Abstract
Rationale
The effects of ethanol on attention and impulsivity have been contradictory.
Objectives
The aim of the present investigation is to study the effects of acute ethanol administration in measures of attention and response control in the five-choice serial reaction time task (5-CSRTT) in two strains of mice, C57BL/6JOlaHsd and CD1.
Materials and methods
Mice were trained in the 5-CSRTT and then were injected intraperitoneally (i.p.) with 0, 0.5, 1 and 2 g/kg ethanol before testing under standard parameters and in a long inter-trial interval (ITI) session, which promotes the emergence of premature responses, a measure of poor inhibitory control. To examine if the effects of ethanol in the 5-CSRTT were due to its actions at GABAA receptors or at NMDA receptors, the GABAA receptor agonist diazepam (1 and 2 mg/kg, i.p.) and the non-competitive NMDA antagonist ketamine (10 and 20 mg/kg, i.p.) were tested in long ITI sessions.
Results
Ethanol did not affect attention or impulsivity in the standard procedure, but increased premature responding in long ITI sessions. The effects of ethanol were mimicked by diazepam in both strains of mice, whereas ketamine increased premature responding only in the CD1 strain.
Conclusions
Ethanol's ability to increase impulsivity in the 5-CSRTT is mediated by both common and different neurotransmitter systems in the two strains of mice and is dependent on the task's parameters. Furthermore, ethanol did not decrease response accuracy, suggesting that attentional mechanisms are preserved after acute ethanol in mice and that the increases in impulsive behaviour are independent of attentional performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethanol ingestion is frequently associated with disruptive and antisocial behaviours like dangerous driving and automobile accidents, injuries, unprotected sex and aggression (Cherpitel 1993, 1999; Ericksen and Trocki 1994). These irrational and detrimental behaviours have been thought to occur as a consequence of ethanol's effects on impulsive behaviour (Critchlow 1986). The term impulsivity is not a unitary construct and can be defined in several ways. For example, impulsivity may refer to the tendency to act without thinking, on the urge of the moment, the inability to delay gratification, distractibility or the difficulty in the inhibition of incorrect or inappropriate responses (Dougherty et al. 2008; Evenden 1999).
Pathological impulsivity is associated with altered functioning of prefrontal–subcortical circuits, particularly the orbitofrontal cortex (Spinella 2004) and ethanol is known to produce long-term effects on prefrontal cortical function (Moselhy et al. 2001; Tarter et al. 2004) and has been reported to impair performance on several information-processing tasks in humans (Maylor and Rabbitt 1993). For instance, ethanol impairs divided attention (Billings et al. 1991; West et al. 1993) and sustained attention in continuous performance tasks (Dougherty et al. 2000; Rohrbaugh et al. 1988). In these tasks, the person is usually required to respond repeatedly to brief stimuli, which involves high demands on attentional capacity.
Studies that have focused on the evaluation of acute ethanol administration on impulsivity have reported different results depending on the aspect of impulsivity being measured (Dougherty et al. 2008). In measures of response inhibition, when the subject is required to withhold an already initiated response, ethanol seems to increase impulsivity in moderate drinkers and in college students (Dougherty et al. 1999, 2000; Mulvihill et al. 1997). On the other hand, in the delayed reward procedure (Thiebot et al. 1985), where subjects are asked to choose between a small reward that is available immediately vs a larger reward that is available after a certain delay, the choice of the small reinforcer being considered as a sign of impulsivity, has shown mixed results: in ethanol abusers, acute ingestion of ethanol increased impulsive responding (Petry 2001), but in healthy adults, ethanol had no effects in impulsivity (Richards et al. 1999) or reduced impulsive choices in college students (Ortner et al. 2003).
In rats, ethanol intoxication decreases performance in sustained attention or vigilance tasks, such as in a two-choice serial reaction time task (Givens 1997; Givens and McMahon 1997) and in a visual signal detection task (Rezvani and Levin 2003), indicating that these are promising animal models for assessing ethanol effects on attentional performance. More recently, however, it has been reported that, in rats, ethanol did not affect the accuracy of responding in an apparently similar five-choice serial reaction time task (5-CSRTT), but acted mainly by slowing the speed or rate of responding at high doses (1.2–1.6 g/kg) while showing no effect at any parameter in small doses (0.4–0.8 g/kg) (Bizarro et al. 2003).
With regard to impulsivity, it has been reported that ethanol increased impulsivity in the delay of reinforcement paradigm in rats (Evenden and Ryan 1999; Olmstead et al. 2006; Poulos et al. 1998; Tomie et al. 1998) while a reduction in the rate of premature responding with high doses of ethanol (1.2, 1.6 g/kg) in the 5-CSRTT has also been reported (Bizarro et al. 2003). These findings suggest that the effects of ethanol are dependent on the dimension of impulsivity measured and of the particular task, confirming that impulsivity is not a unitary construct (Dougherty et al. 2008; Evenden 1999).
Both attention and impulsivity can be assessed in rodents by means of the 5-CSRTT, analogous to continuous performance tests in humans (Carli et al. 1983; Robbins 2002). This task assesses attentional performance by the detection of a brief visual stimulus presented randomly across five spatial locations. The 5-CSRTT provides information about aspects of sustained and divided attention and also measures aspects of inhibitory response control: premature responding into the holes represents a failure of response inhibition where the animal has to withhold responding until the stimulus light is illuminated and provides a measure of impulsivity (Robbins 2002); perseverative responding occurs when the animal continues nose-poking into the holes after a correct detection and represents a measure of compulsivity (Dalley et al. 2008).
There have been numerous pharmacological studies that have used the 5-CSRTT paradigm in the study of attention and response control mechanisms, but studies that test the effects of alcohol in this paradigm are scarce. Acute ethanol treatment has two major sites of action in the nervous system: it is associated with the facilitation of GABAergic inhibitory mechanisms by its action as an indirect agonist at GABAA receptors (Roberto et al. 2004a), while it also acts as an antagonist of glutamatergic N-methyl-d-aspartate (NMDA) receptors, interfering with glutamate transmission (Roberto et al. 2004b; Samson and Harris 1992). For this reason, we compared the effects of diazepam, as an agonist at the benzodiazepine binding site of GABAA receptors, and ketamine, a non-competitive antagonist at NMDA receptors, with the effects of ethanol, to examine which sites of action may be responsible for the hypothesised effects of ethanol in attention and response control in the 5-CSRTT. We carried out these experiments in two mouse strains, the C57BL/6JOlaHsd (that we will denominate as C57), which comes from a behaviourally and pharmacologically well-characterised standard inbred strain (Cabib et al. 2002; Crawley et al. 1997), and the CD1 strain, used because it represents a commonly used genetically heterogeneous stock of mice. The use of these two genetically different strains of mice will help us to test the suitability of the 5-CSRTT for the measurement of the effects of ethanol on attention and impulsivity in mice.
Additionally, in order to obtain information on possible strain differences in activity that might contribute to performance in the 5-CSRTT, we measured locomotor activity.
Materials and methods
Subjects
CD1 (n = 8) and C57BL/6JOlaHsd (C57, n = 7) male mice were bred in the Department of Psychology at the University of Sussex from commercially obtained parents and weighed 25–30 g at the beginning of the experiments. They were housed in groups of two or three per cage on a 12-h light/dark cycle (lights off at 7 p.m.) at a temperature of 19–21°C and 50% humidity. After the last day of testing in the locomotor activity boxes, the mice were food restricted to reduce their body weights to 85% of their free-feeding weight and were kept under food restriction until the end of the study. Water was available ad libitum. After the second experiment with ethanol (under long inter-trial interval [ITI] parameters), one mouse in the C57 strain had to be killed and data from this mouse were not included in the analysis of the long ITI study. All experiments were approved by the institutional ethics committee and were performed under United Kingdom legislation on animal experimentation [Animals (Scientific Procedures) Act, 1986].
Drugs
Ethanol (95%) was diluted with distilled water. Diazepam (Hoffman LaRoche, Basel, Switzerland) was suspended in distilled water containing 0.2% Tween 80. Ketamine (Sigma-Aldrich, Poole, UK) was diluted with distilled water. All drugs were administered intraperitoneally (i.p) 15 min before the test session. Ketamine and diazepam were administered at a volume of 10 mL/kg and ethanol at 20 mL/kg to avoid tissue irritation.
Test apparatus
Locomotor boxes
Locomotor activity was assessed in black Perspex circular runways (internal diameter, 11 cm; external diameter, 25 cm; height, 25 cm) in which the animals were videoed from below through a translucent Perspex floor that detected the moving shadow of the animal. Images were digitised and locomotor activity determined using in-house software written in Matlab (The MathWorks, Natick, MA, USA). Both overall and forward locomotion (whereby animals circulate in one direction for 90° and additional counts for every 45° thereafter) were calculated.
Five-choice serial reaction time task boxes
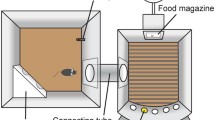
The test apparatus consisted of eight mouse operant chambers (Med Associates, St. Albans, VT, USA). Each chamber was housed in a wooden sound-attenuating outer cabinet with a ventilator fan providing a constant low-level background noise. The left wall of the chamber was curved and contained five apertures with an infrared detector located inside each of them to detect nose-poke responses. The apertures were illuminated by a yellow stimulus light located inside each aperture. The right wall of the chamber contained a receptacle hole with a round access opening where the liquid reinforcer was delivered; 0.01 mL of a 30% condensed milk solution was used as a reinforcer and was delivered in a small cup by means of a dipper. Head entries into the food magazine were recorded by an infrared photo-cell beam crossing the entrance of the receptacle hole that could be illuminated by a yellow stimulus light inside the aperture. Above the food magazine and at the top of the wall, a house-light was located. The presentation of stimuli and the recording of the responses were controlled by a Smart Ctrl Package 8IN/16OUT with an additional interface by MED-PC for Windows (Med Associates, St. Albans, VT, USA).
Behavioural procedures
Locomotor activity
Animals were handled and weighed for 1 week before the start of the experiment. Testing took place between 1000 and 1200 hours in 45-min sessions for four consecutive days to investigate possible differences in locomotor activity in a novel environment as well as the within- and between-sessions habituation.
Habituation to the reinforcement and to the 5-CSRTT boxes
During the first two to three sessions, animals were placed in the boxes for 30 min and the liquid reward was available in the magazine on a continuous schedule. The animal had only to nose-poke in the magazine to receive the condensed milk solution that was available for 10 s, after which the dipper was refilled and available again. The house-light, the magazine light and the stimulus lights in the five holes were turned on during the entire session. Magazine head entries and number of reinforcers earned were recorded. When the animal had earned about 50 reinforcers in two consecutive sessions, then it started the training in the 5-CSRTT.
5-CSRTT training
The session started with the illumination of the house-light and a free delivery of the liquid reinforcer accompanied by the illumination of the food magazine, which signalled reward availability. When the animal nose-poked into the magazine to obtain the reward (dipper on for 3 s), the first trial was initiated. After a fixed interval (ITI), one of the stimulus lights in the holes was turned on for a brief time and the animal was required to nose-poke within a certain period of time (limited hold, LH) into the correct hole in order to obtain the reinforcer. After a correct response, the animal had to nose-poke into the magazine to collect the reward and to initiate the next trial. An incorrect response occurred when the animal made a response in a different hole to the one that had been illuminated, and this was followed by a time out period (TO) during which the lights were turned off for 5 s. Responses made into the holes during this period restarted the TO. An error of omission occurred when the animal failed to respond into any of the holes after the completion of the LH and was also followed by a TO. Any response into the holes during the ITI, which means that the stimulus light had not yet been presented, was registered as a premature response and was followed by a TO. After a TO period, the next trial was restarted by a nose-poke into the magazine. Perseverative responses, that is, further responses into the holes after a correct response and before the collection of the reward, were registered but had no programmed consequences.
At the beginning of the training, the stimulus duration (SD) was set to 30 s and the ITI was set to 2 s, but these parameters were adjusted according to the performance of each animal. When the animal produced two consecutive sessions achieving the performance criteria (>50 correct trials, >80% accuracy and <25% omissions), the stimulus duration was reduced in the following pattern: 30, 20, 10, 5, 2.5, 1.8, 1.4, 1.2, 1, 0.9, 0.8 (baseline) and the LH and the ITI were set at 5 s (see Table 1 for the particular parameters of each successive stage of training). Testing was carried out daily (5–6 days/week), and the session lasted for 100 trials or 30 min, whichever came first. In the long ITI sessions, the ITI was set at 7 s and the duration of the task was increased to 45 min. The 7-s duration for the long ITI sessions was chosen because it has been shown to increase premature responding consistently in rats (Dalley et al. 2007, 2008).
Pharmacological manipulations
After 10 days of stable performance under baseline parameter conditions, mice were treated with vehicle, 0.5, 1 and 2 g/kg ethanol, i.p., 15 min prior to testing. To further analyse the performance in the 5-CSRTT using a long ITI session, mice were treated with vehicle, 1 and 2 g/kg ethanol.
Two further studies under long ITI parameters were performed testing diazepam (0, 1 and 2 mg/kg) and ketamine (0, 10 and 20 mg/kg) 15 min prior to testing. Separate Latin square designs were used for each pharmacological study. During the days between drug testing, mice performed a minimum of 2 days under baseline parameters in the 5-CSRT task to stabilise the performance in case it had been disrupted by the drug testing sessions.
Statistical analysis
The statistical analysis was performed using the ‘Statistical Package for Social Sciences’ (SPSS, version 14.0). Locomotor activity data were analysed by repeated-measures analysis of variance (ANOVA) with strain as the between-subjects factor and days (four levels) or 15-min bins (three levels) as the within-subjects factor. Total number of sessions to achieve criteria of performance in the 5-CSRTT was analysed by a non-parametric Kruskal–Wallis test.
The variables considered in the analysis of the 5-CSRTT were:
-
Total trials: total correct responses + total incorrect + total omissions.
-
Accuracy (percentage of correct responses): correct responses/(correct responses + total incorrect responses) × 100.
-
Percentage of omissions: total omissions/(correct responses + incorrect responses + omissions) × 100.
-
Percentage of premature responding: premature responses/(correct responses + incorrect responses + omissions + premature responses) × 100.
-
Correct latency: latency to nose-poke into the correct hole after the onset of the stimulus (in milliseconds).
-
Magazine latency: latency to collect the reward after a correct response (in milliseconds).
-
Perseverative responses: total number of responses made into the holes after a correct response and before the collection of the reward.
Two-way repeated-measures ANOVA with strain as the between-subjects factor and stage of training or drug dose as the within-subjects factor was used for the analysis of each variable of the 5-CSRTT training and pharmacological manipulation. One-way ANOVAs and paired t tests were used for post hoc analysis. Where sphericity assumptions were violated, the Greenhouse–Geisser correction was applied. The variable ‘premature responding’ was arcsine transformed [x′ = 2arcsine (√x)] and ‘perseverative responding’ was log_10 transformed in order to attain homogeneity of variance and permit valid parametric analysis. A p < 0.05 was required for results to be considered statistically significant.
Results
Locomotor activity
The repeated-measures analysis of the total locomotion across days showed that mice of both groups reduced the amount of activity showing habituation to the environment (effect of day: F (3,39) = 32.81, p = 0.001; Fig. 1a) but there was no main differences between strains. To further study the response of the animals to a new environment, we analysed the locomotor activity data on the first day by splitting the session into 15-min bins. The results showed that both groups of mice decreased the amount of activity during the 45-min session (effect of time: F (2,26) = 76.95, p < 0.001; Fig. 1b) with CD1 mice showing higher levels of activity during the initial 15 min in comparison with the C57 mice (time × strain interaction: F (2,26) = 5.28, p < 0.05; Fig. 1b) and indicating greater initial reactivity to a novel environment, though no significant differences were found between strains in total activity during this session.
5-CSRTT training
CD1 mice required fewer training sessions to achieve criteria for performance under the final baseline parameters of 0.8 s stimulus duration (CD1, 44.5 ± 2.8; C57, 55 ± 3.06; χ² (1) = 6.50, p < 0.05). Both strains showed an increase in accuracy of responding as the difficulty of the sessions increased, displaying higher levels of accuracy as the stimulus was progressively shortened in duration (effect of stage: F (10,130) = 21.68, p < 0.001, e = 0.458; Fig. 2a). No strain differences were found in this measure of execution during training.
Performance of CD1 (open squares) and C57 (filled squares) mice at different stages of training in the 5-CSRTT. a The mean ± SE of the accuracy of responding, b percentage omissions, c percentage of premature responses, d latency to make a correct response, e latency to collect the reward and f perseverative responses. Data shown are for the last day of each successive training stage. *p < 0.05, **p < 0.01; differences between strains
Strain differences appeared in the percentage of omission errors displayed across stages (stage × strain interaction: F (10,130) = 2.31, p < 0.05; Fig. 2b) with C57 mice showing higher levels of omissions with stimulus durations of 20, 5, 1.2 and 0.8 s. A main strain difference appeared in the ANOVA, confirming that C57 mice displayed a higher percentage of omission errors than CD1 mice (F (1,13) = 6.87, p < 0.05).
The repeated-measures ANOVA of the percentage of premature responses showed an interaction between session and strain, showing that, during the first stages of training, CD1 mice performed more premature responding, but this difference between strains was abolished in subsequent stages (stage × strain interaction: F (10,130) = 2.81, p < 0.01; Fig. 2c).
Due to problems with the computer, several missing values appeared during data collection across the first two stages of the training for correct and magazine latency variables, and for this reason, only the data from stage 3 onwards were subjected to statistical analysis for these two variables. The repeated-measures ANOVA showed that the animals decreased correct latencies as the requirements of the task increased (effect of stage: F (8, 104) = 197.42, p < 0.001, e = 0.324; Fig. 2d) and a main effect of strain was found with CD1 animals displaying shorter correct response latencies than the C57 mice (F (1,13) = 8.017, p < 0.05); this effect was especially reliable in the sessions with stimulus duration of 1.8, 1.4 and 1.2.
The repeated-measures ANOVA of the magazine latency data showed variation across the stages of training (F (8,104) = 4.017, p < 0.01, e = 0.277; Fig. 2e) and pointed to differences between strains (F (1,13) = 6.46, p < 0.05); however, one-way ANOVAs failed to reveal strain differences at any single stage of training. No statistical differences were found in the total number of perseverative responses during training (F < 0.15, n.s.; Fig. 2f).
Effects of ethanol in the 5-CSRTT in the sessions with standard conditions
Figure 3 represents the effects of ethanol in the baseline parameter conditions. Ethanol did not affect the total number of trials completed in the 30-min session (F < 2.4, n.s., data not shown) or in accuracy (F < 1.27, n.s.; Fig. 3a). Ethanol dose-dependently increased omissions (effect of dose: F (3,36) = 6.65, p < 0.01, e = 0.607; Fig. 3b) with significant effects emerging at 2 g/kg. The analysis also revealed a main effect of strain (F (1,12) = 8.706, p < 0.05) with C57 consistently showing higher values.
Effects of ethanol on the 5-CSRTT in CD1 (white bars) and in C57 (black bars) mice. a The mean ± SE of accuracy, b percentage omissions, c percentage of premature responses, d correct latency, e magazine latency and f perseverative responses on sessions under baseline standard parameters (stimulus duration, 0.8 s; ITI, 5 s). *p < 0.05 vs same dose, different strain; •p < 0.05, ••p < 0.01 vs saline (dose 0)
Ethanol did not affect the percentage of premature responses in the baseline parameter sessions (F (3,36) < 1.82, n.s.), but a main effect of group was found (F (1,12) = 10.88, p < 0.01; Fig. 3c) attributable to CD1 mice displaying more premature responses than the C57 mice.
A significant effect of dose (F (3,36) = 6.65, p < 0.001; Fig. 3d) indicated that latencies to perform correct responses differed across doses, probably due to a small reduction in latencies at the 0.5-g/kg dose and an increase the 2-g/kg dose in comparison with the saline condition; however, paired t test comparisons failed to find reliable differences between each of the doses and the saline condition.
Magazine latency was also affected by ethanol dose (F (3,36) = 7.52, p < 0.001; Fig. 3e) with the 2-g/kg dose increasing latency to collect the liquid reward.
The analysis of the perseverative responses showed a dose × group interaction (F (3,36) = 3.31, p < 0.05; Fig. 3f) attributable to a decrease in perseverative responses with the dose of 2 g in the CD1 animals who normally showed elevated levels in this variable.
Effects of ethanol in the 5-CSRTT in the long ITI sessions
Figure 4 illustrates the effects of ethanol under the long ITI sessions. There were no differences in number of total trials or in accuracy in the long ITI sessions as a consequence of ethanol injections (F < 1.94, n.s., for both variables), and ethanol did not affect the percentage of omissions (F < 2.29, n.s.; Fig. 4b), though a main effect of strain was observed (F (1,12) = 5.118, p < 0.05), the C57 mice again showing more omissions than the CD1 mice. The effect of ethanol on percentage of premature responses was affected by dose (F (2,24) = 12.6, p < 0.001; Fig. 4c), the 1-g/kg dose in particular increasing premature responding in both groups of mice.
Effects of ethanol on the 5-CSRTT in CD1 (white bars) and in C57 (black bars) mice. a The mean ± SE of accuracy, b percentage omissions, c percentage of premature responses, d correct latency, e magazine latency and f perseverative responses on sessions under long ITI parameters (stimulus duration, 0.8 s; ITI, 7 s). *p < 0.05 vs same dose, different strain; •p < 0.05 vs saline (dose 0)
A dose × strain interaction appeared in the latency to make a correct response (F (2,24) = 3.61, p < 0.05; Fig. 4d) that was attributable to the dose of 2 g/kg producing a larger increase in correct reaction time in C57 animals compared with the saline condition.
A dose × strain interaction appeared again in the magazine latency (F (2,24) = 8.56, p < 0.01; Fig. 4e) due to the increase in the latency to collect the liquid reward with the 2-g/kg dose in comparison with the saline condition in the C57 mice.
Ethanol also modified the number of perseverative responses in the long ITI sessions (dose × group interaction: F (2,24) = 9.48, p = 0.001; Fig. 4f) due to a decrease in perseverative responding at 2 g/kg ethanol in the CD1 mice.
Effects of diazepam in the 5-CSRTT in the long ITI sessions
Figure 5 illustrates the effects of diazepam in the long ITI sessions. Diazepam did not affect the total trials completed in the sessions (F < 3.4, n.s., data not shown). No differences in accuracy were found as a consequence of dose of diazepam (F < 2.89, n.s.; Fig. 5a), but a main effect of strain (F (1,12) = 12.92, p < 0.01) indicated that the CD1 strain performed at higher levels of accuracy than the C57 mice.
Effects of diazepam on the 5-CSRTT in CD1 (white bars) and in C57 (black bars) mice. a The mean ± SE of accuracy, b percentage omissions, c percentage of premature responses, d correct latency, e magazine latency and f perseverative responses on sessions under long ITI parameters (stimulus duration, 0.8 s; ITI, 7 s). *p < 0.05 vs same dose, different strain; •p < 0.05, ••p < 0.01 vs saline (dose 0)
No dose or dose × strain interaction effects were found in percentage omissions (F < 2.3, n.s.), but a main effect of strain was found (F (1,12) = 24.84, p < 0.001; Fig. 5b) where again the C57 displayed higher omission values than the CD1 mice across doses.
The effect of diazepam on percentage of premature responses was affected by dose (F (2,24) = 47.126, p < 0.01; Fig. 5c), and a trend towards a dose × group interaction appeared in the analysis (F (2,24) = 2.99, p = 0.069; Fig. 5c). A main effect of group (F (1,12) = 8.59, p < 0.05) indicates that, in general, C57 mice made more premature responses than CD1 mice. Post hoc analysis showed that the two doses of diazepam (1 and 2 mg/kg) increased premature responding in the long ITI sessions in both groups of mice in comparison with the saline condition.
No statistical differences were found in correct latency, but a trend towards a main effect of group appeared in the analysis (F (1,12) = 3.49, p = 0.086, n.s.; Fig. 5d) with the C57 mice showing the higher values.
An effect of dose on the magazine latency was found (F (2,24) = 5.949, p < 0.01; Fig. 5e), and that was evident for 1 g/kg diazepam, which decreased magazine latency in both groups of mice.
No significant differences were found in the repeated-measures ANOVA for perseverative responses, but a trend towards a main effect of group was found (F (1,12) = 4.19, p = 0.063; Fig. 5f) where CD1 animals showed more perseverative behaviour than the C57.
Effects of ketamine in the 5-CSRTT in the long ITI sessions
Figure 6 illustrates the effects of ketamine in the long ITI sessions. Ketamine did not affect total number of trials (F < 1.5, n.s.) or accuracy (F < 3.2, n.s.). No dose or dose × strain interaction effects were found in percentage omissions (F < 0.49, n.s.; Fig. 6b) but a main effect of strain (F (1,12) = 9.16, p < 0.05) indicated that the C57 mice displayed more omissions than the CD1 mice across doses.
Effects of ketamine on the 5-CSRTT in CD1 (white bars) and in C57 (black bars) mice. a The mean ± SE of accuracy, b percentage omissions, c percentage of premature responses, d correct latency, e magazine latency and f perseverative responses on sessions under long ITI parameters (stimulus duration, 0.8 s; ITI, 7 s). *p < 0.05 vs same dose, different strain; •p < 0.05, vs saline (dose 0)
The two strains showed different responsiveness to ketamine for premature responses. There was a marginally significant dose × group interaction in the percentage of premature responding (F (2,24) = 3.29, p = 0.05; Fig. 6c), and a main effect of group was also found (F (1,12) = 7.86, p < 0.05) that was attributable to the higher number of premature responses in the CD1 mice. Post hoc analysis indicated that the 10-mg/kg dose of ketamine increased premature responding in CD1 mice but had no effect in C57 animals. The higher dose of 20 mg/kg had no significant effects in either strain.
The analysis of the correct latency data revealed a main effect of group (F (1,12) = 8.56, p < 0.05; Fig. 6d) but no dose or dose × group interactions (F < 0.012, n.s.). Overall, the C57 strain showed greater latencies to make correct responses than the CD1 mice.
A dose × strain interaction was found for magazine latency (F (2,24) = 3.72, p < 0.05; Fig. 6e) and a main effect of group appeared in the ANOVA (F (1,12) = 6.47, p < 0.05) attributable to the higher latencies to collect the food reward in the C57 mice. Post hoc analysis did not reveal statistical differences between the doses compared with the saline condition, but the C57 mice showed higher latencies for the doses of 10 and 20 mg/kg in comparison with CD1 mice.
With regard to perseverative responses, ketamine affected the two groups of mice (dose effect: F (2,24) = 7.041, p < 0.01; Fig. 6f), a trend towards a dose × group interaction (F (2,24) = 2.97, p = 0.07; Fig. 6f), suggesting that ketamine dose-dependently increased perseverative responses especially in C57 mice.
Discussion
The main results obtained in the present investigation showed that ethanol had no detrimental effects on attentional performance in the 5-CSRTT, whereas it increased impulsivity in a manner that was dependent on the session conditions; that is, ethanol did not increase premature responding when the animals performed the task under the training session parameters but increased premature behaviour when the mice were confronted by extended ITI sessions where the ‘waiting time’ to perform the correct response was increased.
Strain differences
During training, we observed differences between the two strains used, with the outbred CD1 strain acquiring the task more rapidly than the inbred C57 strain. Nevertheless, both strains of mice performed the task at high levels of accuracy and did not differ in the main variables once they had achieved criterion for performance in the baseline parameters condition, with the exception of the number of omission errors, which were consistently higher in the C57 than in the CD1 strain across the study. Elevated levels of omission errors can reflect sensory, motor or motivational factors (Robbins 2002). In the present investigation, the increased numbers of omissions were not accompanied by increased latencies in performing correct choices or in magazine latency measures during the last session of baseline performance, so the greater number of omissions may indicate that the C57 mice are inferior in their attentional abilities to the CD1 strain. High rates of omissions in a somewhat different version of the 5-CSRTT have previously been reported for a C57BL/6 strain in comparison with the DBA/2 strain of mice in the final stages of the experimental training (Patel et al. 2006). After extensive training, animals reached asymptotic levels of performance, and in this case, the ceiling performance for the CD1 was also higher than for the C57 mice. An alternative explanation may be that the CD1 mice are more responsive to changes in the environment than the C57 mice, as suggested by the results from the locomotor tests where the CD1 mice were more active during the first 15 min in the locomotor boxes, even though no differences were found in total general activity; that CD1 mice showed more initial activity suggests that, in the initial 5-CSRTT training, they may have been more ready to explore the 5-CSRTT apparatus. CD1 mice also emitted more premature responses at initial stages of training, a feature that could conceivably be beneficial since impulsive behaviour at this stage of training allows more experience of the particular contingencies of the task. Such an interpretation may be supported by the reported association between impulsivity and the acquisition of Pavlovian autoshaping in rats (Tomie et al. 1998). Alongside higher levels of impulsive action, CD1 mice also showed lower latencies in correct choices during training, though these differences were abolished following extensive training. Perseverative responses did not differ between the strains during training, but during the course of the pharmacological experiments, the CD1 mice showed a tendency to emit more perseverative responses than C57 mice, the tendency reaching significance at certain stages of the experiment. The group sizes tested in the present experiments were low, and it cannot be excluded that the tendency of CD1 mice to show perseveration might have been statistically reliable with larger numbers of mice.
Effects of ethanol
No differences in total number of trials completed or in accuracy were found as a consequence of the pharmacological manipulations. Some authors have suggested a negative relationship between accuracy and premature responding (Hahn et al. 2002; Patel et al. 2006; Puumala et al. 1996; Puumala and Sirvio 1998), but the results from the present paper indicate that the drug-induced increases in premature responding were independent of accuracy, which was not affected. The independence of these measures would be consistent with different underlying neurobiology, in keeping with observations that lesions of the anterior cingulate cortex increased impulsive responding without affecting attentional performance in the 5-CSRTT (Robbins 2002). Nevertheless, ethanol (2 g/kg) increased omissions in the baseline sessions but not in the long ITI sessions. Perhaps this latter effect may indicate that omissions reflect sedation and that, with a switch from 5 to 7 s during the long ITI sessions, the animal simply has more time to position itself to scan the holes. The increase in magazine latency at the dose of 2 g/kg would be consistent with such an interpretation. Another explanation for this effect could be that, in the long ITI sessions, the animals are more aroused because they are performing the task under non-habitual parameters, minimising the effects of ethanol in this variable. The ethanol (2 g/kg)-induced increase in correct and magazine latencies was especially notable in C57 mice. Perseverative responses were affected differently by 2 g/kg ethanol in the two strains, suggesting different interactions of the drug with the genotype of the animal. Thus, ethanol decreased perseverative responding in the CD1 strain in the baseline parameters and in the long ITI sessions while having no or a very small effect in the C57 mice. This decrease in perseverative behaviour in CD1 mice is likely to be a consequence of a general reduction in the vigour of responding under ethanol, as it was accompanied by increased response latencies and it was an effect not mimicked by the later injections with diazepam or ketamine. With regard to premature responses, as commented above, ethanol did not affect prematures under the baseline conditions in any of the doses tested; nevertheless, 1 g/kg ethanol significantly increased premature responding in the long ITI sessions. This result may suggest that actions that are performed habitually can be insensitive to the effects of ethanol, while non-habitual situations, where the subject is required to adapt its behaviour and respond accordingly to new requirements, make clear the effects of alcohol in inhibitory control mechanisms.
The fact that alcohol did not affect choice accuracy is in accordance with the results from Bizarro et al. (2003) in rats, where they found that alcohol (0.4–1.6 g/kg, i.p.) had no effects in accuracy of responding. In that report, alcohol increased omission errors even more than in the present experiment, resulting in more than 80% omissions at a dose of 1.6 g/kg; correct response latencies were also increased in a dose-related manner. Contrary to the present results, the authors reported a dose-dependent decrease in the rate of premature responding, which they argue could be explained by sedation or an impairment of motor ability (Bizarro et al. 2003). Furthermore, other methodological differences between the present investigation and the results reported from Bizarro et al. (2003) could account for the opposite effects of ethanol found in premature responding, as in the protocol followed by these authors the occurrence of premature responding was not punished with a TO period as it is in our protocol. Our results also differ from reports that alcohol (0.5–1.5 g/kg, i.p.) impaired response accuracy in a two-choice reaction time task (Givens 1997; Givens and McMahon 1997) or in the visual signal detection task in rats (Rezvani and Levin 2003), but the procedural differences between these paradigms, as well as species differences, could account for these discrepancies.
In regard to the increase in impulsive responding after ethanol treatment, our results are in agreement with other reports using different paradigms of impulsivity in rats, including the delay of reinforcement paradigm where ethanol increased impulsive behaviour (Evenden and Ryan 1999; Olmstead et al. 2006; Poulos et al. 1998; Tomie et al. 1998), suggesting that ethanol increases impulsive choice and impulsive action.
Effects of diazepam and ketamine
Diazepam and ketamine, like ethanol, did not affect accuracy of responding or percentage of omissions in the 5-CSRTT in long ITI sessions. The two strains of mice were sensitive to the drug effects on premature responses; both doses of diazepam increasing impulsive behaviour, although the effects were more consistent in the CD1 strain. A trend towards the dose × group interaction suggested a reduced sensitivity to the 2 mg/kg diazepam in the C57 strain.
No differences were found in correct latency but 1 g/kg diazepam decreased magazine latency, an effect that might be explained by an increase in motivation to consume the liquid reward (Robbins 2002) or by a general increase in response vigour that might have also contributed to the increase in premature responding. As mentioned, diazepam did not affect accuracy of responding in the present study, which is discordant with the reported impairment induced by diazepam in sustained attention in rats (Cole 1990; McGaughy and Sarter 1995). Differences in the tasks employed can explain this disparity of results. In the experiment by Cole 1990, a go–no go discrimination task was used, and the author suggested than the impairment in the task was due to an impairment in the ability of the subjects to inhibit or withhold responding rather than to deficits in attention. In the experiment by McGaughy and Sarter (1995), where a visual detection task was used, the benzodiazepine chlordiazepoxide impaired the animal's ability to discriminate between signal and non-signal events, but the authors reported that these effects were due to an increase in the number of omissions. These data indicate that impairment in accuracy as a consequence of low doses of benzodiazepine may not occur in mice. Furthermore, our finding of a lack of effect in accuracy in the 5-CSRTT in mice is in agreement with the results from Greco and Carli (2006), who reported that diazepam (2 mg/kg) had no effect in wild-type mice from a mixed C57BL/6–129SvJ background, while increasing the number of anticipatory responses (Greco and Carli 2006). Additionally, and also in agreement with the present paper, the number of perseverative responses was not affected by diazepam, and diazepam had no effects on the percentage of omissions or the number of trials completed.
To our knowledge, this is the first study that examines the effects of the non-competitive NMDA receptor antagonist, ketamine, in the 5-CSRTT. Contrary to the reported decrease in attentional function induced by the competitive NMDA receptor antagonist, 3-(R)-2-carboxypiperazin-4-propyl-1-l-phosphonic acid (CCP), in rats (Carli et al. 2006), our results indicate that ketamine does not affect accuracy of responding in mice, in agreement with the report by Higgins et al. (2003b) in which no effects in response accuracy in the 5-CSRTT were found after dizocilpine treatment (using a low dose of 0.03 mg/kg). Nevertheless, an impairment in accuracy in the 5-CSRTT with dizocilpine at higher doses (0.06 and 0.1 mg/kg) has also been reported (Higgins et al. 2003b) and a decrease in accuracy and an increase in premature responding and omissions with the dose of 0.06 mg/kg (Grottick and Higgins 2000). It may be that the doses of ketamine used in the present investigation are in a low range because no increase in omissions was found.
Dizocilpine and CPP have also been shown to increase impulsivity in the 5-CSRTT (Carli et al. 2006; Higgins et al. 2003a, b) and in the differential reinforcement of low rate of responding (DRL) procedure (Stephens and Cole 1996). The effects of ketamine in impulsive behaviour are more controversial, it has been reported that ketamine (5 mg/kg) increased impulsivity in the DRL procedure (Floresco et al. 2008) or had no effects in impulsivity measures (10 and 15 mg/kg, i.p.) in the DRL or the bisection procedure in rats (Cheng et al. 2006), but differences in doses can explain the discordance in the results. In our study, ketamine differentially affected both strains of mice. That is, ketamine had no effect in C57 animals but increased premature responding in CD1 mice, especially at the dose of 10 mg/kg, indicating that CD1 mice are more sensitive to NMDA antagonism than the C57 mice in this measure. Nevertheless, in measures of perseverative responding, the C57 strain seems to be more sensitive to the effects of ketamine, showing a trend towards a dose-dependent increase in compulsive behaviour, although not reaching statistical significance. A potential weakness of the current experiment is that all animals received ketamine after having experienced with ethanol and diazepam, so that it cannot be ruled out that a lack of effects of ketamine may reflect adaptations (e.g. tolerance) to the two other drugs. However, both strains received all drugs in the same order, and ketamine differed in its effectiveness between the strains in increasing premature responding.
Neurochemistry of impulsive behaviour
The present results show that alcohol did not affect the accuracy of responding in the 5-CSRTT, but it affected measures of response inhibition. The effects of ethanol on impulsive responding can be explained by two main pharmacological actions: by its agonist action at GABAA receptors or by its antagonism at NMDA receptors. In keeping, the perceptual and subjective effects of alcohol are mimicked by both GABA agonists and NMDA antagonists (Shelton and Balster 1994). For the CD1 strain, the effects of ethanol in premature responding were mimicked by diazepam and ketamine; that is, both pharmacological manipulations lead to the same behavioural outcome. For the C57 strain, while diazepam increased premature responding, ketamine failed to mimic this effect, suggesting that the effects of alcohol on premature responding in C57 mice are exerted by an action at GABAA receptors. Although suggesting that both GABAergic and glutamatergic mechanisms may contribute to impulsivity, the current experiments do not throw light on brain regions in which these effects are exerted. The neural basis of impulsive actions of the kind investigated here is thought to be localised in the prefrontal cortex and in its interaction with subcortical structures such as the striatum (Cole and Robbins 1989; Pattij and Vanderschuren 2008; Winstanley et al. 2006a, b). Excitotoxic lesions of presumably glutamatergic output neurons of the infralimbic cortex produce a selective increase in premature responses without affecting other measures of responding (Chudasama et al. 2003), and premature responding is also increased by infusions of NMDA antagonists into the infralimbic cortex (Murphy et al. 2005). Thus, ketamine's, and perhaps ethanol's, effects may be attributable to their inhibiting prefrontal activity. Given the evidence that impulsivity in the 5-CSRTT has previously been associated with facilitated dopamine (Pattij et al. 2007; van Gaalen et al. 2006) and serotonin neurotransmission (Puumala and Sirvio 1998), it is also possible that the role of GABA and glutamate is in regulating activity in these systems. Results from the present paper also support the notion that impulsivity and compulsivity are mediated by different neural regions as the effects of ethanol, diazepam and especially ketamine in measures reflecting these aspects of response inhibition appear to be unrelated.
The present study concludes that ethanol had no effects on attentional performance in the 5-CSRTT but that it increased motor impulsivity in mice when the subjects were required to wait longer to make an appropriate response, suggesting that the interactions between ethanol and impulsivity depend on the characteristics of the situation. Further research is needed to clarify the neurobiological bases that support such interactions and we conclude that the 5-CSRTT has proved to be a useful paradigm for the study of ethanol and its effects on impulsivity in mice.
References
Billings CE, Demosthenes T, White TR, O'Hara DB (1991) Effects of alcohol on pilot performance in simulated flight. Aviat Space Environ Med 62:233–235
Bizarro L, Patel S, Stolerman IP (2003) Comprehensive deficits in performance of an attentional task produced by co-administering alcohol and nicotine to rats. Drug Alcohol Depend 72:287–295
Cabib S, Puglisi-Allegra S, Ventura R (2002) The contribution of comparative studies in inbred strains of mice to the understanding of the hyperactive phenotype. Behav Brain Res 130:103–109
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Carli M, Baviera M, Invernizzi RW, Balducci C (2006) Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology 31:757–767
Cheng RK, MacDonald CJ, Meck WH (2006) Differential effects of cocaine and ketamine on time estimation: implications for neurobiological models of interval timing. Pharmacol Biochem Behav 85:114–122
Cherpitel CJ (1993) Alcohol and injuries: a review of international emergency room studies. Addiction 88:923–937
Cherpitel CJ (1999) Substance use, injury, and risk-taking dispositions in the general population. Alcohol Clin Exp Res 23:121–126
Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW (2003) Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119
Cole SO (1990) Diazepam-induced impairment of a go-no go successive discrimination. Behav Neural Biol 53:371–377
Cole BJ, Robbins TW (1989) Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res 33:165–179
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124
Critchlow B (1986) The powers of John Barleycorn. Beliefs about the effects of alcohol on social behavior. Am Psychol 41:751–764
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270
Dalley JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–260
Dougherty DM, Moeller FG, Steinberg JL, Marsh DM, Hines SE, Bjork JM (1999) Alcohol increases commission error rates for a continuous performance test. Alcohol Clin Exp Res 23:1342–1351
Dougherty DM, Marsh DM, Moeller FG, Chokshi RV, Rosen VC (2000) Effects of moderate and high doses of alcohol on attention, impulsivity, discriminability, and response bias in immediate and delayed memory task performance. Alcohol Clin Exp Res 24:1702–1711
Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW (2008) A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend 96:111–120
Ericksen KP, Trocki KF (1994) Sex, alcohol and sexually transmitted diseases: a national survey. Fam Plann Perspect 26:257–263
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology (Berl) 146:348–361
Evenden JL, Ryan CN (1999) The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 146:413–421
Floresco SB, Tse MT, Ghods-Sharifi S (2008) Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33:1966–1979
Givens B (1997) Effect of ethanol on sustained attention in rats. Psychopharmacology (Berl) 129:135–140
Givens B, McMahon K (1997) Effects of ethanol on nonspatial working memory and attention in rats. Behav Neurosci 111:275–282
Greco B, Carli M (2006) Reduced attention and increased impulsivity in mice lacking NPY Y2 receptors: relation to anxiolytic-like phenotype. Behav Brain Res 169:325–334
Grottick AJ, Higgins GA (2000) Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res 117:197–208
Hahn B, Shoaib M, Stolerman IP (2002) Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 162:129–137
Higgins GA, Ballard TM, Huwyler J, Kemp JA, Gill R (2003a) Evaluation of the NR2B-selective NMDA receptor antagonist Ro 63-1908 on rodent behaviour: evidence for an involvement of NR2B NMDA receptors in response inhibition. Neuropharmacology 44:324–341
Higgins GA, Enderlin M, Haman M, Fletcher PJ (2003b) The 5-HT2A receptor antagonist M100, 907 attenuates motor and ‘impulsive-type’ behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 170:309–319
Maylor EA, Rabbitt PM (1993) Alcohol, reaction time and memory: a meta-analysis. Br J Psychol 84(Pt 3):301–317
McGaughy J, Sarter M (1995) Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 117:340–357
Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 36:357–368
Mulvihill LE, Skilling TA, Vogel-Sprott M (1997) Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol 58:600–605
Murphy ER, Dalley JW, Robbins TW (2005) Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 179:99–107
Olmstead MC, Hellemans KG, Paine TA (2006) Alcohol-induced impulsivity in rats: an effect of cue salience? Psychopharmacology (Berl) 184:221–228
Ortner CN, MacDonald TK, Olmstead MC (2003) Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol 38:151–156
Patel S, Stolerman IP, Asherson P, Sluyter F (2006) Attentional performance of C57BL/6 and DBA/2 mice in the 5-choice serial reaction time task. Behav Brain Res 170:197–203
Pattij T, Vanderschuren LJ (2008) The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29:192–199
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 191:587–598
Petry NM (2001) Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 154:243–250
Poulos CX, Parker JL, Le DA (1998) Increased impulsivity after injected alcohol predicts later alcohol consumption in rats: evidence for “loss-of-control drinking” and marked individual differences. Behav Neurosci 112:1247–1257
Puumala T, Sirvio J (1998) Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience 83:489–499
Puumala T, Ruotsalainen S, Jakala P, Koivisto E, Riekkinen P Jr, Sirvio J (1996) Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem 66:198–211
Rezvani AH, Levin ED (2003) Nicotine–alcohol interactions and attentional performance on an operant visual signal detection task in female rats. Pharmacol Biochem Behav 76:75–83
Richards JB, Zhang L, Mitchell SH, de Wit H (1999) Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav 71:121–143
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR (2004a) Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci 24:10159–10166
Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR (2004b) Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci 24:1594–1603
Rohrbaugh JW, Stapleton JM, Parasuraman R, Frowein HW, Adinoff B, Varner JL, Zubovic EA, Lane EA, Eckardt MJ, Linnoila M (1988) Alcohol intoxication reduces visual sustained attention. Psychopharmacology (Berl) 96:442–446
Samson HH, Harris RA (1992) Neurobiology of alcohol abuse. Trends Pharmacol Sci 13:206–211
Shelton KL, Balster RL (1994) Ethanol drug discrimination in rats: substitution with GABA agonists and NMDA antagonists. Behav Pharmacol 5:441–451
Spinella M (2004) Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. Int J Neurosci 114:95–104
Stephens DN, Cole BJ (1996) AMPA antagonists differ from NMDA antagonists in their effects on operant DRL and delayed matching to position tasks. Psychopharmacology (Berl) 126:249–259
Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M (2004) Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend 73:121–132
Thiebot MH, Le Bihan C, Soubrie P, Simon P (1985) Benzodiazepines reduce the tolerance to reward delay in rats. Psychopharmacology (Berl) 86:147–152
Tomie A, Aguado AS, Pohorecky LA, Benjamin D (1998) Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology (Berl) 139:376–382
van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ (2006) Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 187:73–85
West R, Wilding J, French D, Kemp R, Irving A (1993) Effect of low and moderate doses of alcohol on driving hazard perception latency and driving speed. Addiction 88:527–532
Winstanley CA, Eagle DM, Robbins TW (2006a) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26:379–395
Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW (2006b) Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex 16:106–114
Acknowledgements
This work was carried out within the framework of the IMAGEN consortium. IMAGEN receives research funding from the European Community's Sixth Framework Programme (LSHM-CT-2007-037286). This paper reflects only the authors' views and the community is not liable for any use that may be made of the information contained therein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliver, Y.P., Ripley, T.L. & Stephens, D.N. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology 204, 679–692 (2009). https://doi.org/10.1007/s00213-009-1500-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1500-0