Abstract
Rationale
Cue exposure therapy, which attempts to limit relapse by reducing reactivity to cocaine-paired cues through repeated exposures, has had limited success.
Objectives
The current experiments examined cocaine cue-induced anxiogenesis and investigated whether a model of cue exposure therapy would reduce reinstatement of cocaine seeking in rats with a history of cocaine self-administration.
Methods
Male rats experienced daily intravenous cocaine self-administration. Rats then experienced exposure to either the self-administration context or the context plus noncontingent presentations of cocaine-paired cues. Immediately following exposure, anxiety-like behavior was measured using elevated plus maze and defensive burying tests. In a second group of rats, self-administration was followed by 7 days of exposure to the context, context + noncontingent cue exposure, lever extinction, or cue + lever extinction. All animals then underwent two contingent cue-induced reinstatement tests separated by 7 days of lever extinction.
Results
Exposure to noncontingent cocaine-paired cues in the self-administration context increased anxiety-like behavior on the defensive burying test. Animals that experienced lever + cue extinction displayed the least cocaine seeking on the first reinstatement test, and lever extinction reduced cocaine seeking below context exposure or context + noncontingent cue exposure. All animals had similar levels of cocaine seeking on the second reinstatement test.
Conclusion
Noncontingent cue exposure causes anxiety, and noncontingent cue and context exposure are less effective at reducing contingent cue-induced reinstatement than lever or lever + cue extinction. These data indicate that active extinction of the drug-taking response may be critical for reduction of relapse proclivity in former cocaine users.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relapse to drug taking after successful abstinence is one of the primary challenges to the successful treatment of addiction. Previous studies have consistently demonstrated that cues associated with drug use can trigger craving (Childress et al. 1988; Ehrman et al. 1992; Kilgus and Pumariega 1994; Robbins et al. 1997) and cause subjective withdrawal symptoms, mood swings, anxiety (Powell et al. 1993a; Sinha et al. 2003), and autonomic responses consistent with stress (Miller and Gold 1994; Tiffany and Carter 1998). When asked, addicts identify drug-paired cues as important contributors to relapse (Heather et al. 1991), and brain response to drug-cue exposure is predictive of treatment success (Kosten et al. 2006), indicating that cues have a dramatic effect on drug abstinence and relapse, perhaps in part due to their ability to increase stress and anxiety in former users.
One approach to addiction treatment has been cue exposure therapy (CET), whereby repeated unreinforced presentations of the stimuli associated with drug use potentially reduces the established learned associations between cues and drug states. The goal of CET is a reduction in cue reactivity, presumably including craving and anxiety, leading to potential decreases in relapse to renewed drug taking upon exposure to such cues. While some initial studies indicated promising results after such treatment (Loeber et al. 2006; Rohsenow et al. 2001), the overall efficacy of CET has been minimal (Franken et al. 1999; Conklin and Tiffany 2002a; Dawe et al. 1993; Powell et al. 1993b). It has been suggested that extinction-based treatments might be improved by incorporating basic research findings from animal models into clinical treatment approaches (Havermans and Jansen 2003; Conklin and Tiffany 2002b; Bouton 2002). However, cue exposure therapy and the extinction techniques employed in preclinical research studies differ in a number of potentially critical ways. Clinical exposure to cues involves repeated noncontingent exposures to drug-related stimuli in a laboratory setting that may or may not be personalized or relevant to the users’ individual drug history. Also, cue exposure therapy seldom involves extinction of the specific instrumental response directed at drug consumption. Extinction of drug seeking in animal models focuses on the instrumental response alone with little regard for mood or affective states, whereby responses previously reinforced by the drug no longer result in any primary reinforcement. Preclinical approaches to extinction have the benefit of most commonly being performed in the drug-taking context. Previous studies of extinction of drug seeking in animal models have not closely modeled CET as it is often administered in a laboratory setting. These models have also paid little attention to alterations in the affective state of the rat following cue exposure, despite evidence that this may be critical to subsequent relapse (Sinha et al. 2006).
The current studies sought to examine the potential contribution of the anxiety-causing properties of cocaine-paired cues to reinstatement and investigate the effects of different extinction procedures on subsequent contingent cue-induced reinstatement of cocaine-seeking behavior. This approach aimed to model different aspects of CET and their efficacy in reducing subsequent drug seeking that occurred in response to contingent cocaine-paired cues. We utilized four separate types of extinction: exposure to cocaine-paired context, exposure to noncontingent cocaine-paired cues in the cocaine-paired context, lever response extinction in cocaine-paired context, and extinction of the lever–cue relationship during lever response extinction in the cocaine-paired context. We then examined the ability of these different types of extinction to decrease cocaine-seeking behavior in a contingent cue-induced reinstatement test. Finally, we trained all rats on lever extinction and conducted a final contingent cue-induced reinstatement test to ensure all rats would extinguish normally and to examine potential carryover effects of the initial extinction experience on subsequent lever extinction and contingent cue-induced cocaine-seeking behavior.
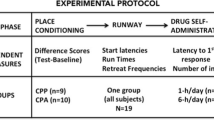
Methods and materials
Subjects and surgery
Male, Sprague–Dawley rats (initial weight, 275–300 g; Charles River, Raleigh, NC, USA) were individually housed in a temperature- and humidity-controlled vivarium on a reverse 12-h light–dark cycle (lights on 1800–0600 hours). Animals received ad libitum water and standard rat chow (Harlan, Indianapolis, IN, USA), with the exception of 2–3 days of food restriction during initial cocaine self-administration. All rats underwent surgery for jugular catheter implantation (Feltenstein and See 2006) using catheters constructed as previously described (Grimm and See 2000). Maintenance of catheter patency and animal health were carried out as previously described (Grimm and See 2000). Procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and approved by the IACUC of the Medical University of South Carolina.
Cocaine self-administration
Rats (n = 50; n = 12 for experiment 1, n = 38 for experiment 2) self-administered cocaine (cocaine hydrochloride dissolved in 0.9 % sterile saline, cocaine provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) during daily 2-h sessions on a fixed ratio-1 (FR 1) schedule of reinforcement according to previously described methods (Buffalari et al. 2012a). Briefly, animals were placed in an operant chamber and allowed to self-administer cocaine whereby a single active lever press resulted in a cocaine infusion and presentation of a light-tone stimulus complex. Inactive lever presses resulted in no programmed consequences. This continued until rats achieved 14 sessions during which they received 10 or more cocaine infusions.
Experiment 1: does exposure to noncontingent cues previously paired with cocaine self-administration increase anxiety-like behavior?
Noncontingent cue + context single exposure
Forty-eight hours after the last cocaine self-administration session, rats (n = 12) were removed from their home cages and placed in the self-administration chamber. Half of the animals (n = 6) remained in the chamber for 30 min with no levers, infusions, or stimuli presentations. The other half (n = 6) experienced the same conditions, with the additional exposure to the noncontingent discrete cues that had been paired with cocaine self-administration. This 30-min exposure involved 23 presentations of the compound light and tone stimulus on a variable interval schedule (range of 10–110 s and average intertrial interval of 40 s) in the absence of cocaine reinforcement. The number and timing of cue presentations were based on the cue presentation data of previous cocaine self-administering groups. At the end of the session, all rats were removed from the operant chamber and immediately sequentially tested on the elevated plus maze and defensive burying task, with a 10-min break in between tests. We did not counterbalance the order of the behavioral tests, as we did not want potential differences in reactivity to the acute shock of the probe to influence elevated plus maze behavior.
Tests of anxiety-like behavior
Elevated plus maze
Animals underwent testing immediately following context or context + noncontingent cue exposure. The elevated plus maze test (Pellow et al. 1985) was carried out according to previously described methods (Cecchi et al. 2002a, b). Animals were placed on the maze and allowed to freely explore for 5 min and then removed and returned to their home cage for 10 min until testing on the shock-probe burying test.
Shock-probe burying paradigm
The defensive burying test was carried out according to previously described methods (Pinel and Treit 1978). Animals were acclimated to the shock-probe chamber without the shock probe present for 1 h per day on the 3 days prior to testing. On test day, the probe was in place and electrified. Animals were allowed to freely explore. Once they were shocked by the probe, latency was calculated as the time until initiation of burying, and duration was the total time spent burying during the entire 15-min test. After 15 min, animals were removed and returned to their home cage.
Data analysis
Active lever responding and total infusions at the end of self-administration and data from tests of anxiety-like behavior including open-arm entries, open-arm time, total arm entries, latency to initiate probe burying, and burying duration were analyzed using paired t tests. Data from anxiety testing viewed via videotape were verified by two independent observers. Differences were considered statistically significant at p < 0.05.
Experiment 2: does exposure to noncontingent cocaine-paired cues and/or contexts reduce subsequent contingent cue-induced reinstatement?
Forty-eight hours after the last self-administration session, rats (n = 38) were divided into four groups, each of which underwent a different type of extinction training for phase 1 of extinction training. Each rat experienced a single 2-h extinction session per day for a total of 7 days. We have previously found that these extinction procedures allow subjects to reach the established criterion for reinstatement testing in an average of 7 days (Buffalari and See 2011; Buffalari et al. 2012a, b).
Lever extinction
Rats (n = 10) were extinguished in a standard manner most commonly used in our laboratory and the reinstatement literature (Millan et al. 2011). Once per day, rats were placed in the operant chambers and parameters were identical to those of self-administration (house light on and levers extended at the start of the session); however, active lever presses had no programmed consequences (no cocaine infusion, no light and tone presentations).
Cue extinction
Rats (n = 10) experienced procedures that were identical to those of lever extinction, with the exception that lever presses resulted in presentation of the light and tone compound stimulus, but no cocaine infusion.
Noncontingent cue + context exposure
Each session was identical to that described in experiment 1. However, after the noncontingent cue presentation ended, rats (n = 8) remained in the operant chamber for an additional 90 min to match the total session time of the other extinction groups. We chose this condition as we wanted the noncontingent cue exposure to be identical to the conditions of experiment 1, yet we also wanted to match the total overall context exposure across groups.
Context exposure
Each session was identical to those described in experiment 1. However, a 120-min session length was used for rats (n = 10) for reasons stated above.
After the final extinction session, all rats were given a contingent cue-induced reinstatement test whereby active lever responses resulted in contingent presentations of the light and tone previously paired with cocaine infusions. All rats were then run on 7 days of lever extinction for phase 2 of extinction training and given a final contingent cue-induced reinstatement test. We ran the additional extinction and reinstatement phase to (a) ensure all rats would extinguish normally and (b) examine any carryover effects the initial extinction experience might have on subsequent lever extinction and contingent cue-induced reinstatement.
Data analysis
Active lever responding and total drug infusions (also representative of total cue presentations) at the end of self-administration were analyzed using one-way ANOVAs. Total drug infusions across the entirety of self-administration were also analyzed using a one-way ANOVA. Data from extinction were analyzed using two-way repeated measure ANOVAs. Lever responding across the two reinstatement tests was compared using a two-way repeated measures ANOVA comparing test (one vs. two) and group (cue extinction, lever extinction, context exposure, noncontingent cue + context exposure). All main effects and interactions were compared using Bonferroni post hoc tests. Differences were considered statistically significant at p < 0.05.
Results
Experiment 1
Self-administration
All animals readily acquired cocaine self-administration and maintained stable levels of responding throughout the maintenance phase of the experiment. Paired t tests revealed no significant differences for lever responding (t (10) = 0.19, p > 0.05) or infusions/cue presentations (t (10) = 0.21, p > 0.05) between groups during self-administration, thus indicating equivalent operant experience, cue presentations, and cocaine intake before extinction and anxiety testing (Table 1). Inactive lever responding declined after day 1 and remained uniformly low throughout the duration of self-administration.
Behavioral anxiety testing
Animals exposed to noncontingent cocaine-paired cues in the self-administration context demonstrated increased anxiety-like behavior in the defensive burying test when compared to animals exposed to the self-administration context alone. Noncontingent cue + context-exposed animals displayed shorter burying latencies (t (10) = 2.3, p < 0.05) and longer burying durations (t (10) = 2.8, p < 0.05) than their context-exposed controls (Fig. 1). There were no significant differences on the elevated plus maze test (data not shown).
Cocaine-paired cues increase anxiety-like behavior. Animals that were exposed to noncontingent cocaine-paired cues at 48 h after the last cocaine self-administration session showed significantly increased anxiety-like behavior on the defensive burying test compared to context-exposed controls. Exposure to cocaine-paired cues a decreased the latency to initiate shock-probe burying and b increased the overall duration of burying (*p < 0.05)
Experiment 2
Self-administration
All animals readily acquired cocaine self-administration and maintained stable levels of responding throughout the maintenance phase of the experiment (Table 1). One-way ANOVA confirmed no significant differences in active lever responding between groups during the last 2 days of self-administration (F (3,33) = 1.89, p > 0.05). Further, total drug infusions and cue presentations were equivalent across the entire self-administration session as well (F (3,33) = 0.96, p > 0.05). Inactive lever responding declined after day 1 and remained uniformly low throughout the duration of self-administration, extinction, and reinstatement.
Extinction
Phase 1
Two-way repeated measures ANOVA revealed a significant effect of day (F (6,96) = 31.19, p < 0.05) on active lever responding during extinction, but no significant effect of group (F (1,16) = 0.96, p > 0.05) and no significant interaction (F (6,96) = 1.63, p > 0.05). Responding on extinction day 1 was significantly different from responding on all other days (ps < 0.05, Fig. 2a).
Lever responding during extinction after varied extinction training procedures. a Responding during extinction phase 1 for cue extinction and lever extinction groups showed no difference between groups, with responding on day 1 of extinction significantly greater than all other days of extinction (*p < 0.05, day 1 of extinction compared to all other days). b Responding on day 1 of extinction phase 2 was significantly higher than all other days for all groups except the cue extinction group (*p < 0.05), and the cue extinction group displayed significantly less responding than all other groups on day 1 of extinction († p < 0.05). Inactive lever responding is shown in the subpanels
Phase 2
Two-way repeated measures ANOVA revealed a significant effect of day (F (6,204) = 17.45, p < 0.05) on active lever responding during extinction and no significant effect of group (F (3,34) = 2.12, p > 0.05), but a significant interaction (F (18,204) = 2.11, p < 0.05). Responding on extinction day 1 was significantly greater than responding on all other days for all groups, except the cue extinction group (ps < 0.05). Further, responding on day 1 for the cue extinction group was significantly less than responding as compared to all other groups (ps < 0.05, Fig. 2b).
Reinstatement
Two-way ANOVA revealed a significant effect of test (F (1,34) = 55.81, p < 0.05), a significant effect of group (F (3,34) = 6.73, p < 0.05), and a significant interaction (F (3,34) = 12.09, p < 0.05). Post hoc analyses revealed that reinstatement on test 1 was greater than test 2 (p < 0.05). Also, rats in the cue extinction group showed reduced reinstatement as compared to all other groups (ps < 0.05) on test 1. Further, rats in the lever extinction group displayed significantly less reinstatement as compared to the context-exposed group (p < 0.05), with a trend towards less responding than those in the noncontingent cue + context exposure group (p = 0.07) on test 1. There were no significant differences between the context-exposed and noncontingent cue + context-exposed group for test 1. Rats in the noncontingent cue + context group and context exposure group had significantly less responding on test 2 than test 1 (ps < 0.05). Finally, there were no significant differences between any groups on test 2 (Fig. 3).
Contingent cue-induced reinstatement after varied extinction training procedures. Animals with a history of cue extinction showed reduced cocaine seeking relative to all other groups on cue test 1 (*p < 0.05). Responding in the lever extinction group on cue test 1 was significantly less than the context-exposed group († p < 0.05). Responding during test 2 was significantly decreased compared to test 1 for both the noncontingent cue + context- and context-exposed groups (Θ p < 0.05). There were no significant group differences following phase 2 of extinction in all groups at cue test 2. Inactive lever responding is shown in the subpanel
Discussion
The present experiments demonstrate that exposure to the context with or without the discrete cues associated with cocaine self-administration does not reduce subsequent drug seeking triggered by contingent cocaine-paired cues. Active extinction of the relationship between the lever press and the cocaine infusion, as well as the relationship between the cocaine and the contingent cues, was most effective at reducing contingent cue-induced reinstatement, as seen in our animals that received combined cue and lever extinction. Simple lever extinction was more effective in reducing reinstatement than noncontingent cue + context or context exposure as well. Noncontingent exposure to cocaine-paired cues in the cocaine context also increased anxiety-like behavior in the defensive burying paradigm beyond exposure to the context alone. These data suggest that current cue exposure therapies may benefit from improved attempts to extinguish the consequences of the specific instrumental response directed at drug-taking behavior and/or attempts to reduce anxiety caused by cue exposure. This evidence also distinguishes the self-administration and reinstatement behavioral paradigm from other paradigms commonly used to study drug abuse such as the straight alley runway task or conditioned place preference, where latent and context extinction are effective (Gabriele et al. 2009; Malvaez et al. 2013).
Despite reports that cue exposure causes stress and anxiety in former cocaine users (Sinha et al. 2003; Fox et al. 2005), few studies have demonstrated that noncontingent exposure to cocaine-paired cues elicits anxiety-like behavior in cocaine-experienced rats. We saw a significant increase in anxiety-like behavior on the defensive burying test after noncontingent exposure to cues. However, our interpretation of this effect as strictly related to anxiety behavior is complicated by the lack of effect in the elevated plus maze. As defense burying involves exposure to a painful and stressful stimulus, it may be useful to consider other factors such as differences in pain reactivity or differences in stress-evoked behavior as opposed to baseline avoidance as measured by the plus maze. These two tests also involve competing stress-coping behaviors, active (burying) vs. passive (avoidance). Previous studies have demonstrated differences in these two paradigms, suggesting that they may measure different components of anxiety (Rodgers 1997). Further, our animals were in a cocaine-withdrawn state, so the anxiety-like behavior we observed was likely an interaction between basal anxiety and that caused by exposure to drug-related stimuli. It is not uncommon to see differences in these two tests, and any of these factors could have contributed to our differences.
Although it has been suggested that stress and anxiety may have an important relationship with reinstatement to drug seeking in animal models and relapse in former drug users (Sinha et al. 2006; Erb 2010), few studies have examined the interaction of stress and cues in promoting drug-seeking behavior. Stress potentiates cue-induced reinstatement of cocaine seeking (Feltenstein and See 2006; Buffalari and See 2009a), and cue-induced reinstatement of drug seeking is related to anxiety-like behavior during early cocaine withdrawal (Buffalari et al. 2012a). Recent reports have shown reduced cue-induced reinstatement after administration of drugs that have anxiolytic properties (Smith and Aston-Jones 2011; Buffalari and See 2009b). Further, guanfacine, an alpha-2 norepinephrine agonist with anxiolytic actions, reduced craving and arousal elicited by cocaine cues in addicts (Fox et al. 2012). Also, stress may interfere with extinction of cocaine responding, particularly when experienced in the extinction context (Kupferschmidt et al. 2009), and this may interfere with subsequent stress-induced reinstatement. Since noncontingent exposure to cues can generate stress, this may have contributed to the ineffectiveness of latent or Pavlovian extinction procedures in extinction learning as revealed during cue test 1. Indeed, examining the first reinstatement test for the context or noncontingent cue + context groups and comparing it directly to extinction day 1 for the contingent cue group (whose testing conditions are identical) demonstrates nearly identical responding, suggesting that the latent and context extinction had little effect in reducing responding. Thus, previous and current data suggest that cues and stress may promote reinstatement and relapse via at least partially overlapping mechanisms, and that reductions in the stress and anxiety caused by cues may be beneficial in the treatment of former cocaine users to prevent relapse.
The lever and cue extinction groups showed no differences during phase 1 of extinction. This was somewhat surprising, as previous studies have demonstrated higher levels of responding during cue extinction compared to lever extinction (Feltenstein and See 2006). However, those differences existed only during early extinction training. We saw a similar pattern in that on the first day of extinction training, the cue extinction group exhibited a trend for higher responding than the lever extinction group. Also, the previous study used fewer days of self-administration, which could affect lever responding during extinction and explain the differences between these two studies. Our animals were trained on a fixed ratio schedule of reinforcement, which generally causes less resistance to extinction. We chose these procedures as they are most common in the literature. However, a different training schedule during self-administration may have altered our results. Despite these differences, our data suggest that the presence of the light and tone cues prompts responding beyond the presence of the lever alone.
Seven days of combined cue and lever extinction significantly decreased contingent cue-induced cocaine seeking compared to all other groups, as seen in the results from cue test 1. Cocaine seeking was decreased in the lever extinction group compared to both noncontingent exposure groups as well. This suggests that active extinction of the lever response is critical for reducing cocaine seeking. Interestingly, cue-induced reinstatement only occurs if discrete cues are presented in a response-contingent manner (Grimm and See 2000; Kruzich et al. 2001), unless the cues are initially entrained under occasion setting conditions, such as discriminative stimulus training (Ciccocioppo et al. 2001; Alleweireldt et al. 2001). In the current study, only lever responding for contingent cues elicited extinction that reduced subsequent reinstatement. Notably, performance of the specific drug-taking behavioral response in the absence of reinforcement is often a key component that is missing from cue exposure paradigms in humans; our data suggest that inclusion of some form of response extinction training may be critical for success. This approach is supported by clinical data demonstrating greater physiological reactivity and craving in cocaine users that manually manipulate drug stimuli when compared to those that are passively exposed to auditory or visual drug cues (Johnson et al. 1998).
We exposed all groups to a second phase of extinction that involved lever extinction training in an attempt (1) to demonstrate that all animals would extinguish under normal lever conditions and reinstate to cues and (2) to examine the effect of serial training that first involved noncontingent exposure to cues or context followed by lever extinction. While not significant, there was a trend for those animals that only experienced lever extinction to respond more on cue test 2 than the cue extinction (p = 0.08), noncontingent cue (p = 0.08), and context (p = 0.09) groups. With a longer exposure time and the elimination of cue test 1 (which essentially served as a cue extinction session for all groups tested), these differences may have been more dramatic. This effect suggests that cue or context exposure that occurs in the absence of extinction of the instrumental response may provide some benefit if followed by some form of extinction of the consequences of the instrumental response. It is important to note that the noncontingent cue group also experienced 90 min of context exposure after the cues and all groups received context exposure during their extinction procedures. It may have been useful to include a “yoked cue group” as a control that received noncontingent cue presentations every time the cue extinction group pressed the active lever and received a contingent cue presentation. Also, the noncontingent cue + context and context groups received extinction in the absence of levers. However, since there were no instances where noncontingent cue and context exposure groups differed, it is unlikely that exposure to noncontingent cues provided any benefit beyond context exposure alone. Further, other behavioral paradigms have demonstrated extinction in the absence of the ability to perform the behavioral response or in the absence of exposure to stimuli associated with the behavioral response. This confirms a difference between the self-administration and other paradigms.
There was a possibility that differences on cue test 1 would have carry over effects on phase 2 of extinction and cue test 2. As animals in the lever extinction group responded less on cue test 1, they received fewer cue extinction trials than animals in the noncontingent cue + context and context exposure groups, which could contribute to their elevated reinstatement on cue test 2. However, there were no significant differences between those three groups throughout phase 2 of extinction. Further, we compared responding on the active lever during cue test 1 (representative of the number of cue extinction trials) to responding during cue test 2 in each of the three groups. There was a positive correlation between total responding across all 7 days of phase 1 extinction in the cue extinction group and responding on contingent cue-induced reinstatement on test 1 (r 2 = 0.66, p < 0.05), which was more dramatic when comparing the responding across the entire extinction period and cue test 2 (r 2 = 0.79, p < 0.05). This suggests that a larger number of cue extinction trials are not necessarily beneficial in reducing contingent cue-induced reinstatement, and that animals which tend to respond more often for cues may do so over an extended period of time.
Varying extinction procedures in preclinical models of relapse have had moderate success in reducing reinstatement of drug seeking. A recent study suggests that a brief “memory retrieval” session (i.e., extinction session) given 10–60 min prior to standard extinction can reduce subsequent cocaine- and heroin-seeking behavior (Xue et al. 2012). This finding extended to reduce craving in heroin addicts, but cocaine addicts were not tested. Previous studies have demonstrated that extinction training in multiple contexts is more effective at reducing reinstatement compared to extinction in a single context (Gunther et al. 1998; Chaudhri et al. 2008). Presentation of previously extinguished cues during further extinction training can also deepen extinction (Gabriele et al. 2009; Kearns et al. 2012), while extinction of the interoceptive drug cues associated with cocaine administration can reduce subsequent extinction and cue-induced reinstatement of cocaine seeking (Mihindou et al. 2012). However, all of these studies included some degree of lever extinction. Previous data suggest that cocaine may impair latent extinction (Gabriele et al. 2009) or extinction that occurs in the absence of performance of the previously reinforced response, represented in our noncontingent cue + context and context groups. This may have contributed to the lack of reinstatement reduction in our context-exposed rats. This lends further support to the importance of instrumental and associative extinction procedures. It should be noted that the use of the term “reinstatement” here is somewhat nontraditional, as other manuscripts in the literature use reinstatement only after extinction of the instrumental response, which here represents only the cue + lever and lever extinction groups. However, the other groups do experience alternate forms of extinction; therefore, for simplicity, we have used the term reinstatement throughout.
Attempts to enhance extinction procedures in clinical treatment have had moderate success. Personalization of drug cues and contexts can enhance craving (Conklin et al. 2010); it is not yet known if using personalized stimuli enhances cue exposure therapy. Pharmacological treatment has been shown to enhance the utility of exposure therapy for patients with generalized anxiety disorder (Davis 2011); however, little efficacy has been shown in addicted populations (Myers and Carlezon 2012). In fact, some studies demonstrate increased cue-induced craving in patients treated with d-cycloserine, an NMDA receptor agonist (Price et al. 2012; Hofmann et al. 2012). Notably, the majority of these experiments used exposure that did not involve performance of the instrumental response to reduce craving. One example in which the user experiences an unreinforced instrumental response is through smoking of denicotinized or very low nicotine cigarettes. Initial data suggest that such procedures may provide treatment benefit, but likely require long-term exposure (Donny et al. 2007). Combined use of more personalized, active exposure procedures in combination with pharmacological treatment and/or procedures that allow for performance of the instrumental response may be better at reducing craving and relapse.
Analysis of cue exposure therapy suggests that a major limitation for this type of treatment is a failure to expose the user to the drug-taking behavior in the absence of the drug experience. Our data demonstrate that this type of training in preclinical models of relapse is more beneficial than noncontingent cue + context or context exposure in reducing subsequent reinstatement to cues. Further, extinction procedures that also reduce the association of discrete drug-paired cues with the drug administration effects provide further benefit in reducing drug seeking. These findings have clinical relevance and suggest specific ways in which cue exposure therapies may be improved.
References
Alleweireldt AT, Weber SM, Neisewander JL (2001) Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav 69:555–560
Bouton ME (2002) The other learning process in substance abuse: comment on Alessi, Roll, Reilly, and Johanson (2002). Exp Clin Psychopharmacol 10:84–86, discussion 101–103
Buffalari DM, See RE (2009a) Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav 98:614–617
Buffalari DM, See RE (2009b) Guanfacine blockade of stress-induced and conditioend cue-induced cocaine-seeking in an animal model of relapse. Soc Neurosci Abstr 387.385
Buffalari DM, See RE (2011) Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 213:19–27
Buffalari DM, Baldwin CK, See RE (2012a) Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 223:179–190
Buffalari DM, Baldwin CK, Feltenstein MW, See RE (2012b) Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav 105:209–214
Cecchi M, Khoshbouei H, Morilak DA (2002a) Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology 43:1139–1147
Cecchi M, Khoshbouei H, Javors M, Morilak DA (2002b) Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience 112:13–21
Chaudhri N, Sahuque LL, Janak PH (2008) Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry 64:203–210
Childress A, Ehrman R, McLellan AT, O'Brien C (1988) Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr 81:74–80
Ciccocioppo R, Sanna PP, Weiss F (2001) Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A 98:1976–1981
Conklin CA, Tiffany ST (2002a) Cue-exposure treatment: time for change. Addiction 97:1219–1221
Conklin CA, Tiffany ST (2002b) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97:155–167
Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP (2010) Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend 111:58–63
Davis M (2011) NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci 13:463–474
Dawe S, Powell J, Richards D, Gossop M, Marks I, Strang J et al (1993) Does post-withdrawal cue exposure improve outcome in opiate addiction? A controlled trial. Addiction 88:1233–1245
Donny EC, Houtsmuller E, Stitzer ML (2007) Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction 102:324–334
Ehrman RN, Robbins SJ, Childress AR, O'Brien CP (1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 107:523–529
Erb S (2010) Evaluation of the relationship between anxiety during withdrawal and stress-induced reinstatement of cocaine seeking. Prog Neuropsychopharmacol Biol Psychiatry 34:798–807
Feltenstein MW, See RE (2006) Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res 174:1–8
Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R (2005) Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology 30:880–891
Fox H, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT et al (2012) Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol 26:958–972
Franken IH, de Haan HA, van der Meer CW, Haffmans PM, Hendriks VM (1999) Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. J Subst Abuse Treat 16:81–85
Gabriele A, Setlow B, Packard MG (2009) Cocaine self-administration alters the relative effectiveness of multiple memory systems during extinction. Learn Mem 16:296–299
Grimm JW, See RE (2000) Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology 28:383–386
Gunther LM, Denniston JC, Miller RR (1998) Conducting exposure treatment in multiple contexts can prevent relapse. Behav Res Ther 36:75–91
Havermans RC, Jansen AT (2003) Increasing the efficacy of cue exposure treatment in preventing relapse of addictive behavior. Addict Behav 28:989–994
Heather N, Stallard A, Tebbutt J (1991) Importance of substance cues in relapse among heroin users: comparison of two methods of investigation. Addict Behav 16:41–49
Hofmann SG, Huweler R, MacKillop J, Kantak KM (2012) Effects of d-cycloserine on craving to alcohol cues in problem drinkers: preliminary findings. Am J Drug Alcohol Abuse 38:101–107
Johnson BA, Chen YR, Schmitz J, Bordnick P, Shafer A (1998) Cue reactivity in cocaine-dependent subjects: effects of cue type and cue modality. Addict Behav 23:7–15
Kearns DN, Tunstall BJ, Weiss SJ (2012) Deepened extinction of cocaine cues. Drug Alcohol Depend 124:283–287
Kilgus MD, Pumariega AJ (1994) Experimental manipulation of cocaine craving by videotaped environmental cues. South Med J 87:1138–1140
Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R et al (2006) Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology 31:644–650
Kruzich PJ, Congleton KM, See RE (2001) Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci 115:1086–1092
Kupferschmidt DA, Tribe E, Erb S (2009) Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav 91:473–480
Loeber S, Croissant B, Heinz A, Mann K, Flor H (2006) Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. Br J Clin Psychol 45:515–529
Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S et al (2013) HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A 110:2647–2652
Mihindou C, Vouillac C, Koob GF, Ahmed SH (2012) Preclinical validation of a novel cocaine exposure therapy for relapse prevention. Biol Psychiatry 70:593–598
Millan EZ, Marchant NJ, McNally GP (2011) Extinction of drug seeking. Behav Brain Res 217:454–462
Miller NS, Gold MS (1994) Dissociation of "conscious desire" (craving) from and relapse in alcohol and cocaine dependence. Ann Clin Psychiatry 6:99–106
Myers KM, Carlezon WA Jr (2012) d-cycloserine effects on extinction of conditioned responses to drug-related cues. Biol Psychiatry 71:947–955
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Pinel JPJ, Treit PJ (1978) Burying as a defensive response in rats. J Comp Physiol Psychol 92:708–712
Powell J, Gray J, Bradley B (1993a) Subjective craving for opiates: evaluation of a cue exposure protocol for use with detoxified opiate addicts. Br J Clin Psychol 32(Pt 1):39–53
Powell J, Dawe S, Richards D, Gossop M, Marks I, Strang J et al (1993b) Can opiate addicts tell us about their relapse risk? Subjective predictors of clinical prognosis. Addict Behav 18:473–490
Price KL, Baker NL, McRae-Clark AL, Saladin ME, Desantis SM, Santa Ana EJ et al (2012) A randomized, placebo-controlled laboratory study of the effects of d-cycloserine on craving in cocaine-dependent individuals. Psychopharmacology (Berl) 226:739–746
Robbins SJ, Ehrman RN, Childress AR, O'Brien CP (1997) Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav 22:157–167
Rodgers RJ (1997) Animal models of 'anxiety': where next? Behav Pharmacol 8:477–496, discussion 497–504
Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA et al (2001) Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction 96:1161–1174
Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ (2003) Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 170:62–72
Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006) Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63:324–331
Smith RJ, Aston-Jones G (2011) alpha(2)adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry 70:712–719
Tiffany ST, Carter BL (1998) Is craving the source of compulsive drug use? J Psychopharmacol 12:23–30
Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C et al (2012) A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 336:241–245
Acknowledgments
This research was supported by National Institute on Drug Abuse grants DA16511 and DA21690 (RES), 1F32 DA025411-01 (DMB), and NIH grant C06 R015455. The authors thank Shannon Ghee and Bernard Smalls for assistance and data collection.
Conflict of interest
The authors have no financial interests in the current data and report no conflicts of interest. The authors have full control of the data and agree to allow journal review of the data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buffalari, D.M., Feltenstein, M.W. & See, R.E. The effects of varied extinction procedures on contingent cue-induced reinstatement in Sprague-Dawley rats. Psychopharmacology 230, 319–327 (2013). https://doi.org/10.1007/s00213-013-3156-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3156-z