Abstract
Rationale
d-Cycloserine (DCS), a partial glutamate N-methyl-d-aspartate (NMDA) receptor agonist, enhances extinction of conditioned fear responding; preliminary data suggest that it may facilitate extinction of drug cue reactivity.
Objective
This study investigates DCS effects on cocaine cue craving and drug use in cocaine-dependent subjects.
Methods
Thirty-two subjects were randomly assigned to receive (1) DCS only, (2) DCS before sessions 1 and 3, placebo (PBO) before session 2, or (3) PBO only 15-min before each of 3 1-h cocaine cue exposure sessions conducted 1 day apart. Craving ratings were obtained before, during, and after sessions. Drug use and cue-induced craving were assessed 1 week after the last cue session.
Results
Repeated presentation of cocaine cues resulted in decreased craving both within and between sessions. DCS did not facilitate extinction learning and may have enhanced craving. The group that received three doses of DCS had significantly higher craving than the PBO group at the baseline ratings taken before sessions 2 and 3, as well as significantly higher cue-induced craving at follow-up. The group that received two doses of DCS did not differ from the PBO group. There were no group differences in postextinction cocaine use.
Conclusions
The reduction of cocaine cue reactivity in the PBO group suggests that the study procedures were sufficient to produce extinction. Under these conditions, DCS did not facilitate extinction and may have enhanced craving. Further studies of glutamatergic agents and extinction in cocaine dependence should include consideration of procedural variables that could have a major impact on study outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cocaine dependence remains a serious problem in the United States and in spite of two decades of intense research, efficacious pharmacotherapies have not been identified. One potential for medication development is the learning processes presumed to contribute to the development and maintenance of cocaine dependence. It has been well established that cocaine-associated environmental cues can elicit drug craving and that exposure to cocaine-related cues is likely to be involved in relapse. As such, extinction of these learned associations could help relapse prevention. The glutamatergic projection from the prefrontal cortex to the nucleus accumbens is altered after repeated exposure to drugs of abuse; these enduring changes are purported to be responsible for the characteristic excessive motivational importance of drug seeking in the transition to addiction (Kalivas and Volkow 2005). Recent developments in the neuroscience of learning have established that extinction involves the learning of new associations which inhibit, override, or, at the very least, compete with initial associative memories rather than replacing them (Bouton 2004). In addition, there is now considerable evidence suggesting that glutamate plays an important role in associative learning and memory and that extinction of conditioned responses can be facilitated by pharmacological manipulation of this system (Myers et al. 2011). These important advances have contributed to renewed interest in developing novel strategies that may help diminish craving and subsequent drug use.
Acute treatment with d-cycloserine (DCS), a partial glutamate N-methyl-d-aspartate (NMDA) receptor agonist active at the glycine binding site, enhances learning processes underlying the acquisition of the extinction of conditioned fear responding in animal models as well as in clinical populations of individuals with anxiety disorders (for a recent meta-analysis, see Norberg et al. 2008). If DCS enhances associative learning in the extinction of response to aversive cues, it may also enhance the learning that occurs during extinction training for appetitively conditioned cues. Support for this comes from preclinical investigations which demonstrate that the medial prefrontal cortex regulates the expression of both fear and drug memories after extinction, but through divergent projections to the amygdala and nucleus accumbens, respectively (Peters et al. 2009; Quirk and Mueller 2008). Recent studies in rodents have shown DCS to facilitate extinction of cocaine conditioned place preference (Botreau et al. 2006; Paolone et al. 2009; Thanos et al. 2009) and impair reacquisition of cocaine self-administration (Kelamangalath et al. 2009; Nic Dhonnchadha et al. 2010), indicating a potential role for DCS in the treatment of addiction. A placebo (PBO)-controlled pilot study in cigarette smokers showed promising results: DCS facilitated within-session extinction of craving and skin conductance responses to smoking cues during exposure therapy sessions in nicotine-dependent individuals (Santa Ana et al. 2009). Although there was no DCS-facilitated reduction of smoking evident at the conclusion of this study, the small sample size and chosen experimental parameters (e.g., a 2-week interval between the two DCS extinction sessions) may have contributed to this finding.
In all of the clinical studies of DCS facilitation of fear extinction, there has been at least 1 week between DCS dosing. However, addicted individuals are most prone to relapse during the initial days of abstinence, so investigation of shorter intervals between DCS dosing and the impact of timing between DCS doses on facilitation of extinction is essential in exploring the therapeutic potential for DCS in the treatment of addictions. Importantly, in our pilot study of DCS and cocaine cue extinction using a similar paradigm (Price et al. 2009), we noted a trend toward elevated craving during cue exposure sessions in DCS- versus PBO-treated participants when DCS was administered 2 h before each of two extinction sessions in a method similar to that used in the successful anxiety disorder trials (Hofmann et al. 2006a, b; Ressler et al. 2004). We reasoned that DCS might be acutely increasing cocaine craving and enhancing response to cocaine cues through stimulation of glutamatergic systems, which are purported to be altered in cocaine-dependent individuals (Baker et al. 2003; Kalivas and Volkow 2005). Because of this finding, DCS was administered 15 min before the beginning of the cue presentation in the current study so that it would be minimally active during the cue exposure and more active during consolidation of memory for extinction learning.
The proposed study will extend initial findings in a proof-of-concept investigation of DCS facilitation of extinction of response to cocaine-related cues in a human laboratory paradigm. In addition, the number of doses and spacing of DCS administration was varied to determine the optimal number and timing of sessions. The protocol was designed to test the following hypotheses: (1) that a DCS dose-dependent facilitation of within- and between-session cue extinction would occur, with more frequent dosing of DCS resulting in more rapid or robust reduction in craving to cocaine cues; and (2) that postextinction follow-up tests would reveal a DCS dose-dependent reduction of cocaine cue-induced craving and measures of drug use.
Methods and materials
Subjects
Men and women aged 18–65 who met DSM-IV criteria for cocaine dependence within the past 3 months were eligible for study participation. The study was approved by the Institutional Review Board of the Medical University of South Carolina. All participants provided written informed consent after being fully informed of potential risks of participation before any study assessments/procedures were undertaken. Both outpatient treatment-seeking and nontreatment-seeking participants were recruited through referrals from local substance abuse treatment clinics or advertisements in the community and were compensated for their participation. All subjects were required to maintain at least 72 h of abstinence from cocaine, alcohol, and all other drugs of abuse except nicotine as confirmed by breathalyzer and urine drug screening (UDS) prior to each extinction training session [positive UDS for tetrahydrocannabinol (THC) was acceptable as long as marijuana use within the last 3 days was denied]. Exclusion criteria included current abuse or dependence on other drugs of abuse (except nicotine and alcohol), a history of or current psychotic disorder, bipolar affective disorder, major depressive disorder or presenting with significant suicidal risk. Subjects with significant hematologic, endocrine (including diabetes mellitus), cardiovascular, pulmonary, renal, gastrointestinal, or neurological disease were also excluded.
Study procedures were conducted between October 2008 and August 2010 at the Clinical Neuroscience Division laboratory at the Medical University of South Carolina or the research clinic of Behavioral Health Services in Pickens, South Carolina, a National Institute on Drug Abuse Clinical Trials Network site. As part of a proof-of-concept study, accrual to this protocol was closed following a planned interim analysis. After giving informed consent, potential participants were screened using the Mini International Neuropsychiatric Interview (Sheehan et al. 2003), a structured interview based on the DSM-IV for assessment of psychiatric and substance use symptoms, and a medical history and physical exam were performed. Qualified participants were asked to think of a time when they craved cocaine and ended up using it. Details surrounding the event were recorded by research assistants and used to construct personalized imagery scripts for use during the cue extinction sessions, based on the methods of Sinha (Sinha 2005). Subjective ratings of craving were assessed using a modification of the within-session rating scale (Childress et al. 1986), a visual analog scale (0–10) assessment of subjective desire to use cocaine. Quantitative cocaine use data for the past 90 days were assessed using the time-line follow-back (TLFB), a calendar-based instrument used to assess daily self-reported substance use (Sobell and Sobell 1992).
Extinction procedures
Experimental sessions consisted of three 10-min periods of cocaine cue exposure over each of three 1-h extinction sessions, conducted on Monday, Wednesday, and Friday. Multimodal cocaine cues consisted of a prerecorded personalized imagery script (~3 min), in vivo handling of paraphernalia and simulated cocaine (~2 min), and video of individuals procuring and using cocaine (~5 min), presented in that order; there were 10 min rest periods between each cue presentation. Craving ratings were collected 20 and 5 min prior to the first cue exposure (baseline), immediately following each cue exposure period, and at 5, 30, and 60 min after the final rest period (recovery). Following the recovery period, if subjects’ desire to use cocaine was elevated (≥20% baseline), counseling was provided by a qualified staff member including suggestions to minimize likelihood of use (e.g., avoid situations that may trigger use) and a reduction in craving was required before release from the facility was granted. Subjects were required to provide a urine sample negative for drugs of abuse (except marijuana) prior to each cue extinction session. An identical cue exposure session was conducted at a 1-week follow-up visit. Drug use during the 1-week period following the extinction procedures was assessed with TLFB and UDS.
DCS administration
In a double-blind fashion, participants were randomly assigned to one of three drug-treatment groups: PBO before extinction sessions on Monday/Wednesday/Friday (PBO/PBO/PBO=PBO-only), 50 mg DCS (DCS/DCS/DCS=DCS-only), or 50 mg DCS/PBO mix (DCS/PBO/DCS=DCS–PBO). Level of change in craving following the administration of the craving induction script during the assessment visit (high vs. low cravers) as well as length of cocaine use history (≤/>5 years) were used as stratification variables to ensure balanced groups. A random number generator was used by a statistician to allocate group designation in a single-block design within each of the four strata. Medication (DCS and PBO) was compounded and packaged by the Institutional Drug Services (IDS) at the Medical University of South Carolina (MUSC) in identical capsules within blister packs. IDS assigned drug-treatment group designation based on stratification variables; all study personnel and study participants were blind to group designation. IDS broke the blind at the planned interim analysis and reconfirmed drug group designations. All participants received PBO before the cue exposure session during the follow-up visit. Medication was administered 15 min prior to the first cue presentation to ensure that it would be active (peak concentration at 2–4 h postadministration) during the memory consolidation phase following extinction procedures.
Statistical analysis
Standard descriptive statistics were summarized for the full sample and compared between treatment groups with a two-sided Kruskal–Wallis test statistic for continuous measures and Pearson’s Chi-square test statistic (Fisher exact test where appropriate) for categorical measures. Nonparametric statistics were used in baseline group comparisons since the size of the groups precludes the assumption of normal distribution. To confirm that cue-induced activation was achieved, change in craving ratings that were taken immediately prior to and following the initial cue exposure were compared via a Wilcoxon’s signed-rank test, and differences in treatment group response to the cue presentation were evaluated with a two-sided Kruskal–Wallis test statistic. To test for within- and between-session extinction of cue-elicited craving, a repeated-measures generalized linear model was developed. Each model consisted of the effects of treatment group and time as well as their corresponding interaction. Separate models were developed to assess within-session effects, adjusting for session-specific baseline craving ratings. Pairwise comparisons of within-session baseline craving rating were made following a significant analysis of variance F test. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA). No correction for multiple testing has been applied to reported p values.
Results
Baseline
The 32 subjects who completed the study were randomized to one of three treatment groups; PBO-only (n = 10), DCS-only (n = 10), and DCS–PBO (n = 12). Demographic and clinical characteristics are presented in Table 1. Subject demographics and cocaine use characteristics were similar between the three treatment groups. Alcohol dependence status was reported for 30 of the 32 subjects, with eight subjects (26.7%) endorsing criteria for current alcohol dependence and ten subjects (33.3%) endorsing past alcohol dependence; the distribution of reported diagnoses across treatment groups was not different (p = 0.44), did not modify the effect of DCS, and was therefore left out of the final analysis model. Although not statistically different, a greater portion of the participants in PBO-only were actively enrolled in a drug treatment program as compared to the two DCS groups (PBO-only, DCS–PBO, and DCS-only, 50% vs. 10% vs. 8.3%, respectively; p = 0.06). Due to this difference, drug treatment program enrollment was included as a covariate in all extinction analysis models.
Cue craving extinction
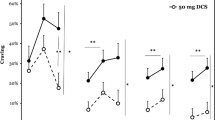
Craving during baseline, cue, and recovery periods for each cue exposure session is shown in Fig. 1. The initial cocaine cue presentation elicited a significant increase in craving from baseline for the entire cohort (Δ = 1.3 ± 0.3; p < 0.01) but with no group differences (PBO-only: Δ = 1.3 ± 0.6; DCS-only: Δ = 1.9 ± 0.7; DCS–PBO: Δ = 0.8 ± 0.4; p = 0.30). As seen in the inset, there was an overall reduction in mean cue-elicited craving across sessions for the entire cohort (p < 0.01); however, the analysis failed to show either a significant main effect of group (p = 0.53) or group by session interaction (p = 0.26).
Craving rating across cue exposure sessions by group. Means and standard errors for baseline, cue-elicited, and recovery craving rating by treatment group during all three extinction training sessions (M/W/F) as well as the 1-week follow-up cue exposure test. Baseline craving ratings are taken as the average of two craving ratings taken 20 and 5 min prior to initial cue onset. Cue-elicited craving was assessed immediately after each of three 10-min cue exposures (followed by 10-min rest periods), over one h cue exposure sessions. Craving during the recovery period was assessed at 5, 30, and 60 min following the final rest period. Inset: Averaged cue-induced craving during each cue exposure session by treatment group
Craving ratings during the cue and recovery periods were assessed within each session to examine the effects of DCS on craving. During session 1 cue exposure, there was an overall reduction in craving across time (p < 0.01), but no differential group response was noted (p = 0.26). However, during the recovery period following cue session 1, a significant group by time interaction (p < 0.05) indicated that craving decreased at a greater rate in the PBO-only group than in the DCS–PBO or DCS-only groups, an effect that remained significant when the two DCS groups (identical at this point in the protocol) were collapsed (p = 0.02). At the initiation of session 2, baseline craving was greater in the DCS-only group as compared to the PBO-only group (p = 0.04), but not the DCS–PBO group (p = 0.13). As in session 1, there was a reduction in craving during session 2 cue exposure (p = 0.04) with no differential group response (p = 0.31); similar results were seen during the recovery period (all p > 0.10). At the initiation of session 3, baseline craving was greater in the DCS-only group as compared to both the PBO-only (p = 0.02) and the DCS–PBO (p = 0.03) groups. Craving during session 3 did not show a significant decrease over time in either the cue exposure (p = 0.49) or recovery period (p > 0.11). Visual inspection of the graphs in Fig. 1 suggest that the DCS-only group had greater cue-induced craving than the other two groups during the extinction procedures, but these differences failed to meet statistical significance.
Follow-up cue reactivity and drug use
The follow-up cue test session was performed 5–12 days after the final extinction session. Baseline craving at follow-up was not statistically greater in the DCS-only group as compared to the PBO-only group (p = 0.06) or the DCS–PBO group (p = 0.11). Mean cue-elicited craving during follow-up was significantly higher in the DCS-only group as compared to the PBO-only group (p < 0.05; see Fig. 1, inset). All of the subjects in the PBO-only group with measurable craving (>0) during the first cue session had a substantial reduction (≥50%) in mean cue-elicited craving at follow-up as compared to session 1; 73% of the subjects in the DCS–PBO group and a smaller proportion (44%) of those in the DCS-only group had similar decreases. There were no statistically significant differences in the proportions of participants positive for postextinction cocaine use (as evidenced by positive UDS or self-report: DCS-only 50.0%, DCS–PBO 58.3%, PBO-only 30.0%, p = 0.46). Additional analysis after excluding the seven subjects enrolled in treatment revealed comparable rates of cocaine use during the postextinction follow-up period between groups (DCS-only 55.6%, DCS–PBO 63.6%, PBO-only 60.0%, p = 0.93).
As part of a proof-of-concept study, the results of this planned interim analysis lead to discontinuation of recruitment into this particular protocol.
Discussion
As expected, repeated administration of multimodal cocaine cues resulted in a decreased craving response to these cues in a laboratory setting. Contrary to our hypotheses, DCS administration did not facilitate extinction learning either within extinction training sessions or over the course of the training protocol. In fact, there is some evidence that DCS administration interfered with the extinction of responses to cocaine-related cues in this paradigm. Both groups that received DCS before session 1 experienced significantly less diminution of craving during the 1-h, postsession recovery period than did the group that received PBO. While baseline craving was not significantly different between groups at the beginning of session one, session 2 baseline craving was significantly higher in the DCS-only group as compared to the PBO group and session 3 baseline craving was significantly higher in the DCS-only group as compared to both the PBO and the DCS–PBO groups, suggesting a lack of attenuation in cocaine craving in the DCS-only group as compared to the other two groups over time. This is supported by the fact that the mean cue-elicited craving scores are significantly higher during the follow-up session in the DCS-only group as compared to the other groups.
There are several possible explanations for these results including the contributions of glutamate function in this population and the extinction parameters employed. For example, the results from this randomized, double-blind, PBO-controlled proof-of-concept study support the findings of a pilot study from our laboratory (Price et al. 2009) that DCS may contribute to elevated cocaine craving. While the dosing parameters were adjusted in the current study to minimize the impact of DCS during cue exposure, the data suggest that there may have been DCS-induced glutamatergic activation. Since glutamate homeostasis is an important mediator of drug-seeking behaviors in addicted individuals (Reissner and Kalivas 2010), administration of an NMDA receptor partial agonist may be more likely to contribute to the motivated drive elicited by drug cues and subsequent anticipatory craving during baseline than to the formation of novel associations and cognitive control expected to occur during extinction procedures. Thus, the mechanism of increased craving in this study may be related to enhanced memory reconsolidation.
It is known that presentation of a conditioned stimulus (CS) can lead to extinction, whereby memories for extinction-related inhibitory learning are formed, and subsequent responding to the CS is attenuated. Alternatively, CS presentations can reactivate the memory for extant learning and, once reactivated, they become unstable for a period of time before they are restabilized (i.e., reconsolidated). The apparent lack of response attenuation in the DCS-only group is inconsistent with the development of extinction-based inhibitory learning but suggests the possibility that DCS, in conjunction with cocaine cue presentations, may have potentiated reconsolidation of memories for the learning originally controlled by the CS (i.e., cue–cocaine associations). Potentiation of memories for this learning would be expected to enhance rather than diminish the responses (e.g., craving) elicited by cocaine cues. This expectation is consistent with the observed outcomes of the present study.
The likelihood of potentiated reconsolidation versus facilitated extinction following CS presentation(s) is dependent—at least in part—on the duration and strength of the CS presentation(s), as well as the durability of the memories for the original learning. When memory reactivation sessions are brief, reconsolidation is more likely to occur and the original associations are preserved; longer sessions induce extinction mechanisms (Eisenberg et al. 2003). Importantly, two recent animal studies have demonstrated unexpected outcomes in potentiated reconsolidation versus facilitated extinction. In one study, infusion of DCS into the baslolateral amygdala of rats shortly before a brief (30 cue presentations over 30 min) reexposure to cocaine-associated stimulus (to achieve memory reactivation without extinction) increased subsequent cocaine-seeking behavior (Lee et al. 2009). By contrast, another rodent study reported DCS administration after two 30-min extinction sessions consisting of 60 presentations each attenuated cue-induced reinstatement of cocaine-seeking behavior in a context-independent manner (Torregrossa et al. 2010), thereby indicating that DCS enhancement of memory reconsolidation may be avoided, and extinction enhanced, with sufficient cue presentations. Notably, in the study conducted by Santa Ana et al. (2009), exploring DCS-facilitated extinction of smoking cue reactivity in humans, the extinction sessions were 4.5 h in length and involved training, review, and practice in techniques that can be used to decrease the response to drug-related cues. In this study, within-session extinction of both the skin conductance and craving response to smoking-related cues was found in the DCS-treated group. In the current study, participants were not instructed to resist craving when presented with cues. Optimization of the use of pharmacologic agents to enhance extinction learning in addictions may require a more robust extinction training paradigm than was used in the current study. However, the extinction of cocaine cue reactivity in the PBO group suggests that the number, length, and intervals between training sessions were sufficient to produce extinction.
In terms of the number of and interval between DCS doses, the findings of this study are of interest since little is known concerning optimal dosing parameters for extinction of craving in drug-dependent populations. Interestingly, preclinical investigations have shown that chronic administration of DCS before conditioned learning occurs failed to facilitate retention of maze-trained memories (Quartermain et al. 1994) or subsequent extinction of a conditioned fear response (Parnas et al. 2005), which is consistent with chronic DCS-induced desensitization of the NMDA receptor complex (Hofmann et al. 2006a). The experimental parameters in our study were remarkably dissimilar from those preclinical studies, as our participants were administered DCS well after real-world conditioned learning had occurred; however, the impact of multiple dosing on a paradoxical antagonistic effect or behavioral desensitization during extinction procedures cannot be ruled out.
Those participants who received three doses of DCS with only a 48-h interval between dosing (DCS-only) showed sustained higher baseline and cue-induced craving throughout the three extinction sessions and follow-up period. This group displayed a clear (though nonsignificant) medication effect, although the effect was not in the expected direction. This suggests that this number and scheduling of dosing may impact associative learning in this population, but a different experimental paradigm must be implemented so that the learning that is facilitated during cue exposure sessions leads to therapeutic gains. Those participants who received only two doses of DCS with a 96-h interval between dosing showed no significant differences in craving or extinction patterns from the PBO-only group. Whether there is a potential medication effect using this dosing schedule is difficult to ascertain because the PBO-only group displayed extinction over the three-session paradigm, leading to a possible floor effect. To further investigate this dosing schedule, an experimental paradigm less likely to produce complete extinction over three sessions would need to be explored.
Several limitations of this study deserve attention. As was stated earlier, this experiment is part of a proof-of-concept study, and the results shown here are from a planned interim analysis, after which accrual to the study was discontinued. Thus, while the cocaine-craving profiles of the groups over time appear to be significantly different, this study was underpowered to detect the interaction of these two variables. A post hoc power analysis demonstrated that we had approximately 20% power to detect an overall difference in response between the groups. In addition, the protocol included cocaine-dependent people who were in treatment for their addiction as well as those who were not. Motivation for abstinence and having a skill set to overcome automatic conditioned responses may increase participant engagement in passive extinction training sessions (see above discussion of protocol for extinction to smoking cues). Because of the small sample size, it is difficult to assess the impact of being in treatment on the DCS response or extinction training. However, this variable was included as a covariate in all extinction analyses, and its lack of impact on the results renders it unlikely that treatment status contributed significantly to group differences in extinction of cocaine craving. The distribution of females between groups was also notably different, with the DCS-only group having 10% and the other two groups having approximately 40% female. However, there are no known sex differences in response to DCS, and analysis of the DCS/PBO/DCS group showed no cue reactivity or extinction differences between males (n = 7) and females (n = 5; data not shown).
In summary, the results from this randomized, double-blind, PBO-controlled proof-of-concept study support the findings of a pilot study from our laboratory (Price et al. 2009) that DCS may contribute to elevated cocaine craving. The nature of the role of glutamate in cocaine dependence as compared to anxiety disorders may explain the fact that our study did not produce the hypothesized results and highlights an important consideration in adapting successful treatments across disorders. Based on our findings, further studies of glutamatergic agents and extinction in cocaine dependence should include consideration of procedural variables that could have a major impact on study outcomes.
References
Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW (2003) Neuroadaptations in cystine–glutamate exchange underlie cocaine relapse. Nat Neurosci 6:743–749
Botreau F, Paolone G, Stewart J (2006) d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res 172:173–178
Bouton ME (2004) Context and behavioral processes in extinction. Learn Mem 11:485–494
Childress AR, McLellan AT, O’Brien CP (1986) Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict 81:655–660
Eisenberg M, Kobilo T, Berman DE, Dudai Y (2003) Stability of retrieved memory: inverse correlation with trace dominance. Science 301:1102–1104
Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW (2006a) Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Arch Gen Psychiatry 63:298–304
Hofmann SG, Pollack MH, Otto MW (2006b) Augmentation treatment of psychotherapy for anxiety disorders with d-cycloserine. CNS Drug Rev 12:208–217
Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413
Kelamangalath L, Seymour CM, Wagner JJ (2009) d-Serine facilitates the effects of extinction to reduce cocaine-primed reinstatement of drug-seeking behavior. Neurobiol Learn Mem 92:544–551
Lee JL, Gardner RJ, Butler VJ, Everitt BJ (2009) d-Cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem 16:82–85
Myers KM, Carlezon WA Jr, Davis M (2011) Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 36:274–293
Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM (2010) d-Cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology 35:357–367
Norberg MM, Krystal JH, Tolin DF (2008) A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 63:1118–1126
Paolone G, Botreau F, Stewart J (2009) The facilitative effects of d-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 202:403–409
Parnas AS, Weber M, Richardson R (2005) Effects of multiple exposures to d-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem 83:224–231
Peters J, Kalivas PW, Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16:279–288
Price KL, McRae-Clark AL, Saladin ME, Maria MM, DeSantis SM, Back SE, Brady KT (2009) d-cycloserine and cocaine cue reactivity: preliminary findings. Am J Drug Alcohol Abuse 35:434–438
Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH (1994) Acute but not chronic activation of the NMDA-coupled glycine receptor with d-cycloserine facilitates learning and retention. Eur J Pharmacol 257:7–12
Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72
Reissner KJ, Kalivas PW (2010) Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol 21:514–522
Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M (2004) Cognitive enhancers as adjuncts to psychotherapy: use of d-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61:1136–1144
Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, Gonsai K, Hill KP, Carroll KM (2009) d-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend 104:220–227
Sheehan D, Janavs J, Baker R, Harnett-Sheehan K, Knapp E, Sheehan M (2003) MINI: Mini International Neuropsychiatric Interview
Sinha R (2005) Imagery script development procedures, unpublished manual
Sobell LC, Sobell MB (1992) Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ (eds) Measuring alcohol consumption: Psychological and biological methods. Humana Press, Totowa, NJ, pp 41–72
Thanos PK, Bermeo C, Wang GJ, Volkow ND (2009) d-Cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav Brain Res 199:345–349
Torregrossa MM, Sanchez H, Taylor JR (2010) d-Cycloserine reduces the context specificity of Pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci 30:10526–10533
Acknowledgments
This study was supported by the National Institutes of Health (Grant Nos. 1R01DA023188-01A1 and 3R01DA023188-02S1). The authors thank Lisa Jenkins, Katherine Shugart, Colleen Reed, and Cullen McWhite at MUSC and Elizabeth Chapman and Margaret Garret at BHSPC for their assistance with study participants. Preliminary analyses of these data were presented in poster format at the 2010 College on Problems of Drug Dependence annual meeting in Scottsdale, AZ.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Price, K.L., Baker, N.L., McRae-Clark, A.L. et al. A randomized, placebo-controlled laboratory study of the effects of d-cycloserine on craving in cocaine-dependent individuals. Psychopharmacology 226, 739–746 (2013). https://doi.org/10.1007/s00213-011-2592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2592-x