Abstract
Rationale
Mice are useful tools for dissecting genetic and environmental factors in relation to the study of attention and impulsivity. The five-choice serial reaction time task (5CSRTT) paradigm has been well established in rats, but its transferability to mice is less well documented.
Objectives
This study aims to summarise the main results of the 5CSRTT in mice, with special focus on impulsivity.
Methods
The 5CSRTT can be used to explore aspects of both attentional and inhibitory control mechanisms.
Results
Different manipulations of the task parameters can lead to different results; adjusting the protocol as a function of the main variable of interest or the standardisation of the protocol to be applied to a large set of strains will be desirable.
Conclusions
The 5CSRTT has proven to be a useful tool to investigate impulsivity in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
A constellation of neuropsychiatric disorders, such as ADHD, personality disorders, mania, Alzheimer’s disease (AD), Parkinson’s disease and substance abuse, has been associated with attentional disruptions and impulsive behaviours (Clark and Robbins 2002; Evenden 1999). Several operant tasks have been developed to assess both attention and impulsivity in rodents, including the now well-established five-choice serial reaction time task (5CSRTT). This technique has been proven to be a useful tool in rat studies and is increasingly used in mouse studies. Mice are particularly useful in the dissection of genetic and environmental factors that might influence behaviour in the task. This review is aimed at presenting the evidence generated from mouse studies and will discuss the nature of the results found among different studies in relation to the particular procedures implemented. The surveyed data suggest that the results obtained may depend on the particular parameters of the test. Hence, information regarding the extent to which the parameters of the task detect (or even produce) differences in impulsivity will be examined and future directions for research suggested.
Measuring attention and impulsivity: 5CSRTT
In 1983, Robbins and colleagues initially reported a test for assessing attention in rats. This paradigm was based on another procedure used to monitor attentional function in humans, the continuous performance task (Carli et al. 1983; Robbins 2002). Briefly, the 5CSRTT assesses attentional performance by the detection of a brief visual stimulus presented pseudorandomly across several spatial locations, in a five-hole box, though variations with nine holes or even one hole have also been used. The 5CSRTT also provides information about aspects of inhibitory response control: premature responding (responding before the light stimulus is presented) into the holes is viewed as a failure of response inhibition where the animal has to withhold responding until the stimulus light is illuminated and provides a measure of impulsivity (Robbins 2002); perseverative responding occurs when the animal continues (unnecessary) nose-poking into the holes after a correct detection and may represent a measure of compulsivity (Dalley et al. 2008). As indicated by other excellent reviews, attention and impulsivity are not unitary constructs (Evenden 1999; Robbins 2002; Winstanley 2007), and only one specific form of impulsivity is measured by the 5CSRTT. This impulsivity subtype was initially described as ‘motor’ impulsivity (Winstanley 2007), but has been more recently characterised as ‘waiting’ impulsivity (Robinson et al. 2009).
The flexibility and non-aversive nature of the 5CSRTT makes it suitable for several testing purposes and its use has been well described in rats (Robbins 2002). However, due to the availability of techniques to manipulate the mouse genome, it is important to be able to perform these studies in mice. A complete analysis of all the variables of the 5CSRTT is beyond the scope of the present review; instead, we will focus on premature responses, which provide our main impulsivity measure, and other variables will be introduced as secondary measures.
The focus of interest when approaching the study of attentional and impulsive phenotypes in the mouse can be subdivided into different domains which we will categorise into four main topics. Of increasing interest in recent years have been the (1) exploration of the genetic basis of attention and impulsivity, (2) discovery of neurochemical pathways mediating processes of attention, (3) neuropharmacological assessment of drugs and their role in impulsivity and attention and (4) examination of the role of affective states in attention and impulsivity. Although the early reports using mice focused mainly on attentional function, later studies have emphasised other variables such as premature or perseverative responding in the analysis, expanding the use of the task to study aspects of inhibitory control.
Evidence from the mouse: test parameters influence the results
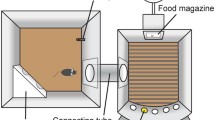
The possible trial sequence that a mouse has to follow to obtain a reinforcer is illustrated in Fig. 1, a similar protocol to that described for rats (Robbins 2002). Considering all variables together, attention is measured mainly by accuracy of performance and omissions and also by a measure of processing speed—the reaction time or latency to perform a correct response. Accuracy (percentage of responses that are correct) provides a conservative measure of attention, and if latencies or total trials completed are not impaired, we can assume that any disruptions of the task are true attentional deficits. Taking the reaction time for a correct response, increases in correct latency in the absence of changes in another reaction time measure (i.e. latency to retrieve the reward) suggest that the animal’s locomotor function and motivation for the reward are unaffected; thus, this measure is likely to reflect a true slowing of processing speed. The average latency to make an incorrect response is rarely reported, presumably because it is affected similarly to the latency to perform a correct response (Amitai and Markou 2010). The second common measure of attention, omissions (failures to respond), can also reflect failures of signal detection and/or motivational/motor deficits (Davies et al. 2007; Humby et al. 1999). On the other hand, inhibitory control variables, premature and perseverative responses, can also affect attentional performance and can be associated with each other, as has been argued by Dalley et al. (2011). The aforementioned considerations, therefore, illustrate a crucial point about the 5CSRTT: it is critical to consider the various measures of the task in combination before a final interpretation is made.
Sequence of a session in a 5CSRTT chamber. Different steps may lead to a reward or to a timeout where no reinforcer (R) is available. Correct responses made into the illuminated hole are rewarded by the presentation of a reinforcer in the magazine entry. Omissions (failure to respond to the signalled stimulus within a concrete period of time), incorrect responses (nose-pokes into a non-designated hole) and premature responses (responses into the apertures during the ITI, prior to the stimulus presentation) are generally followed by a period of ‘timeout’, perseverative responses (responding repeatedly into the apertures after a correct detection and before collecting the reward) can also be punished by a timeout in some protocols. Specifically, mice have to nose-poke into the magazine to start a new trial, and then withhold responding during the ITI (in seconds) until the stimulus is presented. If the animal makes a response into one of the holes during this interval, a premature response is recorded. When the ITI period is terminated, the animal is required to nose-poke into the illuminated hole within a limited time (correct response) in order to obtain a reinforcer in the magazine entry. The times to make the correct response and to collect the reinforcer are also recorded (correct latency and magazine latency, respectively). If the animal nose-pokes in a non-illuminated hole, this is recorded as an incorrect response. Both correct and incorrect responses provide a measure of attention (accuracy), usually assessed by the percentage of correct responses (correct/correct + incorrect). However, the animal can also display no response, and this is recorded as an omission. At the same time, some animals tend to nose-poke repeatedly into the holes after a correct response, this measure being considered a form of compulsivity and recorded as a perseverative response. An additional measure of compulsivity, unfortunately not usually reported, could be responses during timeout (i.e. nose-pokes made during the timeout interval) (Amitai and Markou 2010). To signal appropriateness of behaviour, incorrect, omission, premature and perseverative responses can be followed by a timeout, generally signalled by a period of darkness (or by illuminating the chamber, depending on the protocol); during timeout, no reinforcer can be obtained. Finally, the total number of trials completed (TT) can be examined, providing a measure of motivation. A camera located inside the box may be useful to detect scanning strategies not easily inferred with the analysis of the variables (Humby et al. 1999)
5CSRTT parameters are well defined in rat studies (Robbins 2002). However, the 5CSRTT protocol used in mice varies and the use of different procedures, according to the particular question asked, can make comparison between studies and laboratories sometimes difficult.
Pre-training
A number of stages of pre-training in the 5CSRTT, starting with behavioural shaping, gradually introduce the subjects to the different aspects of the 5CSRTT. As reported by Humby et al. (2005), animals perform behavioural shaping to learn how nose-pokes in the magazine lead to a reinforcer, during a small number of sessions, often no more than two. Studies often limit this pre-training session to 50 reinforcers, but the number of total trials will be increased in subsequent stages of the training. Secondly, during the proper 5CSRTT training, nose-pokes into the signalled holes are required to obtain the reward. Stimuli are initially presented for a longer period of time (usually starting from 30 s) and are subsequently reduced according to specific criteria (de Bruin et al. 2006; Hoyle et al. 2006; Humby et al. 1999). Next, parameters are adjusted according to the performance of each animal, also depending on the study. For instance, when using animals modelling AD-like attentional deficits, the standard task was adjusted to less restrictive conditions: the duration of the session was set to 50 trials or 1 h (which allows the analysis of possible deficits in sustained attention), the inter-trial interval (ITI) was set at 20 s and then a larger-than-usual punishment of 10 s and a less attentionally demanding stimulus duration of 4 s were used (Romberg et al. 2011). In Relkovic et al. (2010), animals were trained to baseline performance at 0.8 s stimulus duration, but only 30 total trials were required to be completed and criteria of 80% of accuracy and <25% of omissions were established. In contrast, when the assessment of attention was the main priority, the number of trials was set at 50, since overtraining could be a confounding factor (Wrenn et al. 2006): attention rather than the ability to learn is being assessed. However, daily sessions of 30 min duration or limited to 100 trials (whichever comes first) are the most commonly used (Greco et al. 2005; Patel et al. 2006). Testing is routinely carried out daily (5–6 days/week) (Hoyle et al. 2006; Oliver et al. 2009; Patel et al. 2006). Animals can also be tested during the dark phase (Pattij et al. 2007), although is important to note that the time at which animals are trained, tested and fed should be constant throughout the experiment (Bari et al. 2008), since circadian changes can lead to different results (Yan et al. 2011).
Another aspect of the protocol that needs particular attention is whether punishment is used. In some studies, omissions (failures to respond when a stimulus is presented) and incorrect responses are recorded and punished with a timeout in which no reinforcer is available for a set period of time; in most studies, as originally set up in rats, premature responses are also punished by a timeout (Humby et al. 1999). The use of the timeout has the consequence of suppressing inappropriate behaviour and thus narrowing the sequence of actions needed to obtain the reward (Bari et al. 2008). Some studies perform the timeout punishment less rigorously, using a 2-s period (e.g. Greco and Carli 2006; Hoyle et al. 2006; Pozzi et al. 2010), whereas others use 4 s (Kerr et al. 2004; Young et al. 2004) or even 10 s (Romberg et al. 2011; Wrenn et al. 2006); 5 s is the most common timeout interval used (e.g. Bailey et al. 2010; Davies et al. 2007; Humby et al. 1999; Lambourne et al. 2007; Loos et al. 2009; Oliver et al. 2009; Pattij et al. 2007; Relkovic et al. 2010; Yan et al. 2011). The timeout might be signalled by a period of darkness (e.g. Hoyle et al. 2006; Oliver et al. 2009; Walker et al. 2011) or might be signalled by the illumination of the house light (Davies et al. 2007; Humby et al. 1999; Kerr et al. 2004; Lambourne et al. 2007; Wrenn et al. 2006; Young et al. 2004). Responses in the holes during the timeout period may restart the timeout (Greco and Carli 2006). Some studies add the possibility of avoiding/terminating this timeout through a panel push (Davies et al. 2007; Lambourne et al. 2007). To what extent do these variations in the timeout procedure lead to the same consequences for behaviour? The importance of this topic is discussed in the study by Hoyle et al. (2006) in which two experiments with different protocols were carried out. In the first experiment, where premature responses were not punished and time available to complete a response (limited hold; LH) was long (LH = 5 s), mice showed high levels of premature responding and low levels of accuracy; after modifying task parameters so that time allowed for responding was shortened (LH = 2 s) and premature responses were punished by a timeout, mice presented normal anticipatory responding and accuracy levels, but more omissions (Hoyle et al. 2006). Similarly, Bizarro et al. (2003), using rats, found, in the absence of a timeout, that acute alcohol decreased premature responding, whereas Oliver et al. (2009), using mice and a timeout of 5 s after a premature response, found an increase in premature responses after acute ethanol treatment under long ITI conditions. As noted in Amitai and Markou 2010, the absence of a timeout decreases the incentive to withhold premature responding. However, punishment for premature responding is not always necessary, and the punishment of perseverative responses has been reported to disrupt training (Bari et al. 2008). In particular, during the training period or when only attentional assessment is relevant, some studies (de Bruin et al. 2006; Lee et al. 2002) prefer to use a protocol in which premature responses are not punished.
Similarly, the duration of the LH (time available to perform the response after the stimulus presentation) has also been proven to be important. Both Patel et al. (2006) and Hoyle et al. (2006) argued that a long LH offers more time to make an incorrect response, and they proposed that some of these supposedly ‘incorrect’ responses are, actually, impulsive responses—the animal that failed to detect the stimulus presentation ‘thinks’ that the stimulus has not yet been presented and, consequently, makes a response into a random hole. Thus, a long LH diminishes accuracy and could hypothetically increase premature responses (false incorrect), especially when the subjects are not punished (perhaps resembling more compulsive responses). However, when the LH is reduced and premature responses are punished, although the omission rate is increased, animals make fewer anticipatory responses and display normal accuracy (Hoyle et al. 2006).
Therefore, as discussed, the task may be adjusted, depending on the main variable of interest, by modifying the training length, the baseline criteria and/or the task parameters (Bari et al. 2008). It needs to be borne in mind that such methodological differences in the protocol may account for apparently different findings across laboratories.
5CSRTT in mice: a summary of main findings
As outlined above, different test parameters can lead to different outcomes. We will divide this section into the different steps and modifications that can be implemented in order to vary task demands. After a period of extensive training in the task and upon the stabilisation of performance under baseline parameters, a variety of behavioural manipulations can be designed to affect specific aspects of attention and impulse control.
In order to investigate stable differences between groups, or the impact of certain drugs, animals are commonly tested under the particular standard conditions used during training. A complementary approach, following training under standard conditions, is to introduce probe sessions from time to time in which particular parameters are varied. One example is the use of ITIs (the time that the animal has to wait before the stimulus is presented) that are varied from the ITI used during training. Variations that have been reported in the literature involve lengthening the ‘waiting time’—long ITI condition, by shortening it—short ITI condition or by making the presentation of the stimulus unpredictable—variable ITI (vITI) condition. Another option might consist of varying the attentional load (e.g. by the manipulation of the characteristics of the stimuli—i.e. short stimulus duration (short SD) condition.
Acquisition of the task/training
Generally, mice display no problems in their ability to perform the basic task and to advance across the different stages of training in the 5CSRTT (Davies et al. 2007; Hoyle et al. 2006). Under low attentional demands, mice display high levels of accuracy and short reaction times (de Bruin et al. 2006; Marston et al. 2001) with no particular problems arising from premature responding (Humby et al. 1999). The task has been proven suitable for the testing of mice genetically manipulated to mimic Alzheimer disease-related dysfunctions (3xTgAD; Romberg et al. 2011). Even aged animals (mice of 14, 20 and 27 months old overexpressing human caspase 3, implicated in cell death following neurodegeneration), showed no difficulties in learning the task and did not show differences in performance in comparison to their age-matched wild-type (WT) control littermates (Kerr et al. 2004). As previously described for rats (Dalley et al. 2002), mice are able to reach high levels of performance. Indeed, comparing mice and rats, under baseline conditions, the level of premature responding in mice is lower than that seen typically in rats (Humby et al. 1999); on the other hand, the omission rate can be higher in mice (Oliver et al. 2009; Wrenn et al. 2006). This impairment may be associated with different motivational processes among different strains of mice and/or a more acute decrement in vigilance within the session in mice, compared to rats (Dalley et al. 2004), but it may also be related to satiation, a common problem with the procedure in mice (de Bruin et al. 2006). For this reason, the murine version sometimes consists of shorter sessions than are typically used with rats (Wrenn et al. 2006).
Choice of the reinforcer used, liquid reinforcer or food pellets, will help to rule out this confounding variable. When delivering pellets (preferred in order to facilitate comparisons with rats’ performance (de Bruin et al. 2006; Patel et al. 2006), sessions will be of shorter duration. On the other hand, liquid reinforcement allows sessions to have a higher number of total trials (100 TT or 30 min; Oliver et al. 2009). In the case of liquid reinforcers, the volume used might be reduced to avoid satiation but also to appropriately restrict body weight; type of reinforcer, on the other hand, can also be used as a motivational factor.
Although the performance of mice generally rivals that of rats, some differences in training are seen in mice with specific mutations or depending on the strain. For example, in the study by Greco and Carli (2006), investigating the effects of deletion of neuropeptide Y2 receptors in memory, attention and inhibitory response control, the less anxious Y2−/− mice took twice as many sessions as WT mice to nose-poke consistently into an illuminated hole, showing lower accuracy and more premature responding during the training (as well as in long ITI and variable SD sessions) in comparison to the Y2+/+ mice, suggesting a possible role for anxiety in the learning of the 5CSRTT (Greco and Carli 2006). Also, a Prader–Willi syndrome (PWS) mouse model, the PWS-IC+/−, with learning impairments thought to arise from attentional deficits, took twice as many sessions and had impaired accuracy, increased omissions and elevated correct reaction times than their WT controls, but no differences were found in premature responses or motivation (latency to collect and consume the reinforcer) (Relkovic et al. 2010).
Baseline performance under standard conditions
Once the criteria of stability of performance are reached, data from the last days of training can be used to provide a baseline index of execution. Generally, baseline performance is calculated from the values obtained over the last 2 days (Romberg et al. 2011; Relkovic et al. 2010), 3 days (Greco et al. 2005; Humby et al. 1999; Kerr et al. 2004; Pattij et al. 2007), 4 days (El-Kordi et al. 2009) or even 10 days (Davies et al. 2007) of training in the 5CSRTT in which asymptotic performance at the final SD is reached (e.g. 1 s; Loos et al. 2010).
Baseline performance has been generally used to study the differences between strains (Greco et al. 2005; see Table 2): for example, F1 C57BL/6xDBA/2 vs C57BL/6x129Sv (Humby et al. 1999), C57BL/6 vs DBA/2 (Loos et al. 2010; Patel et al. 2006) and C57BL/6JOlaHsd vs 129S2/SvHsd vs DBA/2OlaHsd (Pattij et al. 2007) and to compare different genetic manipulations. Some examples include studies of the effects of X-monosomy on visuospatial attention (Davies et al. 2007), a mouse model of PWS (Relkovic et al. 2010), studies of mice with overproduction of corticoid-releasing hormone (van Gaalen et al. 2003), caspase 3 mutant mice (Kerr et al. 2004) and transgenic mice for the human FTDP-17 tauV337M mutation (Lambourne et al. 2007). Baseline rates have also been used to examine the role of nicotinic α5 (Bailey et al. 2010) and α7 (Young et al. 2004) subunits in attention. Lastly, pharmacological challenges are also generally implemented under standard conditions, as we will explore later.
Challenge condition
Altering the duration of the ITI: short, long and variable ITI
In order to provoke impulsive responding, a number of experimenters have varied the ITI away from the training conditions. Both shortened (0.5, 1.5, 3.0 or 4.5 s) (Humby et al. 1999; Lambourne et al. 2007; Wrenn et al. 2006) or lengthened (5, 6, 7, 8 or 10 s) ITIs have been used, usually by interleaving occasional long ITI sessions within baseline training sessions; in order to allow sufficient time to complete the same number of trials per session, session duration is usually increased to 45 min. Increasing the ITI from a baseline value of 5 to 7 s in the long ITI session has been shown consistently to increase premature responding in both rats (Dalley et al. 2007; Dalley et al. 2008; Fletcher et al. 2007) and mice (Oliver et al. 2009).
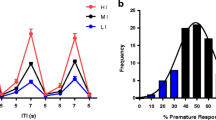
Altering the length of the ITI itself has little effect on attentional functioning in the rat, and similar findings apply to mice (de Bruin et al. 2006; Robbins 2002) (see Fig. 2).
a Effects of increasing the ITI on 5CSRTT performance in C57Bl/6JOlaHsd mice. Once stable baseline levels have been achieved, C57Bl/6JOlaHsd mice were tested in five long ITI sessions (L1–L5) in which the stimulus predictability was disrupted by increasing the length of the ITI from 5 to 10s. The number of premature responses was accentuated when mice were confronted with a long ITI session, but this effect was diminished by repeated sessions (Oliver et al. 2009). b Effects of a vITI on 5CSRTT performance in ethanol-treated and control C57BL/6J mice. Once stable baseline levels had been achieved, the animals were tested in four vITI sessions (Day 14, Day 28, Day 42 and Day 56) in which the stimulus predictability was disrupted by varying the ITI from 2 to 15 s. The number of premature responses exacerbated at longer ITIs. Premature responding decreased over sessions (Walker et al. 2011)
Nevertheless, although the main effects of increasing the ITI in mice are seen in impulsivity measures, Marston et al. (2001) additionally described impairments in attention that were, however, strain-dependent: lengthening the ITI caused greater accuracy deficits in C57BL/6J than in 129P2/OlaHsd mice. In detail, when increasing the ITI, C57BL/6J mice seem to show greater impairments in accuracy in comparison to 129P2/OlaHsd mice, whereas when reducing the SD, 129P2/OlaHsd mice were more affected. At the same time, Yan et al. (2011), using a mouse model with cognitive and inhibitory control deficits that resemble diagnostic features of ADHD (inattentiveness, impulsivity and compulsivity; NK1R−/−), reported that long ITI sessions increased omissions, perseverative responses and latency to collect the reward in NK1R−/− mice in comparison to their WT control group. On the other hand, the same authors report that, using a vITI procedure, perseverative responding and premature responding were increased in the NK1−/− mice, and accuracy diminished, indicating that the long ITI and vITI conditions may give rise to rather different outcomes.
In the vITI condition, the stimulus can be presented using different inter-trial delays, for example, 4–6–8–10s, in a semi-random fashion, within a single session. By disrupting the temporal predictability of the stimulus onset, the possibility of mice using temporal mediating strategies is minimised. Although mainly implemented in a single session, it may also be interesting to repeat the vITI procedure more than once to study the evolution of the behaviour and analyse how impulsive responses show adaptation over time and/or repeated testing (Walker et al. 2011). In rats, when introducing a vITI condition, at the longest ITI values, levels of premature responding are increased (Fletcher et al. 2007). The same phenomenon is seen in mice (Walker et al. 2011; de Bruin et al. 2006; see Fig. 3), regardless of the strain (Relkovic et al. 2010). Increasing the ITI (from 5 to 6, 7, 8 s) produced significant differences in impulsive responding in young mutant tau V337M mice, the deficit being more acute when the animals were older (Lambourne et al. 2007), though changes in accuracy or correct response latency did not occur. In a study comparing 40,XX and 39,XO mice (Davies et al. 2007), both groups showed a similar increase in the levels of premature responding in a vITI session. In a later study by the same authors, premature responding was especially higher in 40,XY in comparison with 39,XY*O mice (Davies et al. 2009). Consistent with these findings, Greco and Carli (2006) also found premature responses to be increased by vITI, especially in the less anxious Y2−/− mice, whereas no additional effects on accuracy or omissions were reported. Although not consistently reported, impairments in attention can accompany increases in premature responding when using the vITI condition (Yan et al. 2011). Humby et al. (1999) report that vITI also resulted in an increase in omissions and a decrease in correct reaction times, in C57BL/6xDBA/2 mice. Although vITI is usually implemented as a challenge session, Hoyle et al. (2006) trained animals under a vITI, so that they could be compared for their abilities in coping with temporally unpredictable stimuli. Results under this training procedure showed that fewer reinforcers were obtained and higher correct response latencies were seen, in comparison with the animals trained in a fixed ITI protocol.
The diagram exemplifies the complex relationship between the 5CSRTT variables. Some of the variables covary but, at the same time, can be controlled by independent mechanisms. Symbols indicate positive (plus sign) or negative (minus sign) correlations (Spearman’s r) from data from 12 BXD recombinant inbred strains and their progenitors (C57Bl/6J and DBA/2J), during the baseline and the three long ITI challenge sessions (Peña-Oliver, in preparation). Firstly, from the bottom left to the right, we can see that accuracy shows a negative correlation with omissions; that is, strains that perform more accurately during the challenge sessions, also show fewer omissions (r = −0.790). Furthermore, the BXD study also suggests a negative correlation between accuracy and correct latency, during the long ITI condition. As expected, strains that perform more accurately take less time to make a correct response (r = −0.507). At the same time, accuracy, during baseline but also during the long ITI sessions, correlates with inhibitory control variables in the 5CSRTT: negatively with premature responding, as reported by others (Dalley et al. 2008; Greco et al. 2005), but not in agreement with Loos et al. (2010), and positively with perseverative responses, but not in a model of mice 3xTgAD where animals with diminished accuracy also increased perseverative responses, similar to rat models of AD and patients (Romberg et al. 2011). Focussing on omissions, values during baseline, second and third long ITI positively correlate with premature responding, but not with perseverative responses. In the bottom central part of the figure, we can see that the inhibitory control variables have a negative correlation between them: strains with higher number of perseverative responses, show lower premature responding. Those results indicate that perseverative and premature responses might be under different mechanisms, as also suggested in other studies (Greco et al. 2005; Oliver et al. 2009; Loos et al. 2010). Moreover, it is also interesting to assess the stability of those variables over time (symbols inside the boxes), not only during the standard conditions but also under challenge sessions. Accuracy and, especially, perseverative responses, tend to remain stable over time. Omissions and premature responses, on the other hand, show increments during long ITI sessions compared with baseline conditions, as also reported in other studies (Walker et al. 2011). However, premature responses tend to decrease over time, whereas omissions show a less consistent pattern; indeed, the rate of omissions shown during the baseline does not predict long ITI performance. Above all, as we highlight, special care needs to be taken with motivational, sedation and motor impairment since they can affect overall 5CSRTT performance, as also reported by Bari et al. (2008). If motivation is decreased, fewer trials will be completed by the end of the session, and the latency to collect the reward and the number of head pokes into magazine will increase and decrease, respectively. In the case of sedation, an increase in response latencies and reward collection will be seen. More concrete to this study, a positive correlation is seen between magazine latency during a long ITI and perseverative responses (+0.608) and correct latency (+0.559); and this last measure correlating negatively with number of total trials (−0.650). In detail, strains that during the long ITI take more time to collect the reward are also more compulsive and completed fewer total trials. Although the high magazine latency may come from lack of motivation, the increase in number of perseverative responses suggests that delay in retrieving the reinforcer is attributable to a longer time spent repeatedly nose-poking: high responding into the stimulus hole may indicate a high motivation or excitatory effects towards the potential reward (or perhaps, insecurity as to whether the nose-poke was effective). In sum, a cautious approach would be required in the analysis of 5CSRTT variables
Under the standard ITI procedure, the stimulus light informs about the correct location for a response. Since the ITI is fixed, timing of the response may be mediated by either the light onset or by internal timing. Under the vITI procedure, stimulus onset informs both about the location of nose-poke and the appropriate time of responding. If the animal has been trained using a fixed ITI, and thus had the opportunity to employ internal timing to solve the delay aspect of the task, introduction of a vITI requires a change in strategy to use only the stimulus onset and to ignore internal timing. In contrast, under the long ITI condition, as in the standard configuration, the stimulus informs about the place, but a possible strategy is for the animal to adjust its internal timing to the new contingencies (wait 7 s not 5 s), a strategy that is not available for the vITI procedure. The standard and long ITI procedures may thus have elements in common with differential reinforcement of low rates procedures, in which the animal is required to estimate the passage of time to perform efficiently (Ripley et al. 2001; Stephens and Voet 1994). We may speculate that, in the vITI condition, because the animals cannot rely on internal timing, they will pay more attention to the stimulus and, for that reason, in the vITI condition, animals may also perform with higher accuracy. On the other hand, under the long ITI condition, animals might still use internal timing, appropriately adjusted, and thus rely less on stimulus detection; greater deficits in accuracy might then be expected. To test this notion, we performed correlational analysis using unpublished data from our laboratory from 46 mice (C57BL/6J and DBA2/J obtained from Jackson Laboratories, C57BL/6J from Charles River and C57BL/6OlaHsd from Harlan, UK). Since in these experiments we used a 10-s ITI in the long-ITI version of the task, we compared the performance of these mice with mice of the same strains performing the vITI version, selecting the 10-s ITI data from the vITI sessions for comparison. Figure 4 illustrates this comparison. Accuracy and percentage of premature responding in the 10-s condition of the vITI session were only weakly negatively correlated (Spearman’s rho = −0.325, p = 0.03), whereas these two variables in the long ITI condition appear highly negatively correlated (Spearman’s rho = −0.673, p < 0.0001). Correlations between accuracy and percentage of premature responses were significantly different between the variable and long ITI conditions (z = 3.276, p < 0.0116), suggesting that the increase in premature responding obtained using the two procedures is achieved by different mechanisms.
Scatter plot of accuracy and percentage of premature responding for the long ITI and vITI conditions for C57BL/6 and DBA/2J strains (C57BL/6J and DBA/2J from Jackson laboratories, C57BL/6J from Charles River laboratories and C57BL/6OlaHsd from Harlan; pooled data, n = 46) (Peña-Oliver and Stephens, unpublished data). Levels of premature responding are strongly negatively correlated with levels of accuracy when animals are tested under the long ITI condition (Spearman’s rho = −0.673, p < 0.0001). In contrast, levels of premature responding and accuracy show only a low negative correlation in the vITI condition (Spearman’s rho = −0.325, p = 0.03). Regression lines for the long ITI (dotted line) and the vITI condition (straight line) are significantly different (z = 3.276, p < 0.0116)
The foregoing discussion raises the possibility that differences in impulsivity between mouse strains or resulting from pharmacological treatment or lesions may arise from differences in internal time estimation or ‘internal clock’ (Coull et al. 2011; Wittmann and Paulus 2008). We recently examined this possibility in substrains of C57BL/6J mice that differ in the expression of alpha-synuclein, a protein involved in the regulation of dopamine function (Abeliovich et al. 2000; Anwar et al. 2011). Mice lacking alpha-synuclein (either snca KO mice or the C57BL/6JOlaHsd substrain that exhibits a spontaneous loss of chromosomal material carrying the snca gene) showed lower levels of premature responding than WT C57BL/6J mice, indicating lower levels of impulsivity, but there were no differences among the groups in their overall timing behaviour, leading us to conclude that, at least in this example, differences in impulsivity in the 5CSRTT were not caused by differences in timing behaviour (Peña-Oliver et al. 2011).
Altering the characteristics of the stimulus: attentional challenge
When the assessment of attentional function is the main goal of the study, due to a ceiling effect as a consequence of extensive training, it is sometimes difficult to discriminate between groups when comparing performance under baseline parameters. The use of testing sessions specifically designed to increase the attentional load are thus sometimes useful in exploring differences between strains or to elucidate pharmacological actions. Typically, attentional challenges are achieved by reducing or varying the stimulus duration (SD; 0.2, 0.4, 0.6 and 0.8 s) or reducing stimulus brightness (percentage of reduction = 52, 30, 21 and 12), or imposing a tone distracter. As well as increasing attentional demands, such manipulations may also lead to changes in response patterns leading to premature responding.
Reducing or varying the stimulus duration
Reduction in stimulus duration has been reported to produce effects on impulsive responding. For example, when stimulus duration was reduced from 1 to 0.3 s, premature responses were increased in three inbred strains tested, the C57BL/6JOlaH, DBA/2N and 129SvHsd (Pattij et al. 2007). Reducing the stimulus duration to 0.5 s caused an increase in premature responding in DBA/2 mice, whereas C57BL/6 (substrain unspecified) showed higher accuracy (Patel et al. 2006). Another author to report an increase in premature responding as a consequence of reducing the stimulus duration was de Bruin et al. (2006), who described in C57NL/6Jx129sv F2 mice (B6129F2), an increase not only in impulsivity when the SD was reduced from 2 to 1 s, but also in perseverative responding when the SD was further reduced to 0.6 s. But these manipulations of stimulus duration generally have greater effects on measures of attention. For instance, in de Bruin et al. (2006), an increase in omissions and a decrease in accuracy were also reported, and these were more evident with further reductions of the SD to 0.5 s. Marston et al. (2001) described more profound deficits in accuracy in 129P2/OlaHsd mice than in C57BL/6J in sessions employing short SD.
In another study, with the 3xTgAD mice, the reduction in SD to 0.6 s caused a decrease in accuracy accompanied by an increase in perseverative responses into the holes, deficits consistent with rat models of AD and AD patients (Lawrence and Sahakian 1995; Romberg et al. 2011). Moreover, these same 3xTgAD mice, that showed no problems sustaining attention during the less attentionally demanding condition (1.5 s), experienced a decrease in performance across the short SD session, possibly due to impairments in vigilance, as shown by an increase in omissions towards the end of the session. Generally, in the short SD session, there is a decrease in accuracy accompanied by an increase in the number of omissions (Relkovic et al. 2010), but these two variables can help to discriminate between strains, as in Humby et al. (1999), in where C57BL/6x129sv showed an increase in omissions at 0.4 s SD, while C57BL/6xDBA mice increased the omissions only when the SD was set at 0.2 s, suggesting better attentional abilities in the latter strain. Sometimes the increase in omissions is not accompanied by a decrease in accuracy, as in Wrenn et al. (2006) when the stimulus duration was reduced to 0.8 s. Since this reduction (0.8 s) is not as low as that used in other studies, and taking into account that they also found an increase in latency to collect the reinforcer, perhaps this attentional disruption was more related to motivational factors.
The short SD challenge has also been used to study the potential cognition enhancer, erythropoietin, in reversing the increment in omissions, with positive results in C57BL/6NCrl mice (El-Kordi et al. 2009; see Table 1, section H). Surprisingly, mice can learn to perform at very low SDs: Bailey et al. (2010) used one of the lowest SD challenges (0.125 s), reporting that nicotinic alpha5 KO mice were less accurate than their WT control group under this protocol. Another study investigating the genetics of attention used an extremely short SD of 0.1 s, but in a one-choice procedure, and found that the procedure could discriminate between strains (Davies et al. 2009).
A few studies have employed a variable SD presentation (SDs 0.25, 0.5, 1.0, 2.0 and 4.0). Under this modification, Y2−/− mice (anxious phenotype), showed less accuracy and more premature responses than the less anxious WT control group (Greco and Carli 2006). Moreover, a variable SD can also be used during standard training in a two-choice serial reaction time task (Lee et al. 2002). In that investigation, the SD was varied (1, 2 or 5 s) according to the days of the week. As expected, and in accordance with other results, when animals were presented the highest SD (5 s), the rate of omission was lower, as well as the correct response latency.
Reducing stimulus brightness (52%, 30%, 21% and 12% of full)
Reducing stimulus brightness has also been shown to discriminate between levels of attentional ability in mice: Humby et al. (1999) reported an increase in correct latency in C57x129sv and C57BL/6xDBA/2 mice and described a larger impairment in accuracy and omissions in the former group. The authors suggested that the differences in disruption of attentional variables could be due to different strategies in the two strains or to differences in visual acuity. No differences in other variables were reported.
Imposition of white noise distractor
The main effects seen as a consequence of the white noise distractor challenge are decrements in accuracy (Davies et al. 2007) or in omissions (Humby et al. 1999), but no effects in premature responding have been described (Davies et al. 2007; Humby et al. 1999; Wrenn et al. 2006). Nevertheless, de Bruin et al. (2006) found no disruptive effects of this challenge on attention or premature responding but a reduction in the number of perseverative responses.
Pharmacological manipulations
Often, once animals acquire stable performance under baseline parameters, the effects of a series of drugs are tested. Results of some of the key drugs are summarised in Table 1, based upon the findings from mouse studies.
Dopamine
We will limit this review to the impact of different drugs on impulsive behaviour. As can be seen in Table 1, little is known with respect to the possible role of dopamine in the modulation of inhibitory control and visuospatial attention in mice (Table 1, section A). Loos et al. (2010) studied the effects of the psychostimulant amphetamine and the DA uptake inhibitor, GBR12909, in the 5CSRTT (standard condition) and a go/no-go task in C57BL/6J and DBA2/J mice. As in rats (Harrison et al. 1997; Robbins 2002), amphetamine increased premature responses, but only in C57BL/6J mice (which were showing lower baseline levels of premature responding in comparison with DBA/2J mice). On the other hand, amphetamine had no effects on attentional measures. GBR12909, given at the highest dose (10 mg/kg), decreased accuracy and increased premature responses in C57BL/6J mice, compared with saline. In summary, Loos et al. (2010) found that amphetamine and GBR12909 modulate inhibitory control mechanisms in C57BL/6J but not in DBA2/J mice. Moreover, amphetamine seems to increase impulsivity without affecting accuracy, while a higher dose of GBR12909 increased premature responding and decreased accuracy in C57BL/6J mice. Nevertheless, inconsistent results were found when d-amphetamine was tested in a model of ADHD in mice (NK1R−/−; Yan et al. 2011). As mentioned in the previous section, those animals presented increased perseverative and omission rate during a long ITI challenge, and also increased premature and decreased accuracy during the vITI. When d-amphetamine was administered under the long ITI condition, it decreased the number of perseverative responses and omissions. In contrast, when the psychostimulant was administered on a vITI condition, it increased premature responding.
Glutamate
In a study performed by Greco et al. (2005), the role of glutamate neurotransmission in impulsivity and attention was investigated using DBA/2N and C57BL/6N mice (see Table 1, section B). PCP, a non-competitive glutamate receptor antagonist, and LY379268, an mGluR2/3 receptor agonist, were tested. PCP, when given at a 1.5 mg/kg dose, on standard conditions, increased the number of premature responses only in the animals that were already more impulsive (DBA/2N mice) and caused disruptions in accuracy, compared to C57BL/6N. Surprisingly, when administering LY379268, premature responses were diminished, but only in the mice that showed low levels during the baseline (C57BL/6N). Three aspects of this study need to be considered: first, PCP increased impulsivity only in baseline high-impulsive mice, whereas LY379168 reduced premature responses only in baseline low-impulsive mice; second, strain contributed to the different effects of PCP and LY379168; and third, perseverative and premature responses seemed to be controlled by different mechanisms, since PCP only affected perseverative responses in DBA/2N but not C57BL/6N mice. Furthermore, accuracy and premature responding might be associated, since PCP increased premature responding and reduced accuracy in DBA/2N mice. However, this apparent relationship should be treated with caution, since the effects of administering LY379168 were limited to premature responding without modifying accuracy (Greco et al. 2005).
A similar result is reported by Pozzi et al. (2010); again, PCP impaired inhibitory response control in DBA/2 mice, but this time increased not only premature but also perseverative responding. This result was accompanied by a decrease in accuracy, in agreement with Greco et al. (2005). However, while Greco et al. (2005) found LY379168 to decrease premature responding only in C57BL/6N mice, in contrast, in the study by Pozzi et al. (2010), the 5-HT2A antagonist, M100907, was able to reverse the effects induced by PCP, by increasing accuracy and preventing perseverative and premature deficits, in both C57BL/6N and DBA2/N mice. Thus, this study adds evidence supporting a role for glutamate and serotoninergic neurotransmission in attention and impulsivity. Similar results were also described in rats, where M100907 reduced and SB242084 increased premature responding in a long ITI session; no treatments in this study significantly impaired accuracy (Fletcher et al. 2007).
In keeping with NMDA receptor blockade increasing impulsive behaviour, Oliver et al. (2009) found that the NMDA receptor antagonist ketamine (10 and 20 mg/kg) increased premature responses in CD1 mice, but not in the C57BL/6JOlaHsd strain. No disruptions in perseverative responding or in accuracy were seen.
Ethanol possesses some pharmacological effects as an antagonist of NMDA receptors, but no effects of ethanol were seen during baseline conditions of the task; when the mice were confronted by long ITI sessions, ethanol (1 g/kg) increased premature responding in both C57BL/6JOlaHsd and CD1 mice (Oliver et al. 2009), in contrast to a study reported in rats, where ethanol 1.2 and 1.6 g/kg resulted in a reduction of impulsivity (Bizarro et al. 2003). In a later study (Walker et al. 2011), we found that chronic ethanol treatment induced no impairments in impulsivity in baseline conditions of the task. However, when given a vITI challenge, ethanol-treated C57BL/6J mice took more sessions to diminish premature responding (after repeated testing) in comparison to control mice. Even though no differences in impulsive responding were seen between groups during the first challenge, the ethanol-treated mice remained impulsive for longer. As in Oliver et al. (2009), the disruption in premature responding was not accompanied by disruptions in attentional ability. Alcohol withdrawal may increase glutamatergic transmission, leading to hyperexcitation, which dissipates over time, perhaps explaining the temporary nature of the learning deficits (Stephens and Duka 2008). If the vITI procedure requires a switch in strategy from the use of internal timing to predict stimulus onset under baseline conditions to one in which the timing of the response is externally cued by light onset, these observations might suggest that chronic alcohol impairs the ability to flexibly switch strategies to fit the new requirements, consistent with human data on alcoholic patients (Duka et al. 2011).
Additionally, ethanol may have also resulted in a decrease in sensitivity to TO punishment, taking into account that, when animals are not trained under TO periods, they acquire the task more slowly (Christakou et al. 2004)
GABAergic system
Oliver et al. (2009) reported premature responses to be increased in strains C57BL/6JOlaHsd and CD1, after diazepam administration, with no disruptions in perseverative responding or in accuracy. In this experiment, diazepam mimicked the effects of ethanol in both strains.
GABAergic pharmacological manipulations have also been used to test the hypothesis of impulsivity being associated with anxiety (see Table 1, section D). The same anxiolytic drug diazepam increased premature responding in an anxious group of mice (van Gaalen et al. 2003). The opposite result was found by Greco and Carli (2006) who reported that diazepam increased premature responses in Y2−/− and WT mice, but this increase was greater in the animals that presented a less anxious phenotype (Y2−/−). In the same study, the anxiogenic compound FG7142 decreased premature responding again in the non-anxious Y2−/− mice. No effects on perseverative responding were found with any of these compounds. These results seem to indicate the existence of a possible relationship between anxiety and impulsivity, low levels of anxiety being indicative of higher impulsivity in the 5CSRTT, as proposed by Loos et al. (2009).
Interesting results were found by Davies et al. (2009) where the neurosteroid dehydroepiandrosterone sulphate (DHEAS), a compound with activity at both GABAA and NMDA receptors and hypothesised to influence ADHD endophenotypes (attention, motor impulsivity and activity), proved to enhance attentional functioning but showed no effects on inhibitory control (see Table 1, section G). These findings are not easy to integrate with the above, possibly because DHEAS has a number of additional actions (Yadid et al. 2010).
Cholinergic mechanisms
Administration of nicotine increased the number of premature responses and decreased correct latency in nicotinic α5 KO and WT mice when tested under a short SD session (Bailey et al. 2010).
But apart from the study of impulsivity, most investigations using the 5CSRTT have been focused in the evaluation of attentional function (see Table 1, section E). In the very first study using the 5CSRTT in mice, Humby et al. (1999) studied the role of other cholinergic compounds, such as the muscarinic antagonist scopolamine, describing disruptions in accuracy and omissions but reporting no effects in premature or perseverative responding. In line with this report, as replicated in subsequent studies (de Bruin et al. 2006; Siegel et al. 2011), ACh mechanisms proved to be important for attentional functioning in mice, but not for inhibitory control. Specifically, Romberg et al. (2011) investigated the impact of donepezil, a cholinesterase inhibitor, in attentional performance of 3xTgAD mice, a mouse model with cholinergic deficits used as a model of human AD. Donepezil selectively increased accuracy of responding, reducing decrements in vigilance throughout the session, while no effects in omissions or perseverative responding were reported.
With regard to nicotine, several studies have described an enhancement in attentional performance in mice in the 5CSRTT, in line with effects in humans and rats (Hahn et al. 2002; Hahn and Stolerman 2002) (see Table 1, section F). Specifically, nicotine improved attention in comparison to the vehicle-treated mice (Young et al. 2004; de Bruin et al. 2006), the effect persisting after chronic nicotine treatment (Pattij et al. 2007). Nevertheless, when another protocol (limited LH and punished premature responding; Hoyle et al. 2006) and manipulations (short SD condition; Bailey et al. 2010) were implemented, nicotine failed to show the mentioned attentional benefits. If anything, nicotine caused a general impairment in omissions, exacerbated in nicotinic alpha7 KO mice, suggesting that alpha7 nAChR may be involved in mediating the effects of nicotine in the task (Hoyle et al. 2006). Thus, the beneficial effects of nicotine are restricted to certain conditions (Bailey et al. 2010). Consistent with a role for alpha7 nicotinic receptors in attentional performance, KO mice for α7 nAChR were unable to perform the task equally to the WT (Young et al. 2004). Similarly, mice lacking apolipoprotein E (Apoe −/−) could not acquire the task performance criteria in terms of attention (Siegel et al. 2011), which suggested that apolipoprotein E may alter ACh neurotransmission and, consequently, impair cognition.
So far, pharmacological experiments strongly support the hypothesis that results depend on parameters of the task. Figure 3 exemplifies the complex relationship between the 5CSRTT variables. All those variables build an intrinsic structure where one variable is associated with others but, at the same time, are also controlled by independent mechanisms. Moreover, we emphasise the need to test drugs in other paradigms of attention and impulsivity in order to draw a more complete picture (Dalley et al. 2008; Pattij and Vanderschuren 2008).
Looking for candidate genes
As introduced in the first paragraphs of the present review, the use of the 5CSRTT in mice allows the study of the contribution of both genetic and environmental factors, and their interactions, in the study of impulsivity, compulsivity and attention. The publications reviewed here are an example of the increasing number of investigations using different inbred strains with the aim of unravelling the genetics of impulsivity (Humby et al. 1999; Isles et al. 2004; Loos et al. 2009, 2010; Patel et al. 2006; Pattij et al. 2007) (see Table 2). For instance, Isles et al. (2004) used the strategy of testing four inbred strains to investigate the genetic contribution to impulsive behaviour by using a delayed-reinforcement paradigm, which evaluates impulsive choice. Furthermore, Loos et al. (2009) measured locomotor activity and impulsivity in the 5CSRTT in 12 different inbred strains of mice: after repeated testing in a vITI condition, the authors concluded that both genetic and environmental factors contribute to the stability of impulsivity over time. The authors reported genetic correlations between impulsivity and the expression of the genes Frzb, Snx5 and BC056474 in dorsal mPFC (Loos et al. 2009). The Frzb gene inhibits the Wnt signalling pathway, which has shown an important role in axon path finding (Bovolenta et al. 2006) and synapse structure and function (Ataman et al. 2008), and the Snx5 in intracellular trafficking (Otsuki et al. 1999) and in response to ethanol treatment (Kerns et al. 2005).
Inbred strains of mice represent a powerful tool to study the contribution of genetic factors in behaviour, and taking this approach a step further, the BXD recombinant inbred strains of mice have proven to be an invaluable tool for behavioural genetics (Chesler et al. 2003; Crabbe et al. 1999). BXD mice were derived from the cross of C57BL/6J and DBA2/J mice, two strains that differ in a variety of behavioural traits (see present review, also Crawley et al. 1997; Phillips et al. 1998). Because this inbred panel is composed of genetically identical individuals (within each strain), they can be repeatedly tested and data collected from different laboratories can be compared and added to a large database to allow multi-trait analysis. Using web QTL database (http://www.webqtl.org; Chesler et al. 2003), data collected from Affymetrix microarrays in the BXD strains can be used to carry out genetic correlation analysis of gene expression with any other trait of interest, such as with behavioural traits, i.e. impulsivity or compulsivity in the 5CSRTT (Chesler et al. 2003).
In our laboratory, we have collected data from 12 BXD recombinant inbred strains and their progenitor C57BL/6J and DBA/2J mice in the 5CSRTT (using long ITI probe sessions; Peña-Oliver, in preparation) with the aim of finding candidate genes responsible of the impulsive phenotype.
Conclusions
Mice are just as good as rats in the 5CSRTT. The results presented illustrate that mice are capable of learning the complex 5CSRTT, and show many similarities to rats. Findings across laboratories are reproducible, provided that the same procedures are used. However, variations in procedure and differences between strains can give rise to quite marked differences in outcome. Thus, bear in mind to choose the strain and task parameters depending on the question being asked. The 5CSRTT paradigm is based on appetitive learning and, therefore, the confounding effects of stress are less likely to affect the animal performance, especially in stress-reactive strains. This offers the opportunity to test transgenic and knockout mice with similar background as animal models of human psychiatric and neurological diseases. However, understanding the meaning of the different variables and the way they interact is crucial to understanding the mechanisms that lead to different phenotypes. New research approaches, such as the use of inbred strains, will bring us a step closer to the discovery of the genetics of impulsivity and attention.
References
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252
Amitai N, Markou A (2010) Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry 68:5–16
Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, Bolam JP, Chandra SS, Cragg SJ, Wade-Martins R, Buchman VL (2011) Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci 31:7264–7274
Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V (2008) Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron 57:705–718
Bailey CD, De Biasi M, Fletcher PJ, Lambe EK (2010) The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci 30:9241–9252
Bari A, Dalley JW, Robbins TW (2008) The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3:759–767
Bizarro L, Patel S, Stolerman IP (2003) Comprehensive deficits in performance of an attentional task produced by co-administering alcohol and nicotine to rats. Drug Alcohol Depend 72:287–295
Bovolenta P, Rodriguez J, Esteve P (2006) Frizzled/RYK mediated signalling in axon guidance. Development 133:4399–4408
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Chesler EJ, Wang J, Lu L, Qu Y, Manly KF, Williams RW (2003) Genetic correlates of gene expression in recombinant inbred strains: a relational model system to explore neurobehavioral phenotypes. Neuroinformatics 1:343–357
Christakou A, Robbins TW, Everitt BJ (2004) Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci 24:773–780
Clark L, Robbins T (2002) Decision-making deficits in drug addiction. Trends Cogn Sci 6:361
Coull JT, Cheng RK, Meck WH (2011) Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36:3–25
Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK (1999) Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci 22:173–179
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124
Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW (2002) Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology 26:716–728
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784
Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW (2007) Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology 32:1195–1206
Dalley JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–260
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–694
Davies W, Humby T, Isles AR, Burgoyne PS, Wilkinson LS (2007) X-monosomy effects on visuospatial attention in mice: a candidate gene and implications for Turner syndrome and attention deficit hyperactivity disorder. Biol Psychiatry 61:1351–1360
Davies W, Humby T, Kong W, Otter T, Burgoyne PS, Wilkinson LS (2009) Converging pharmacological and genetic evidence indicates a role for steroid sulfatase in attention. Biol Psychiatry 66:360–367
de Bruin NM, Fransen F, Duytschaever H, Grantham C, Megens AA (2006) Attentional performance of (C57BL/6Jx129Sv)F2 mice in the five-choice serial reaction time task. Physiol Behav 89:692–703
Duka T, Trick L, Nikolaou K, Gray MA, Kempton MJ, Williams H, Williams SC, Critchley HD, Stephens DN (2011) Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics. Biol Psychiatry. doi:10.1016/j.biopsych.2011.04.006
El-Kordi A, Radyushkin K, Ehrenreich H (2009) Erythropoietin improves operant conditioning and stability of cognitive performance in mice. BMC Biol 7:37
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology (Berl) 146:348–361
Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA (2007) Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 195:223–234
Greco B, Carli M (2006) Reduced attention and increased impulsivity in mice lacking NPY Y2 receptors: relation to anxiolytic-like phenotype. Behav Brain Res 169:325–334
Greco B, Invernizzi RW, Carli M (2005) Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU(2/3) receptor agonist LY379268. Psychopharmacology (Berl) 179:68–76
Hahn B, Stolerman IP (2002) Nicotine-induced attentional enhancement in rats: effects of chronic exposure to nicotine. Neuropsychopharmacology 27:712–722
Hahn B, Shoaib M, Stolerman IP (2002) Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 162:129–137
Harrison AA, Everitt BJ, Robbins TW (1997) Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 133:329–342
Hoyle E, Genn RF, Fernandes C, Stolerman IP (2006) Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 189:211–223
Humby T, Laird FM, Davies W, Wilkinson LS (1999) Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci 11:2813–2823
Humby T, Wilkinson L, Dawson G (2005) Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Curr Protoc Neurosci Chapter 8: Unit 8 5H
Isles AR, Humby T, Walters E, Wilkinson LS (2004) Common genetic effects on variation in impulsivity and activity in mice. J Neurosci 24:6733–6740
Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, Williams RW, Miles MF (2005) Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. J Neurosci 25:2255–2266
Kerr LE, McGregor AL, Amet LE, Asada T, Spratt C, Allsopp TE, Harmar AJ, Shen S, Carlson G, Logan N, Kelly JS, Sharkey J (2004) Mice overexpressing human caspase 3 appear phenotypically normal but exhibit increased apoptosis and larger lesion volumes in response to transient focal cerebral ischaemia. Cell Death Differ 11:1102–1111
Lambourne SL, Humby T, Isles AR, Emson PC, Spillantini MG, Wilkinson LS (2007) Impairments in impulse control in mice transgenic for the human FTDP-17 tauV337M mutation are exacerbated by age. Hum Mol Genet 16:1708–1719
Lawrence AD, Sahakian BJ (1995) Alzheimer disease, attention, and the cholinergic system. Alzheimer Dis Assoc Disord 9(Suppl 2):43–49
Lee B, Tumu P, Paul IA (2002) Effects of LP-BM5 murine leukemia virus infection on errors and response time in a two-choice serial reaction time task in C57BL/6 mice. Brain Res 948:1–7
Loos M, van der Sluis S, Bochdanovits Z, van Zutphen IJ, Pattij T, Stiedl O, Smit AB, Spijker S (2009) Activity and impulsive action are controlled by different genetic and environmental factors. Genes Brain Behav 8:817–828
Loos M, Staal J, Schoffelmeer AN, Smit AB, Spijker S, Pattij T (2010) Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res 214:216–224
Marston HM, Spratt C, Kelly JS (2001) Phenotyping complex behaviours: assessment of circadian control and 5-choice serial reaction learning in the mouse. Behav Brain Res 125:189–193
Oliver YP, Ripley TL, Stephens DN (2009) Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology (Berl) 204:679–692
Otsuki T, Kajigaya S, Ozawa K, Liu JM (1999) SNX5, a new member of the sorting nexin family, binds to the Fanconi anemia complementation group A protein. Biochem Biophys Res Commun 265:630–635
Patel S, Stolerman IP, Asherson P, Sluyter F (2006) Attentional performance of C57BL/6 and DBA/2 mice in the 5-choice serial reaction time task. Behav Brain Res 170:197–203
Pattij T, Vanderschuren LJ (2008) The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29:192–199
Pattij T, Janssen MC, Loos M, Smit AB, Schoffelmeer AN, van Gaalen MM (2007) Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav 6:579–587
Peña-Oliver Y, Buchman V, Dalley J, Robbins TW, Schumann G, Ripley T, King S, Stephens D (2011) Deletion of alpha-synuclein decreases impulsivity in mice. Genes, Brain and Behaviour (in press)
Phillips TJ, Huson MG, McKinnon CS (1998) Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J Neurosci 18:3023–3034
Pozzi L, Greco B, Sacchetti G, Leoni G, Invernizzi RW, Carli M (2010) Blockade of serotonin 2A receptors prevents PCP-induced attentional performance deficit and CREB phosphorylation in the dorsal striatum of DBA/2 mice. Psychopharmacology (Berl) 208:387–399
Relkovic D, Doe CM, Humby T, Johnstone KA, Resnick JL, Holland AJ, Hagan JJ, Wilkinson LS, Isles AR (2010) Behavioural and cognitive abnormalities in an imprinting centre deletion mouse model for Prader–Willi syndrome. Eur J Neurosci 31:156–164
Ripley TL, Horwood JM, Stephens DN (2001) Evidence for impairment of behavioural inhibition in performance of operant tasks in tPA−/− mice. Behav Brain Res 125:215–227
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, Robbins TW, Dalley JW (2009) Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ‘waiting’ versus ‘stopping’. Behav Brain Res 196:310–316
Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM (2011) Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: rescue by donepezil (Aricept). J Neurosci 31:3500–3507
Siegel JA, Benice TS, Van Meer P, Park BS, Raber J (2011) Acetylcholine receptor and behavioral deficits in mice lacking apolipoprotein E. Neurobiol Aging 32:75–84
Stephens DN, Duka T (2008) Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 363:3169–3179
Stephens DN, Voet B (1994) Differential effects of anxiolytic and non-anxiolytic benzodiazepine receptor ligands on performance of a differential reinforcement of low rate (DRL) schedule. Behav Pharmacol 5:4–14
van Gaalen MM, Stenzel-Poore M, Holsboer F, Steckler T (2003) Reduced attention in mice overproducing corticotropin-releasing hormone. Behav Brain Res 142:69–79
Walker SE, Peña-Oliver Y, Stephens DN (2011) Learning not to be impulsive: disruption by experience of alcohol withdrawal. Psychopharmacology (Berl) 217(3):433–442
Winstanley CA (2007) The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann N Y Acad Sci 1121:639–655
Wittmann M, Paulus MP (2008) Decision making, impulsivity and time perception. Trends Cogn Sci 12:7–12
Wrenn CC, Turchi JN, Schlosser S, Dreiling JL, Stephenson DA, Crawley JN (2006) Performance of galanin transgenic mice in the 5-choice serial reaction time attentional task. Pharmacol Biochem Behav 83:428–440
Yadid G, Sudai E, Maayan R, Gispan I, Weizman A (2010) The role of dehydroepiandrosterone (DHEA) in drug-seeking behavior. Neurosci Biobehav Rev 35:303–314
Yan TC, Dudley JA, Weir RK, Grabowska EM, Peña-Oliver Y, Ripley TL, Hunt SP, Stephens DN, Stanford SC (2011) Performance deficits of NK1 receptor knockout mice in the 5-choice serial reaction-time task: effects of d-amphetamine, stress and time of day. PLoS One 6:e17586
Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J (2004) Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology 29:891–900
Acknowledgements
Work in Stephens’ lab during the writing of this review was supported by MRC grant G1000008, EU FP7 grant “IMAGEN”, and EU InterReg grant “AlcoBinge”. This paper reflects only the authors’ views and the Community is not liable for any use that may be made of the information contained therein.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanchez-Roige, S., Peña-Oliver, Y. & Stephens, D.N. Measuring impulsivity in mice: the five-choice serial reaction time task. Psychopharmacology 219, 253–270 (2012). https://doi.org/10.1007/s00213-011-2560-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2560-5