Abstract
Rationale
Individuals vary considerably in the extent to which they attribute incentive salience to food-associated cues.
Objectives
We asked whether individuals prone to attribute incentive salience to a food cue are also prone to attribute incentive properties to a stimulus associated with a drug of abuse—cocaine.
Methods
We first identified those rats that attributed incentive salience to a food cue by quantifying the extent to which they came to approach and engage a food cue. We then used a conditioned place preference procedure to pair an injection of 10 mg/kg cocaine (i.p.) with one distinct floor texture (grid or holes) and saline with another. Following 8 days of conditioning, each rat was given a saline injection and placed into a chamber that had both floors present. We measured the time spent on each floor, and also 50-kHz ultrasonic vocalizations, which have been associated with positive affective states.
Results
Rats that vigorously engaged the food cue (“sign trackers”) expressed a preference for the cocaine-paired floor compared to those that did not (“goal trackers”). In addition, sign trackers made substantially more frequency-modulated 50-kHz vocalizations when injected with cocaine and when later exposed to the cocaine cue.
Conclusions
Rats prone to attribute incentive salience to a food cue are also prone to attribute incentive motivational properties to a tactile cue associated with cocaine. We suggest that individuals prone to attribute incentive salience to reward cues will have difficulty resisting them and, therefore, may be especially vulnerable to develop impulse control disorders, including addiction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
If an otherwise neutral stimulus [conditional stimulus (CS) or cue] is associated with a biologically significant stimulus (unconditional stimulus, US), the CS not only acquires the ability to evoke a conditional response (CR, Pavlov 1927), but under some circumstances, it also is attributed with incentive salience, acquiring the ability to act as an incentive stimulus (Berridge and Robinson 2003; Bindra 1978; Rescorla 1988). An incentive stimulus has the ability to: (1) attract attention and elicit approach towards it, thus biasing choice; (2) reinforce the acquisition of new behaviors; and (3) evoke conditioned motivational states that support reward seeking even when the reward is not present (Berridge and Robinson 2003; Cardinal et al. 2002; Everitt et al. 2000; Milton and Everitt 2010; Robinson and Flagel 2009; Wyvell and Berridge 2001). There are, however, large individual differences in the extent to which a CS is attributed with incentive salience (Beckmann et al. 2011; dela Cruz et al. 2009; Flagel et al. 2009; Mahler and de Wit 2010; Robinson and Flagel 2009). For example, in rats, if a spatially discrete cue is paired with delivery of food reward, the cue itself becomes attractive, eliciting approach towards it, serves as an effective conditioned reinforcer, and readily reinstates reward-seeking behavior only in a subset of rats (Robinson and Flagel 2009; Yager and Robinson 2010). These rats are called sign trackers (STs), a term based on one of their behavioral propensities—to approach the cue or “sign” that predicts reward (Hearst and Jenkins 1974; Tomie et al. 2008). Other rats do not find this food cue attractive, but upon CS presentation they learn to immediately go to the location where the reward will be delivered (i.e., the “goal”), and in these animals, the cue is not a very effective conditioned reinforcer nor very effective in reinstating reward-seeking behavior (Robinson and Flagel 2009; Yager and Robinson 2010). These animals are called goal trackers (GTs, Boakes 1977), based on their propensity to approach to the location where a reward will be delivered. Thus, the CS acts as a potent incentive stimulus in some rats (STs) but not in others (GTs).
Not only can cues associated with natural rewards acquire incentive motivational properties but so can cues associated with drugs of abuse. Indeed, drug cues are thought to play a central role in promoting potentially maladaptive behavior, such as the persistent drug-seeking behavior and relapse seen in addiction (Caggiula et al. 2001; Cardinal et al. 2002; de Wit and Stewart 1981; Grimm et al. 2001; Robinson and Berridge 2003; Stewart et al. 1984). However, in order for drug cues to exert strong control over motivated behavior, they must be attributed with incentive salience. It is important to know, therefore, whether individuals prone to attribute incentive salience to food cues (STs) are also prone to attribute incentive salience to cues associated with drugs of abuse. A cue associated with cocaine delivery in an instrumental self-administration setting, when both cue presentation and cocaine delivery are contingent upon an action, can acquire incentive motivational properties (Caggiula et al. 2001; Di Ciano and Everitt 2004; Grimm et al. 2001). Furthermore, we previously reported that a discrete cue associated with self-administered cocaine acquires greater incentive value in STs than GTs (Saunders and Robinson 2010), as does cocaine itself (Saunders and Robinson 2011). However, there are many complex interacting psychological processes controlling behavior in an instrumental setting (Konorski and Miller 1937; Skinner 1935), and it is difficult to tease them apart. In the present study, we sought to determine if there are individual differences in the attribution of incentive salience to a cocaine cue if the cocaine cue acquired such properties solely through classic Pavlovian conditioning—when the CS and US are paired independent of an animal's action.
To do this we used a modification of a conditioned place preference (CPP) procedure described by Cunningham et al. (1993, 2006a, 2011; see also Tzschentke 2007; Vezina and Stewart 1987a). With this procedure, noncontingent (i.e., experimenter administered) drug administration is paired with a single tactile cue (the surface of the floor; CS+) and saline with a different tactile cue (CS−), in a dark chamber. Thus, the tactile stimulus is the only environmental cue that is reliably associated with drug administration. In this situation, learning the CS–US association is due to Pavlovian conditioning. To test whether the CS+ acquired conditioned motivational properties, rats are placed into the chamber on a drug-free test day with both the CS+ and CS− floors present and the time spent on each recorded. In this situation, it is thought that the expression of a preference for the floor paired with cocaine is mediated by the acquired reinforcing properties of the floor (i.e., by conditioned reinforcement, Cunningham et al. 2006b; also see White et al. 2005). In rats, another independent measure of whether a stimulus has unconditional or conditional positive affective/motivational properties is whether it evokes 50-kHz ultrasonic vocalizations (USVs, Knutson et al. 2002; Ma et al. 2010; Panksepp and Burgdorf 2000). Therefore, in addition to quantifying a place preference in STs and GTs, we also quantified 50-kHz USVs both following saline or cocaine administration during training and then again on the drug-free preference test day.
Methods
Subjects and housing
A total of 112 adult male Sprague–Dawley rats (200–250 g) were purchased from either Charles River (experiment 1) or Harlan (experiments 1 and 2). They were housed in pairs for the duration of the experiment and were gently handled daily. Testing occurred during the dark phase of a reverse light/dark cycle (12:12 h, lights off at 7 a.m.). Food and water were freely available in the home cage (i.e., the rats were not food deprived). Banana-flavored sucrose pellets (45 mg, BioServe, #F0059, Frenchtown, NJ, USA) were used during Pavlovian conditioned approach (PCA) training, and, therefore, rats were given 20 pellets in their home cages for 2 days immediately prior to testing. All experiments followed the principles of laboratory animals care specified by Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research National Research Council (2003).
Pavlovian conditioned approach
Apparatus
Med associates conditioning chambers (20.5 × 24.1 cm floor area, 29.2 cm high; St. Albans, VT) were equipped with retractable levers located on either the left or right side (counterbalanced across rats) of a central food magazine (3 cm above a stainless steel grid floor), into which banana-flavored sucrose pellets could be delivered by an automatic feeder. There was also a white LED light located behind the slot through which the lever protruded. Illumination of a red house light, located near the top of the opposite side of the chamber, signaled the start of the conditioning session and was the only source of illumination except during CS presentation (during which the white LED backlight behind the lever was illuminated). Head entries were detected by breaks of an infrared photobeam inside the food magazine, and lever contacts were recorded when the lever was deflected.
Procedure
Rats were trained using a PCA procedure described previously (e.g., Flagel et al. 2007). Rats underwent one magazine training session, in which 25 banana-flavored sucrose pellets were delivered according to a variable time (VT) 30 (0–60 s) schedule. The lever was retracted during this session. Subsequently, rats underwent 5 days of Pavlovian conditioning (one session/day), during which they received 25 8-s presentations of the illuminated lever, followed immediately by delivery of a sucrose pellet. Rats in this study ate all the pellets during pretraining and PCA sessions. Lever–pellet pairings were presented according to a VT 90 (30–150 s) schedule; sessions lasted on an of average 37.5 min. Importantly, there was no behavioral requirement for the rats to receive the sucrose pellet. Rats were removed from the chamber at the end of each session and returned to their home cages.
PCA index
We have previously reported that, under these conditions, some rats tend to learn a sign-tracking CR (STs; they approach the lever during the CS period), whereas others learn a goal-tracking CR (GTs; they tend to approach the food magazine during the CS period). To quantify these individual differences in the nature of the CR that developed as a function of Pavlovian training, we used a PCA index developed for this purpose. The PCA index is a score from −1.0 to 1.0, calculated as the average of (a) response bias [(number of lever presses − number of CS magazine entries)/(number of lever presses + number of CS magazine entries)], (b) approach probability difference [(number of trials with at least one lever press − number of trials with at least one CS magazine entry)/25], and (c) latency difference [(latency to approach magazine during the CS − latency to approach lever)/8]. We operationally defined STs as rats with an average PCA score from 0.5 to 1.0 for the last 3 days of conditioning and GTs as rats with a score of −0.5 to −1.0. Analysis of a large population of rats (>1,800) has determined that, using these criteria, approximately 30% and 35% of rats screened in this manner are classed as GTs and STs, respectively (unpublished data). The remaining intermediate group of animals (with scores ranging from −0.49 to 0.49), which did not show consistent ST or GT CRs, was not tested in subsequent experiments.
Cocaine-induced conditioned place preference
Apparatus
Chambers were constructed with black acrylic (47.5 cm length × 15.5 width). Floors were either smooth metal (for the habituation day) or textured with patterned “hole” or “grid” floor halves (Cunningham et al. 1993, 2006a; Fidler et al. 2004). The walls and floor of the conditioning chamber were spray painted with low-reflective camouflage paint (Krylon Products Group, Cleveland, OH, USA). This facilitated video capture from cameras (SPE-57, CCTV Specialty Bullet Cameras, Lake Worth, FL, USA) positioned 12 cm above the open ceiling of the chamber. Pairs of fluorescent light tubes (32 W) were covered with red filtering shields (McMaster-Carr, Elmhurst, IL, USA), and placed centrally over each chamber. A speaker emitting ambient white noise was located near the ceiling of the testing room to mask extraneous laboratory noises.
Experiment 1: CPP without a pretest
Place conditioning began within 1 week following the last PCA session. Only STs and GTs (n = 10 and 12, respectively) were used in this experiment. Each day, the rats were moved, in their home cages, to the testing room, weighed, and left for a 45-min period before testing began. On the first day (habituation), rats were injected with 0.9% saline (1 ml/kg, i.p.) and placed into chambers containing the smooth floors for 30 min. For the following 8 days, rats were injected i.p. with 10 mg/kg cocaine or saline, on alternating days, immediately before being placed into chambers containing either grid or hole textures for 30 min. The cocaine-paired (CS+) and saline-paired (CS−) floor textures were counterbalanced among STs and GTs, as was the order of cocaine/saline treatment. Each pair of CS+ and CS− days represented a trial; thus, there were four trials during the 8-day conditioning period of this experiment. On the final test day, each rat was given a saline injection and placed into the chamber with both floor types available and left in the chamber for 30 min.
Experiment 2: CPP with a pretest
This experiment was conducted similarly as above, with two major exceptions: (1) rats were given a pretest to measure any preexisting floor bias and (2) an “unpaired” group was included as a control group for use in making USV comparisons (see USV section below). During the pretest, which occurred after the habituation day described above (and before conditioning trials), rats were given a saline injection and placed into the conditioning chamber containing both floor types for 30 min. During conditioning, rats in the “unpaired” group received only saline injections before being placed into the chambers but received cocaine or saline injections 2, 4, or 6 h after removal from the chambers (i.e., in their home cages) on alternating days. “Paired” rats received only saline injections after removal from the chambers. Rats were otherwise treated as described above in experiment 1.
Ultrasonic vocalization recording
USVs emitted by rats in the chambers were recorded using ultrasonic microphones (PCB Electronics, NYC, NY, USA) with a flat frequency response up to 150 kHz. Microphones were placed on the center of the wall 15 cm above the floor of the chamber. Recordings were obtained from all rats receiving cocaine, but because of the equipment limitations, recordings were obtained from only a subset of rats receiving saline. Signals were amplified and digitized at 200-kHz sampling rate with 16-bit resolution through a DAQ board (National Instruments, Austin, TX, USA). Digitized waveforms were stored on a computer for later analysis.
Data analysis
PCA behavior was compared using repeated measures analysis of variance (ANOVA) and Tukey's post hoc comparisons. Video captured during CPP conditioning trials was analyzed using video tracking software (Topscan, Clever Sys., Inc. Reston, VA, USA). Sonograms for USVs were generated and analyzed with Saslab Pro (Avisoft, Berlin, Germany) with a 512-point FFT and 75% overlap frame spectrogram setup. A trained observer, blind to treatment conditions, classified the USVs into two major categories: flat or frequency modulated (FM), based on the presence or absence of rapid fluctuation in frequency (in kilohertz). The primary dependent variables were time spent on the CS+ side during the final test day and number of FM and flat USVs. ANOVA was used to determine the effects of phenotype (ST, GT), paired floor (GRID+, HOLE+), and conditioning (paired, unpaired). Repeated measures ANOVA was also used to examine group differences across conditioning trials. Significant interactions (p < 0.05) were further analyzed using Tukey's post hoc comparisons.
Results
Pavlovian conditioned approach
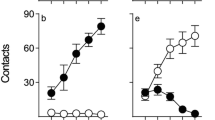
As we have reported previously (Flagel et al. 2007), under these conditions, rats varied considerably in the topography of the CR they acquired. Figure 1 shows the performance of rats classed as STs (n = 28) or GTs (n = 25) based on the PCA index described in the “Methods” section. To be classed as ST, a rat had to engage the lever–CS at least twice as much as the food magazine and vice versa to be classed as GT. Thus, Fig. 1 shows lever-directed (panels a–c) vs. food magazine-directed behavior (panels d–f) in STs and GTs, as a function of training session. Repeated measures ANOVA revealed significant phenotype × day interaction [Fs(4, 204) = 27.0 − 39.9, ps <0.001] for each measure (probability of approach, number of contacts, and latency to approach). Post hoc analysis indicated significant differences between STs and GTs on days 2–5 of training for all measures (ps <0.001). Within-groups comparisons revealed that responses on day 5 had changed significantly compared to day 1 for all measures in STs but only for food magazine-directed behaviors in GTs (ps <0.001).
Sign tracking (left panels) and goal tracking (right panels) during Pavlovian conditioned approach training. Top, middle, and bottom panels display the number of contacts, probability of contacting, and latency, respectively. Rats were categorized as sign or goal trackers based on the PCA index established by our laboratory (see “Methods” section). Data are represented as the mean (+SEM). See text for description of the statistics
To determine whether GTs discriminated the CS and non-CS periods, we measured head entries during the non-CS period (when the lever was not extended). Figure 2 shows the frequency of head entries during the CS and non-CS periods in STs and GTs. GTs entered the magazine more frequently than STs during the non-CS period [F(1, 51) = 8.7, p < 0.01]. GTs clearly discriminated the CS and non-CS periods, evidenced by a decrease in the non-CS entries across days of training concurrent with an increase in CS entries.
Cocaine-induced CPP
Experiment 1: CPP without a pretest
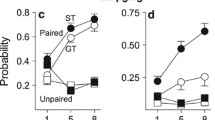
Figure 3 shows that STs (n = 10) developed a preference for the cocaine-paired tactile cue but GTs (n = 12) did not. Data are represented as the mean time spent on the grid floor, subdivided by whether cocaine was paired with the grid floor (GRID+) or hole floor (HOLE+). A significant interaction between phenotype (ST/GT) and paired floor (GRID+/HOLE+) indicated differential floor preferences between STs and GTs [F(1, 18) = 4.50, p < 0.05]. Tukey's post hoc analysis of this interaction revealed that the difference between GRID+ and HOLE+ subgroups was only significant for STs (p < 0.05), indicating that only STs developed a preference for the cocaine-paired floor. The inset displays the same data, collapsed across GRID+ and HOLE+ subgroups and displayed as time spent on the cocaine-paired floor, regardless of floor type. STs spent significantly more time on the cocaine-paired floor [t(20) = 2.26, p < 0.05].
Sign trackers (n = 10), but not goal trackers (n = 12), developed a preference for a cocaine-paired tactile cue. Data are represented as the mean (+SEM) time spent on the grid floor, subdivided by whether cocaine was paired with the grid floor (GRID+) or hole floor (HOLE+). The inset displays the same data, collapsed across GRID+ and HOLE+ subgroups and displayed as time spent on the cocaine-paired floor, regardless of floor type. Asterisks indicate significant differences for the indicated groups, p < 0.05
Experiment 2: CPP with pretest
In experiment 2, rats were given a preference test before conditioning occurred, so that any unconditioned floor preference test could be corrected for during data analysis. When analyzed as a change in time spent on the CS+ floor, STs (n = 13), but not GTs (n = 9), increased preference for the cocaine-paired floor as a result of conditioning [t(20) = 2.39, p < 0.05; Fig. 4]. This replicates the results from experiment 1: STs, but not GTs, developed a significant preference for a cocaine-paired floor.
Ultrasonic vocalizations
FM and flat 50-kHz USVs (abbreviated later as FM USVs and flat USVs) emitted during training trials are shown in Fig. 5. STs emitted more FM USVs during training [effect of phenotype—F(1, 20) = 7.85, p < 0.05]. The number of USVs emitted was trial dependent; post hoc analysis of the effect of trial [F(3, 60) = 7.20, p < 0.001] revealed that the number of FM USVs increased significantly between trial 1 and trials 3 and 4 (ps <0.05). The lack of a phenotype × trial interaction (p > 0.05) indicated that this increase occurred similarly in STs and GTs. For flat USVs, there were no significant differences between STs and GTs, and post hoc analysis of the effect of trial [F(3, 60) = 2.91, p < 0.05] did not reveal significant differences between specific trials. In summary, STs made more FM USVs during cocaine conditioning than GTs and the number of these calls was significantly increased by the end of conditioning in both STs and GTs.
Sign trackers (ST) displayed more 50-kHz frequency-modulated ultrasonic vocalizations during cocaine pairings than goal trackers (GT) during conditioning (panel A), while flat USVs did not differ significantly between STs and GTs (panel B). Cocaine pairings (CS+) are denoted by filled symbols; while open symbols denote saline pairings (CS−). Data are represented as the mean (±SEM) number of USVs during training trails 1–4. See text for description of statistics
On the CPP test day, the majority of USVs (>80%) occurred within the first 5 min of testing. For FM USVs, ANOVA using data from this time period revealed a significant phenotype × conditioning interaction [F(1, 27) = 4.55, p < 0.05; Fig. 6]. Tukey's post hoc comparisons indicated that this effect was driven by more USVs in ST-paired rats relative to all other groups (Fig. 6, ps < 0.05). There were no significant differences in the number of flat calls. This indicates that only in STs was there an effect of conditioning on the frequency of FM USVs. No 22-kHz USVs were observed during the training trials or on the CPP test day.
Sonograms (top) demonstrate examples 50-kHz frequency-modulated ultrasonic vocalizations (FM USVs, left) compared to flat calls (right). For each sonogram, time (in seconds) is represented on the ordinate, wave frequency on the abscissa. Relative sound intensity (in decibels) is represented by red and blue shading. Graph (bottom) depicts mean (+SEM) FM and flat USVs during the first 5 min of the preference test day. Asterisk indicates significant differences (p < 0.05) between indicated groups
Discussion
Some rats (STs) attribute much more incentive salience to a spatially discrete food cue than do others (GTs), as indicated by their propensity to approach and engage it and their willingness to work for it (Flagel et al. 2007; Robinson and Flagel 2009; Yager and Robinson 2010). Here we asked whether STs are also prone to attribute incentive salience to a cue associated with a drug of abuse—cocaine. To do this, we used a CPP procedure to assess the acquired reinforcing properties of a tactile stimulus paired with noncontingent cocaine administration in STs and GTs, as well as the ability of the cocaine cue to evoke 50-kHz USVs. In two independent experiments, STs developed a preference for a tactile cue associated with cocaine and GTs did not. In addition, only STs emitted conditioned FM 50-kHz USVs when placed into the environment containing the cocaine cue. We conclude that animals prone to attribute incentive salience to a food cue are also prone to attribute incentive salience to a cue associated with cocaine. In this study the attribution of incentive salience to a cocaine cue occurred solely through classic Pavlovian conditioning, that is when the CS and US were paired independent of an animal's action. Thus, some individuals appear to be inherently prone to attribute incentive salience to cues associated with multiple classes of rewards.
One goal of the present experiment was to determine if STs and GTs vary in the extent to which they attribute incentive salience to a cocaine cue when incentive motivational value is acquired through classic Pavlovian conditioning. With the procedure used here, learning the CS (tactile cue)–US (cocaine) association clearly involves Pavlovian conditioning because cocaine injections were administered by an experimenter independent of an animal's behavior. However, inferring what psychological process is responsible for expression of a CPP on the test day is more complicated (Cunningham et al. 2006b; Vezina and Stewart 1987b; White et al. 2005). It is often assumed that a place preference is due to Pavlovian attraction—animals are attracted (“pulled”) to the location where a reward was received. This may be true if the procedure involves chambers that differ in multiple aspects, including visual, tactile, spatial, and olfactory features, and if such features can be detected from a distance. However, in the present experiment, the tactile cues were not confined to a specific spatial location during training, and the CS+ tactile stimulus was the only stimulus in the environment that was reliably associated with cocaine. In addition, the tactile cues could not be detected from afar because testing occurred in the dark (red light conditions). Thus, on the test day, a rat would encounter the cocaine floor only by chance, as it explored the chamber. If the cocaine-associated floor was attributed with incentive salience, it would have acquired the ability to act as a conditioned reinforcer and contact with it would reinforce actions necessary for the rat to maintain contact with it. Therefore, in the present experiment, a preference for the cocaine-associated floor is most likely due to its acquired reinforcing properties. This interpretation is supported by studies showing that spatial location is important for configurations using visual cues but not important when tactile cues are used (Cunningham and Patel 2007; Cunningham et al. 2006b). Indeed, some have noted that when tactile cues are used, calling it a “place” preference may be a misnomer (Cunningham et al. 2006b; van der Kooy 1987). The dissociation between CPP procedures using visual vs. tactile cues is further supported by neurobiological studies showing that the involvement of specific brain areas in CPP depends on the type of cues used (Gremel and Cunningham 2008; White et al. 2005).
The finding that a cocaine cue acquired conditioned reinforcing properties in STs, but not GTs, is consistent with previous studies in which a cocaine cue acquired incentive motivational properties in an instrumental setting. Saunders and Robinson (2010) allowed rats to self-administer cocaine intravenously, and each injection of cocaine was paired with a discrete light stimulus. This situation involves many hundreds of light–cocaine pairings over many days of training. They found that the cocaine cue acquired greater incentive motivational properties in STs than GTs, which was assessed in two ways. Firstly, removal of the cue greatly decreased the rate of self-administration behavior in STs but not in GTs, suggesting that the cue was important for motivating instrumental responding in the former but not the latter. Furthermore, following extinction of self-administration behavior, the cocaine cue was more effective in reinstating cocaine seeking in STs than GTs. The test for reinstatement was conducted under extinction conditions (i.e., no cocaine was available), but as in most tests of reinstatement, presentation of the cue was contingent upon the action that formerly delivered cocaine (Epstein et al. 2006; Meil and See 1996; Weiss et al. 2000). Therefore, taken together, these studies indicate that a cocaine cue acts as a more effective conditioned reinforcer in STs than GTs, and this is seen using either instrumental or Pavlovian training procedures. Note, however, that only four CS–US pairings were necessary to see this effect in the present study, suggesting that the hundreds of pairings used in a self-administration setting may not be necessary, although this issue requires further investigation. It is possible, for example, that learning the light–cocaine association is retarded in a typical self-administration setting, because continuous high blood levels of cocaine are not conducive to forming associations between the CS and US. If so, more widely spaced injections may lead to faster learning of the Pavlovian association.
The lack of the development of a cocaine CPP in GTs is not due to a general learning deficit. STs and GTs do not differ in their rates of learning in the PCA task, in learning to self-administer food (Morrow et al. 2011; Robinson and Flagel 2009; Yager and Robinson 2010), in the acquisition of cued and contextual fear responses (Morrow et al. 2011), or in learning to self-administer cocaine in the absence of cues (Saunders and Robinson 2011).
However, there are other possible reasons why STs and GTs differed in developing a cocaine CPP: They may differ in (1) their pharmacological sensitivity to cocaine, (2) the unconditioned/stimulus effects of cocaine (the ability to detect presence of cocaine and thereby make cocaine cue associations), or (3) the primary reinforcing effect of cocaine. However, previous studies suggest that STs and GTs do not differ in sensitivity to 10 mg/kg cocaine as measured by the locomotor response to i.p. injection. At some doses GTs actually show a greater locomotor response, which makes a lower pharmacological sensitivity to cocaine an unlikely explanation for the lack of development of CPP (Flagel et al. 2008). Second, it also appears that the unconditioned stimulus effects of cocaine do not differ between STs and GTs. For example, both STs and GTs readily acquire cocaine self-administration behavior (Saunders and Robinson 2010, 2011). This indicates that cocaine functions as an effective reinforcer in both STs and GTs and that they are equally capable of learning an act–outcome (cocaine) association. One study did report that at low (but not high) doses, STs more readily acquired cocaine self-administration behavior (Beckmann et al. 2011). However, in this study, an autoshaping procedure was used, and a cue was presented along with the cocaine injections, thereby confounding the unconditioned effects of cocaine with conditioned reinforcing effects of its associated cue. Importantly, we have shown that STs and GTs do not differ in learning cocaine self-administration when there is no cue present (Saunders and Robinson 2011). In addition, in unpublished studies, we have found that that STs and GTs do not differ in their initial orienting response to a discrete Pavlovian cocaine cue (while they do differ in approach the cue). Together, these findings suggest that GTs are able to detect the delivery of cocaine and learn cue–cocaine associations but are less prone than STs only in attributing of incentive value to the cue.
The results reported here could also be explained if GTs have a reduced sensitivity to the unconditioned, primary reinforcing effects of cocaine or that the euphorigenic or “liking” response to cocaine is reduced in GT. That a single injection of cocaine elicited more FM USVs in STs in the current study is consistent with this explanation. Also, Saunders and Robinson (2011) recently reported that although there was no difference between STs and GTs in the rate of cocaine self-administration when they were tested on a FR-1 schedule, suggesting that cocaine was equally reinforcing, when transferred to a progressive ratio schedule STs showed significantly higher “breakpoints” than GTs; indeed, they worked almost twice as hard. However, two important caveats to this explanation should be mentioned. The first caveat is that the interoceptive cues produced by cocaine itself may have been responsible for the differences in ST and GT breakpoints. Supporting this, Saunders and Robinson (2011) found that following extinction of self-administration behavior, a cocaine “prime” reinstated more robust cocaine-seeking behavior in ST than GTs. The second caveat is that the incentive value of drug cues can be distinguished from their primary reinforcing properties (Palmatier et al. 2006; Wyvell and Berridge 2000), that is, an alteration of the primary reinforcing effect of a drug does not necessarily alter the conditioned reinforcing properties of the cues associated with it.
Therefore, regardless of the proximal explanation for the lack of the development of a CPP in GTs, the available evidence suggests that multiple classes of cocaine cues (tactile or visual, discrete or continuous, external or interoceptive) are attributed with greater incentive salience in STs than GTs, and this is seen using very different training procedures (instrumental vs. Pavlovian) and different tests to assess motivational properties. Similar individual variation is seen not only using quite different tests but with different rewards (food and cocaine), suggesting that the propensity to attribute incentive salience to reward cues represents a stable “trait,” rather than a transitory “state” evident only under very specific conditions. This hypothesis is further supported by evidence that it is heritable (Flagel et al. 2010), modified by early experience (Lomanowska et al. 2011), and associated with biological differences (Flagel et al. 2007, 2009, 2010, 2011).
In rats, the emission of USVs within the 50-kHz range has been associated with the delivery and anticipation of various rewards, including brain stimulation reward, psychostimulant administration, olfactory sexual stimuli, as well as during cocaine CPP (Bialy et al. 2000; Burgdorf et al. 2007; Knutson et al. 1999; Maier et al. 2010; Panksepp et al. 2002). Based on the spectral–temporal shape of vocalizations within the 50-kHz range, there are two main categories—flat vs. FM (Wright et al. 2010). It is primarily the FM 50-kHz USVs that are emitted during mating and other positive social interactions (Burgdorf et al. 2008; Panksepp and Burgdorf 2003; Wang et al. 2008), following the administration of cocaine or amphetamine (Ahrens et al. 2009; Ma et al. 2010), or in anticipation of such drugs (Maier et al. 2010). Our findings are consistent with this distinction between FM vs. flat 50-kHz USVs. During the Pavlovian training, a cocaine injection evoked more unconditional FM 50-kHz USVs in STs than GTs. This is the first demonstration of a different unconditioned response between STs and GTs. Inasmuch as FM USVs are an index of a positive affective/motivational state, as suggested by Panksepp (Burgdorf et al. 2011; Panksepp and Burgdorf 2000, 2003), the initial affective response to an unconditioned reward may predict the attribution of incentive value to cues associated with that reward. A recent study supports this association, the USVs on the first day of cocaine self-administration was positively correlated with how rapidly cocaine self-administration was acquired (Browning et al. 2011).
Successive injections of cocaine resulted in an increase (sensitization) in FM 50-kHz USVs, as described previously for both amphetamine (Ahrens et al. 2009) and cocaine (Ma et al. 2010). Interestingly, STs and GTs did not differ in the rate of sensitization of FM 50-kHz USVs. Therefore, neither the unconditioned USV response to cocaine nor the expression of CPP was related to the magnitude of sensitization of USVs produced by repeated injections of cocaine. This supports an established literature that shows that although conditioning factors have a powerful impact on drug sensitization, the expression of conditioned responses are often dissociable from sensitization (Anagnostaras et al. 2002; Brown et al. 2011; Crombag et al. 2000, 2001; Cunningham et al. 2002; Rowlett et al. 1994; Veeneman et al. 2011). Nevertheless, on the CPP test day, only STs showed a conditioned increase in FM USVs. These latter data provide additional support that the cocaine cue acquired greater positive motivational value in STs than GTs.
In conclusion, the propensity to attribute incentive salience to a food cue predicted, prior to any drug experience, which rats later developed a preference for a tactile cue associated with cocaine, and emitted 50-kHz FM USVs in the presence of the cocaine cue. Taken together with previous studies, the available evidence suggests that some rats (STs) are prone to attribute incentive motivational properties to very different classes of reward cues (food vs. drug), that this trait is evident when incentive value is acquired in either an instrumental or Pavlovian setting, and it is revealed by performance on a number of different tests of incentive motivation. Individuals who are prone to attribute incentive salience to reward cues will have greater difficulty resisting them, and in such individuals, these cues may tend to motivate maladaptive behavior, including continued drug-taking and drug-seeking behavior even in the face of negative consequences, and relapse. Therefore, such individuals may be especially prone to develop impulse control disorders, including addiction.
References
Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T (2009) Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav Brain Res 197:205–209
Anagnostaras SG, Schallert T, Robinson TE (2002) Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology 26:703–715
Beckmann JS, Marusich JA, Gipson CD, Bardo MT (2011) Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res 216:159–165
Berridge KC, Robinson TE (2003) Parsing reward. Trends Neurosci 26:507–513
Bialy M, Rydz M, Kaczmarek L (2000) Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci 114:983–990
Bindra D (1978) How adaptive behavior is produced: a perceptual-motivation alternative to response reinforcement. Behav Brain Res 1:41–91
Boakes R (1977) Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H (eds) Operant-Pavlovian interactions. Lawrence Erlbaum Associates, Hillsdale, pp 67–97
Brown RM, Short JL, Lawrence AJ (2011) Identification of brain nuclei implicated in cocaine-primed reinstatement of conditioned place preference: a behaviour dissociable from sensitization. PLoS One 5:e15889
Browning JR, Browning DA, Maxwell AO, Dong Y, Jansen HT, Panksepp J, Sorg BA (2011) Positive affective vocalizations during cocaine and sucrose self-administration: a model for spontaneous drug desire in rats. Neuropharmacology 61:268–275
Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J (2007) Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res 182:274–283
Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J (2008) Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol 122:357–367
Burgdorf J, Panksepp J, Moskal JR (2011) Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev. doi:10.1016/j.neubiorev.2010.11.011
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530
Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352
Crombag HS, Badiani A, Maren S, Robinson TE (2000) The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res 116:1–22
Crombag HS, Badiani A, Chan J, Dell'Orco J, Dineen SP, Robinson TE (2001) The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effect on acute drug responsiveness and on conditioned responding. Neuropsychopharmacology 24:680–690
Cunningham CL, Patel P (2007) Rapid induction of Pavlovian approach to an ethanol-paired visual cue in mice. Psychopharmacology (Berl) 192:231–241
Cunningham CL, Niehus JS, Noble D (1993) Species difference in sensitivity to ethanol's hedonic effects. Alcohol 10:97–102
Cunningham CL, Tull LE, Rindal KE, Meyer PJ (2002) Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 160:414–424
Cunningham CL, Gremel CM, Groblewski PA (2006a) Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1:1662–1670
Cunningham CL, Patel P, Milner L (2006b) Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav Neurosci 120:1115–1132
Cunningham CL, Groblewski PA, Voorhees CM (2011) Place conditioning. In: Olmstead MC (ed) Animal models of drug addiction, neuromethods, vol 53. Humana Press, New York
de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 75:134–143
dela Cruz AM, Herin DV, Grady JJ, Cunningham KA (2009) Novel approach to data analysis in cocaine-conditioned place preference. Behav Pharmacol 20:720–730
Di Ciano P, Everitt BJ (2004) Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology 47(Suppl 1):202–213
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16
Everitt BJ, Cardinal RN, Hall G, Parkinson JA, Robbins TW (2000) Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: Aggleton JP (ed) The amygdala: a functional analysis. Oxford University Press, New York, pp 353–390
Fidler TL, Bakner L, Cunningham CL (2004) Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacol Biochem Behav 77:731–743
Flagel SB, Watson SJ, Robinson TE, Akil H (2007) Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 191:599–607
Flagel SB, Watson SJ, Akil H, Robinson TE (2008) Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res 186:48–56
Flagel SB, Akil H, Robinson TE (2009) Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology 56(Suppl 1):139–148
Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H (2010) An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology 35:388–400
Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H (2011) A selective role for dopamine in stimulus-reward learning. Nature 469:53–57
Gremel CM, Cunningham CL (2008) Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J Neurosci 28:1076–1084
Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142
Hearst E, Jenkins HM (1974) Sign tracking: the stimulus-reinforcer relation and directed action. Psychonomic Society, Austin
Knutson B, Burgdorf J, Panksepp J (1999) High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav 66:639–643
Knutson B, Burgdorf J, Panksepp J (2002) Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull 128:961–977
Konorski J, Miller S (1937) On two types of conditioned reflex. J Gen Psychol 16:264–272
Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW (2011) Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res 220:91–99
Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL (2010) Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res 212:109–114
Mahler SV, de Wit H (2010) Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS One 5:e15475
Maier EY, Ahrens AM, Ma ST, Schallert T, Duvauchelle CL (2010) Cocaine deprivation effect: cue abstinence over weekends boosts anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res 214:75–79
Meil WM, See RE (1996) Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol 7:754–763
Milton AL, Everitt BJ (2010) The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci 31:2308–2319
Morrow JD, Maren S, Robinson TE (2011) Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res 220:238–243
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF (2006) Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 184:391–400
Panksepp J, Burgdorf J (2000) 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res 115:25–38
Panksepp J, Burgdorf J (2003) “Laughing” rats and the evolutionary antecedents of human joy? Physiol Behav 79:533–547
Panksepp J, Knutson B, Burgdorf J (2002) The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction 97:459–469
Pavlov IP (1927) Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford University Press, London
Rescorla RA (1988) Pavlovian Conditioning—Its Not What You Think It Is. Am Psychol 43:151–160
Robinson TE, Berridge KC (2003) Addiction. Annu Rev Psychol 54:25–53
Robinson TE, Flagel SB (2009) Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry 65:869–873
Rowlett JK, Gibson TR, Bardo MT (1994) Dissociation of buprenorphine-induced locomotor sensitization and conditioned place preference in rats. Pharmacol Biochem Behav 49:241–245
Saunders BT, Robinson TE (2010) A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry 67:730–736
Saunders BT, Robinson TE (2011) Individual variation in the motivational properties of cocaine. Neuropsychopharmacology 36(8):1668–1676
Skinner BF (1935) Two types of conditioned reflex and a pseudo-type. J Gen Psychol 12:66–77
Stewart J, de Wit H, Eikelboom R (1984) Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev 91:251–268
Tomie A, Grimes KL, Pohorecky LA (2008) Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev 58:121–135
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462
van der Kooy D (1987) Place conditioning: a simple and effective method for assessing the motivational properties of drugs. In: Bozarth MA (ed) Methods of assessing the reinforcing properties of abused drugs. Springer, New York, pp 229–240
Veeneman MM, Boleij H, Broekhoven MH, Snoeren EM, Guitart Masip M, Cousijn J, Spooren W, Vanderschuren LJ (2011) Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 214:863–876
Vezina P, Stewart J (1987a) Conditioned locomotion and place preference elicited by tactile cues paired exclusively with morphine in an open field. Psychopharmacology (Berl) 91:375–380
Vezina P, Stewart J (1987b) Morphine conditioned place preference and locomotion: the effect of confinement during training. Psychopharmacology (Berl) 93:257–260
Wang H, Liang S, Burgdorf J, Wess J, Yeomans J (2008) Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One 3:e1893
Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O (2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A 97:4321–4326
White NM, Chai SC, Hamdani S (2005) Learning the morphine conditioned cue preference: cue configuration determines effects of lesions. Pharmacol Biochem Behav 81:786–796
Wright JM, Gourdon JC, Clarke PB (2010) Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl) 211:1–13
Wyvell CL, Berridge KC (2000) Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20:8122–8130
Wyvell CL, Berridge KC (2001) Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci 21:7831–7840
Yager LM, Robinson TE (2010) Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res 214:30–34
Acknowledgments
The authors would like to thank Drs. Chris Cunningham and Carrie Ferrario for their consultations on apparatus and experimental design, as well as David Haidar, Matea Mustafaj, and Rebeca Kelley for their excellent technical support. We also thank Ben Saunders for the helpful comments on an earlier version of this article. All research was conducted in concordance with current local and national US laws. This research is supported by Grant R37 DA04294 from NIDA to TER. PJM was supported by Grant T32 DA007268.
Conflict of interest
The authors declare no financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paul J. Meyer and Sean T. Ma contributed equally to this work.
Rights and permissions
About this article
Cite this article
Meyer, P.J., Ma, S.T. & Robinson, T.E. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology 219, 999–1009 (2012). https://doi.org/10.1007/s00213-011-2429-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2429-7