Abstract
Rationale
Nitric oxide (NO)-mediated transmission in the dorsolateral periaqueductal gray matter (dlPAG) has been involved in the expression of anxiety-like behaviors. Ethanol withdrawal sensitizes the dlPAG and results in increased anxiety-like responses.
Objectives
The objective of the study was to test the hypothesis that NO in the dlPAG is involved in the expression of ethanol withdrawal-induced anxiety.
Methods
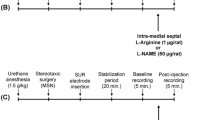
Male Wistar rats were implanted with guide cannulae aimed at the dlPAG. The animals were forced to consume a liquid diet containing ethanol 6–8 % (v/v) for 15 days as their only source of diet. Six days after surgery and 24 h after ethanol discontinuation, the animals received microinjections of the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO), nonselective nitric oxide synthase inhibitor N G-nitro-L-arginine methyl ester (L-NAME), selective neuronal nitric oxide synthase inhibitor 1-(2-[trifluoromethyl]phenyl) imidazole (TRIM), or selective inducible nitric oxide synthase (iNOS) inhibitor N-([3-(aminomethyl)phenyl]methyl) ethanimidamide dihydrochloride (1400W) into the dlPAG. Ten minutes later, the animals were tested in the light/dark box.
Results
Carboxy-PTIO (1 nmol), L-NAME (200 nmol), TRIM (20 nmol), and 1400W (0.3 and 1 nmol) decreased the anxiogenic-like effects of ethanol withdrawal in rats in the light/dark box test. The NO precursor L-arginine reversed the effects of L-NAME.
Conclusions
NO production in the dlPAG may play a role in the modulation of ethanol withdrawal-induced anxiety-like behavior in rats. Furthermore, iNOS-mediated NO synthesis in the dlPAG is predominantly involved in the behavioral expression of anxiety-like behavior during ethanol withdrawal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dorsolateral periaqueductal gray (dlPAG) is part of a longitudinally organized neural system involved in numerous physiological functions, including cardiovascular regulation, pain modulation, and behavioral responses to aversive stimuli (Bandler and Shipley 1994). Electrical stimulation of the human dorsal PAG produces anxiety, terror, desire to flee, palpitation, and hyperventilation (Amano et al. 1978; Nashold et al. 1969). Besides, chemical or electrical stimulation of the dlPAG in cats and rodents results in similar autonomic, somatic, and behavioral responses recognized as defensive reactions (Bandler and Carrive 1988; Bittencourt et al. 2005). These responses resemble many of the effects observed during abrupt ethanol discontinuation or withdrawal in rats (Cabral et al. 2006; Kliethermes 2005). The dlPAG has also been involved in the anxiolytic effects of different classes of drugs, including benzodiazepines (Russo et al. 1993; Schenberg and Graeff 1978), that are clinically used to alleviate the symptoms of ethanol withdrawal (Chick and Nutt 2012; Clapp 2012).
Ethanol withdrawal has been shown to function as an unconditioned stressor promoting unconditioned withdrawal responses and activation of several brain structures, in particular those involved in the modulation and expression of anxiety- and defensive-related behaviors such as the hypothalamus, amygdala, and dlPAG (Bonassoli et al. 2011; Knapp et al. 2007; Vilpoux et al. 2009). Rats subjected to ethanol withdrawal and electrical stimulation of the dlPAG exhibited reductions of the stimulation thresholds required to elicit freezing and escape responses and number and duration of ultrasonic vocalizations (Cabral et al. 2006). In this context, the dlPAG has been recognized as the mesencephalic output underlying both the expression of unconditioned anxiety elicited by anxiety-evoked situations and those promoted by ethanol withdrawal (Brandão et al. 1999; Leite and Nobre 2012). The dlPAG has a dense connection with a set of hypothalamic subnuclei (e.g., anterior hypothalamic nucleus, ventromedial hypothalamic nucleus, and dorsal premammillary nucleus) that influence defensive responses (Cameron et al. 1995; Canteras and Swanson 1992). The dlPAG also shares direct and reciprocal connections with the central nucleus of amygdala (CeA) (Rizvi et al. 1991), which has been proposed to be critical for mediating ethanol withdrawal-related behaviors (Gilpin 2012). The CeA amygdala sends inhibitory projections to the hypothalamus and PAG, which could account for the increased anxiety observed in ethanol withdrawn rats.
Accumulating evidence suggests that an enhanced glutamatergic transmission may play a role in dlPAG neuronal excitability in response to ethanol withdrawal. Microinjection of N-methyl-D-aspartate (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptor antagonists into the PAG was shown to block the susceptibility of rats to develop audiogenic seizures during ethanol withdrawal (Long et al. 2007; Yang et al. 2003) and to decrease voluntary alcohol drinking in both low-anxiety and high-anxiety alcohol-withdrawn rats (Leite and Nobre 2012).
Activation of NMDA receptors by glutamate has been shown to generate nitric oxide (NO) (Garthwaite et al. 1988). Nitric oxide is synthesized by nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzymes, referred to as NO synthases (NOSs), that catalyze the conversion of L-arginine (L-Arg) to L-citrulline and NO (Bredt and Snyder 1994). The physiological actions of NO are mainly mediated by the stimulation of soluble guanylate cyclase, which in turn leads to an increase in the levels of cyclic guanosine 3′,5′-monophosphate (cGMP) (Schuman and Madison 1991). Nitric oxide synthase exists in three isoforms: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) (Bredt et al. 1990; Förstermann et al. 1995; Guix et al. 2005; Murphy et al. 1993). The nNOS and eNOS isoforms have been characterized as constitutively expressed, and their activity is Ca2+–calmodulin dependent. iNOS is a Ca2+–calmodulin-independent enzyme that is regulated by de novo synthesis following immunological or inflammatory stimulation (Lyons et al. 1992; Zhou et al. 2009).
Functional studies have indicated increases in NO production and NOS activity during ethanol withdrawal. Systemic administration of N G-nitro-L-arginine methyl ester (L-NAME), a nonspecific inhibitor of NOS (Moncada et al. 1997), attenuated many signs of ethanol withdrawal and decreased ethanol intake in alcohol-preferring rats (Adams et al. 1995; Uzbay et al. 1997; 2000). More7over, L-NAME pretreatment in rats blocked the increase in L-citrulline concentrations in the striatum during ethanol withdrawal (Gören et al. 2001). Ethanol intoxication and withdrawal was also shown to induce inflammatory processes in the brain by stimulating intracellular signaling pathways that trigger the induction of cytokines and cyclooxygenase-2 (COX-2) and iNOS expression (Pascual et al. 2007).
The dlPAG has been histologically characterized by the presence of a well-delimited population of nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d)- and NOS-positive neurons (Onstott et al. 1993; Vincent and Kimura 1992). Nitric oxide in this brain area has been systematically shown to be involved in the modulation of anxiety-like behavior in rodents (for review, see Guimarães et al. 2005). Intra-dlPAG injections of nNOS inhibitors (Guimarães et al. 1994), guanylate cyclase inhibitors (Aguiar et al. 2006; De Oliveira and Guimarães 1999; Guimarães et al. 2005), and a NO scavenger (Aguiar et al. 2006) exerted anxiolytic-like effects, whereas NO donors administered into the dlPAG produced flight and defensive reactions in rats (De Oliveira et al. 2000a). Additionally, NOS expression and activity increases in the dlPAG in rats subjected to restraint stress (De Oliveira et al. 2000b, 2001; Kishimoto et al. 1996; Smalls and Okere 2012) and in rats that express fear response to a predator (Chiavegatto et al. 1998). Recently, Bonassoli et al. (2011) found that NADPH-d-positive neurons in the dlPAG are activated 24 and 48 h after ethanol discontinuation. These authors suggested that NO in the dlPAG might be involved in the development and expression of ethanol withdrawal-induced anxiety.
To our knowledge, experiments that have used direct application of NO-interfering compounds into the dlPAG during ethanol withdrawal have not been conducted. Given that anxiety-related behavior may be associated with an increase in NO transmission in the dlPAG, we hypothesized that ethanol withdrawal mediates similar increases in NO transmission, ultimately driving increased anxiety during ethanol withdrawal. Therefore, the aim of the present study was to test the hypothesis that NO in the dlPAG is involved in the expression of ethanol withdrawal-induced anxiety-like behavior in rats. Because NO production can occur as a result of activity of either constitutive nNOS or iNOS, we tested the effects of the selective nNOS and iNOS inhibitors 1-(2-[trifluoromethyl]phenyl) imidazole (TRIM) and 1400W, respectively, directly injected into the dlPAG in rats during ethanol withdrawal.
Materials and methods
Animals
Male Wistar rats (Rattus norvegicus), weighing 250–300 g, were housed in groups of three per cage under a 12/12-h light/dark cycle (lights on at 7:00 AM) at 23 ± 1 °C and given free access to water. The procedures were conducted in accordance with the Brazilian Society of Neuroscience and Behavior Guidelines for the Care and Use of Laboratory Animals and approved by the local committee on animal ethics (CEAE 031/2010). All efforts were made to minimize animal suffering.
Drugs
The following drugs and doses were used: the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO; 1, 3, and 10 nmol) (Aguiar et al. 2006; Gualda et al. 2011), the NO precursor L-Arg (100 nmol) (Forestiero et al. 2006; Spiacci et al. 2008), the nonselective NOS inhibitor L-NAME (50, 100, and 200 nmol) (Calixto et al. 2008), the selective nNOS inhibitor TRIM (40, 80, and 160 nmol) (Hall and Behbehani 1998), and the selective iNOS inhibitor N-([3-(aminomethyl)phenyl]methyl) ethanimidamide dihydrochloride (1400W; 0.1, 0.3, and 1.0 nmol) (Kalinchuk et al. 2006). All of the drugs were purchased from Sigma (St. Louis, MO, USA) and dissolved in sterile isotonic saline immediately before use. The selected doses were based on previous studies that used intracerebral injections.
Chronic ethanol administration
Chronic ethanol administration consisted of a procedure of forced dietary fluid intake by drinking an alcohol-containing liquid diet (Bonassoli et al. 2011; Cabral et al. 2006). The animals had access to bottles (180 ml each, i.e., 17.1 g of Sustagen per 60 ml water per rat per day) that contained only a dietary base composed of Sustagen M (chocolate flavor; Mead Johnson, São Paulo, Brazil). This diet is a ready-to-feed liquid formula that provides protein, carbohydrates, fat, vitamins, and mineral salts, corresponding to 1.1 kcal/ml. The liquid diet was the only source of food available daily to the animals, and it was prepared daily and presented to the animals at the same time each day (2:00 PM). Initially, a liquid diet without ethanol was provided to rats for 2 days. The animals were then randomly assigned to experimental groups: control and ethanol withdrawal groups. For control animals, the liquid diet without ethanol was provided until the end of the treatment. For ethanol withdrawal groups, on the first 2 days of treatment, the ethanol concentration was 6 % (v/v), which was then increased to 8 % (v/v) until the end of treatment. The animals received ethanol for 15 consecutive days, followed by 24 h of ethanol withdrawal, in which ethanol was withdrawn from the diet by replacing it with a diet that did not contain ethanol. This method of ethanol administration has been shown to reliably induce ethanol dependence with blood ethanol concentrations (BEC) of 80 to 132 mg% during ethanol consumption (Baldwin et al. 1991; Cabral et al. 2006). Using the same protocol, we have previously detected BEC of 80 ± 14 mg% in ethanol-treated rats and very low amount of ethanol (4.0 ± 2.0 mg%) in the 24-h ethanol-withdrawn rats (Bonassoli et al. 2011).
The body weights of the rats were recorded every 2 days, and ethanol intake was measured daily and is expressed in gram per kilogram per animal.
Surgery and drug administration
Six days before behavioral testing, the animals were anesthetized with 45 mg/kg pentobarbital, i.p. (Thiopentax; Cristália, São Paulo, Brazil) and fixed in a stereotaxic frame (David Kopf, Tujunga, CA, USA). Stainless-steel guide cannulae (outer diameter 0.7 mm) were implanted directly into the dlPAG, with bregma serving as the reference for each stereotaxic plane (anterior/posterior, 1.7 mm; medial/lateral, 0.7 mm; dorsal/ventral, 4.5 mm), according to the atlas of Paxinos and Watson (1997). The tip of the guide cannula was positioned 1 mm above the dlPAG and fixed to the skull using acrylic resin and two stainless-steel screws. Afterwards, a stylet was introduced inside the guide cannula to reduce the incidence of occlusion. At the end of surgery, the animals were injected intramuscularly with an antibiotic solution (Pentabiótico, 1 mg/kg; Fort Dodge, São Paulo, Brazil) to prevent possible infection.
Intracerebral injections were performed with a thin dental needle (outer diameter 0.3 mm) that was 1 mm longer than the guide cannula and was connected to a 2-μl microsyringe (7002H, Hamilton, Reno, NV, USA). The needles were carefully inserted to the guide cannulae, and a volume of 0.3 μl was injected over 30 s using a Hamilton microsyringe (Reno, NV, USA) controlled by an infusion pump (BI200 Insight Equipment, Ribeirão Preto, Brazil). A polyethylene-10 catheter was interposed between the upper end of each dental needle and microsyringe. For the combined treatments, the animals received a first microinjection of either saline or L-NAME (200 nmol), followed by a second microinjection of L-Arg (100 nmol) or saline 10 min later.
Behavioral testing
The animals were randomly divided into different experimental groups according to the pharmacological treatments. Six days after surgery and 24 h after ethanol withdrawal, the animals were transported to a dimly illuminated (40 lux), sound-attenuated, and temperature-controlled (23 ± 1 °C) room and remained undisturbed for at least 1 h prior to testing. They were then individually evaluated in the light/dark box followed by the open field test 10 min after the last drug administration. After each trial, the light/dark box and open field were cleaned with a 70 % alcohol solution.
To confirm the behavioral effects of ethanol withdrawal, two additional nonoperated experimental groups were included: (1) a control nonoperated group that received a liquid diet without ethanol and (2) an ethanol withdrawal nonoperated group that received a liquid diet with ethanol. All of the behavioral procedures were identical to the procedures for the dlPAG-operated rats.
All of the behavioral sessions were performed during the diurnal phase (between 8:00 AM and 12:00 PM) and videotaped for later analysis using a video-tracking analysis system (ANY-maze version 1.9; Stoelting, Wood Dale, USA).
Light/dark box test
The apparatus consisted of a wooden box (80 × 40 × 20 cm) divided into two equal-size compartments (40 × 40 × 20 cm) by a barrier that had a doorway (8 × 12 cm). One of the compartments was black, and the other compartment was white and well illuminated. The animals were placed in the middle of the lit compartment, facing away from the dark compartment, and were allowed to freely explore the box for 5 min. The latency (in seconds) to enter the dark compartment with all four paws, number of transitions, and time (in seconds) spent in the light compartment were manually scored.
Open field test
After light/dark box testing, each animal was immediately exposed to the open field apparatus, which consisted of a wooden black box (80 × 80 × 50 cm), for 10 min. The software detected the position of the animal in the open field and calculated the distance traveled (in meters).
Histology
After the behavioral tests, the rats were anesthetized with an overdose of pentobarbital (Thiopentax; Cristália, São Paulo, Brazil) and transcardially perfused with saline followed by a 10 % formalin solution. The brains were removed and immersed in a 10 % formalin solution for a minimum of 3 days. Using a freezing microtome, 40-μm coronal brain sections were then cut (Criocut CM 1850; Leica, Bensheim, Germany). The sections were mounted on gelatin-coated slides and stained with Nissl’s dye. In some adjacent sections, NADPH-diaphorase histochemistry was performed in order to visualize NOS-expressing neurons. The injection sites were identified using the Paxinos and Watson (1997) atlas.
Statistical analysis
The data are expressed as mean ± standard error of the mean (SEM). Between-group differences in body weight and ethanol consumption were analyzed using repeated-measures analysis of variance (ANOVA), with group as the independent factor and day (1 to 15) as the repeated measure.
The data obtained in the light/dark box and open field test were analyzed using Student’s t test or one-way ANOVA. Post hoc comparisons were performed using Tukey’s test. Values of p < 0.05 were considered statistically significant.
Results
The total daily liquid diet intake of the rats ranged from 6.4 to 12.6 g/kg/day over the 15-day period. The body weights of the animals ranged from 262.5 to 282.7 g at the beginning of the treatment to 304.5–323.0 g after ethanol withdrawal. No differences in body weights were observed among the experimental groups (F 14, 149 = 1.33, p = 0.19). A significant group × day interaction was found (F 14, 149 = 1.79, p < 0.001). From day 7, all experimental groups exhibited increase of weight when compared to the first day.
As shown in Fig. 1, ethanol withdrawal decreased the latency (t 16 = 2.57, p = 0.021) and percent time in the light compartment (t 16 = 5.33, p < 0.0001) in the light/dark box in ethanol-withdrawn rats compared with controls. A decrease was also found in the number of transitions in ethanol-withdrawn rats compared with controls, although the difference did not reach statistical significance (t 16 = 1.74, p = 0.10). A significant difference in the distance traveled was observed in ethanol-withdrawn rats compared with controls (t 10 = 2.79, p = 0.019).
Figure 2 shows representative diagrams and photomicrographs of the injection sites in the dlPAG. The animals that received microinjections outside the dlPAG were excluded from the statistical analysis.
a Diagram of the dlPAG, modified from the Paxinos and Watson (1997) atlas. b, c Photomicrographs of a coronal section (40 μm) from a rat brain with a guide cannula implanted into the dlPAG. Notice the presence of NADPH-diaphorase-positive neurons that delimit the dorsal column of the dlPAG. Aq aqueduct of Sylvius. d Representative diagrams showing the localization of microinjection sites inside (filled circles) and outside of the dlPAG (open circles). Numbers on each section indicate the distance from bregma (Paxinos and Watson 1997)
The ANOVA revealed that the NO scavenger carboxy-PTIO (1 nmol) increased the latency to the first entry in the dark compartment (F 3, 43 = 2.91, p = 0.05) and percent time spent in the light compartment of the light/dark box (F 3, 43 = 2.72, p = 0.05) compared with the saline group (Fig. 3), indicating that this treatment decreased ethanol withdrawal-induced anxiety-like behavior in rats. No significant difference was found among the experimental groups with regard to the number of transitions between the two compartments in the light/dark box or distance traveled in the open field test (Table 1).
Effects of carboxy-PTIO (1 nmol) injected into the dlPAG of rats tested in the light/dark box. The data are expressed as mean ± SEM (n = 8–15 per group). Rats that received injections of carboxy-PTIO outside the dlPAG (n = 7) are represented as an OUT group. Asterisks indicate the significant difference from saline (Sal) group (*p < 0.05, ANOVA followed by Tukey’s test)
As shown in Fig. 4, L-NAME (200 nmol) increased the latency to the first entry in the dark compartment (F 3, 46 = 3.24, p = 0.03) and percent time spent in the light compartment of the light/dark box (F 3, 46 = 7.93, p < 0.001) compared with the saline group. A trend to decrease the number of transitions in the light/dark box was detected (F 3, 46 = 2.24, p = 0.10). No significant effect was observed in distance traveled in the open field test (Table 1). The effects of L-NAME on the latency and percent time spent in the light compartment were reversed by L-Arg (F 3, 30 = 5.45, p = 0.005, and F 3, 30 = 4.03, p = 0.017, respectively), implicating the involvement of NO in the observed effects. No changes were detected in the number of transitions and distance traveled in the open field test.
Effects of L-NAME (50–200 nmol) or L-NAME (200 nmol) followed by saline or L-Arg (100 nmol) injected into the dlPAG of rats tested in the light/dark box. Rats that received injections of active compounds outside the dlPAG (L-NAME, n = 6 and L-NAME combined treatment groups, n = 3) are represented as OUT groups. The data are expressed as mean ± SEM (n = 8–15 per group) and were analyzed by ANOVA followed by Tukey’s test. Asterisks indicate the significant difference from Sal or Sal + Sal control groups (*p < 0.05, **p < 0.001) and number signs (# p < 0.05, ## p < 0.001) indicate the significant difference from the L-NAME (200 nmol) + Sal group
TRIM (20 nmol) increased the percent time in the light compartment (F 3, 42 = 3.19, p = 0.034; Fig. 5). A trend to increase the number of transitions was also detected with TRIM (20 nmol) (F 3, 42 = 2.58, p = 0.07). ANOVA revealed no difference in the latency or distance traveled in the open field test when compared with controls.
Effects of TRIM (20–80 nmol) injected into the dlPAG of rats tested in the light/dark box. Rats that received injections of TRIM outside the dlPAG (n = 10) are represented as an OUT group. The data are expressed as mean ± SEM (n = 8–15 per group) and were analyzed by ANOVA followed by Tukey’s test. Asterisk indicates the significant difference from Sal group (*p < 0.05)
Figure 6 shows the effects of injections of the selective iNOS inhibitor 1400W into the dlPAG. 1400W at 0.3 and 1 nmol significantly increased the percent time spent in the light compartment of the light/dark box compared with the saline group (F 3, 39 = 6.26, p = 0.0016). No differences were found with regard to the latency to the first entry in the dark compartment, number of transitions in the light/dark box, or distance traveled in the open field test when compared with the saline group.
Effects of 1400W (0.1–1.0 nmol) injected into the dlPAG of rats tested in the light/dark box. Rats that received injections of 1400W outside the dlPAG (n = 8) are represented as an OUT group. The data are expressed as mean ± SEM (n = 7–15 per group). Asterisk indicates the significant difference from Sal group (*p < 0.05, ANOVA followed by Tukey’s test)
Discussion
The dlPAG has been shown to be involved in the expression of anxiety-like behavior during ethanol withdrawal in rats (Bonassoli et al. 2011; Cabral et al. 2006; Long et al. 2007; Yang et al. 2003). Our results suggest that these behaviors may be attributable to increases in NO levels in the dlPAG. In the present study, direct dlPAG administration of carboxy-PTIO and L-NAME, a NO scavenger and a nonselective NOS inhibitor, respectively, decreased the anxiogenic-like effects of ethanol withdrawal in rats subjected to the light/dark box. Because L-Arg, a NO precursor, reversed the effects of L-NAME, these findings support the hypothesis that NO production in the dlPAG plays a role in the modulation of ethanol withdrawal-induced anxiety-like behavior in rats. Our results further demonstrated that iNOS-mediated NO synthesis in the dlPAG is predominantly involved in the behavioral expression of anxiety during ethanol withdrawal.
Abrupt cessation of chronic ethanol administration increases anxiety-like behavior in rodents subjected to different behavioral tasks, which may reflect different aspects of human ethanol dependence (for review, see Kliethermes 2005). Anxiety-like behaviors in the light/dark box have been suggested to be an index of acute anxiety 10 to 48 h after ethanol discontinuation (Costall et al. 1988; Kliethermes et al. 2004; Kliethermes 2005). Anxiogenic-like effects have been characterized by a decrease in the latency to enter the dark compartment and time spent in the light compartment (Bourin and Hascoët 2003). The number of transitions between the light and dark compartments of the light/dark box has been reported to be an index of activity/exploration and, in general, this parameter is decreased during ethanol withdrawal (Kliethermes et al. 2004; Costall et al. 1993). Accordingly, we found that ethanol-withdrawn rats exhibited decreases in latency and the percent time spent in the light side of the light/dark box 24 h after abrupt ethanol discontinuation compared with control animals. A statistical trend to decrease the number of crossings in the light/dark box and a significant decrease in the distance traveled in the open field were also detected in the ethanol-withdrawn rats compared with controls. These results are in agreement with previous reports (Bonassoli et al. 2011) showing that ethanol withdrawal significantly decreased the traveled by rats exposed to the open field test. Trends to increase the number of transitions in the light/dark box, on the other hand, were observed following TRIM (20 nmol), L-Arg (100 nmol), or L-Arg + L-NAME administrations into the dlPAG. The increase in the number of transitions in the light/dark box may indicate a decrease in the anxiogenic-like effect induced by ethanol withdrawal.
The effects of NO in the dlPAG appear to occur at the level of mediator release because inhibition of endogenous NO by the NO scavenger carboxy-PTIO or NOS inhibitors decreased anxiogenic-like behavior induced by ethanol withdrawal. Curiously, two distinct effect profiles of NO-interfering drugs were observed in ethanol-withdrawn rats subjected to the light/dark box. The effects of the NO scavenger carboxy-PTIO and TRIM were evident only at the lowest doses tested (i.e., 1 and 20 nmol, respectively), and the effects of the nonselective NOS inhibitor L-NAME and selective iNOS inhibitor 1400W were obtained with higher doses (i.e., 200 and 0.1–0.3 nmol, respectively). The reasons for these discrepant results are unclear. The absence of effects of the higher TRIM doses is consistent with previous studies that found inverted U-shaped dose–response curves for NOS inhibitors injected into the dlPAG (Guimarães et al. 1994; Tonetto et al. 2009). Local dlPAG injection of carboxy-PTIO (2 nmol) into dlPAG resulted in anxiolytic-like effects in the Vogel conflict test while a similar treatment (carboxy-PTIO, 1 and 3 nmol) failed to prevent defensive reaction induced by NMDA injection in the dlPAG (Aguiar et al. 2006). Carboxy-PTIO, in addition to its potent activity as a NO scavenger, has been shown to enhance the effects of the NO donor 3-morpholinosylnomine hydrochloride in cultured endothelial cells by inducing peroxynitrite formation (Pfeiffer et al. 1997). Peroxynitrite may in fact exert similar physiological effects as NO, such as the induction of NO-like relaxation of vascular smooth muscles (Liu et al. 1994), inhibition of platelet aggregation (Moro et al. 1994), and stimulation of soluble guanylyl cyclase (Mayer et al. 1995). Therefore, if a carboxy-PTIO-induced increase in peroxynitrite occurs in the dlPAG in ethanol-withdrawn rats, then this effect may counteract the scavenger properties of carboxy-PTIO. Nevertheless, we cannot exclude the possible participation of other molecules in the NO-cGMP cascade and downstream effectors in ethanol withdrawal-induced anxiety-like behavior. cGMP acts as a second messenger, amplifying signals received at postsynaptic receptors and activating effector molecules that result in gene expression changes and specific neuronal responses. These aspects deserve further investigation and will be matter of future work in our laboratory.
Anxiolytic- and antidepressant-like effects have been largely attributed to the selective inhibition of nNOS by 7-nitroindazole (Yildiz et al. 2000), TRIM (Volke et al. 2003), and N-propyl-L-arginine (Montezuma et al. 2012). Preclinical and clinical findings, such as the increased expression of nNOS in limbic regions in stressed animals (De Oliveira et al. 2001) and depressed patients (De Oliveira et al. 2008), have also helped to link nNOS with anxiety-like behavior and a depressive phenotype in the Flinders sensitive rat line (Wegener and Volke 2010). A recent study found that iNOS inhibitors induced an antidepressant-like effect (Montezuma et al. 2012). Despite the presence of NOS isoforms in the brain, the role of eNOS in experimental anxiety, including ethanol withdrawal-induced anxiety-like behavior in rats, is still unknown. One reason for this lack of knowledge is the lack of high-affinity eNOS inhibitors on the market. The selectivity of some nNOS compounds has been attributed to the presence of extra charge–charge interactions caused by nNOS’s extended conformation, whereas the selectivity of high-affinity iNOS inhibitors may be explained by the formation of an iNOS-specific subpocket upon binding (Oliveira et al. 2012). Our results suggest that both the nNOS and iNOS isoforms contribute to the action of NO, with iNOS as the likely main source for NO in the dlPAG during ethanol withdrawal. This is consistent with the pharmacological potency of both TRIM and 1400W in inhibiting NOS isoforms. TRIM has been described as a selective nNOS inhibitor in the rat brain under normal physiological conditions (Handy et al. 1996). However, the ability of TRIM to inhibit both the nNOS (K i = 27 μM) and iNOS (K i = 28.2 μM) isoforms is equivalent (Handy et al. 1995). 1400W has been shown to be an irreversible and specific iNOS inhibitor (K i = 7 nM, i.e., 5,000- and 200-fold more potent against iNOS than eNOS and nNOS, respectively) (Garvey et al. 1997). In the present study, TRIM was less effective than 1400W in decreasing ethanol withdrawal-induced anxiety-like behavior. Additionally, the actions of both 1400W and L-NAME in ethanol-withdrawn rats yielded similar behavior profiles, indicating that the iNOS isoform in the dlPAG may play a predominant role in the modulation of anxiety during ethanol withdrawal.
In the brain, NO production can occur as a result of the activity of either dominant constitutive nNOS or iNOS, and NO production can be induced by both under stressful conditions. In general, iNOS overexpression has been associated with the presence of inflammatory and infectious processes (Brown 2007; Guix et al. 2005). However, several studies have pointed to a constitutive expression of iNOS in the brain, and raised the possibility of astrocyte-derived NO participating in physiological process (for review, see Amitai 2010). iNOS has been detected in the hippocampus of young and aged rats under basal circumstances, in close proximity to newly born cells (Adachi et al. 2010; Pinnock et al. 2007). iNOS expression has also been described in the neocortex, striatum, amygdala, hypothalamus, and brain stem, where it could be involved in the modulation of autonomic activity and synaptic transmission (Amitai 2010). Recently, Montezuma et al. (2012) demonstrated that selective iNOS inhibition or knockdown induced antidepressant-like effects in rats, suggesting that iNOS-mediated NO synthesis may be involved in the modulation of stress-induced behavioral effects. Additionally, increased levels of iNOS and COX-2 have been described in the hippocampus, cortex, and cerebellum in rats 24 h after a cyclic pattern of ethanol exposure and withdrawal. These findings were associated with the development of motor and cognitive deficits (Pascual et al. 2007). Elevated glutamate levels have been directly associated with increased iNOS expression and glial activation in the hippocampus of rats subjected to an ethanol binge drinking model (Ward et al. 2009). In the present study, 1400W administered into the dlPAG decreased anxiety-like behavior induced by ethanol withdrawal. Altogether, these results support the hypothesis that NO production in the dlPAG is involved in the modulation of anxiety during ethanol withdrawal.
Behavioral signs of ethanol withdrawal in rodents are consequences of neuroadaptative processes elicited by the interruption of ethanol consumption following prolonged ethanol exposure (Cowen and Lawrence 2006; Kliethermes 2005). During ethanol withdrawal, excitatory transmission increases in an attempt to maintain homeostasis in the face of the ethanol-mediated enhancement of γ-aminobutyric acid (GABA) inhibition (De Witte et al. 2003; Koob 2004). Accumulating evidence suggests that the NMDA glutamate receptor is upregulated during ethanol withdrawal, which has been related to the occurrence of signs of ethanol withdrawal, such as seizures, convulsions, and anxiety-like behavior (Grant et al. 1990; Kotlinska and Bochenski 2008; Nagy et al. 2005). Increases in glutamatergic function have been shown to occur during ethanol withdrawal in brain structures related to the control and expression of anxiety, such as the hippocampus (Dahchour and De Witte 2003; Whittington et al. 1995), basolateral amygdala (Christian et al. 2012; Läck et al. 2007; McCool et al. 2010), and bed nucleus of stria terminalis (Kash et al. 2009). Increased glutamatergic transmission has also been detected in the hippocampus in benzodiazepine-withdrawn rats (Das et al. 2008; Van Sickle et al. 2004). Souza-Pinto et al. (2007) showed that the inhibition of glutamatergic neurotransmission in the dlPAG reduces the anxiety-like effects of diazepam withdrawal in rats, a finding that implicates involvement of excitatory transmission in the dlPAG in the modulation of the aversive state induced by benzodiazepine withdrawal. Given the similar pharmacological mechanisms of action of benzodiazepines and ethanol, similar neurochemical effects may also occur in the dlPAG during ethanol withdrawal. However, this assumption remains to be further investigated.
The mechanisms by which NO influences dlPAG function during ethanol withdrawal are unknown. Intracellular NO signaling involves the activation of guanylate cyclase, but it also interacts with mitogen-activated protein kinases, apoptosis-related proteins, and the mitochondrial respiratory chain (Guix et al. 2005). In the dlPAG, complex interactions between NO and glutamatergic, GABAergic, or serotoninergic systems have been shown to modulate anxiety-like behavior (Guimarães et al. 2005; Moreira et al. 2012), blood pressure (Chaitoff et al. 2012; Hall and Behbehani 1998), and nociception (Hamalainen and Lovick 1997). Recently, an interaction between NO and the cannabinoid system in the dlPAG in the modulation of defensive behavior has also been proposed (Lisboa and Guimarães 2012). Given the putative important role that anxiety plays in the etiology of ethanol addiction, future studies are needed to solve the specific molecular mechanisms through which NO transmission in the dlPAG contributes to anxiety-like behavior during ethanol withdrawal.
In conclusion, the present study showed that inhibition of iNOS in the dlPAG decreases ethanol withdrawal-induced anxiety-like behavior in rats, an observation supporting the involvement of NO in the dlPAG in the modulation and expression of anxiety-like behavior.
References
Adachi M, Abe M, Sasaki T, Kato H, Kasahara J, Araki T (2010) Role of inducible or neuronal nitric oxide synthase in neurogenesis of the dentate gyrus in aged mice. Metab Brain Dis 25:419–424
Adams ML, Sewing BN, Chen J, Meyer ER, Cicero TJ (1995) Nitric oxide-related agents alter alcohol withdrawal in male rats. Alcohol Clin Exp Res 19(1):195–199
Aguiar DC, Moreira FA, Guimarães FS (2006) Flight reactions induced by injection of glutamate N-methyl-d-aspartate receptor agonist into the rat dorsolateral periaqueductal gray are not dependent on endogenous nitric oxide. Pharmacol Biochem Behav 83(2):296–301
Amano K, Tanikawa T, Iseki H, Kawabatake H, Notani M, Kawamura H, Kitamura K (1978) Single neuron analysis of the human midbrain tegmentum. Rostral mecencephalic reticulotomy for pain relief. Appl Neurophysiol 41:66–78
Amitai Y (2010) Physiologic role for “inducible” nitric oxide synthase: a new form of astrocytic-neuronal interface. Glia 58(15):1775–1781
Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT (1991) CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 103(2):227–232
Bandler R, Carrive P (1988) Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res 439(1–2):95–106
Bandler R, Shipley MT (1994) Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 7(9):379–389
Bittencourt AS, Nakamura-Palacios EM, Mauad H, Tufik S, Schenberg LC (2005) Organization of electrically and chemically evoked defensive behaviors within the deeper collicular layers as compared to the periaqueductal gray matter of the rat. Neuroscience 133(4):873–892
Bonassoli VT, Milani H, de Oliveira RMW (2011) Ethanol withdrawal activates nitric oxide-producing neurons in anxiety-related brain areas. Alcohol 45(7):641–652
Bourin M, Hascoët M (2003) The mouse light/dark box test. Eur J Pharmacol 463(1–3):55–65
Brandão ML, Anseloni VZ, Pandossio JE, De Araujo JE, Castilho VM (1999) Neurochemical mechanisms of the defensive behaviour in the dorsal midbrain. Neurosci Biobehav Rev 23:863–875
Bredt DS, Snyder SH (1994) Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63:175–195
Bredt DS, Hwang PM, Snyder SH (1990) Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347(6295):768–770
Brown GC (2007) Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans 35(Pt 5):1119–1121
Cabral A, Isoardi N, Salum C, Macedo CE, Nobre MJ, Molina VA (2006) Fear state induced by ethanol withdrawal may be due to the sensitization of the neural substrates of aversion in the dPAG. Exp Neurol 200(1):200–208
Calixto AV, Duarte FS, Moraes CKL, Faria MS, De Lima TCM (2008) Nitric oxide involvement and neural substrates of the conditioned and innate fear as evaluated in the T-maze test in rats. Behav Brain Res 189(2):341–349
Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD (1995) The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol 351:568–584
Canteras NS, Swanson LW (1992) The dorsal premammillary nucleus: an unusual component of the mammillary body. Proc Natl Acad Sci USA 89:10089–10093
Chaitoff KA, Toner F, Tedesco A, Maher TJ, Ally A (2012) Effects of inducible nitric oxide synthase blockade within the periaqueductal gray on cardiovascular responses during mechanical, heat, and cold nociception. Neurol Sci 33(1):69–78
Chiavegatto S, Scavone C, Canteras NS (1998) Nitric oxide synthase activity in the dorsal periaqueductal gray of rats expressing innate fear responses. Neuroreport 9(4):571–576
Chick J, Nutt DJ (2012) Substitution therapy for alcoholism: time for a reappraisal? J Psychopharmacol (Oxford) 26(2):205–212
Christian DT, Alexander NJ, Diaz MR, McCool BA (2012) Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology 65:134–142
Clapp P (2012) Current progress in pharmacologic treatment strategies for alcohol dependence. Expert Rev Clin Pharmacol 5(4):427–435
Costall B, Kelly ME, Naylor RJ (1988) The anxiolytic and anxiogenic actions of ethanol in a mouse model. J Pharm Pharmacol 40(3):197–202
Costall B, Domeney AM, Kelly ME, Tomkins DM, Naylor RJ, Wong EH (1993) The effect of the 5-HT3 receptor antagonist, RS-42358-197, in animal models of anxiety. Eur J Pharmacol 234:91–99
Cowen MS, Lawrence AJ (2006) Alcoholism and neuropeptides: an update. CNS Neurol Disord Drug Targets 5(2):233–239
Dahchour A, De Witte P (2003) Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol 459(2–3):171–178
Das P, Lilly SM, Zerda R, Gunning WT 3rd, Alvarez FJ, Tietz EI (2008) Increased AMPA receptor GluR1 subunit incorporation in rat hippocampal CA1 synapses during benzodiazepine withdrawal. J Comp Neurol 511(6):832–846
De Oliveira RW, Guimarães FS (1999) Anxiolytic effect of methylene blue microinjected into the dorsal periaqueductal gray matter. Braz J Med Biol Res 32(12):1529–1532
De Oliveira RM, Aparecida Del Bel E, Mamede-Rosa ML, Padovan CM, Deakin JF, Guimarães FS (2000a) Expression of neuronal nitric oxide synthase mRNA in stress-related brain areas after restraint in rats. Neurosci Lett 289(2):123–126
De Oliveira RW, Del Bel EA, Guimarães FS (2000b) Behavioral and c-fos expression changes induced by nitric oxide donors microinjected into the dorsal periaqueductal gray. Brain Res Bull 51(6):457–464
De Oliveira RM, Del Bel EA, Guimarães FS (2001) Effects of excitatory amino acids and nitric oxide on flight behavior elicited from the dorsolateral periaqueductal gray. Neurosci Biobehav Rev 25(7–8):679–685
De Oliveira RMW, Guimaraes FS, Deakin JF (2008) Expression of neuronal nitric oxide synthase in the hippocampal formation in affective disorders. Braz J Med Biol Res 41:333–341
De Witte P, Pinto E, Ansseau M, Verbanck P (2003) Alcohol and withdrawal: from animal research to clinical issues. Neurosci Biobehav Rev 27(3):189–197
Forestiero D, Manfrim CM, Guimarães FS, de Oliveira RMW (2006) Anxiolytic-like effects induced by nitric oxide synthase inhibitors microinjected into the medial amygdala of rats. Psychopharmacology (Berl) 184(2):166–172
Förstermann U, Gath I, Schwarz P, Closs EI, Kleinert H (1995) Isoforms of nitric oxide synthase. Properties, cellular distribution and expressional control. Biochem Pharmacol 50(9):1321–1332
Garthwaite J, Charles SL, Chess-Williams R (1988) Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336(6197):385–388
Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ (1997) 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem 272(8):4959–4963
Gilpin NW (2012) Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol 46(4):329–337
Gören MZ, Aricioglu-Kartal F, Yurdun T, Uzbay IT (2001) Investigation of extracellular l-citrulline concentration in the striatum during alcohol withdrawal in rats. Neurochem Res 26(12):1327–1333
Grant KA, Valverius P, Hudspith M, Tabakoff B (1990) Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol 176(3):289–296
Gualda LB, Martins GG, Müller B, Guimarães FS, Oliveira RMW (2011) 5-HT1A autoreceptor modulation of locomotor activity induced by nitric oxide in the rat dorsal raphe nucleus. Braz J Med Biol Res 44(4):332–336
Guimarães FS, de Aguiar JC, Del Bel EA, Ballejo G (1994) Anxiolytic effect of nitric oxide synthase inhibitors microinjected into the dorsal central grey. Neuroreport 5(15):1929–1932
Guimarães FS, Beijamini V, Moreira FA, Aguiar DC, de Lucca ACB (2005) Role of nitric oxide in brain regions related to defensive reactions. Neurosci Biobehav Rev 29(8):1313–1322
Guix FX, Uribesalgo I, Coma M, Muñoz FJ (2005) The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 76(2):126–152
Hall CW, Behbehani MM (1998) Synaptic effects of nitric oxide on enkephalinergic, GABAergic, and glutamatergic networks of the rat periaqueductal gray. Brain Res 805(1–2):69–87
Hamalainen MM, Lovick TA (1997) Role of nitric oxide and serotonin in modulation of the cardiovascular defence response evoked by stimulation in the periaqueductal grey matter in rats. Neurosci Lett 229(2):105–108
Handy RL, Wallace P, Gaffen ZA, Whitehead KJ, Moore PK (1995) The antinociceptive effect of 1-(2-trifluoromethylphenyl) imidazole (TRIM), a potent inhibitor of neuronal nitric oxide synthase in vitro, in the mouse. Br J Pharmacol 116(5):2349–2350
Handy RL, Harb HL, Wallace P, Gaffen Z, Whitehead KJ, Moore PK (1996) Inhibition of nitric oxide synthase by 1-(2-trifluoromethylphenyl) imidazole (TRIM) in vitro: antinociceptive and cardiovascular effects. Br J Pharmacol 119(2):423–431
Kalinchuk AV, Stenberg D, Rosenberg PA, Porkka-Heiskanen T (2006) Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur J Neurosci 24(5):1443–1456
Kash TL, Baucum AJ 2nd, Conrad KL, Colbran RJ, Winder DG (2009) Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology 34(11):2420–2429
Kishimoto J, Tsuchiya T, Emson PC, Nakayama Y (1996) Immobilization-induced stress activates neuronal nitric oxide synthase (nNOS) mRNA and protein in hypothalamic-pituitary-adrenal axis in rats. Brain Res 720(1–2):159–171
Kliethermes CL (2005) Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev 28(8):837–850
Kliethermes CL, Cronise K, Crabbe JC (2004) Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res 28(7):1012–1019
Knapp DJ, Overstreet DH, Angel RA, Navarro M, Breese GR (2007) The amygdala regulates the antianxiety sensitization effect of flumazenil during repeated chronic ethanol or repeated stress. Alcohol Clin Exp Res 31(11):1872–1882
Koob GF (2004) A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol 68(8):1515–1525
Kotlinska J, Bochenski M (2008) The influence of various glutamate receptors antagonists on anxiety-like effect of ethanol withdrawal in a plus-maze test in rats. Eur J Pharmacol 598(1–3):57–63
Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA (2007) Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol 98(6):3185–3196
Leite LE, Nobre MJ (2012) The negative effects of alcohol hangover on high-anxiety phenotype rats are influenced by the glutamate receptors of the dorsal midbrain. Neuroscience 28(213):93–105
Lisboa SF, Guimarães FS (2012) Differential role of CB1 and TRPV1 receptors on anandamide modulation of defensive responses induced by nitric oxide in the dorsolateral periaqueductal gray. Neuropharmacology 62(8):2455–2462
Liu S, Beckman JS, Ku DD (1994) Peroxynitrite, a product of superoxide and nitric oxide, produces coronary vasorelaxation in dogs. J Pharmacol Exp Ther 268(3):1114–1121
Long C, Yang L, Faingold CL, Steven Evans M (2007) Excitatory amino acid receptor-mediated responses in periaqueductal gray neurons are increased during ethanol withdrawal. Neuropharmacology 52(3):802–811
Lyons CR, Orloff GJ, Cunningham JM (1992) Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem 267(9):6370–6374
Mayer B, Schrammel A, Klatt P, Koesling D, Schmidt K (1995) Peroxynitrite-induced accumulation of cyclic GMP in endothelial cells and stimulation of purified soluble guanylyl cyclase. Dependence on glutathione and possible role of S-nitrosation. J Biol Chem 270(29):17355–17360
McCool BA, Christian DT, Diaz MR, Läck AK (2010) Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol 91:205–233
Moncada S, Higgs A, Furchgott R (1997) International Union of Pharmacology nomenclature in nitric oxide research. Pharmacol Rev 49(2):137–142
Montezuma K, Biojone C, Lisboa SF, Cunha FQ, Guimarães FS, Joca SRL (2012) Inhibition of iNOS induces antidepressant-like effects in mice: pharmacological and genetic evidence. Neuropharmacology 62(1):485–491
Moreira FA, Aguiar DC, Resstel LB, Lisboa SF, Campos AC, Gomes FV, Guimarães FS (2012) Neuroanatomical substrates involved in cannabinoid modulation of defensive responses. J Psychopharmacol 26(1):40–55
Moro MA, Darley-Usmar VM, Goodwin DA, Read NG, Zamora-Pino R, Feelisch M (1994) Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci U S A 91(14):6702–6706
Murphy S, Simmons ML, Agullo L, Garcia A, Feinstein DL, Galea E (1993) Synthesis of nitric oxide in CNS glial cells. Trends Neurosci 16(8):323–328
Nagy J, Kolok S, Boros A, Dezso P (2005) Role of altered structure and function of NMDA receptors in development of alcohol dependence. Curr Neuropharmacol 3(4):281–297
Nashold BS, Wilson WP, Slaughter DG (1969) Sensations evoked by stimulation in the midbrain of man. J Neurosurg 30:14–24
Oliveira BL, Moreira IS, Fernandes PA, Ramos MJ, Santos I, Correia JD (2012) Insights into the structural determinants for selective inhibition of nitric oxide synthase isoforms. J Mol Model. doi:10.1007/s00894-012-1677-8
Onstott D, Mayer B, Beitz AJ (1993) Nitric oxide synthase immunoreactive neurons anatomically define a longitudinal dorsolateral column within the midbrain periaqueductal gray of the rat: analysis using laser confocal microscopy. Brain Res 610(2):317–324
Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C (2007) Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci 25(2):541–550
Paxinos G, Watson C (1997) The rat brain in stereotaxic co-ordinates, 4th edn. Academic, San Diego
Pfeiffer S, Leopold E, Hemmens B, Schmidt K, Werner ER, Mayer B (1997) Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Radic Biol Med 22(5):787–794
Pinnock SB, Balendra R, Chan M, Hunt LT, Turner-Stokes T, Herbert J (2007) Interactions between nitric oxide and corticosterone in the regulation of progenitor cell proliferation in the dentate gyrus of the adult rat. Neuropsychopharmacology 32:493–504
Rizvi TA, Ennis M, Behbehani M, Shipley MT (1991) Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol 303:121–131
Russo AS, Guimarães FS, De Aguiar JC, Graeff FG (1993) Role of benzodiazepine receptors located in the dorsal periaqueductal grey of rats in anxiety. Psychopharmacology (Berl) 110(1–2):198–202
Schenberg LC, Graeff FG (1978) Role of the periaqueductal gray substance in the antianxiety action of benzodiazepines. Pharmacol Biochem Behav 9(3):287–295
Schuman EM, Madison DV (1991) A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science 254(5037):1503–1506
Smalls SL, Okere CO (2012) Acute restraint increases varicosity density and reduces the inter-varicosity distance in NADPH diaphorase-containing neurons in the rat dorsolateral periaqueductal gray matter. Neurosci Lett 511(1):23–27
Souza-Pinto LFS, Castilho VM, Brandão ML, Nobre MJ (2007) The blockade of AMPA-kainate and NMDA receptors in the dorsal periaqueductal gray reduces the effects of diazepam withdrawal in rats. Pharmacol Biochem Behav 87(2):250–257
Spiacci A Jr, Kanamaru F, Guimarães FS, Oliveira RMW (2008) Nitric oxide-mediated anxiolytic-like and antidepressant-like effects in animal models of anxiety and depression. Pharmacol Biochem Behav 88(3):247–255
Tonetto LL, Terzian AL, Del Bel EA, Guimarães FS, Resstel LB (2009) Inhibition of the NMDA receptor/Nitric Oxide pathway in the dorsolateral periaqueductal gray causes anxiolytic-like effects in rats submitted to the Vogel conflict test. Behav Brain Funct 5:40
Uzbay IT (2001) L-NAME precipitates catatonia during ethanol withdrawal in rats. Behav Brain Res 119(1):71–76
Uzbay IT, Erden BF, Tapanyigit EE, Kayaalp SO (1997) Nitric oxide synthase inhibition attenuates signs of ethanol withdrawal in rats. Life Sci 61(22):2197–2209
Van Sickle BJ, Xiang K, Tietz EI (2004) Transient plasticity of hippocampal CA1 neuron glutamate receptors contributes to benzodiazepine withdrawal-anxiety. Neuropsychopharmacology 29(11):1994–2006
Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M (2009) Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res 33:945–969
Vincent SR, Kimura H (1992) Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46(4):755–784
Volke V, Wegener G, Bourin M, Vasar E (2003) Antidepressant- and anxiolytic-like effects of selective neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole in mice. Behav Brain Res 140:141–147
Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte LD, Dexter D (2009) Neuro-inflammation induced in the hippocampus of 'binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem 111(5):1119–1128
Wegener G, Volke V (2010) Nitric oxide synthesis inhibitors as antidepressants. Pharmaceuticals 3:273–279
Whittington MA, Lambert JD, Little HJ (1995) Increased NMDA receptor and calcium channel activity underlying ethanol withdrawal hyperexcitability. Alcohol Alcohol 30(1):105–114
Yang L, Long C, Randall ME, Faingold CL (2003) Neurons in the periaqueductal gray are critically involved in the neuronal network for audiogenic seizures during ethanol withdrawal. Neuropharmacology 44(2):275–281
Yildiz F, Erden BF, Ulak G, Utkan T, Gacar N (2000) Antidepressant-like effect of 7-nitroindazole in the forced swimming test in rats. Psychopharmacology (Berl) 149:41–44
Zhou D, Lee H, Rothfuss JM, Chen DL, Ponde DE, Welch MJ (2009) Design and synthesis of 2-amino-4-methylpyridine analogues as inhibitors for inducible nitric oxide synthase and in vivo evaluation of [18F]6-(2-fluoropropyl)-4-methyl-pyridin-2-amine as a potential PET tracer for inducible nitric oxide synthase. J Med Chem 52(8):2443–2453
Acknowledgments
This work was supported by grants from Fundação Araucária (006/2011, 15245) and CNPq, Brazil. We thank M.A. Trombelli for the technical support.
Conflict of interest
There is no conflict of interest with any organization regarding the material presented in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonassoli, V.T., Contardi, E.B., Milani, H. et al. Effects of nitric oxide synthase inhibition in the dorsolateral periaqueductal gray matter on ethanol withdrawal-induced anxiety-like behavior in rats. Psychopharmacology 228, 487–498 (2013). https://doi.org/10.1007/s00213-013-3049-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3049-1