis, p < 0.05 from saline and saline + rapamycin and p < 0.01 from ketamine; Fig. 7b).
Carousel maze I. Significant difference between OBX animals and controls was found in non-cognitive parameters. a The number of shocks OBX animals received during the first session was significantly lower than in controls. Sham controls lacked the escape reaction. Ketamine-treated OBX rats received significantly less shocks than saline-treated OBX animals. b Between-session decline in number of shocks received by sham controls was not as prominent as in OBX rats. OBX rats learned to avoid the sector more effectively as seen from dropping number of shocks. Ketamine + rapamycin treatment of OBX rats resulted in increased number of shocks delivered compared to OBX rats treated solely with ketamine. c The distance walked by OBX animals was significantly longer than in controls, indicating the loss of motivation to solve the task by sham controls, resulting in behavior resembling learned helplessness. Ketamine + rapamycin co-application led to decreased distance walked in OBX rats when compared to ketamine-treated OBX rats. All values represent group means ± SEM. *p < 0.05
Two-way ANOVA analysis showed significant effect of bulbectomy in the distance walked between sham controls and OBX rats, F (1, 89) = 37.77, p < 0.0001, even during the first session. Post hoc test detected a significant difference between ketamine-treated OBX group and ketamine + rapamycin-treated OBX group (p < 0.05; Fig. 7c). Sham-operated controls had reduced ambulation, corresponding to diminished escape response after shock delivery.
The numbers of entrances and maximum time avoided during the last session were analyzed by two-way ANOVA. In parameter the number of entrances into forbidden sector, the significant effect of interaction between the effects of bulbectomy and treatment was discovered, F (3, 90) = 9.912, p = 0.0367, as well as the effect of bulbectomy, F (1, 90) = 2.008, p = 0.0022. The post hoc test detected significant difference between ketamine + rapamycin-treated OBX rats and all other OBX groups (p < 0.01 from saline and ketamine and p < 0.05 from saline + rapamycin; Fig. 8a). The next cognitive parameter evaluated was the maximum time avoided within one session. The significant effect of both bulbectomy and treatment was detected, F (1, 90) = 24.50, p < 0.0001 for bulbectomy and F (3, 90) = 4.053, p = 0.0094 for the treatment. The significant difference was again found between ketamine + rapamycin-treated OBX rats and all other OBX groups (p < 0.05 from all other treatment groups; Fig. 8b). Data of cognitive parameters and of number of shocks from fourth session were transformed using common logarithm.
Carousel maze II. In cognitive parameters, the substantial difference was found between OBX and sham controls as well. a Number of entrances (errors) made by sham controls in last session reflects cognitive deficit. Administration of ketamine + rapamycin resulted in increased number of entrances, approaching the performance of sham controls, when compared to all other OBX groups. b During the last session, OBX animals spent significantly greater portion of time outside the sector than sham controls, as indicated by the maximum time avoided. The maximum time avoided of ketamine + rapamycin-treated OBX rats was significantly reduced compared to all other OBX groups. All values represent group means ± SEM. *p < 0.05, **p < 0.01
BDNF
Two-way ANOVA analysis showed significant difference in BDNF content between sham controls and OBX animals in prefrontal cortex, F (1, 60) = 16.85, p = 0.0001, for effect of bulbectomy. Neither treatment effect nor the effect of interaction was detected. Post hoc test did not reveal difference between groups (Fig. 9a). Hippocampal content of BDNF was comparable between control and experimental groups; neither bulbectomy effect nor treatment effect was significant (Fig. 9b).
mTOR
Measurements of absorbance at 450 nm were expressed in percentage of baseline absorbance at time 0 (before drug application), defined as 100 % absorbance. Two-way ANOVA detected significant effects of treatment F (3, 72) = 4.36, p = 0.007 and bulbectomy F (1, 72) = 15.7, p = 0.0002 as well as their interaction F (3, 72) = 3.454, p = 0.0209. Post hoc test revealed significant difference in OBX group between saline-treated and ketamine-treated rats (p < 0.05), ketamine-treated and saline +rapamycin-treated rats (p < 0.001), and saline + rapamycin- and ketamine + rapamycin-treated animals (p < 0.05; Fig. 10).
mTOR. Treatment applied affected level of phosphorylated mTOR in OBX rats that where more responsive to the treatment. The level of mTOR was significantly increased in OBX rats when compared to controls. Ketamine treatment increased the mTOR level in OBX animals when compared to saline-treated or saline + rapamycin-treated OBX rats. However, ketamine + rapamycin co-application did not reduce the mTOR level and did not eliminate the effect of ketamine. All values represent group means ± SEM. *p < 0.05, ***p < 0.001
Discussion
The objective of the present study was to evaluate an antidepressant effect of a single ketamine injection in a variety of behavioral tests involving various degrees of stress and to investigate the role of mTOR signaling pathway in mediating its antidepressant effect. Since antidepressant effect of ketamine was reported to last up to 7 days, all tests were terminated within 1 week postinjection. In addition, we examined the changes in BDNF and mTOR levels triggered by different treatments applied.
The main results of our research may be summarized as follows: (1) OBX rats significantly differed from sham-operated controls in behavioral tests (except for PA see below), (2) treatment used had significant effect on depressive phenotype only—OBX animals were more responsive to the treatment, (3) rapamycin blocked the effect of ketamine in task-dependent manner; and (4) intensity of stressor (mild vs. severe) used in the behavioral tests had opposite effect on controls and on OBX animals.
Open field test
Activity of OBX rats in the OF arena was significantly increased compared to sham-operated animals during first 2 min. This increase in locomotion (Fig. 2a) was accompanied with increased hyperactivity (Fig. 2b) and increased vertical activity (Fig. 2c) measured by a number of rearings. Enhanced locomotor activity reflects hyper-arousal of OBX animals as a response to a novel environment. Therefore, the difference between control and experimental groups was the most prominent during the first 2 min and gradually decreased as animals habituated to the OF apparatus. The horizontal activity of OBX animals normalized to that of sham-operated controls in the final 2 min of the experiment regardless of treatment used (Fig. 3a, b). However, vertical activity of OBX animals remained substantially elevated compared to controls even during the last 2 min (Fig. 3c). Treatment did not normalize the increased activity of OBX rats in the OF expressed in terms of distance walked, hyperactivity, and rearing. However, there was a tendency of ketamine-treated OBX animals to reduce their activity when compared with OBX animals treated with saline or saline + rapamycin with the latter being significant in rearing activity during the last 2 min.
Increased exploration in open field following olfactory bulbectomy is one of the first reported behavioral changes (Sieck and Baumbach 1974) evident as soon as 1 week after surgery (Hendriksen et al. 2015). Hyperactivity, as a consequence of novelty exposure, is probably caused by increased release of glutamate in the striatum (Ho et al. 2000) and normalized by chronic treatment with antidepressants. The rearing and locomotion can be dissociated by pharmacological manipulation (Balcells-Olivero and Vezina 1997), indicating that the two behavioral parameters might differ in the neural substrates regulating their activity. It was suggested that unlike locomotion, rearing reflects not only exploratory activity but also emotionality of animals (Gironi Carnevale et al. 1990). If there is sufficient time for OBX animals to habituate to the new environment, they are able to reduce their hyperactivity to the level of sham controls. It corresponds with gradual decrease of elevated glutamate levels in striatum after habituation to the open field lasting at least 10 min (Ho et al. 2000). Hyperactivity is the most frequently detected alternation in behavior, and our data are consistent with literature findings (Table 1). Interestingly, it was documented that animals with higher locomotor and rearing responses to novelty showed higher rate of habituation with repeated testing (Thiel et al. 1999).
Elevated plus maze
The EPM is a standard test for evaluation of anxiety or anxiolytic effect of compounds administered (Dawson and Tricklebank 1995; Carobrez and Bertoglio 2005), where the time spent in open arms is indicative of lowered anxiety (Hogg 1996). In the EPM test, OBX and sham controls significantly differed in both parameters tested, distance walked and time spent in open arms. Ketamine administration resulted in reduction of novelty-induced hyperlocomotion when compared with OBX animals injected with saline (Fig. 4a). In the parameter time spent in open arms, ketamine exerted strong anxiolytic effect that was completely blocked by prior rapamycin administration (Fig. 4b). In spite of increased ambulation of saline-treated OBX rats, the animals were able to partially adjust their behavior as a reaction to aversive stimuli represented by open arms and avoid them.
After olfactory bulbectomy, a degeneration of inhibitory projection from olfactory lobes to amygdala occurs, resulting in reduced inhibitory input and subsequent disinihibition of the amygdala. Prolonged disinhibition of amygdala was reported also after chronic stress, which is relevant to depressive disorders (Liu et al. 2014). Amygdala plays a crucial role in processing of emotional stimuli, memory, and decision making. Therefore, it is not surprising that disinhibition of amygdala may result in sensitization to stress (McNish and Davis 1997) and may account for altered behavioral changes in OBX animals. Hyperexcitability of amygdala in OBX animals correlates with increased 2-deoxyglucose uptake indicating an increased demand for energy by axon terminals (Shibata and Watanabe 1994). It is compatible with the hypothesis that limbic hyperactivity in the amygdala, during evaluation of emotional stimuli, combined with prefrontal hypoactivity leading to a failure of cortical control, might cause negative emotional biases in depressed patients (Drevets 1999; Phillips et al. 2003; DeRubeis et al. 2008). OBX rats appear to be sensitive to increasingly aversive environments (Primeaux and Holmes 1999). This may explain partial avoidance of open arms in EPM by OBX animals treated with saline and differences between three cognitive tests used. The results from literature reported increased anxiety, decreased anxiety, and no change in anxiety scores in OBX animals (Table 1). However, anxiety phenotype of sham controls detected in EPM in our study is consistent with results obtained from cognitive test.
Cognitive tests
The most widely used test for assessment of memory function in OBX rats is PA learning based on the association formed between an aversive stimulus and a specific environmental context. The PA is the only test from whole behavioral assay used where the difference between OBX animals and sham controls was not found, whereas the treatment used affected the performance. The saline-treated OBX rats displayed significantly shorter latency to enter dark compartment compared to all other experimental groups (Fig. 5). Twenty-four hours later, no difference was found (data not shown). These results are in opposition to the results from the MWM. However, the locomotor, cognitive, and motivational variables are confounded in the passive avoidance test (Bizot and Thiébot 1996). Therefore, interpretation of obtained results as a cognitive deficit in OBX rats injected with saline may be misleading. Our results are in accordance with literature. Memory deficit in passive avoidance task after bulbectomy is frequently detected (Table 2).
In spatial learning assessed in MWM, OBX animals displayed significantly longer escape latencies when compared to sham-operated controls regardless of treatment applied. Both groups improved with each subsequent day; however, the rate of acquisition was faster in control group (Fig. 6). The results are in agreement with findings from other studies assessing cognitive functions after bulbectomy (Kelly et al. 1997; Song and Leonard 2005). Nevertheless, several studies reported no change in acquisition between control and OBX groups (Table 2). But, there are some evidence that deficit in MWM is transient and with prolonged time from surgery it spontaneously recovers (van Rijzingen et al. 1995).
The most intriguing results were obtained from active place avoidance in CM. The OBX phenotype was again significantly distinct from controls, but this time controls exhibited cognitive deficits. Cognitive parameters, number of entrances into the forbidden sector and the maximum time avoided, were assessed in the last fourth session. Both parameters were affected by the treatment administered. Prior rapamycin administration suppressed the effect of ketamine, leading to impaired cognitive coordination, memory, and learning expressed in terms of increased number of entrances (Fig. 8a) and decreased maximum time outside the forbidden sector (Fig. 8b) exhibited by ketamine + rapamycin-treated OBX rats. Their level of performance approximated that of sham controls. Number of shocks received correlates with the number of entrances; however, it illustrates well that sham animals lacked the escape reaction. Between-session decline in number of shocks received by sham controls was not as prominent as in OBX rats (Fig. 7b). On the other side, the OBX animals exhibited increased sensitivity to shocks even during the first session (Fig. 7a) and it intensified with subsequent sessions (Fig. 7b). Ketamine-treated OBX animals reacted to the shocks to a greater extent than all other experimental groups in first session (Fig. 7a). In the last session, prior rapamycin administration to ketamine-treated OBX resulted in lack of escape reaction (Fig. 7b). To the best of our knowledge, there are no data available about bulbectomy experiments conducted in CM test to compare it with our results.
Afore-mentioned results indicate that OBX animals can adjust their behavior to increasingly aversive environments. The number of shocks received during the first session had opposing impact on control and experimental groups. OBX rats learned to avoid the sector more effectively. The sham-operated controls resigned to solving the task, and they let themselves to be dragged passively to the forbidden sector. In some, it even resulted in behavior resembling learned helplessness, marked not only by cognitive deficits but also by shorter distance walked (Fig. 8c). The active place avoidance in CM is very demanding and challenging task which requires constant attention. The design of the experiment was very stressful for the animals that underwent two 20-min sessions a day, 2 days in a row. For successful solving of stressful cognitive tasks, the amygdala plays a crucial role and may account for the differences between cognitive tests.
The amygdala is crucial for mediating behavioral response to stress (Regev et al. 2012) and therefore influences learning under stressful conditions (Kim et al. 2001). Although the amygdala-lesioned animals showed deteriorated performances in PA, they executed spatial learning tasks as well as the control animals (Takashina et al. 1995). It was demonstrated that stress and glucocorticoids cause changes in learning strategy. Stress impaired hippocampal-dependent learning and led to the switch from spatial learning to the stimulus–response learning. Amygdala mediates stress effects on hippocampal LTP and learning, resulting in impaired performance in the MWM (Kim et al. 2001). These findings are in accordance with our results obtained from MWM. Hyperactive amygdala negatively affects spatial learning. In line with this reasoning, the performance of OBX rats in PA test should not be affected. However, the results from passive avoidance might not express cognitive deficits solely (Bizot and Thiébot 1996). The decreased latency displayed by saline-treated OBX rats may reflect lack of impulse control (Kamei et al. 2007) and/or defensive behavior (Stock et al. 2001) that were observed in OBX rats.
CM is highly hippocampal-dependent spatial test requiring hippocampal integrity since even unilateral inactivation leads to deficits in cognitive functions (Cimadevilla et al. 2001). Therefore, the amygdala hyperexcitability coupled with extremely stressful task should deteriorate performance in CM in OBX animals. During episode of stress, corticotropin-releasing factor (CRF) is released into the amygdala where activation of local CRF receptor-containing neurons results in stress-induced alternations in behavior. Stress-induced activation of basolateral amygdala by CRF could shift the balance between glutamate-mediated excitation and GABA-mediated inhibition toward greater excitability and override the effect of prefrontal inhibition. The ability to cope with the aversive stimulus is reduced (Shekhar et al. 2005). Habituation to repeated stress exposure is an adaptive response, wherein repeated exposure reduces hypothalamic–pituitary–adrenal (HPA) axis activation. Stress of sufficient intensity may be able to block the habituation process and lead to maladaptive consequences (Herman 2013). This might account for cognitive impairment of sham controls in active place avoidance task. OBX phenotype is marked by disinhibited amygdala. Therefore, excessive exposure to severe stress might blunt the effect of CRF release on already hyperactivated amygdala and consequently on the behavior in OBX animals. Complementary explanation is that the arena-bound olfactory stimuli, owing to bulb ablation, were not perceived by OBX rats and the absence of conflicting spatial representations partly rescued the cognition.
In our previous study, the effect of elevated levels of CRF and corticosterone on cognitive functions in rats was studied in our laboratory (Rezacova et al. 2011). Rats were exposed to elevated CRF levels applied by i.c.v. osmotic mini-pump for a period of 28 days. This manipulation resulted in increased release of corticosterone. Activation of glucocorticoid receptors by corticosterone constitutes the negative feedback loop on the secretion of CRF from hypothalamus. However, after prolonged stress exposure, hypothalamus becomes insensitive to high levels of glucocorticoids (GCs), resulting in feedback resistance. The dysregulation of the HPA axis results in impaired HPA axis habituation, subsequently leading to cognitive impairment. Elevated levels of CRF and GC resulted in permanent cognitive deficit in CM, while the performance in MWM assessing working memory was not affected (Rezacova et al. 2011). CRF regulates adaptive mechanisms during stress response, but its high levels lead to anxiety (Heinrichs and Koob 2004). These previous results are in accordance with results obtained from the present study and support the hypothesis that impairment in cognitive coordination displayed by sham controls may be caused by stress-induced activation of amygdala and impaired stress response. However, we did not evaluate any physiological marker of stress; therefore, we made assumption based solely on the nature of and phenomenology of the task.
MWM and CM spatial tests differ in their complexity, severity of stressor applied, and spatiotemporal design. The mild and severe stresses had opposite effects on control and OBX animals. In addition, the distinct features of the spatial tasks used might explain these discrepancies. MWM requires very precise navigation since the hidden platform constitutes less than 8 % of MWM surface. On the other hand, to-be-avoided sector in CM makes more than 16 % of the maze surface. OBX rats conducted to MWM were losing motivation to search the pool if they were not to find the platform within the first few seconds, marked by gradually decreasing velocity and shifting to random search of the pool (personal observation). In CM, passive strategy would result in excessive number of shocks received, and therefore, it might increase motivation to avoid the sector. The time for acquisition of the task may play a role, too. In MWM, the rats spent in the maze the maximum of 32 min (often a lot less) spread between 4 days. In CM, the rats underwent two 20-min sessions a day. During the first session in CM, no difference in cognitive parameters was found (data not shown) between two phenotypes tested contrary to difference in number of shocks received, indicating the escape deficits in control animals (Fig. 7a, b). It suggests that if the aversive enough stressor is applied to OBX animals for extended period of time, the OBX animals are capable to cope with the task. On the other hand, severity of stressor hindered control animals from proper information processing required for task solving. However, the resistance of OBX rats to severe stress experienced during CM testing remains to be investigated. The feedback control of HPA axis activity by prelimbic region of medial prefrontal cortex (mPFC) (Radley et al. 2006) via GC-mediated inhibition (Akana et al. 2001), together with its crucial role in cognitive functions, (Granon and Poucet 2000) might play a role. With numerous connections between mPFC and amygdala (Vertes 2004), amygdala is recruited in stress response pathway (Akana et al. 2001). In the present study, we did not examine any molecular changes in amygdala to support our conclusions. Analysis of molecular changes in amygdala will be the subject of the future study.
Treatment effects
It was suggested that activation of mTOR signaling pathway promotes therapeutic effect of rapid-acting antidepressants (Dutta et al. 2015; Krystal et al. 2013). Assumption was based on the discovery that inhibition of this pathway diminishes the antidepressant effect of ketamine in behavioral tasks such as novelty-suppressed feeding (Li et al. 2011), sucrose consumption test (Li et al. 2011), and reverses ketamine-induced dendritic outgrowth and synaptogenesis (Li et al. 2010, 2011). The effect of rapamycin-induced inhibition of protein synthesis is observed 24 h but not 1 h after its application (Iijima et al. 2012). Therefore, rapamycin has profound effect on long-term memory formation and inhibits fear/spatial memory consolidation and retrieval, leaving acquisition unaffected (Blundell et al. 2008; Deli et al. 2012; Fifield et al. 2015).
Experimental testing started 24 h after drug application. With increasing severity of stressors applied in the tests, the treatment effect was becoming more significant. In OF, the treatment did not affect the performance. In EPM, ketamine showed antidepressant and anxiolytic properties when applied to OBX rats; ketamine normalized the distance walked and increased the time spent in open arms. Pretreatment by rapamycin eliminated the anxiolytic effect of ketamine in EPM. In cognitive tests, the ketamine treatment had positive effect in PA and CM tests, whereas in MWM, no effect of treatment was detected. This inconsistency may arise from task-specific demands, amygdala’s hyperactivity, and from spatiotemporal design (as described above).
However, it should be noted that OBX animals treated with saline + rapamycin and ketamine + rapamycin in PA achieved the same results as ketamine-injected OBX rats. The treatment in passive avoidance might have suppressed the impulsivity and enhanced defensive behavior. In CM, ketamine + rapamycin administration led to cognitive impairment not observed in saline-, ketamine-, or saline + rapamycin-treated OBX rats. The performance of ketamine + rapamycin-injected OBX animals approached that of controls. It suggests that ketamine + rapamycin application resulted in impaired stress response manifested by cognitive deficits in CM.
Rapamycin application exhibits task specificity, and in some tasks, it eliminates ketamine antidepressant effect, while in others, it does not. It appears that rapamycin affects the anxiety by itself. Indeed, Hadamitzky et al. showed that systemic rapamycin injection enhanced electrical activity in amygdala accompanied by increased anxiety-like behavior in EPM (Hadamitzky et al. 2014). Prenatal rapamycin administration increased anxiety-like behavior during postnatal development and adulthood (Tsai et al. 2013). This may account for avoidance of open arms in EPM and avoidance of shocks in saline + rapamycin-treated OBX animals. Balance between mTOR inhibition and activation is essential to maintain neuronal health and function in order to adequately respond to stress challenge (Polman et al. 2012). In CM, co-application of ketamine, that has anxiolytic effect and enhances mTOR activity, together with rapamycin, which has diametrically opposite effect on anxiety and mTOR pathway, may distort this delicate balance and result in impaired stress response. High-intensity stress may suppress mTOR so that it cannot further stimulate some stress responses (Aramburu et al. 2014) and it may be manifested as learned helplessness-like behavior seen in sham controls and ketamine + rapamycine-treated OBX rats.
The treatment applied had minimum effect on performance of sham controls. Even more surprising is the finding that treatment had no effect upon mTOR level in sham animals. It seems as if ketamine influenced mTOR level preferably in OBX group. From the results of EPM test, it seems that both phenotypes (OBX and sham) differ in baseline lever of anxiety—saline-treated sham controls exhibiting higher anxiety scores than saline-treated OBX rats. The drug application therefore was more efficient in phenotype with lowered anxiety level. Increased anxiety may negatively influence performance under stressful condition as in CM test. The reason for increased anxiety in sham controls needs to be thoroughly investigated.
The depression and anxiety disorders share some common features beside treatment medication (SSRI, tricyclics, and MAOIs for the treatment of both diseases). Several studies pointed to the relationship between anxiety and greater amygdala reactivity, indicative of its defective function in anxiety disorders (Brühl et al. 2011; Hyde et al. 2011; Shah et al. 2009). Anxiolytics enhancing GABAergic transmission (Sanders and Shekhar 1995; Nuss 2015) or/and reducing NMDAR-mediated glutamate transmission (Sajdyk and Shekhar 1997) influence excitability of amygdala and restore its inhibitory tone and normal activity (Paulus et al. 2005). Increased anxiety-like behaviors may play a key role in onset of affective disorders. Most behavioral tests for assessment of antidepressant and anxiolytic properties of various compounds use some kinds of stressor (open spaces, high illumination, shocks, cold water, social defeat, etc.). If animals are capable of coping with stressor-induced anxiety, they probably do better in other tasks as well, e.g., cognitive tests. This prompts a question as to what extent is antidepressant effect of ketamine attained by its anxiolytic properties. Can anxiolytic and antidepressant effects of ketamine be separated from each other? Or can administration of anxiogenic drugs suppress the effect of antidepressant treatment? Of course, brain circuits and role of neurotransmitters involved in regulation of anxiety are much more complex and not yet fully understood.
BDNF
The BDNF level in the prefrontal cortex of OBX animals was significantly reduced when compared to sham-operated controls (Fig. 9a). This finding is in agreement with our previous results showing substantial difference between OBX groups and sham controls in behavioral tasks assessing executive functions relaying on prefrontal cortex. Interestingly, no difference in hippocampal BDNF content between experimental and control groups was detected (Fig. 9b). It suggests that the difference between two phenotypes in behavioral tests was at least partly associated with imbalance of BDNF in prefrontal cortex. However, Hellweg et al. reported an increase in BDNF level in hippocampus and frontal cortex after bulbectomy in mice (Hellweg et al. 2007). Possible explanation for this controversy may lay in differences in experimental design. In the present study, the rats underwent several stressful behavioral tasks, while in Hellweg’s study, mice were subjected to open field before BDNF analysis. It was documented that increased level of GC down-regulates BDNF expression (Dwivedi et al. 2006) and it hints at differential impact of behavioral procedures on BDNF levels between two studies. Lack of treatment effect on BDNF level was not surprising since BDNF level was analyzed a week after treatment administration when the effect of a single i.p. application of ketamine is very unlikely. Therefore, we may not draw any conclusions about ketamine effect on BDNF expression.
In animal models of stress, it was repeatedly shown that acute and chronic stresses decrease the expression of BDNF in hippocampus (Roceri et al. 2002; Grønli et al. 2006). In clinical and postmortem studies, BDNF levels were found to be lower in depressed patients than healthy volunteers in hippocampus and PFC (Brunoni et al. 2008; Yu and Chen 2011). Chronic treatment with antidepressant as well as application of ketamine increase level of BDNF in the brain (Garcia et al. 2008a; Autry et al. 2011; Yang et al. 2013). However, the BDNF knockout did not induce depressive-like behavior in male mice (Groves 2007; Monteggia et al. 2007), and few studies reported no change in BDNF level in depressed patients (Fernandes et al. 2009; Gustafsson et al. 2009). Similarly, ketamine’s antidepressant action was not always positively correlated with increased BDNF levels (Garcia et al. 2008b; Machado-Vieira et al. 2009; Rybakowski et al. 2013). Even if not being an etiological factor for onset of depression, imbalance in BDNF expression is co-existing circumstance accompanying the illness.
mTOR
OBX animals were more responsive to the treatment than sham controls; their peripheral level of phosphorylated mTOR was significantly elevated (Fig. 10). The difference between OBX and sham animals in mTOR level was not detected before drug administration (0 min, data not shown), indicating that bulbectomy resulted in increased susceptibility to treatment, outcome corroborated by behavioral analyses. Ketamine administration led to increases in mTOR level in OBX animals. This ketamine-induced activation of mTOR signaling pathway was reported previously in preclinical (Yang et al. 2013) and clinical studies (Denk et al. 2011).
It is rather surprising that ketamine + rapamycin treatment did not suppress the rise in mTOR level (Fig. 10). However, its co-application resulted in impaired behavior in some behavioral tests used—especially those where animals were exposed to severe stress. The stress might play a pivotal role in regulation of mTOR signaling pathway and in energy metabolism, balancing catabolic and anabolic cell processes. Indeed, alternations in mTOR signaling were observed after chronic stress. Chronic exposure to stress decreases phosphorylation level of mTOR in amygdala (Chandran et al. 2013) and ERK in frontal cortex and hippocampus of rats (First et al. 2011). Postmortem studies revealed a significant reduction in mTOR protein expression (Jernigan et al. 2011) and altered kinase activity of Akt (Karege et al. 2007; Dwivedi et al. 2010) in depressive patients. The imbalance in on-off switch to cell metabolism induced by ketamine + rapamycin application might have been intensified by stress encountered during behavioral testing resulting in cognitive deficits. It prompts a question if sole dysfunction of mTOR pathway is responsible for manifestation of depressive symptoms.
Conclusions
The olfactory bulbectomy induced changes in phenotype, which corresponded to depressive-like behavior. OBX led to increased horizontal and vertical activities and decreased anxiety when assessed in OF and EPM. The severity of stress applied in cognitive tests had differential effects upon OBX animals and sham controls. Neuronal mechanisms responsible for this dichotomy remain to be elucidated. The activation and/or inhibition of mTOR pathway by ketamine and rapamycin, respectively, did not anticipate effect upon behavior of animals. It suggests that ketamine antidepressant effect is not exclusively mediated by this signaling pathway and/or that dysfunction of this pathway is not the only causal factor for manifestation of depressive symptoms. The present study has shown the depression as a complex phenomenon affecting a whole variety of behaviors and neural structures, indicating that there probably is not one single mechanism and cause accountable for the onset and progression of illness.
References
Abdallah CG, Sanacora G, Duman RS, Krystal JH (2015) Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66:509–523
Akana SF, Chu A, Soriano L, Dallman MF (2001) Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinol 13(7):625–637
Aleksandrova IY, Kuvichkin VV, Kashparov IA, Medvinskaya NI, Nesterova IV, Lunin SM, Samokhin AN, Bobkova NV (2004) Increased level of beta-amyloid in the brain of bulbectomized mice. Biochemistry (Mosc) 69(2):176–180
Aramburu J, Ortells MC, Tejedor S, Buxadé M, López-Rodríguez C (2014) Transcriptional regulation of the stress response by mTOR. Sci Signal 7(332):re2
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64(2):238–258
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 475(7354):91–95
Balcells-Olivero M, Vezina P (1997) Effects of naltrexone on amphetamine-induced locomotion and rearing: acute and repeated injections. Psychopharmacology (Berlin) 131(3):230–238
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354
Bizot JC, Thiébot MH (1996) Impulsivity as a confounding factor in certain animal tests of cognitive function. Brain Res Cogn Brain Res 3(3–4):243–250
Blundell J, Kouser M, Powell CM (2008) Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem 90(1):28–35
Bobkova NV, Medvinskaya NI, Kamynina AV, Aleksandrova IY, Nesterova IV, Samokhin AN, Koroev DO, Filatova MP, Nekrasov PV, Abramov AY, Leonov SV, Volpina OM (2014) Immunization with either prion protein fragment 95–123 or the fragment-specific antibodies rescue memory loss and neurodegenerative phenotype of neurons in olfactory bulbectomized mice. Neurobiol Learn Mem 107:50–64
Borre Y, Sir V, de Kivit S, Westphal KG, Olivier B, Oosting RS (2012a) Minocycline restores spatial but not fear memory in olfactory bulbectomized rats. Eur J Pharmacol 697(1–3):59–64
Borre Y, Bosman E, Lemstra S, Westphal KG, Olivier B, Oosting RS (2012b) Memantine partly rescues behavioral and cognitive deficits in an animal model of neurodegeneration. Neuropharmacology 62(5–6):2010–2017
Brühl AB, Rufer M, Delsignore A, Kaffenberger T, Jäncke L, Herwig U (2011) Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res 1378:72–83
Brunoni AR, Lopes M, Fregni F (2008) A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 11(8):1169–1180
Carlini VP, Machado DG, Buteler F, Ghersi M, Ponzio MF, Martini AC, Schiöth HB, de Cuneo MF, Rodrigues AL, de Barioglio SR (2012) Acute ghrelin administration reverses depressive-like behavior induced by bilateral olfactory bulbectomy in mice. Peptides 35(2):160–165
Carobrez AP, Bertoglio LJ (2005) Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29(8):1193–1205
Chandran A, Iyo AH, Jernigan CS, Legutko B, Austin MC, Karolewicz B (2013) Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog Neuropsychopharmacol Biol Psychiatry 40:240–245
Cimadevilla JM, Wesierska M, Fenton AA, Bures J (2001) Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociation of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci U S A 98(6):3531–3536
Dawson GR, Tricklebank MD (1995) Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci 16(2):33–36
Deli A, Schipany K, Rosner M, Höger H, Pollak A, Li L, Hengstschläger M, Lubec G (2012) Blocking mTORC1 activity by rapamycin leads to impairment of spatial memory retrieval but not acquisition in C57BL/6J mice. Behav Brain Res 229(2):320–324
Denk MC, Rewerts C, Holsboer F, Erhardt-Lehmann A, Turck CW (2011) Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am J Psychiatry 168(7):751–752
DeRubeis RJ, Siegle GJ, Hollon SD (2008) Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci 9(10):788–796
Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67(8):793–802
Douma TN, Borre Y, Hendriksen H, Olivier B, Oosting RS (2011) Simvastatin improves learning and memory in control but not in olfactory bulbectomized rats. Psychopharmacology (Berlin) 216(4):537–544
Drevets WC (1999) Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci 877:614–637
Duman RS (2014) Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci 16(1):11–27
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62(1):35–41
Dutta A, McKie S, Deakin JF (2015) Ketamine and other potential glutamate antidepressants. Psychiatry Res 225(1–2):1–13
Dwivedi Y, Rizavi HS, Pandey GN (2006) Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience 139(3):1017–1029
Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN (2010) Modulation in activation and expression of phosphatase and tensin homolog on chromosome ten, Akt1, and 3-phosphoinositide-dependent kinase 1: further evidence demonstrating altered phosphoinositide 3-kinase signaling in postmortem brain of suicide subjects. Biol Psychiatry 67(11):1017–1025
Fernandes BS, Gama CS, Kauer-Sant’Anna M, Lobato MI, Belmonte-de-Abreu P, Kapczinski F (2009) Serum brain-derived neurotrophic factor in bipolar and unipolar depression: a potential adjunctive tool for differential diagnosis. J Psychiatr Res 43(15):1200–1204
Fifield K, Hebert M, Williams K, Linehan V, Whiteman JD, Mac Callum P, Blundell J (2015) Time-dependent effects of rapamycin on consolidation of predator stress-induced hyperarousal. Behav Brain Res 286:104–111
First M, Gil-Ad I, Taler M, Tarasenko I, Novak N, Weizman A (2011) The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and ERK expression. J Mol Neurosci 45(2):246–255
Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008a) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32(1):140–144
Garcia LS, Comim CM, Valvassori SS, Réus GZ, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008b) Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic Clin Pharmacol Toxicol 103(6):502–506
Gironi Carnevale UA, Vitullo E, Sadile AG (1990) Post-trial NMDA receptor allosteric blockade differentially influences habituation of behavioral responses to novelty in the rat. Behav Brain Res 39(2):187–195
Granon S, Poucet B (2000) Involvement of the rat prefrontal cortex in cognitive functions: a central role for the prelimbic area. Psychobiology 28(2):229–237
Grønli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, Ursin R, Portas CM (2006) Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav 85(4):842–849
Groves JO (2007) Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry 12(12):1079–1088
Gustafsson G, Lira CM, Johansson J, Wisén A, Wohlfart B, Ekman R, Westrin A (2009) The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Res 169(3):244–248
Hadamitzky M, Herring A, Keyvani K, Doenlen R, Krügel U, Bösche K, Orlowski K, Engler H, Schedlowski M (2014) Acute systemic rapamycin induces neurobehavioral alterations in rats. Behav Brain Res 273:16–22
Heinrichs SC, Koob GF (2004) Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311(2):427–440
Hellweg R, Zueger M, Fink K, Hörtnagl H, Gass P (2007) Olfactory bulbectomy in mice leads to increased BDNF levels and decreased serotonin turnover in depression-related brain areas. Neurobiol Dis 25(1):1–7
Hendriksen H, Meulendijks D, Douma TN, Bink DI, Breuer ME, Westphal KG, Olivier B, Oosting RS (2012) Environmental enrichment has antidepressant-like action without improving learning and memory deficits in olfactory bulbectomized rats. Neuropharmacology 62(1):270–277
Hendriksen H, Korte SM, Olivier B, Oosting RS (2015) The olfactory bulbectomy model in mice and rat: one story or two tails? Eur J Pharmacol 753:105–113
Herman JP (2013) Neural control of chronic stress adaptation. Front Behav Neurosci 7:61
Hijazi Y, Bodonian C, Bolon M, Salord F, Boulieu R (2003) Pharmacokinetics and haemodynamics of ketamine in intensive care patients with brain or spinal cord injury. Br J Anaesth 90(2):155–160
Ho YJ, Chang YC, Liu TM, Tai MY, Wong CS, Tsai YF (2000) Striatal glutamate release during novelty exposure-induced hyperactivity in olfactory bulbectomized rats. Neurosci Lett 287(2):117–120
Hogg S (1996) A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 54(1):21–30
Hozumi S, Nakagawasai O, Tan-No K, Niijima F, Yamadera F, Murata A, Arai Y, Yasuhara H, Tadano T (2003) Characteristics of changes in cholinergic function and impairment of learning and memory-related behavior induced by olfactory bulbectomy. Behav Brain Res 138(1):9–15
Hyde LW, Gorka A, Manuck SB, Hariri AR (2011) Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia 49(4):651–656
Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA Jr (2012) Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37(6):1526–1533
Iijima M, Fukumoto K, Chaki S (2012) Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behav Brain Res 235(2):287–292
Ivanova M, Belcheva S, Belcheva I, Stoyanov Z, Tashev R (2014) Modulatory effect of VIP injected into hippocampal CA1 area on anxiety in olfactory bulbectomized rats. Acta Neurobiol Exp (Wars) 74(3):317–327
Jaako-Movits K, Zharkovsky A (2005) Impaired fear memory and decreased hippocampal neurogenesis following olfactory bulbectomy in rats. Eur J Neurosci 22(11):2871–2878
Jarosik J, Legutko B, Unsicker K, von Bohlen Und Halbach O (2007) Antidepressant-mediated reversal of abnormal behavior and neurodegeneration in mice following olfactory bulbectomy. Exp Neurol 204(1):20–28
Jarosik J, Legutko B, Werner S, Unsicker K, von Bohlen Und Halbach O (2011) Roles of exogenous and endogenous FGF-2 in animal models of depression. Restor Neurol Neurosci 29(3):153–165
Jastrzębska-Więsek M, Siwek A, Partyka A, Szewczyk B, Sowa-Kućma M, Wasik A, Kołaczkowski M, Wesołowska A (2015) Antidepressant-like activity of EMD 386088, a 5-HT6 receptor partial agonist, following systemic acute and chronic administration to rats. Naunyn Schmiedebergs Arch Pharmacol 388(10):1079–1088
Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B (2011) The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35(7):1774–1779
Jindal A, Mahesh R, Bhatt S (2015) Type 4 phosphodiesterase enzyme inhibitor, rolipram rescues behavioral deficits in olfactory bulbectomy models of depression: involvement of hypothalamic-pituitary-adrenal axis, cAMP signaling aspects and antioxidant defense system. Pharmacol Biochem Behav 132:20–32
Kamei J, Hirose N, Oka T, Miyata S, Saitoh A, Yamada M (2007) Effects of methylphenidate on the hyperemotional behavior in olfactory bulbectomized mice by using the hole-board test. J Pharmacol Sci 103(2):175–180
Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, Malafosse A (2007) Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry 61(2):240–245
Kelly JP, Wrynn AS, Leonard BE (1997) The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther 74(3):299–316
Kim JJ, Lee HJ, Han JS, Packard MG (2001) Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci 21(14):5222–5228
Krystal JH, Sanacora G, Duman RS (2013) Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73(12):1133–1141
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994):959–964
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69(8):754–761
Liu RJ, Aghajanian GK (2008) Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 105(1):359–364
Liu ZP, Song C, Wang M, He Y, Xu XB, Pan HQ, Chen WB, Peng WJ, Pan BX (2014) Chronic stress impairs GABAergic control of amygdala through suppressing the tonic GABAA receptor currents. Mol Brain 7:32
Machado DG, Cunha MP, Neis VB, Balen GO, Colla A, Grando J, Brocardo PS, Bettio LE, Capra JC, Rodrigues AL (2012a) Fluoxetine reverses depressive-like behaviors and increases hippocampal acetylcholinesterase activity induced by olfactory bulbectomy. Pharmacol Biochem Behav 103(2):220–229
Machado DG, Cunha MP, Neis VB, Balen GO, Colla AR, Grando J, Brocardo PS, Bettio LE, Dalmarco JB, Rial D, Prediger RD, Pizzolatti MG, Rodrigues AL (2012b) Rosmarinus officinalis L. hydroalcoholic extract, similar to fluoxetine, reverses depressive-like behavior without altering learning deficit in olfactory bulbectomized mice. J Ethnopharmacol 143(1):158–169
Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA Jr (2009) Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry 70(12):1662–1666
Mar A, Spreekmeester E, Rochford J (2000) Antidepressants preferentially enhance habituation to novelty in the olfactory bulbectomized rat. Psychopharmacology (Berlin) 150(1):52–60
Marsden WN (2013) Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry 43:168–184
McGrath C, Norman TR (1998) The effect of venlafaxine treatment on the behavioural and neurochemical changes in the olfactory bulbectomised rat. Psychopharmacology (Berlin) 136(4):394–401
McGrath C, Norman TR (1999) (+)-S-20499—a potential antidepressant? A behavioural and neurochemical investigation in the olfactory bulbectomised rat. Eur Neuropsychopharmacol 9(1–2):21–27
McNish KA, Davis M (1997) Olfactory bulbectomy enhances sensitization of the acoustic startle reflex produced by acute or repeated stress. Behav Neurosci 111(1):80–91
Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ (2007) Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry 61(2):187–197
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60
Mucignat-Caretta C, Bondí M, Caretta A (2004) Animal models of depression: olfactory lesions affect amygdala, subventricular zone, and aggression. Neurobiol Dis 16(2):386–395
Mucignat-Caretta C, Bondí M, Caretta A (2006) Time course of alterations after olfactory bulbectomy in mice. Physiol Behav 89(5):637–643
Muir KW, Lees KR (1995) Clinical experience with excitatory amino acid antagonist drugs. Stroke 26:503–513
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ (2013a) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170(10):1134–1142
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013b) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74(4):250–256
Nakagawasai O, Hozumi S, Tan-No K, Niijima F, Arai Y, Yasuhara H, Tadano T (2003) Immunohistochemical fluorescence intensity reduction of brain somatostatin in the impairment of learning and memory-related behaviour induced by olfactory bulbectomy. Behav Brain Res 142(1–2):63–67
Nowak G, Szewczyk B, Wieronska JM, Branski P, Palucha A, Pilc A, Sadlik K, Piekoszewski W (2003) Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res Bull 61(2):159–164
Nuss P (2015) Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat 11:165–175
Opal MD, Klenotich SC, Morais M, Bessa J, Winkle J, Doukas D, Kay LJ, Sousa N, Dulawa SM (2014) Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol Psychiatry 19(10):1106–1114
Ostrovskaya RU, Gruden MA, Bobkova NA, Sewell RD, Gudasheva TA, Samokhin AN, Seredinin SB, Noppe W, Sherstnev VV, Morozova-Roche LA (2007) The nootropic and neuroprotective proline-containing dipeptide noopept restores spatial memory and increases immunoreactivity to amyloid in an Alzheimer’s disease model. J Psychopharmacol 21(6):611–619
Pandey DK, Rajkumar R, Mahesh R, Radha R (2008) Depressant-like effects of parthenolide in a rodent behavioural antidepressant test battery. J Pharm Pharmacol 60(12):1643–1650
Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB (2005) Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry 62(3):282–288
Phillips ML, Drevets WC, Rauch SL, Lane R (2003) Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry 54(5):515–528
Pochwat B, Sowa-Kucma M, Kotarska K, Misztak P, Nowak G, Szewczyk B (2015) Antidepressant-like activity of magnesium in the olfactory bulbectomy model is associated with the AMPA/BDNF pathway. Psychopharmacology (Berlin) 232(2):355–367
Polman JA, Hunter RG, Speksnijder N, van den Oever JM, Korobko OB, McEwen BS, de Kloet ER, Datson NA (2012) Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology 153(9):4317–4327
Primeaux SD, Holmes PV (1999) Role of aversively motivated behavior in the olfactory bulbectomy syndrome. Physiol Behav 67(1):41–47
Pudell C, Vicente BA, Delattre AM, Carabelli B, Mori MA, Suchecki D, Machado RB, Zanata SM, Visentainer JV, de Oliveira Santos Junior O, Lima MM, Ferraz AC (2014) Fish oil improves anxiety-like, depressive-like and cognitive behaviors in olfactory bulbectomised rats. Eur J Neurosci 39(2):266–274
Radley JJ, Arias CM, Sawchenko PE (2006) Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci 26(50):12967–12976
Rajkumar R, Pandey DK, Mahesh R, Radha R (2009) 1-(m-Chlorophenyl)piperazine induces depressogenic-like behaviour in rodents by stimulating the neuronal 5-HT(2A) receptors: proposal of a modified rodent antidepressant assay. Eur J Pharmacol 608(1–3):32–41
Regev L, Tsoory M, Gil S, Chen A (2012) Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry 71(4):317–326
Rezacova L, Svoboda J, Stuchlik A, Vales K (2011) Differential effects of stable elevated levels of corticotropin-releasing hormone and systemic corticosterone on various types of rat learning. Neuro Endocrinol Lett 32(1):64–76
Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA (2002) Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry 7(6):609–616
Rybakowski JK, Permoda-Osip A, Skibinska M, Adamski R, Bartkowska-Sniatkowska A (2013) Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved? Hum Psychopharmacol 28(1):87–90
Sajdyk TJ, Shekhar A (1997) Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res 764(1–2):262–264
Sanders SK, Shekhar A (1995) Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav 52(4):701–706
Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL (2009) Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci 34(4):296–302
Shekhar A, Truitt W, Rainnie D, Sajdyk T (2005) Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 8(4):209–219
Shibata S, Watanabe S (1994) Facilitatory effect of olfactory bulbectomy on 2-deoxyglucose uptake in rat amygdala slices. Brain Res 665(1):147–150
Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, Lim KO (2014) Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 155:123–129
Sieck MH, Baumbach HD (1974) Differential effects of peripheral and central anosmia producing techniques on spontaneous behavior patterns. Physiol Behav 13(3):407–425
Song C, Leonard BE (2005) The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29(4–5):627–647
Song C, Zhang XY, Manku M (2009) Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyl-eicosapentaenoate treatment. J Neurosci 29(1):14–22
Stepanichev M, Markov D, Pasikova N, Gulyaeva N (2016) Behavior and the cholinergic parameters in olfactory bulbectomized female rodents: difference between rats and mice. Behav Brain Res 297:5–14
Stock HS, Hand GA, Ford K, Wilson MA (2001) Changes in defensive behaviors following olfactory bulbectomy in male and female rats. Brain Res 903(1–2):242–246
Stuchlik A, Rehakova L, Telensky P, Vales K (2007) Morris water maze learning in Long-Evans rats is differentially affected by blockade of D1-like and D2-like dopamine receptors. Neurosci Lett 422:169–174
Stuchlík A, Petrásek T, Prokopová I, Holubová K, Hatalová H, Valeš K, Kubík S, Dockery C, Wesierska M (2013) Place avoidance tasks as tools in the behavioral neuroscience of learning and memory. Physiol Res 62(Suppl 1):S1–S19
Swiech L, Perycz M, Malik A, Jaworski J (2008) Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta 1784(1):116–132
Szapacs ME, Mathews TA, Tessarollo L, Lyons WE, Mamounas LA, Andrews AM (2004) Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Methods 140:81–92
Takashina K, Saito H, Nishiyama N (1995) Preferential impairment of avoidance performances in amygdala-lesioned mice. Jpn J Pharmacol 67(2):107–115
Thiel CM, Müller CP, Huston JP, Schwarting RK (1999) High versus low reactivity to a novel environment: behavioural, pharmacological and neurochemical assessments. Neuroscience 93(1):243–251
Tsai PT, Greene-Colozzi E, Goto J, Anderl S, Kwiatkowski DJ, Sahin M (2013) Prenatal rapamycin results in early and late behavioral abnormalities in wild-type C57BL/6 mice. Behav Genet 43(1):51–59
van der Stelt HM, Breuer ME, Olivier B, Westenberg HG (2005) Permanent deficits in serotonergic functioning of olfactory bulbectomized rats: an in vivo microdialysis study. Biol Psychiatry 57(9):1061–1067
van Rijzingen IM, Gispen WH, Spruijt BM (1995) Olfactory bulbectomy temporarily impairs Morris maze performance: an ACTH(4–9) analog accellerates return of function. Physiol Behav 58(1):147–152
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51(1):32–58
Volpina OM, Koroev DO, Volkova TD, Kamynina AV, Filatova MP, Zaporozhskaya YV, Samokhin AN, Aleksandrova IY, Bobkova NV (2015) A fragment of the receptor for advanced glycation end products restores the spatial memory of animals in a model of Alzheimer’s disease. Russ J Bioorg Chem 41(6):638–644
Wieronska JM, Szewczyk B, Branski P, Palucha A, Pilc A (2002) Antidepressant-like effect of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist in the olfactory bulbectomized rats. Amino Acids 23(1–3):213–216
Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ (2005) Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav 82(1):200–206
Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ (2013) Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci 118(1):3–8
Yoshihara Y, De Roo M, Muller D (2009) Dendritic spine formation and stabilization. Curr Opin Neurobiol 19(2):146–153
Yu H, Chen ZY (2011) The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin 32(1):3–11
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8):856–864
Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71(11):939–946
Zueger M, Urani A, Chourbaji S, Zacher C, Roche M, Harkin A, Gass P (2005) Olfactory bulbectomy in mice induces alterations in exploratory behavior. Neurosci Lett 374(2):142–146
Acknowledgments
This study was supported by grant IGAMZCR NT13403-4/2012 and institutional support RVO:67985823 and NIMH-CZ ED2.1.00/03.0078 and the European Regional Development Fund. We would like to thank Michaela Fialova for the technical assistance and Barbara Stuchlikova for the graphical assistance with the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were in accordance with Czech and European legislation regarding treatment of laboratory animals (Directive 86/609/EEC).
Rights and permissions
About this article
Cite this article
Holubova, K., Kleteckova, L., Skurlova, M. et al. Rapamycin blocks the antidepressant effect of ketamine in task-dependent manner. Psychopharmacology 233, 2077–2097 (2016). https://doi.org/10.1007/s00213-016-4256-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4256-3