Abstract
Rationale
Individuals vary in the extent to which they attribute incentive salience to reward cues. Discrete food and drug (cocaine and opioid) cues become more attractive, eliciting approach toward them, and more “wanted,” in that they serve as more effective conditioned reinforcers, in some rats (sign-trackers, STs), than in others (goal-trackers, GTs).
Objectives
We asked whether there is similar variation in the extent to which a cue associated with a drug from another class, nicotine, acquires incentive motivational properties.
Methods
First, a Pavlovian conditioned approach procedure was used to identify rats that attribute incentive salience to a food cue (i.e., STs and GTs). We then measured the extent to which a cue (a light) paired with intravenous nicotine injections acquired two properties of an incentive stimulus: (1) the ability to elicit approach toward it, and (2) the ability to act as a conditioned reinforcer.
Results
In contrast to previous findings with food, cocaine, and opioid cues, we found that the nicotine cue was equally attractive in STs and GTs, eliciting dose-dependent approach behavior in both. However, the nicotine cue was a more effective conditioned reinforcer in STs than in GTs.
Conclusions
We suggest the dissociation between these two measures of incentive salience attribution may be related to the fact that when present (as in the test of Pavlovian approach), nicotine can act as a potent “incentive amplifier,” and by this action, nicotine may render cues especially salient for all animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cues associated with drugs of abuse can acquire potent incentive motivational properties (incentive salience), which is problematic in the context of addiction because such cues can both facilitate ongoing drug-taking behavior and, during periods of abstinence, may precipitate relapse, despite an expressed desire to stop using (Bossert et al. 2013; Caggiula et al. 2001; Crombag et al. 2008; Milton and Everitt 2010; Robinson and Berridge 1993; Stewart et al. 1984; Tomie et al. 2008). There is, however, considerable individual variation in the extent to which drug cues bias attention toward them (e.g., Field and Cox 2008) and arouse motivation for them (Mahler and de Wit 2010; Styn et al. 2013; Saunders and Robinson 2013, for review). In a series of preclinical studies, we have suggested that one source of this variation arises from variation in the propensity to attribute incentive salience to reward cues (Flagel et al. 2009; Robinson et al. 2014; Saunders and Robinson 2013).
If a discrete localizable cue (the conditioned stimulus, CS) is paired with delivery of a food reward (the unconditioned stimulus, US), for some rats (sign-trackers, STs; Hearst and Jenkins 1974) the food cue itself becomes attractive eliciting approach and engagement with it, and desired, in that STs will work avidly to obtain it. For other rats (goal-trackers, GTs; Boakes 1977), the food cue itself is less attractive, its presentation instead elicits approach to the location of food delivery, and it is a less effective conditioned reinforcer. Thus, while the cue is an equally effective CS in STs and GTs—it reliably evokes a conditioned response in both—it becomes a more attractive and “wanted” incentive stimulus in STs than in GTs (Flagel et al. 2007; Meyer et al. 2012a; Robinson and Flagel 2009; Yager and Robinson 2010). Importantly, the propensity to attribute incentive salience to a food cue predicts the extent to which drug cues acquire motivational properties (for reviews, see Flagel et al. 2009; Robinson et al. 2014; Saunders and Robinson 2013).
Most previous studies examining individual variation in the extent to which drug cues can motivate behavior have focused on cues associated with cocaine. For example, a cocaine-associated cue is more attractive, eliciting more avid approach behavior, in STs than in GTs (Flagel et al. 2010; Yager and Robinson 2013), and also more desired, in the sense that it acts as a more potent conditioned reinforcer in STs than in GTs (Meyer et al. 2012b; Saunders and Robinson 2010; Yager and Robinson 2013). In addition, a discrete cocaine cue produces greater reinstatement of drug-seeking behavior in STs than in GTs, and this is true whether the cocaine cue acquired its motivational properties in an instrumental (i.e., traditional self-administration paradigm) setting, or using Pavlovian conditioning procedures (Saunders and Robinson 2010; Saunders et al. 2013; Yager and Robinson 2013). More recently, we found similar variation in the motivational properties of an opioid (remifentanil) cue. A discrete light cue associated with intravenous injections of remifentanil is both more attractive and a more effective conditioned reinforcer in STs than in GTs (Yager et al. 2015).
Here we asked whether this variation extends to an additional class of drug that is widely used, nicotine. Nicotine is especially interesting because it is thought to be a relatively weak primary reinforcer, in the sense that it is not as avidly self-administered as a number of other drugs, unless cues accompany nicotine delivery (Caggiula et al. 2001, 2002; Chaudhri et al. 2006b; Rupprecht et al. 2015; c.f. Deroche-Gamonet et al. 2002). Therefore, using rats, we asked whether individuals that vary in their propensity to attribute incentive salience to a food cue (i.e., STs vs. GTs) also vary in how avidly they approach and/or work for presentation of a nicotine cue.
Methods
Subjects
Male Sprague–Dawley rats (initial N = 200; Harlan, Haslett, Michigan) weighing 250–275 g upon arrival were individually housed in a climate-controlled colony room on a 12-h light/12-h dark cycle (lights on at 0800 h). All testing occurred during the light phase of the cycle. Food and water were available ad libitum (i.e., rats were not food-restricted at any time). Rats were given 1 week to acclimate to the colony room before testing began, during which time the experimenter handled them several times. All procedures were approved by the University of Michigan Committee on the Use and Care of Animals.
Pavlovian training using food as the US
Apparatus
Behavioral testing was conducted in 16 standard (22 × 18 × 13 cm) test chambers (Med Associates Inc., St. Albans, VT, USA) located in sound-attenuating cabinets equipped with a ventilating fan to mask background noise. Each chamber was equipped with an illuminated retractable lever located 6 cm above a stainless steel grid floor to either the left or right of a centrally located food magazine, placed 3 cm above the floor. A red house light was located on the wall opposite of the food cup and remained illuminated throughout the testing session.
Pavlovian training procedures
Rats were first trained using a Pavlovian conditioned approach (PCA) procedure as described previously (Flagel et al. 2007; Yager and Robinson 2013). For 2 days prior to the start of training, 25 banana-flavored pellets (45 mg; BioServe) were placed into the home cage to familiarize the rats with this food. Approximately 1 week after arrival, rats underwent one magazine training session during which the lever remained retracted and 25 pellets were delivered into the food magazine according to a variable time (VT) 30 s (0–60 s) schedule. Subsequently, rats underwent 5 days of Pavlovian conditioning (1 session/day). Each session consisted of 25 trials during which an illuminated lever (lever-CS) was inserted into the chamber for 8 s, after which a single 45-mg banana-flavored pellet (the US) was delivered into the food magazine. CS-US pairings occurred on a VT 90 s (30–150 s) schedule. Importantly, no instrumental response was required by the rat to initiate delivery of the food pellet. Lever deflections, magazine entries, latency to the first lever deflection, and latency to the first magazine entry during each CS presentation were recorded using Med Associates software.
Quantification of behavior using an index of pavlovian conditioned approach (PCA)
Following completion of Pavlovian training, animals were classed into three groups: (1) those that preferentially interacted with the lever-CS (‘sign-trackers’, STs), (2) those that preferentially interacted with the food magazine during the lever-CS presentation (‘goal-trackers’, GTs), and (3) those that had no strong preference for the lever-CS or food magazine (‘intermediate group’, IG). The extent to which behavior was directed toward the lever-CS or the food magazine was quantified using a composite Pavlovian conditioned approach (PCA) index, based on performance during days 4 and 5 of training, as described previously (Lomanowska et al. 2011; Meyer et al. 2012a). The PCA index score incorporated three measures of conditioned approach behavior: (1) the probability of contacting either the lever-CS or food magazine during a trial [(number of trials with a lever press/total number of trials) − (number of trials with a magazine contact / total number of trials)], (2) the response bias for contacting the lever-CS or food magazine during a trial [(#lever deflections − #food magazine entries) / (#lever deflections + #food magazine entries)], and (3) the mean latency to contact the lever or enter the food magazine during a trial [(magazine contract latency − lever deflection latency) / 8]. The following formula was then used to calculate the PCA index score: [(probability difference score + response bias score + latency difference score) / 3]. The average of the three measures of approach produces values ranging from −1.0 to +1.0, where a score of +1 indicates an animal made a ST conditioned response (CR) on every trial, a score of −1 that an animal made a GT CR on every trial, and a score of 0 that an animal distributed ST and GT responses equally. For purposes of classification, rats with scores of −1.0 to −0.3 were operationally defined as GTs and rats with scores of +0.3 to +1.0 were defined as STs. Rats that were within the range of −0.29 to +0.29, whose behavior vacillated between the lever-CS and food magazine, were classified as intermediates (IGs) and were not used further because we were interested in comparing rats that differed strongly in their propensity to attribute incentive salience to food cues (Meyer et al. 2012a).
Pavlovian approach using nicotine as the US
Surgery
Following Pavlovian training using food as the US, chronic indwelling catheters were implanted into the jugular vein of STs and GTs, as described previously (Crombag et al. 2000). Catheter patency was tested before the first training session and again after the last training session by intravenous injection of 0.2 ml of methohexital sodium (10 mg/ml in sterile water; JHP Pharmaceuticals). Rats were removed from the study if they failed to become ataxic within 5 s of injection (STs, n = 1; GTs, n = 4).
Apparatus
Behavioral testing was conducted in chambers identical to those used to screen animals for ST and GT, except the food magazine and lever were removed from the chamber and two stimulus lights were placed on the left and right sides of the wall opposite the white house light, 13.5 cm above the stainless steel grid floor. The side of the stimulus light designated to serve as a CS (i.e., to be paired with nicotine infusion) was counterbalanced between rats. A syringe pump, located outside the sound attenuating chamber and connected to rats’ catheter back ports, delivered nicotine infusions. The infusion tubing was suspended into the chamber via a swivel mechanism, which allowed rats’ free movement in the chamber.
Pavlovian training procedures
Pavlovian training procedures were similar to those described previously (Yager and Robinson 2013). Prior to training, rats were assigned to either Paired (CS and US presented together) or Unpaired groups (US explicitly not paired with presentation of the CS). Before Pavlovian training began, rats were first habituated to the presentation of the stimulus light (light-CS) and infusion procedure to decrease otherwise high levels of responding to what were novel stimuli (Uslaner et al. 2006). The habituation session consisted of 25 trials (VT 90 s schedule) during which both stimulus lights were simultaneously illuminated for 10 s and coincided with activation of the infusion pump and an intravenous (IV) infusion of saline (50 μl delivered over 2.8 s). Starting the next day, rats underwent 15 days of Pavlovian conditioning using nicotine as the US. Each session consisted of eight trials (CS-US presentations) occurring on a VT schedule with a mean of 900 s (840–960 s). This long inter-trial interval (ITI) was chosen since nicotine, as opposed to a food pellet, has relatively long-lasting neurobiological and interoceptive effects. Thus, in order to have more discrete CS-US pairings, a long ITI is necessary (see Uslaner et al. 2006 for discussion). For rats in the Paired groups, each light-CS presentation was paired with an intravenous infusion of 7.5, 15, or 25 μg/kg of a nicotine solution (bitartrate salt, calculated on the weight of the base and dissolved in 0.9 % saline, pH-adjusted to 7.2–7.4, delivered in 50 μl over 2.8 s). Independent groups of rats were used for each dose of nicotine tested. Each trial consisted of illumination of the CS for 10 s and nicotine delivery coincided with the onset of the CS. No action was required by the rat to initiate either illumination of the light or the nicotine injection. These testing parameters were selected because we have previously shown, using identical methods, that when cocaine is used as the US, rats will approach the CS (Flagel et al. 2010; Uslaner et al. 2006; Yager and Robinson 2013). Rats in the Unpaired group received non-contingent infusions of 25 μg/kg nicotine that were explicitly not paired with illumination of the CS (nicotine was administered on a VT schedule with a mean of 180 s after the CS was extinguished). We opted to only use the highest dose of nicotine for the Unpaired animals as this was the dose that produced the most robust behavior in the Paired rats.
Video analysis
Video was scored offline by an observer blind to the experimental condition for two different conditioned responses (CRs), as described previously (Yager and Robinson 2013). (1) Conditioned orientation: an orienting response was scored if the rat made a head and/or body movement in the direction of the CS during the CS period, regardless of whether the rat approached the CS. (2) Conditioned approach: an approach response was scored if during the CS period a rat moved toward the CS, bringing its nose to within 1 cm of the light. Due to the location of the cue light within the chamber, a rat had to rear, lifting both paws off the floor, in order to bring its nose within 1 cm of the light. It is important to note that if an approach response was scored on a given trial, an orientation response would also be scored, as orientation always preceded approach. However, an orientation response could occur in the absence of an approach response. The probability of an orientation or approach CR was determined by counting the number of trials in which an animal oriented toward or approached the CS and dividing by the total number of trials. Thus, for example, a probability of 0.5 means that a rat approached (or oriented) on half the trials in a session.
Test for conditioned reinforcement
One week following the last training session with nicotine as the US, all rats underwent a single 40-min test for conditioned reinforcement. During this test, the cue light was relocated to the middle of the front wall and was flanked by two nose poke ports. Responses into one of the ports (active) resulted in illumination of the nicotine cue (light-CS) for 2 s. Responses into the other port (inactive) had no consequence. No nicotine was delivered during this test.
Statistical analysis
Linear mixed-models (LMM) analysis was used for all repeated measures data (Verbeke and Molenberghs 2000). The covariance structure was explored and modeled for each dependent variable. Analysis of variance was used to analyze dose-response data for conditioned orientation, conditioned approach, and to compare responding during conditioned reinforcement. When main effects were found, post hoc comparisons were made using Fisher’s LSD test. Statistical significance was set at p < 0.05.
Results
Individual variation in Pavlovian conditioned approach behavior to a food cue
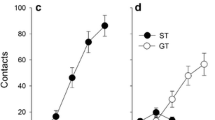
As expected from previous studies (Flagel et al. 2007; Meyer et al. 2012a; Robinson and Flagel 2009; Saunders and Robinson 2012), two distinct phenotypes emerged as a result of Pavlovian training using food as the US. Figure 1 shows the performance of rats classified as STs or GTs based on the PCA index as described in the “Methods” section. Across days of training, STs came to reliably and rapidly approach the lever-CS (Fig. 1a, c), and they vigorously engaged it (Fig. 1b). In contrast, GTs rarely approached the lever-CS, but upon its presentation, they instead reliably and rapidly approached the food cup (Fig. 1d, f), which they vigorously engaged (Fig. 1e). Of the 200 rats screened for this experiment, 59 were classed STs (30 %), 63 IGs (32 %), and 78 GTs (39 %). This distribution of PCA index scores is similar to previous reports (Meyer et al. 2012a).
Behavior directed toward the lever-CS (sign-tracking) versus behavior directed toward the food cup (goal-tracking), during the CS presentation (STs, n = 15; GTs, n = 16). Data are presented as means ± SEM for (a) probability of approaching the lever-CS during the 8-s CS period [#trials with a lever-CS contact / #trials per session], (b) number of lever contacts, (c) latency to first lever contact after CS presentation, (d) probability of approaching the food magazine during the 8-s CS period [#trials with a food cup entry / #trials per session], (e) number of food magazine entries during the 8-s CS period, and (f) latency to the first food cup entry after CS presentation. For all measures, there was a significant effect of group (ST or GT), session, and a group × session interaction (p’s < 0.001)
A nicotine cue is equally attractive to STs and GTs
When a drug is used as the US, rats rarely physically engage the CS. Instead, a sign-tracking CR consists of approach to the vicinity of the CS, and sniffing and investigation of it (Flagel et al. 2010; Uslaner et al. 2006; Yager and Robinson 2013). Thus, when using nicotine as the US, we scored a CS-directed approach response (a ST CR) if a rat brought its nose to within 1 cm of the light-CS during the CS period, which required it to rear. In contrast, conditioned orientation was defined as a head and/or body movement in the direction of the light-CS upon CS presentation, regardless of whether an animal approached it.
Conditioned orientation (7.5 μg/kg)
As can be seen in Fig. 2a, when 7.5 μg/kg nicotine was used as the US, neither Paired STs nor GTs acquired a conditioned orientation response (group, session, interaction effects n.s.). However, both STs and GTs oriented significantly more relative to their respective Unpaired control groups [effect of pairing; STs, F(1, 50.86) = 45.75, p < 0.001; GTs, F(1, 51.73) = 20.78, p < 0.001].
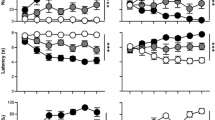
Probability of conditioned orientation to a cue associated with a non-contingent intravenous injection of nicotine. Data are illustrated as the mean ± SEM. (a) Conditioned orientation in rats that received 7.5 μg/kg nicotine (Paired STs n = 12, GTs n = 12). (b) Conditioned orientation in rats that received 15 μg/kg nicotine (Paired STs n = 14, GTs n = 15) (c) Conditioned orientation in rats that received 25 μg/kg nicotine (Paired STs n = 15, GTs n = 16; Unpaired STs n = 8, GTs n = 8). (d) Dose–response function for the probability of conditioned orientation on the final day of training where each data point represents an independent group of rats. UP = Unpaired. Number sign indicates a significant difference between STs and UP rats; @ indicates a significant difference between GTs and UP rats. p < 0.05
Conditioned approach (7.5 μg/kg)
Consistent with the fact that neither STs nor GTs acquired a conditioned orientation response, as an orientation must precede an approach response, neither Paired STs nor GTs acquired a conditioned approach response when using 7.5 μg/kg nicotine as the US (Fig. 3a; group, session, interaction effects n.s.). Furthermore, approach behavior did not differ between Paired and Unpaired groups.
Probability of conditioned approach to a cue associated with a non-contingent intravenous injection of nicotine. Data are illustrated as the mean ± SEM. (a) Conditioned approach in rats that received 7.5 μg/kg nicotine (Paired STs n = 12, GTs n = 12). (b) Conditioned approach in rats that received 15 μg/kg nicotine (Paired STs n = 14, GTs n = 15). (c) Conditioned approach in rats that received 25 μg/kg nicotine (Paired STs n = 15, GTs n = 16; Unpaired STs n = 8, GTs n = 8). (d) Dose–response function for the probability of conditioned approach on the final day of training where each data point represents an independent group of rats. UP = Unpaired. Number sign indicates a significant difference between STs and UP rats; @ indicates a significant difference between GTs and UP rats. p < 0.05
Conditioned orientation (15 μg/kg)
Figure 2b illustrates that when using 15 μg/kg nicotine as the US, both Paired STs and GTs acquired a conditioned orientation response, as indicated by a significant increase in the probability of orientation behavior across sessions [F(2, 27) = 14.76, p < 0.001], and the two groups did not differ. In addition, both STs and GTs showed a significant increase in probability of orienting to the nicotine cue across sessions, relative to their respective Unpaired control groups [pairing × session interaction; STs, F(2, 20) = 2.67, p = 0.03; GTs, F(2, 21) = 7.56, p = 0.003].
Conditioned approach (15 μg/kg)
Figure 3b shows that both Paired STs and GTs acquired a conditioned approach response across sessions when using 15 μg/kg nicotine as the US [F(2, 50.65) = 6.04, p = 0.004], and the two groups did not differ. Furthermore, both STs and GTs approached the nicotine cue more than their respective Unpaired control groups [effect of pairing; STs, F(1, 44.45) = 4.77, p = 0.03; GTs, F(1, 23.39) = 7.44, p = 0.01].
Conditioned orientation (25 μg/kg)
Figure 2c shows that when using 25 μg/kg nicotine as the US, both Paired STs and GTs acquired a conditioned orientation response, as indicated by a significant increase in the probability of orienting behavior across sessions [F(2, 64.54) = 42.39, p < 0.001], and the two groups did not differ. In addition, both STs and GTs showed a significant increase in conditioned orientation to the nicotine cue across sessions, relative to their respective Unpaired control groups [pairing × session interaction; STs, F(2, 48.75) = 14.9, p < 0.001; GTs, F(2, 46.65) = 9.17, p < 0.001].
Conditioned approach (25 μg/kg)
Figure 3c illustrates the probability of conditioned approach across training sessions when using 25 μg/kg nicotine as the US. Figure 3c shows that both STs and GTs acquired a conditioned approach response [effect of session, F(2, 59.95) = 15.81, p < 0.001], and the two groups did not differ in this response. In addition, both STs and GTs approached the nicotine cue more than their respective Unpaired control groups [effect of pairing; STs, F(1, 21.31) = 8.1, p = 0.01; GTs, F(1, 25.62) = 7.2; p = 0.01]. Importantly, neither STs nor GTs in the Unpaired groups developed an orienting or an approach CR.
Dose–response analysis
Figures 2d and 3d summarize the dose–response functions for the probability of conditioned orientation and conditioned approach on the final day of training. For conditioned orientation, a two-way analysis of variance (ANOVA) revealed that there were no differences between STs and GTs, and the probability of this CR increased as a function of dose in both groups [F(2, 78) = 16.49, p < 0.001]. Similarly, as shown in Fig. 3d, the nicotine cue elicited similar approach behavior in STs and GTs and the probability of an approach CR increased as a function of dose in both groups [F(2, 78) = 13.62, p < 0.001]. We separately analyzed conditioned approach dose–response data for STs and GTs and included Unpaired control animals in this analysis. A one-way ANOVA showed a significant effect of treatment group for both STs and GTs [STs, F(3, 45) = 6.15, p = 0.001; GTs, F(3, 47) = 6, p = 0.002]. However, post hoc analysis (Fisher’s LSD) revealed that, on the final day of testing, Paired STs differed from Unpaired STs at both 15 and 25 μg/kg (p’s < 0.05) but not at the lowest dose (p = 0.87). However, Paired GTs only differed from Unpaired GTs at the highest dose tested [7.5 μg/kg, p = 0.4; 15 μg/kg, p = 0.15; 25 μg/kg, p = 0.01].
Latency to approach (25 μg/kg)
We saw the most consistent change in approach behavior across sessions when using 25 μg/kg nicotine as the US. Thus, we also analyzed the latency to approach the nicotine cue at this dose. As can be seen in Fig. 4, the latency to approach the nicotine cue decreased across sessions [F(2, 31.03) = 13.95, p < 0.001], and this did not differ between groups.
A nicotine cue is a more effective conditioned reinforcer in STs than GTs
Following 1 week of abstinence, all rats underwent a single test for conditioned reinforcement. Figure 5 shows the mean difference in nose pokes into the active minus inactive port during the conditioned reinforcement test. As can be seen in Fig. 5, when 7.5 or 15 μg/kg nicotine was used as the US during Pavlovian conditioning, Paired STs and GTs did not differ in the extent to which they would work for the nicotine cue [7.5 μg/kg, t(22) = 1.04, p = 0.31; 15 μg/kg, t(27) = 0.51, p = 0.62], and there were no differences in the degree to which STs and GTs responded in the active versus inactive port [group × port interaction; 7.5 μg/kg, F(1, 22) = 1.09, p = 0.31; 15 μg/kg, F(1, 27) = 0.26, p = 0.62]. However, when 25 μg/kg nicotine was used during training, STs responded more for presentation of the nicotine cue than GTs [t(29) = 2.15, p = 0.04] and showed more robust conditioned reinforcement as indicated by a significant group × port interaction [F(1, 29) = 4.606, p = 0.04]. Importantly, there were no group differences in the number of inactive responses, indicating that this effect was driven by a difference in the number of active responses. For rats in the Unpaired condition, there were no significant differences between groups. We also separately analyzed conditioned reinforcement dose–response data for STs and GTs. Across doses, for GTs, there were no significant differences between the number of active minus inactive nose pokes [F(2, 42) = 1.11, p = 0.34]. However, the degree to which STs worked for presentation of the nicotine cue varied as a function of dose [F(2, 40) = 3.35, p = 0.046]. Post hoc analysis (Fisher’s LSD) revealed that STs that were trained with 25 μg/kg nicotine made significantly more nose pokes into the active than the inactive port than STs trained with 7.5 μg/kg (p = 0.015). STs that were trained with 15 μg/kg did not differ for STs trained with either 7.5 or 25 μg/kg (p’s > 0.05).
Performance during the test for conditioned reinforcement. During this test, a nose poke into the active port resulted in presentation of the cue either previously paired or unpaired with non-contingent nicotine delivery for 2 s. Data represent the mean ± SEM difference in nose pokes into the active minus inactive port during the conditioned reinforcement test where each data point represents an independent group of rats (UP STs n = 8, GTs n = 8; 7.5 μg/kg nicotine STs n = 12, GTs n = 12; 15 μg/kg nicotine STs n = 14, GTs n = 15; 25 μg/kg nicotine STs n = 15, GTs n = 16). UP = Unpaired. Asterisk indicates a significant group difference between STs and GTs. p < 0.05
Discussion
We previously reported that individuals prone to attribute incentive salience to a food cue are also more prone to attribute motivational properties to both cocaine and opioid (remifentanil) cues (Flagel et al. 2010; Meyer et al. 2012b; Saunders and Robinson 2010; Saunders et al. 2013; Yager et al. 2015; Yager and Robinson 2013), based on tests of attractiveness, conditioned reinforcement, and conditioned motivation (Milton and Everitt 2010). Here we asked whether there is similar individual variation in the extent to which a light cue associated with intravenous injections of nicotine acquires motivational properties. With training, the nicotine cue did become attractive, eliciting orientation towards it and approach into close proximity with it. However, in contrast to studies using cocaine or remifentanil as the US, the nicotine cue was equally attractive in STs and GTs, eliciting dose-dependent approach behavior in both. Therefore, by this measure it would seem that the nicotine cue was attributed with incentive salience to the same extent in STs and GTs. However, the incentive motivational properties of the nicotine cue were also assessed using a different test—the ability to act as a conditioned reinforcer. On this test, the nicotine cue was a more effective conditioned reinforcer in STs than in GTs, at least at the highest dose tested, consistent with studies with cocaine and remifentanil.
There has long been evidence that classically conditioned food cues can become attractive, eliciting approach behavior (Brown and Jenkins 1968; Davey and Cleland 1982; Hearst and Jenkins 1974; Zener 1937), but it was only recently established that classically conditioned drug cues can also elicit approach behavior; i.e., a sign-tracking CR (Uslaner et al. 2006). The first demonstration was by Tomie and colleagues (Tomie 2001; Tomie et al. 2003) who reported that rats would approach a cue associated with a sweetened ethanol solution. Although Tomie included a number of controls suggesting otherwise, there was some concern as to whether rats approached the cue because it was associated with the drug (ethanol) or because it was associated with the sweet solution. Supporting Tomie’s original reports, Krank et al. (2008) later reported that rats also learned to approach an unsweetened ethanol solution. Initial attempts to determine if rats would learn to approach a cue associated with intravenous (IV) drug (cocaine) delivery were unsuccessful (Kearns and Weiss 2004), and there are a number of reasons why this may have been the case (see Uslaner et al. 2006 for discussion). Nevertheless, there are now several studies reporting that rats will approach a cue associated with an IV injection of cocaine (Aragona et al. 2009; Flagel et al. 2010; Uslaner et al. 2006; Yager and Robinson 2013) or various opioids (Madsen and Ahmed 2014; Peters and De Vries 2013; Yager et al. 2015). The results reported here add nicotine to this list.
However, the main goal of the present experiment was to determine if there is individual variation in the extent to which a classically conditioned nicotine cue acquires incentive salience. We found that the nicotine cue was equally attractive to STs and GTs but differed in its ability to serve as a conditioned reinforcer. We next discuss what might account for this difference between measures of conditioned approach versus conditioned reinforcement, when nicotine serves as the US. Caggiula and others have argued that the ability of nicotine to motivate behavior involves three dissociable processes: (1) the ability to act as a primary reinforcer, (2) the ability to transform a neutral stimulus into a conditioned reinforcer, and (3) the ability of nicotine to act as a “reinforcement enhancer” or an “incentive amplifier” (Balfour et al. 2000; Bevins and Palmatier 2004; Caggiula et al. 2009; Chaudhri et al. 2006a; Liu et al. 2007; Palmatier et al. 2007, 2013; Rupprecht et al. 2015). Consideration of these dissociable processes may inform the results here.
First, although nicotine acts as a primary reinforcer, it is a relatively weak one, in the sense that nicotine supports only low levels of self-administration behavior in the absence of associated cues (Caggiula et al. 2002; Chaudhri et al. 2007; Donny et al. 2003; Le Foll and Goldberg 2006; Rupprecht et al. 2015; Sorge et al. 2009). Several self-administration studies have shown that when a cue is paired with nicotine delivery, rats will readily self-administer nicotine, but removal of the nicotine-paired cue dramatically decreases self-administration behavior (Caggiula et al. 2001, 2002; Sorge et al. 2009). This suggests that cues associated with nicotine delivery are at least as important as nicotine itself in maintaining self-administration behavior (e.g., Balfour et al. 2000; Rupprecht et al. 2015). It may be for this reason that the nicotine cue becomes especially salient in all animals, eliciting approach behavior. However, this explanation does not account for the difference we found in the ability of the nicotine cue to serve as a conditioned reinforcer.
In addition to nicotine acting as a primary reinforcer, and establishing cues as conditioned reinforcers (Palmatier et al. 2008, 2007), nicotine can also directly amplify the incentive properties of cues and thus has been termed an incentive amplifier (Bevins and Palmatier 2004; Caggiula et al. 2009; Palmatier et al. 2013, 2012; Rupprecht et al. 2015). For example, systemic injections of nicotine can enhance the ability of a conditioned stimulus to serve as a conditioned reinforcer (Guy and Fletcher 2014a; Olausson et al. 2004; Palmatier et al. 2007) and to attract (Guy and Fletcher 2014a; Palmatier et al. 2013), effects that may be dependent upon dopamine (Guy and Fletcher 2014b; Palmatier et al. 2014). Nicotine can even enhance the incentive properties of unconditioned stimuli (Chaudhri et al. 2007; Donny et al. 2003). Importantly, nicotine amplifies the incentive value of cues “on-the-fly,” as discontinuation of nicotine treatment reverses the enhancement of approach behavior (Guy and Fletcher 2014a). This property of nicotine, the ability to enhance the incentive motivational properties of cues, may help in interpretation of our results. During Pavlovian training using nicotine as the US, nicotine may have acted as an incentive amplifier, enhancing the motivational properties of the cue. This may have had the effect of making the cue an especially attractive stimulus, thus eliciting approach in both STs and GTs. Consistent with this hypothesis, other incentive amplifiers, such as amphetamine, yohimbine, and stress (Feltenstein and See 2006; Robbins 1978), have been found to increase the incentive value of reward-associated cues to the same extent in STs and GTs (Meyer et al. 2014). However, during the conditioned reinforcement test, no nicotine was “on board,” so its action as an incentive amplifier would not be present. Under these conditions, STs worked more avidly for presentation of the nicotine cue, suggesting that they did attribute more incentive salience to it than GTs. In other words, the incentive amplifying effects of nicotine may have masked any differences between STs and GTs as measured by conditioned approach, because during this test, nicotine was on board, whereas it was not during the test of conditioned reinforcement.
It is important to note that rats in the Unpaired group, which received non-contingent IV infusions of nicotine that were explicitly not paired with presentation of the cue light, did not acquire a conditioned approach CR, nor did the cue act as a conditioned reinforcer. At first, this may seem to be inconsistent with a report that non-contingent nicotine delivery increased responding for a visual stimulus that was not associated with any other reward besides illumination of the cue light (Donny et al. 2003). Based on these data, it might be assumed that in the present study, rats that received unpaired CS-US pairings during Pavlovian training would also approach the cue light if nicotine generally amplifies the incentive value of cues. However, in the study conducted by Donny et al. (2003), rats had to actively work for presentation of the visual stimulus, which is quite different than the situation here. Additionally, previous work has shown that rats find light stimuli inherently reinforcing and will sustain instrumental responding for a light stimulus even in the absence of any other reinforcer (Olsen and Winder 2009; Stewart 1960). Thus, in the Donny et al. (2003) study, nicotine may have acted to enhance the reinforcing properties of the visual stimulus, but in this study, nicotine was not present during the conditioned reinforcement test.
One potential limitation of this study is the range of nicotine doses used as the US. We selected doses that have been reported to support nicotine self-administration (Matta et al. 2007; Peartree et al. 2012; Rose and Corrigall 1997). However, while 25 μg/kg is a typical dose used in nicotine self-administration studies and sits near the peak of the dose–response curve, the other doses we selected (7.5 and 15 μg/kg) are both on the ascending limb of the dose–response curve. While these nicotine doses can function as reinforcers, they may only do so in a subset of animals; thus, making it difficult to achieve conditioning with these doses. We think it is unlikely, however, that a higher dose of nicotine (>25 μg/kg) would result in differences in approach behavior between STs and GTs as their approach responses to the three doses we tested were identical.
In summary, we report that the propensity to attribute incentive salience to a food cue predicts the extent to which a nicotine cue serves as a conditioned reinforcer, but not the extent to which it becomes attractive. This dissociation in the ability of a nicotine cue to motivate behavior under different conditions may be due to its ability to amplify the incentive value of cues, as we have found that other incentive amplifiers, such as amphetamine and stress, potentiate the motivational properties of food cues equally in STs and GTs (Meyer et al. 2014). These data also highlight the importance of studying multiple properties of an incentive stimulus, because they are psychologically and neurobiologically dissociable (Cardinal et al. 2002; Everitt and Robbins 2005; Milton and Everitt 2010). In addition, the form of the CS is also an important factor (Holland et al. 2014; Meyer et al. 2014; Saunders et al. 2014), but this has only been studied in relation to food and cocaine cues—not with other drugs.
The present data are also interesting in light of recent studies in human smokers. For example, Mahler and de Wit (2010) found that smokers who reported the highest craving when presented with food cues when food-deprived also reported the highest craving when presented with smoking cues during abstinence, a finding that has recently been replicated (Styn et al. 2013). Thus, some people may also be more susceptible to the motivating effects of nicotine-associated cues during abstinence (i.e., no nicotine on board), making them more likely to relapse. In conclusion, it appears that there is considerable individual variation in the ability of discrete cues associated with drugs from many different classes (cocaine, opioids, alcohol, and nicotine) to acquire motivational properties (Robinson et al. 2014; Saunders and Robinson 2013; Tomie and Sharma 2013), which may prove important in the development of individualized treatment approaches.
References
Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM (2009) Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci 30:1889–99
Balfour DJ, Wright AE, Benwell ME, Birrell CE (2000) The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res 113:73–83
Bevins RA, Palmatier MI (2004) Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev 3:143–58
Boakes R (1977) Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwits H (eds) Operant-pavlovian interactions. Lawrence Erlbaum Associates, Hillsdale, pp 67–97
Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013) The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 229:453–76
Brown PL, Jenkins HM (1968) Auto-shaping of the pigeon’s key-peck. J Exp Anal Behav 11:1–8
Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009) The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv 55:91–109
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2002) Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 163:230–7
Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav R 26:321–352
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF (2006a) Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 189:27–36
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF (2007) Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 190:353–62
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006b) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 184:353–66
Crombag HS, Badiani A, Maren S, Robinson TE (2000) The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res 116:1–22
Crombag HS, Bossert JM, Koya E, Shaham Y (2008) Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci 363:3233–43
Davey GC, Cleland GG (1982) Topography of signal-centered behavior in the rat: effects of deprivation state and reinforcer type. J Exp Anal Behav 38:291–304
Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV (2002) Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci 15:1363–70
Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 169:68–76
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489
Feltenstein MW, See RE (2006) Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res 174:1–8
Field M, Cox WM (2008) Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend 97:1–20
Flagel SB, Akil H, Robinson TE (2009) Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology 56:139–148
Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PEM, Akil H (2010) An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacol 35:388–400
Flagel SB, Watson SJ, Robinson TE, Akil H (2007) Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 191:599–607
Guy EG, Fletcher PJ (2014a) The effects of nicotine exposure during Pavlovian conditioning in rats on several measures of incentive motivation for a conditioned stimulus paired with water. Psychopharmacology (Berl) 231:2261–71
Guy EG, Fletcher PJ (2014b) Responding for a conditioned reinforcer, and its enhancement by nicotine, is blocked by dopamine receptor antagonists and a 5-HT(2C) receptor agonist but not by a 5-HT(2A) receptor antagonist. Pharmacol Biochem Behav 125:40–7
Hearst E, Jenkins HM (1974) Sign tracking: the stimulus-reinforcer relation and directed action. Monograph of the Psychonomic Society, Austin
Holland PC, Asem JS, Galvin CP, Keeney CH, Hsu M, Miller A, Zhou V (2014) Blocking in autoshaped lever-pressing procedures with rats. Learn Behav 42:1–21
Kearns DN, Weiss SJ (2004) Sign-tracking (autoshaping) in rats: a comparison of cocaine and food as unconditioned stimuli. Learn Behav 32:463–476
Krank MD, O’Neill S, Squarey K, Jacob J (2008) Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology (Berl) 196:397–405
Le Foll B, Goldberg SR (2006) Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology (Berl) 184:367–81
Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF (2007) Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology (Berl) 194:463–73
Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW (2011) Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res 220:91–99
Madsen HB, Ahmed SH (2014) Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol. doi:10.1111/adb.12134
Mahler SV, de Wit H (2010) Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS One 5, e15475
Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190:269–319
Meyer PJ, Cogan ES, Robinson TE (2014) The conditioned stiumulus as a determinant of the incentive properties of reward cues. PLoS One 9, e98163
Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE (2012a) Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One 7, e38987
Meyer PJ, Ma ST, Robinson TE (2012b) A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 219:999–1009
Milton AL, Everitt BJ (2010) The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci 31:2308–2319
Olausson P, Jentsch JD, Taylor JR (2004) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 171:173–8
Olsen CM, Winder DG (2009) Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacol 34:1685–94
Palmatier MI, Coddington SB, Liu X, Donny EC, Caggiula AR, Sved AF (2008) The motivation to obtain nicotine-conditioned reinforcers depends on nicotine dose. Neuropharmacology 55:1425–30
Palmatier MI, Kellicut MR, Brianna Sheppard A, Brown RW, Robinson DL (2014) The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav 126:50–62
Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF (2007) Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl) 195:235–43
Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB (2013) The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 226:247–59
Palmatier MI, O’Brien LC, Hall MJ (2012) The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology (Berl) 219:1119–31
Peartree NA, Sanabria F, Thiel KJ, Weber SM, Cheung TH, Neisewander JL (2012) A new criterion for acquisition of nicotine self-administration in rats. Drug Alcohol Depend 124:63–9
Peters J, De Vries TJ (2013) Pavlovian conditioned approach, extinction, and spontaneous recovery to an audiovisual cue paired with an intravenous heroin infusion. Psychopharmacology (Berl) 231:447–53
Robbins TW (1978) The acquisition of responding with conditioned reinforcement: effects of pipradrol, methylphenidate, d-amphetamine, and nomifensine. Psychopharmacology (Berl) 58:79–87
Robinson TE, Berridge KC (1993) The neural basis of drug craving- An incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Robinson TE, Flagel SB (2009) Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry 65:869–873
Robinson TE, Yager LM, Cogan ES, Saunders BT (2014) On the motivational properties of reward cues: individual differences. Neuropharmacology 76:450–9
Rose JE, Corrigall WA (1997) Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl) 130:28–40
Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC (2015) Behavioral mechanisms underlying nicotine reinforcement. Curr Top Behav Neurosci 24:19–53
Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE (2014) A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacol 39:2816–23
Saunders BT, Robinson TE (2010) A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry 67:730–736
Saunders BT, Robinson TE (2012) The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci 36:2521–32
Saunders BT, Robinson TE (2013) Individual variation in resisting temptation: implications for addiction. Neurosci Biobehav R 37:1955–75
Saunders BT, Yager LM, Robinson TE (2013) Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci 33:13989–4000
Sorge RE, Pierre VJ, Clarke PB (2009) Facilitation of intravenous nicotine self-administration in rats by a motivationally neutral sensory stimulus. Psychopharmacology (Berl) 207:191–200
Stewart J (1960) Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol 53:187–193
Stewart J, Dewit H, Eikelboom R (1984) Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev 91:251–268
Styn MA, Bovbjerg DH, Lipsky S, Erblich J (2013) Cue-induced cigarette and food craving: a common effect? Addict Behav 38:1840–1843
Tomie A (2001) Autoshaping and drug-taking. In: Mowrer RR, Klein SB (eds) Handbook of contemporary learning theories. Lawrence Erlbaum Associates, Mahwah, pp 409–439
Tomie A, Festa ED, Sparta DR, Pohorecky LA (2003) Lever conditioned stimulus-directed autoshaping induced by saccharin-ethanol unconditioned stimulus solution: effects of ethanol concentration and trial spacing. Alcohol 30:35–44
Tomie A, Grimes KL, Pohorecky LA (2008) Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev 58:121–135
Tomie A, Sharma N (2013) Pavlovian sign-tracking model of alcohol abuse. Curr Drug Abuse Rev 6:201–19
Uslaner JM, Acerbo MJ, Jones SA, Robinson TE (2006) The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res 169:320–324
Verbeke G, Molenberghs G (2000) Linear mixed models for longitudinal data. Springer, New York
Yager LM, Pitchers KK, Flagel SB, Robinson TE (2015) Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacol 40:1269–1277
Yager LM, Robinson TE (2010) Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res 214:30–34
Yager LM, Robinson TE (2013) A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 226:217–28
Zener K (1937) The significance of behavior accompanying conditioned salivary secretion for theories of the conditioned response. Am J Psychol 50:384–403
Acknowledgments
This research was supported by National Institute on Drug Abuse grants to L.M.Y (F31 DA030799) and T.E.R. (P01 DA031656).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yager, L.M., Robinson, T.E. Individual variation in the motivational properties of a nicotine cue: sign-trackers vs. goal-trackers. Psychopharmacology 232, 3149–3160 (2015). https://doi.org/10.1007/s00213-015-3962-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-3962-6