Abstract
Rationale
Several recent studies have focused on glutamate modulating agents for symptoms relief in schizophrenia, especially negative symptoms which are resistant to conventional therapies.
Objectives
We aimed to assess the efficacy and tolerability of riluzole, an anti-glutamate agent with neuroprotective properties, as an adjunct to risperidone in improving negative symptoms of schizophrenia.
Methods
In this randomized double-blind placebo-controlled parallel-group study, 50 patients with chronic schizophrenia and a score of ≥20 on the negative subscale of positive and negative syndrome scale (PANSS) were enrolled in the active phase of their illness. Participants were equally randomized to receive riluzole (100 mg/day) or placebo in addition to risperidone (up to 6 mg/day) for 8 weeks. Participants were rated by PANSS every 2 weeks. The primary outcome of this study was the difference in the decrease of PANSS negative subscale score from baseline to the study endpoint between the two groups.
Results
By the study endpoint, riluzole-treated patients showed significantly greater improvement in the negative symptoms (P < 0.001) as well as the PANSS total and general psychopathology subscale scores (P = 0.001 and P < 0.001; respectively) compared to the placebo group. Treatment group was the only significant predictor of changes in negative symptom in this trial (β = −0.56, P < 0.001). No significant difference was observed between two groups in the frequency of side effects.
Conclusion
These preliminary findings suggest that riluzole may be a safe and effective medication for the treatment of negative symptoms in patients with chronic schizophrenia. Further research and replication of study findings is warranted.
Clinical trial registry name and registration number
Iranian registry of clinical trials www.irct.ir, IRCT201107281556N26
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Negative symptoms of schizophrenia include deficits in social and emotional functioning, blunted affect, and lack of spontaneity. These symptoms are resistant to current antipsychotic medications and become more prominent over time, hence having a greater contribution to poor quality of life and functional disability than positive symptoms in patients with schizophrenia (Fenton and McGlashan 1991). Negative schizophrenic symptoms can be categorized into primary negative symptoms that are due to the illness itself and secondary ones which result from positive, depressive, or extrapyramidal symptoms as well as medications effects (Murphy et al. 2006). Since no completely effective treatment for negative symptoms has been developed yet, many researchers are striving to find novel therapeutic agents based on underlying mechanistic defects in schizophrenia.
Various pathophysiological mechanisms have been proposed for schizophrenia including dopaminergic and glutamatergic models (Stone et al. 2007). Although the dopaminergic model of schizophrenia explains positive symptoms of the disease, it cannot provide a reasonable justification for negative symptoms (Crow 1981). Of note, current antipsychotics are developed based on the dopaminergic hypothesis and act primarily through blocking dopamine D2 receptors, a fact which may explain why currently available treatments are incapable of targeting the negative symptoms. On the other hand, the glutamatergic hypothesis of schizophrenia provides accountability for both positive and negative symptoms as well as neurocognitive deficits (Coyle 2006; Laruelle et al. 2005). Glutamate, the principal excitatory neurotransmitter in the brain, plays a key role in synaptic plasticity and higher cortical functions and several lines of evidence suggest glutamatergic system dysfunctions as an underlying psychopathologic mechanism in schizophrenia (Javitt 2012). Two main families of glutamate receptors are metabotropic and ionotropic receptors. The latter category includes N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainite receptors. It has been demonstrated that chronic blockade of NMDA receptors results in a hyperglutamatergic state via rebound increase in neurotransmitter release (Moghaddam et al. 1997) and decreases prefrontal cortical function which leads to symptoms similar to negative and cognitive symptoms seen in chronic stages of schizophrenia (Jentsch et al. 1997; Jentsch and Roth 1999). Altogether, these findings suggest that treatment strategies which target glutamatergic pathways and reduce its neurotransmission may be of benefit in the treatment of negative symptoms of schizophrenia.
Riluzole is a glutamate modulating agent with neuroprotective and anticonvulsant properties originally developed for treatment of amyotrophic lateral sclerosis (Miller et al. 2012). Riluzole enhances glutamate reuptake (Frizzo et al. 2004) and inhibits glutamate release from nerve cell terminals (Martin et al. 1993). It also interferes with postsynaptic effects of glutamate by noncompetitive blockade of glutamate receptors (Doble 1996; Du et al. 2007). Several clinical and preclinical studies support the beneficial effects in improving different neuropsychiatric disorders, particularly those with a hyperglutamatergic state as their possible underlying pathology (Zarate and Manji 2008). Encouraging evidences regarding the use of riluzole have been reported in treating mood disorders (Sanacora et al. 2007; Zarate et al. 2004; Zarate et al. 2005), generalized anxiety disorders (Mathew et al. 2005), obsessive–compulsive disorders (Coric et al. 2005; Grant et al. 2007), and autism spectrum disorders (Ghaleiha et al. 2013). Interestingly, it has been suggested that inhibiting glutamate release at presynaptic nerve terminals may be helpful for cognitive improvement of patients with schizophrenia (Moghaddam 2004). Lamotrigine, an anti-glutamate agent, has been reported to improve behavioral and cognitive deficits in both animal and human studies (Anand et al. 2000; Moghaddam and Adams 1998). An experimental study showed that administration of 10 mg/kg of riluzole in mice showed has the ability of depressing MK-810 and amphetamine-induced hyperlocomotion as pharmacological models of schizophrenia (Lourenco Da Silva et al. 2003).
Based on the existing evidence, we hypothesized that riluzole with its glutamate modulating properties and relative safe profile could be helpful in improving the negative symptoms of schizophrenia. To the best of our knowledge, no well-designed clinical trial has been published to date on the probable efficacy of riluzole in patients with schizophrenia, particularly on their negative symptoms. In the present study, we aimed to evaluate the efficacy and tolerability of riluzole as an adjunctive treatment to risperidone for reducing negative symptoms of patients with chronic schizophrenia.
Methods and materials
Trial design
This was a parallel-group, placebo-controlled, double-blind clinical trial in which patients were treated and followed for 8 weeks. The trial protocol was registered at the Iranian Clinical Trials Registry (IRCT201107281556N26; www.irct.ir), approved by the institutional review board (IRB) of Tehran University of Medical Sciences and performed in accordance with the Declaration of Helsinki and its subsequent revisions. After a complete description of study details, written informed consent was obtained from the eligible participant and/or the legal representative. Patients were informed of their right to withdraw from the project at any time without any negative effect on their relationship with health care providers.
Participants
In-patients of both genders aged 18–50 years with a DSM IV-TR diagnosis of schizophrenia were eligible to participate if they were in the active phase of the illness and had a minimum score of 60 on the positive and negative syndrome scale (PANSS) (Kay et al. 1987), a score of ≥20 on the PANSS negative subscale, and a minimum disease duration of 2 years (chronic schizophrenia). Patients were included only if they could satisfactorily comply with the trial requirements. Diagnosis was based on a structured clinical interview for DSM-IV-TR Axis I Disorders and was confirmed with chart review and senior physician interview.
Patients with significant depression, defined as a score ≥14 on the 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton 1960) or a score of ≥4 on depression item of PANSS, were excluded from the study because high degrees of depression could make the pure interpretation of negative symptoms changes unreliable. We also excluded patients with diagnosis of any other DSM-IV psychiatric disorder based on a structured diagnostic interview so we could attribute the outcomes only to the schizophrenic symptoms. Other exclusion criteria were serious somatic disorders, alcohol or substance (other than nicotine) dependence, mental retardation (intelligence quotient<70), inability to communicate, history of hypersensitivity to riluzole or risperidone, pregnancy, lactation, HIV infection, and hepatic or kidney disease. Women in reproductive age were included only if they were using reliable contraception. Due to hepatotoxic nature of riluzole, individuals who were receiving potential hepatotoxic medications were not allowed to take part in the study. Since we aimed to evaluate pharmacological effects of riluzole and risperidone in this study, patients were also excluded if they had received any oral antipsychotic drug during the last week, any depot antipsychotic medication during the last month, or electroconvulsive therapy during the last 2 weeks prior to their enrollment. We did not change or discontinue patients’ drugs before their entry. Instead, we selected the participants from patients who had discontinued their medication due to other reasons such as lack of supportive care or incompliance with their treatment. In order to decrease the risk of drug interactions and adverse events, participants were not allowed to use antidepressants, mood stabilizers, sedating antihistamines, or other antipsychotics during the course of this trial.
Study settings
This study was a multicenter clinical trial conducted from August 2011 to April 2013 at three academic hospitals: Roozbeh Hospital (Tehran University of Medical Sciences, Tehran, Iran), Razi Hospital (University of Social Welfare and Rehabilitation Sciences, Tehran, Iran), and Qods Hospital (Kurdistan University of Medical Sciences, Sanandaj, Iran). In four consecutive visits, patients were evaluated every 2 weeks after the baseline/screening session. There were no ethnical or regional restrictions for participation as the patients were referred from different regions of Iran to these three large referral hospitals.
Intervention
In addition to risperidone (Risperdal®, Janssen Pharmaceuticals) which was administered to all patients, one group received Riluzole (Rliutek®, Sanofi-Aventis) and the other group received placebo from the beginning of this study. Risperidone was started with a dose of 2 mg/day and then based on the clinical response, was increased weekly in increments of 2 mg to a maximum dose of 6 mg/day (2 mg tid). Riluzole starting dosage was 50 mg/day for the first week followed by 100 mg/day (50 mg bid) for the subsequent 7 weeks. Patients were not allowed to receive any behavior intervention therapy during the course of the trial.
Outcomes
The efficacy assessment measure used in this study was PANSS and thus each patient was rated at baseline/screening session and weeks 2, 4, 6, and 8. PANSS is a 30-item rating scale consisting of validated subscales to examine positive (seven items), negative (seven items) and general psychopathological (16 items) symptoms of schizophrenia. These three subscales are summed up in the PANSS total score (Kay et al. 1987). PANSS has been widely used for measuring the treatment efficacy and severity of symptoms in schizophrenia and has been applied in several studies in Iran (Akhondzadeh et al. 2011; Khodaie-Ardakani et al. 2013; Modabbernia et al. 2013; Noroozian et al. 2013; Rezaei et al. 2013). Four trained raters were responsible for rating the patients with an inter-reliablity of >90 % on PANSS total scores. The raters were previously invloved in several trials of schizophrenia and were experienced in implementing the PANSS. In order to evaluate the depressive symptoms, HDRS was also filled at baseline and the study endpoint. This scale contains 17 questions (measured either on five-point or three-point scales) which evaluate the severity of depression-related symptoms (Hamilton 1960). The primary outcome of this study was the difference in the decrease of PANSS negative subscale score from week 0 to week 8 between the two study groups. The difference between the two study arms on PANSS total and other subscales scores were considered as secondary outcome measures.
Safety
Participants and involved nurses were strongly encouraged to immediately inform the research team about any unexpected symptom or complaint during the study. A thorough physical examination was performed and vital signs were recorded at the screening session and each post-baseline visit. A complete blood count (CBC) was taken and serum aminotransferases were measured at baseline and every 4 weeks subsequently. In addition to each post-baseline visit, side effects were recorded 1 week after start of medication through open-ended questioning followed by a complete side effects checklist. The side effects checklist was a 25-item questionnaire covering a broad range of warning symptoms. Extrapyramidal symptoms rating scale (ESRS) (part one: parkinsonism, dystonia, dyskinesia; sum of 11 items) (Chouinard and Margolese 2005) was also administered at baseline and weeks 1, 2, 4, 6, and 8 in order to evaluate extrapyramidal symptoms. Behavior appraisal and side effects checklist were completed by independent raters. In case of encountering any side effect, an expert psychiatrist was responsible for making decisions regarding whether to continue treatment, decrease dosage, or discontinue the drugs.
Randomization
Patients were randomly and equally assigned to two groups (riluzole or placebo) in a 1:1 ratio by means of random allocation method. An independent person who was not involved elsewhere in the research project generated the randomization codes by Excel software. The assignments were kept in sequentially numbered, sealed, opaque envelopes and were opened sequentially only after participant details were written on the envelope. Aluminum foil inside the envelope rendered the envelope impermeable to intense light. Separate persons were responsible for rating and random allocation of the patients.
Blinding
Study medications were packed in identical containers and were dispensed by an investigational drug pharmacist. Placebo tablets and their ingredients were identical to riluzole tablets in shape, size, texture, color, taste, and odor. Participants, nurses, and the physicians who referred the patients as well as the research investigators and the raters were all blind to the treatment assignments.
Sample size and statistical methods
Based on previous trials, we assumed a final difference of 5 between the two groups on the PANSS negative subscale with a standard deviation of 5, a power of 90 %, a two-sided significance level of 5 %, and an attrition rate of 10 %. Therefore, a total sample size of 50 was calculated. All analyses were based on the intention-to-treat sample and were performed using the last observation carried forward procedure. The mean score change from baseline to the study endpoint on PANSS, HDRS, and ESRS were compared between two groups using independent sample T test. Cohen’s d effect sizes were determined by dividing the mean difference of the two groups at each time point by their pooled standard deviation. The effect of time × treatment interaction was assessed by general linear model repeated measures considering the treatment group (riluzole vs. placebo) as the between-subject factor and the study measurements as the within-subject variables (time). If Mauchly’s test of sphericity was significant, Greenhouse–Geisser correction for degrees of freedom was used. Multiple linear regression analysis was used to predict the change in PANSS negative subscale scores (as our primary outcome) by assigning change in PANSS positive subscale, HDRS, and ESRS scores as well as the treatment group. Continuous variables were described as mean (standard deviation, SD) and categorical variable in number (in percent). Mean differences were reported as mean difference (MD) (95 % confidence intervals, 95 % CI). IBM SPSS Statistic 20 (IBM Corporation) was used for data analysis and a P value of <0.05 was considered statistically significant.
Results
Participants
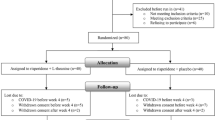
After screening for the eligibility criteria, 50 patients were recruited and a final number of 48 patients completed the trial (riluzole = 24, placebo = 24) (Fig. 1). Baseline characteristics of the participants as well as the baseline PANSS, HDRS, and ESRS scores were not significantly different between the two study groups (Table 1). Mean dose of risperidone administered throughout the study was 4.35 (0.69) and 4.41 (0.51) mg/day in the riluzole and the placebo groups, respectively.
Outcomes
PANSS
PANSS negative subscale
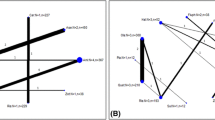
By the study endpoint, reduction of the PANSS negative subscale scores was significantly higher in the riluzole-treated patients than the placebo group [MD (95 % CI) = 5.72(3.17 to 8.26), t(48) = 4.52, P < 0.001] (Cohen’s d = 1.28, r = 0.53). The behavior of the two treatment groups was different across time as demonstrated by a significant effect for time × treatment interaction [F(1.95, 93.90) = 13.74, P < 0.001] (Fig. 2). When the PANSS negative subscale change was predicted by multiple linear regression analysis, it was found that the treatment group (riluzole vs. placebo) was the strongest and the only significant predictor of any changes in negative symptoms over the course of this trial (β = −0.56, t = −4.48, P < 0.001). Changes in the PANSS positive subscale (β = 0.10, t = 0.85, P = 0.39), HDRS (β = 0.02, t = 0.16, P = 0.87), and ESRS (β = −0.18, t = −1.48, P = 0.14) scores could not significantly predict the change in the PANSS negative subscale scores. Considering 50% reduction on negative symptoms score as response to treatment, number needed to treat (NNT) was 3.
PANSS positive subscale
Of the scores on PANSS, the positive subscale was not significantly different between the two groups at the end of the trial [MD (95 % CI) = 1.96 (−1.10 to 5.02), t(48) = 1.28, P = 0.20] (Cohen’s d = 0.36, r = 0.17). Repeated measure analysis demonstrated no significant effect for time × treatment interaction [F(2.16, 104.07) = 1.50, P = 0.22] showing that the behavior of the two groups was similar across time on this subscale (Fig. 3).
PANSS general psychopathology subscale
The riluzole group showed significantly greater improvement than the placebo group on PANSS general psychopathology subscale scores by week 8 [MD (95 % CI) = 7.72 (3.52 to 11.91), t(48) = 1.93, P = 0.001] (Cohen’s d = 1.04, r = 0.46). Behavior of the two groups was different across time as demonstrated by significant effect of time × treatment interaction [F(1.91, 91.85) = 9.83, P < 0.001] in repeated measure analysis (Fig. 4).
PANSS total score
At the study endpoint, patients in the Riluzole group experienced significantly greater improvement in the PANSS total scores than the placebo group [MD (95 % CI) = 15.28 (8.97 to 21.58), t(48) = 4.87, P < 0.001] (Cohen’s d = 1.37, r = 0.56). Repeated measure analysis showed significant effect for time × treatment interaction [F(1.82, 87.85) = 15.85, P < 0.001] as well (Fig. 5).
HDRS
There was no significant difference between the two groups in the HDRS score change from baseline to the study endpoint [MD (95 % CI) = −0.20 (−1.37 to 0.97), t(48) = −0.34, P = 0.73] (Cohen’s d = −0.09, r = −0.04). Repeated measure analysis did not show significant effect for time × treatment interaction [F(1.00, 48.00) = 0.11, P = 0.73].
Adverse events
No serious adverse event or death was reported in this trial. ESRS score changes were not significantly different between the two study groups [MD (95 % CI) = 1.24(−0.81 to 3.29), t(48) = 1.21, P = 0.23] (Cohen’s d = 0.34, r = 0.16) and the effect of time × treatment interaction was not statistically significant in repeated measure analysis [F(2.78, 133.71) = 0.96, P = 0.40]. Summarized in Table 2, no significant difference was detected between the two groups in the frequency of side effects based on the checklist. Similarly, CBC elements and serum aminotransferase levels were not significantly different between the two groups during the trial and at the study endpoint (Table 3).
Discussion
To the best of our knowledge, this study represents the first clinical trial investigating the efficacy and safety of riluzole in patients with schizophrenia. In line with our hypothesis, we showed that riluzole, as an adjunctive treatment to risperidone, was effective in alleviating a part of schizophrenic symptoms. Riluzole-treated patients experienced significant reduction in PANSS negative subscale scores compared with the placebo group. It should be noted that improvement of negative symptoms in clinical studies may result from changes in secondary negative symptoms. The improvement seen in these studies can be purely attributed to primary negative symptoms only if the changes in confounding factors are minimal (Hanson et al. 2010; Murphy et al. 2006). The most important manifestations which cause secondary negative symptoms include positive, depressive, and extrapyramidal symptoms, all of which were not significantly different between two groups in our study. Moreover, treatment group was the only significant predictor of negative symptoms in this trial. Altogether, these results suggest that improvement of negative symptoms in the riluzole group can be mostly attributed to reduction of primary negative symptoms. However, high degrees of positive symptoms in both groups makes such an interpretation relatively indefinite, a point which should be controlled in the future studies. In addition to the PANSS negative subscale as the study primary outcome, significant improvement was seen in the PANSS total and general psychopathology subscale scores in the riluzole versus the placebo-treated patients. This is particularly interesting and suggestive of greater advantages for riluzole in addition to improving negative symptoms in patients with chronic schizophrenia. However, we cannot compare these results to other studies since no report has been published yet on clinical response to riluzole in schizophrenic patients.
Recently, accumulating evidence supports the pathologic involvement of hyperactive glutamatergic neurons of various brain regions in schizophrenia-related symptoms (Krystal et al. 2003; Paz et al. 2008). Glutamate excess in the prefrontal cortex has been shown to impair some cognitive functions (Moghaddam and Adams 1998) and inhibition of glutamate release may improve cognitive symptoms of schizophrenia (Moghaddam 2004). In addition to interacting with post-synaptic glutamate receptors, riluzole inhibits glutamate release and increases its reuptake at pre-synaptic nerve terminals (Doble 1996). Through activation of the NMDA and AMPA receptors, increased levels of glutamate leads to excessive Ca2+ influx into neurons which subsequently results in excitotoxicity and cell death (Arundine and Tymianski 2003; Danysz and Parsons 2002). Riluzole interrupts this deleterious cascade by controlling Ca2+ channels and thus decreases the glutamate excitotoxicity (Wang et al. 2004). Interestingly, stimulation of AMPA glutamate receptors are linked to dopamine release in the prefrontal cortex (Jedema and Moghaddam 1994; Jedema and Moghddam 1996) and riluzole increases AMPA trafficking as well (Du et al. 2007). The promising results from this trial further support the concept that glutamatergic system intervention, particularly with anti-glutamate agents, can be helpful in improving negative symptoms of schizophrenia. Other mechanisms of action which can explain favorable effects of riluzole are related to immune system dysfunctions and neurotrophic factors dysregulation in schizophrenia (Durany and Thome 2004; Muller and Schwarz 2010). Riluzole has been shown to decrease inflammation and axonal damage in the nervous system and to increase the synthesis of neurotrophic factors as well (Gilgun-Sherki et al. 2003; Mizuta et al. 2001).
Treatment with riluzole was generally well-tolerated in our study and this is consistent with previous safety reports on the drug (Lacomblez et al. 2002). Most of the observed adverse events in this trial did not require any intervention as they tended to be mild and transient. No significant difference was observed between the two protocols in the frequency of extrapyramidal symptoms or other side effects. Although mild elevation in liver enzymes, especially alanine transaminase (ALT), has been previously reported in some riluzole trials (Grant et al. 2010; Lacomblez et al. 2002; Miller et al. 2012; Zarate and Manji 2008), no laboratory adverse event was seen in our study. However, the drug should be prescribed with caution to patients suffering from hepatic diseases or receiving concomitant hepatotoxic drugs. Although our study provided a valid assessment of riluzole effects on primary negative symptoms, it also had some limitations. Since this was the first clinical trial of riluzole in schizophrenic patients, we had no guide to make an absolute decision about the optimal dosage and previous trials of riluzole in other neuropsychiatric disorders were used as our guides. Sample size of this study was relatively small and the observational period was short. Therefore, the results should be confirmed in larger and more extended trials. It is better to confirm the results of this study in stabilized schizophrenic patients with more controlled symptoms other than the negative ones. Patients with schizophrenia can probably benefit from cognitive-enhancing properties of riluzole, but we did not evaluate cognitive functions in this study. In conclusion, riluzole adjuvant therapy showed promising therapeutic outcomes for management of negative symptoms in patients with schizophrenia. Nevertheless, long-term efficacy and safety and optimal dosing of riluzole require further investigations.
References
Akhondzadeh S, Ghayyoumi R, Rezaei F, Salehi B, Modabbernia AH, Maroufi A, Esfandiari GR, Naderi M, Ghebleh F, Tabrizi M, Rezazadeh SA (2011) Sildenafil adjunctive therapy to risperidone in the treatment of the negative symptoms of schizophrenia: a double-blind randomized placebo-controlled trial. Psychopharmacology (Berl) 213:809–815
Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH (2000) Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry 57:270–276
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell calcium 34:325–337
Chouinard G, Margolese HC (2005) Manual for the extrapyramidal symptom rating scale (ESRS). Schizophr Res 76:247–265
Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, Saksa J, Wu YT, Gueorguieva R, Sanacora G, Malison RT, Krystal JH (2005) Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry 58:424–428
Coyle JT (2006) Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol 26:365–384
Crow TJ (1981) Positive and negative schizophrenia symptoms and the role of dopamine. Br J Psychiatry 139:251–254
Danysz W, Parsons CG (2002) Neuroprotective potential of ionotropic glutamate receptor antagonists. Neurotox Res 4:119–126
Doble A (1996) The pharmacology and mechanism of action of riluzole. Neurology 47:S233–S241
Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA Jr, Manji HK (2007) The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology 32:793–802
Durany N, Thome J (2004) Neurotrophic factors and the pathophysiology of schizophrenic psychoses. Eur Psychiatry 19:326–337
Fenton WS, McGlashan TH (1991) Natural history of schizophrenia subtypes. II. Positive and negative symptoms and long-term course. Arch Gen Psychiatry 48:978–986
Frizzo ME, Dall’Onder LP, Dalcin KB, Souza DO (2004) Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol 24:123–128
Ghaleiha A, Mohammadi E, Mohammadi MR, Farokhnia M, Modabbernia A, Yekehtaz H, Ashrafi M, Hassanzadeh E, Akhondzadeh S (2013) Riluzole as an adjunctive therapy to risperidone for the treatment of irritability in children with autistic disorder: a double-blind, placebo-controlled. Randomized Trial, Paediatr Drugs. doi:10.1111/bdi.12108
Gilgun-Sherki Y, Panet H, Melamed E, Offen D (2003) Riluzole suppresses experimental autoimmune encephalomyelitis: implications for the treatment of multiple sclerosis. Brain Res 989:196–204
Grant P, Lougee L, Hirschtritt M, Swedo SE (2007) An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 17:761–767
Grant P, Song JY, Swedo SE (2010) Review of the use of the glutamate antagonist riluzole in psychiatric disorders and a description of recent use in childhood obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 20:309–315
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hanson E, Healey K, Wolf D, Kohler C (2010) Assessment of pharmacotherapy for negative symptoms of schizophrenia. Curr Psychiatry Rep 12:563–571
Javitt DC (2012) Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr Bull 38:911–913
Jedema HP, Moghaddam B (1994) Glutamatergic control of dopamine release during stress in the rat prefrontal cortex. J Neurochem 63:785–788
Jedema HP, Moghddam B (1996) Characterization of excitatory amino acid modulation of dopamine release in the prefrontal cortex of conscious rats. J Neurochem 66:1448–1453
Jentsch JD, Redmond DE, Jr., Elsworth JD, Taylor JR, Youngren KD, Roth RH (1997) Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277: 953–955.
Jentsch JD, Roth RH (1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Khodaie-Ardakani MR, Seddighi S, Modabbernia A, Rezaei F, Salehi B, Ashrafi M, Shams-Alizadeh N, Mohammad-Karimi M, Esfandiari GR, Hajiaghaee R, Akhondzadeh S (2013) Granisetron as an add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res 47:472–478
Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003) NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology 169:215–233
Lacomblez L, Bensimon G, Leigh PN, Debove C, Bejuit R, Truffinet P, Meininger V (2002) Long-term safety of riluzole in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 3:23–29
Laruelle M, Frankle WG, Narendran R, Kegeles LS, Abi-Dargham A (2005) Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther 27 Suppl A: S16–S24
Lourenco Da Silva A, Hoffmann A, Dietrich MO, Dall’Igna OP, Souza DO, Lara DR (2003) Effect of riluzole on MK-801 and amphetamine-induced hyperlocomotion. Neuropsychobiology 48:27–30
Martin D, Thompson MA, Nadler JV (1993) The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur J Pharmacol 250:473–476
Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM (2005) Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry 162:2379–2381
Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane database of systematic reviews (Online) 3: CD001447.
Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Kuno S (2001) Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neurosci Lett 310:117–120
Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SM, Tajdini M, Ghaleiha A, Akhondzadeh S (2013) Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia: an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs 27:57–65
Moghaddam B (2004) Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology 174:39–44
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. Journal Neurosci 17:2921–2927
Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352.
Muller N, Schwarz MJ (2010) Immune System and Schizophrenia. Curr Immunol Rev 6:213–220
Murphy BP, Chung YC, Park TW, McGorry PD (2006) Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review. Schizophr Res 88:5–25
Noroozian M, Ghasemi S, Hosseini SM, Modabbernia A, Khodaie-Ardakani MR, Mirshafiee O, Farokhnia M, Tajdini M, Rezaei F, Salehi B, Ashrafi M, Yekehtaz H, Tabrizi M, Akhondzadeh S (2013) A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology (Berl) 228(4):595–602
Paz RD, Tardito S, Atzori M, Tseng KY (2008) Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur neuropsychopharmacol 18:773–786
Rezaei F, Mohammad-Karimi M, Seddighi S, Modabbernia A, Ashrafi M, Salehi B, Hammidi S, Motasami H, Hajiaghaee R, Tabrizi M, Akhondzadeh S (2013) Memantine add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 33:336–342
Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, Krystal JH (2007) Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry 61:822–825
Stone JM, Morrison PD, Pilowsky LS (2007) Glutamate and dopamine dysregulation in schizophrenia—a synthesis and selective review. J Psychopharmacol 21:440–452
Wang SJ, Wang KY, Wang WC (2004) Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes). Neuroscience 125:191–201
Zarate CA Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK (2004) An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry 161:171–174
Zarate CA Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK (2005) An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 57:430–432
Zarate CA, Manji HK (2008) Riluzole in psychiatry: a systematic review of the literature. Expert Opin Drug Metab Toxicol 4:1223–1234
Acknowledgements
This study was supported by a grant from Tehran University of Medical Sciences to Prof. Shahin Akhondzadeh (grant no. 14037). This study was Dr. Maryam Sabzabadi’s postgraduate thesis toward the Iranian Board of Psychiatry.
Conflict of interest
No conflict of interest exists for any of the authors associated with the manuscript and there was no source of extra-institutional commercial funding. The funding organization had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript and the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farokhnia, M., Sabzabadi, M., Pourmahmoud, H. et al. A double-blind, placebo controlled, randomized trial of riluzole as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia. Psychopharmacology 231, 533–542 (2014). https://doi.org/10.1007/s00213-013-3261-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3261-z