Abstract
Rationale
Caffeine, an antagonist of adenosine A1 and A2A receptor, is the most widely used psychoactive substance in the world. Evidence indicates that caffeine interacts with the neuronal systems involved in drug reinforcing. Although adenosine A1 and/or A2A receptor have been found to play important roles in the locomotor stimulation and probably reinforcing effect of caffeine, the relative contribution of the A1 and/or A2A receptors to the acute and chronic motor activation and reinforcing effects of caffeine has not been completely investigated.
Objective
The roles of adenosine A1 and/or A2A receptor and the association of phospho-Thr75-dopamine- and cAMP-regulated phosphoprotein of molecular weight 32 kDa (DARPP-32) in the motor activation and reinforcing effects of caffeine, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), a selective A1 antagonist, and 5-amino-7-(β-phenylethyl)-2-(8-furyl) pyrazolol [4,3-e]-1,2,4-triazolol [1,5-c] pyrimidine (SCH58261), a selective A2A receptor antagonist were examined.
Methods
Locomotor stimulation and behavioral sensitization of caffeine, DPCPX, and SCH58261 were studied in C57BL/6 male mice following acute and chronic administration. Conditioned place preference (CPP) paradigm was used to evaluate the drug-seeking potential of these compounds. Furthermore, the expression of phospho-Thr75-DARPP-32 in striatal membrane from behaviorally sensitized mice was analyzed by Western blot.
Results
Caffeine and SCH58261 but not DPCPX induced CPP and locomotor sensitization in C57BL/6 mice. The locomotor sensitization after chronic treatment was associated with increased DARPP-32 phosphorylation at Thr75 in the striatum.
Conclusion
Caffeine-induced reinforcing effect and behavioral sensitization are mediated by antagonism at adenosine A2A receptor. These effects are associated with phosphorylation of DARPP-32 at Thr75 in the striatum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caffeine is the most widely used psychoactive substance in the world. Although there has been some debate about the abuse potential of caffeine, a recent literature review of human caffeine withdrawal has provided sufficient evidence to warrant the inclusion of caffeine withdrawal as a disorder in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (Juliano and Griffiths 2004). There is evidence to indicate that caffeine interacts with the neuronal systems involved in drug addiction. For example, caffeine, like amphetamine and cocaine, can induce dopamine release in the shell of the nucleus accumbens (Solinas et al. 2002) which is considered the main mechanism involved in the rewarding and motor-activating properties of these drugs (Wise and Bozarth 1987; Pontieri et al. 1995). On the contrary, a recent study by De Luca et al. (2007) failed to observe any changes in the dopamine release in the nucleus accumbens after caffeine administration. The explanation for this discrepancy has been offered in a recent review by Ferré (2008) who further suggested that more mapping analyses are needed to resolve these differences. In animal behavioral model, caffeine causes motor sensitization (Cauli et al. 2003; Simola et al. 2006; Tronci et al. 2006), conditioned place preference (Bedingfield et al. 1998; Patkina and Zvartau 1998), and cross-sensitization to locomotion elicited by nicotine and amphetamine (Celik et al. 2006; Simola et al. 2006).
Blockade of adenosine receptors is significantly affected by normal human caffeine consumption (Fredholm et al. 1999). Adenosine acts on four subtypes of receptors, A1, A2A, A2B, and A3, which have been characterized and cloned in several species (Fredholm et al. 2005). Caffeine is a nonselective competitive blocker of A1 and A2A receptors. The expression level of A3 receptor is low and the inhibition of A2B and A3 receptors requires high concentrations of caffeine. In contrast, A1 and A2A receptors are activated at low basal concentration of adenosine and are the primary targets of caffeine. Adenosine A1 receptors are widely distributed throughout the brain with high levels expressed in the hippocampus, cerebral cortex, cerebellum, and hypothalamic nuclei (Goodman and Synder 1982; Rivkees et al. 1995). In contrast, the expression of adenosine A2A receptor in the brain is limited to regions heavily innervated by dopamine-containing fibers, such as the striatum, nucleus accumbens, and olfactory tubercle (Jarvis and Williams 1989; Parkinson and Fredholm 1990; Ongini and Fredholm 1996; Wooten 2001). The anatomical colocalization of A2A and D2 receptors and the direct functional interactions between them (Fink et al. 1992; Garrett and Holtzman 1994; Fenu and Morelli 1998; Casas et al. 1999; Fenu et al. 2000) support a role for dopamine in the behavioral effects of caffeine. Several studies have demonstrated that the lack of adenosine A2A receptors diminishes the reinforcing efficacy of cocaine and attenuates the nicotine-induced rewarding effect and amphetamine-induced behavioral sensitization (Chen et al. 2003; Bastia et al. 2005; Castane et al. 2006; Soria et al. 2006).
Although adenosine A1 and/or A2A receptor have been found to be involved in the locomotor stimulatory and probably reinforcing effects of caffeine, the relative role of the A1 or A2A receptors to the acute and more importantly the chronic motor activation and reinforcing effects of caffeine has not been completely studied (El Yacoubi et al. 2000; Halldner et al. 2004). For example, a correlation between central motor stimulation and affinities for A1 receptor has been observed for ten methylxanthines in imprinting control region mice (Snyder et al. 1981). Other studies indicated that the selective A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) did not alter locomotor activity (Griebel et al. 1991b). Selective A2A receptor antagonists, SCH58261 and CGS21197, were found to stimulate locomotor activity in rats (Griebel et al. 1991a; Svenningsson et al. 1997). Caffeine no longer exerted locomotor activation in A2A receptor knockout mice (Ledent et al. 1997). Furthermore, recent studies in mice with genetic inactivation of A1, A2A, or both receptors indicated that A1 receptor elicited locomotor stimulation but not inhibition of caffeine (Halldner et al. 2004). On the other hand, coadministration of a selective A1 and a selective A2A antagonist at doses that did not elicit motor stimulation increased locomotor activity implying a behavioral synergism between A1 and A2A antagonists (Kuzmin et al. 2006).
Blockade of adenosine A2A receptors reduces basal cAMP production, resulting in increased phosphorylation of dopamine- and cAMP-regulated phosphoprotein of molecular weight 32 kDa (DARPP-32) at Thr75 (Lindskog et al. 2002). DARPP-32 is a signal transduction molecule that plays an important role in neuronal plasticity (Gould and Manji 2005). Several studies have shown that DARPP-32 participates in the generation and expression of behavioral sensitization to psychostimulants (Nairn et al. 2004). Sensitization of locomotor activity is the most commonly studied paradigm and indicates the incentive motivational properties of drugs which is believed to contribute to the intensification of drug craving and compulsive drug-seeking behavior (Robinson and Berridge 2000). It has been observed that repeated administration of cocaine and amphetamine resulted in psychomotor sensitization and conditioned place preference and was associated with increased DARPP-32 phosphorylation at Thr75 (Bibb et al. 2001; Lin et al. 2002; Scheggi et al. 2004; Chen and Chen 2005).
There are only a small number of studies using conditioned place preference (CPP) paradigm to examine the reinforcing effect of caffeine (Steigerwald et al. 1988; Brockwell et al. 1991; Bedingfield et al. 1998; Patkina and Zvartau 1998). Only one study using the CPP model examined the reinforcing property of a selective A2A receptor antagonist, KW6002 (Harper et al. 2006). Studies using selective A1 or A2A receptor antagonists may provide a better resolution regarding the functional role of A1 or A2A receptor in mediating the reinforcing effect and behavioral sensitization following chronic caffeine administration. In this study, the CPP paradigm was used to investigate the reinforcing behavioral effect of DPCPX and SCH58261. In addition, locomotor activation was studied following acute administration of caffeine, DPCPX and SCH58261, and locomotor sensitization was also studied after chronic administration of caffeine and SCH58261. Furthermore, phosphorylation of DARPP-32 at Thr75 was evaluated in striatum to correlate with the behavioral changes. Our results indicate that caffeine and SCH58261 exerted locomotor stimulation and sensitization in C57BL/6 mice. Only A2A receptor selective antagonist, SCH58261, but not A1 receptor selective antagonist, DPCPX, demonstrated conditioned place preference in this mouse strain. The locomotor sensitization of caffeine and SCH58261 was associated with increased DARPP-32 phosphorylation at Ther75 in the striatum.
Materials and methods
Animals
Male C57BL/6 mice, purchased from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan), were established at the Laboratory Animal Center, Tzu Chi University. Mice weighing 25–35 g were used in the present study. All experimental procedures were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of Tzu Chi University. Every effort was made to minimize the suffering and the number of animals used.
Drugs
Caffeine, DPCPX, and SCH58261 were purchased from Sigma-RBI (Taipei, Taiwan). Caffeine was dissolved in saline whereas DPCPX and SCH58261 were dissolved in dimethyl sulfoxide (DMSO). All drugs were administered i.p. with the dosages specified in each experiment.
Evaluation of locomotor activity
Motor activity was monitored in a quiet and isolated room lit with two fluorescent lamps fixed symmetrically on the ceiling. The test field was surrounded by black curtain which uniformly reduced the illumination to a dimly lit condition. Each mouse was placed individually in an opaque acrylic test cage (30 × 30 × 30 cm) with a plate over the floor. Observation of the activity was carried out by a video camera mounted vertically above the center of the test field at a distance that allowed an unobstructed monitoring of the locomotor activity simultaneously for four to six mice. The recorded images were transferred to the interface of a computer for processing. The track data were stored in a special format and analyzed by TrackMot software (Diagnostic & Research Instruments Co., Taoyuan, Taiwan). All animals were used only once.
Acute locomotor effect of caffeine, SCH58261, and DPCPX
Caffeine can cause anxiety (Fredholm et al. 1999) and that a novel environment can elicit anxiety in rodents (Merali et al. 2003), which may exacerbate the caffeine-induced anxiety and mask the stimulation of locomotor activity or the development of behavioral sensitization. In preliminary experiments (results not shown), we found that 2 h of habituation were needed to allow the animals to maintain the locomotor activity at steady-state level. The activity was summated consecutively every 10-min interval to facilitate calculation and comparison. The activity for the last 10 min of the habituation period was designated zero time or basal activity. Each mouse was then administered caffeine (5, 10, 20, or 30 mg/kg, i.p.), SCH58261 (1.5, 3.75, or 6 mg/kg, i.p.), DPCPX (3 or 6 mg/kg i.p.), or vehicles (0.9% saline or DMSO) and the locomotor activity was recorded for an additional 60 min. No difference in motor activity was found between control mice administered 0.05 ml of DMSO, the maximum amount administered, and mice received saline injection. The total drug-induced locomotor activity (distance traveled) following the injection of the test drugs was also accumulated for 60 min for subsequent comparison.
Chronic locomotor effects of caffeine and SCH58261
Maximum acute motor activation dosages of caffeine and SCH58261 were used in the chronic sensitization experiments. Mice were administered caffeine (20 mg/kg, i.p.) and saline or SCH58261 (3.75 mg/kg, i.p.) and DMSO daily for 14 days. Three days after the last scheduled administration, the locomotor activity of an acute dosage of caffeine, SCH58261, or vehicle was recorded for 60 min following 2-h habituation. The locomotor activities produced by an acute dosage of caffeine (20 mg/kg, i.p.) or SCH58261 (3.75 mg/kg, i.p.) between caffeine-treated and saline-treated groups or SCH58261-treated and DMSO-treated groups after washout period were compared to assess the locomotor sensitization effect.
Conditioned place preference
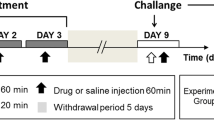
The test apparatus consisted of two main conditioning compartments (30 × 24 × 24 cm; L × W × H) separated by a central compartment (10 × 24 × 24 cm) with removable partitions. Both conditioning compartments had different visual cues (black or white stripes) but with uniform smooth floor. On day 1 (preconditioning phase), mice were allowed to explore the three compartments for 20 min while baseline side preference was established. A “preference score” of preconditioning phase was calculated by dividing the time spent in the least-preferred conditioning compartment into the total time spent in both conditioning compartments (CS+/CS− + CS+). The least-preferred compartment in the preconditioning phase was designated as the conditioned stimulus (CS+) side, which was paired with caffeine, SCH58261, or DPCPX. The preferred compartment was paired with saline or DMSO injections. The preference score reflects shifts in compartment preference independently from the time an animal may spend in the nonconditioning center compartment and represents an effective measure of compartment preference. On day 2 to 4 (conditioning phase), mice were confined to the conditioned stimulus compartment for 20 min when the animals were injected with caffeine, SCH58261, or DPCPX. Four hours later, animals were confined to the other side for 20 min when the animals were injected with vehicles. On day 5 (postconditioning phase), mice were placed in the central compartment and allowed to move freely in all three compartments for 20 min and a “preference score” of postconditioning phase was calculated.
Western blotting
Mice were administered caffeine (20 mg/kg, i.p.), SCH58261 (3.75 mg/kg, i.p.) or vesicles (saline or DMSO, i.p.) daily for a total of 14 days. Three days after the last scheduled administration, caffeine (20 mg/kg, i.p.) or SCH58261 (3.75 mg/kg, i.p.) was administered to vehicle- as well as caffeine- or SCH58261-pretreated mice. Mice were killed by decapitation at 10, 30, or 60 min after drug treatment. The brains were then removed and the striata were dissected under a microscope on an ice-cold surface and homogenized in the lysis buffer (0.5 mM dithiothreitol, 0.2 mM ethylenediaminetetraacetic acid, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2.5 mM MgCl2, 75 mM NaCl, 0.1 mM Na3VO4, 50 mM NaF, 0.1% Triton X-100, and a cocktail tablet containing protease inhibitors (Roche, Mannheim, Germany). After centrifugation at 12,000 rpm for 30 min, the supernatant was removed and stored at −80°C until assayed. Protein concentrations were determined using the Bio-Rad protein assay kit. Eighty micrograms of protein from each sample were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by electrophoretic transfer to polyvinylidene difluoride membranes. The membranes were immunoblotted using primary antibodies for phospho-Thr75 DARPP-32 (1:500; Cell Signaling; Beverly, MA, USA), total DARPP-32 (1:1,000; Cell Signaling), or actin (1:5,000; Chemicon; Temecula, CA, USA) and followed with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz; Santa Cruz, CA, USA). Finally, the protein bands were visualized on the X-ray film using the enhanced chemiluminescence detection system (NEN, Boston, MA, USA). The intensity of each band on the X-ray film was estimated using a densitometer (Molecular Dynamics, Personal Densitometer ST; Sunnyvale, CA, USA).
Statistical analysis
The net locomotor activity was calculated for every 10-min recording. In addition, total drug-induced locomotor activities for the entire duration of 60 min following drug administration were summated. Data were expressed as mean ± standard error of the mean (SEM). Data were analyzed for statistical significance using the computer program Prism for two-way analysis of variance (ANOVA) or repeated measures ANOVA followed by Tukey’s post hoc test. In addition, mean ± SEM of total locomotor activity (60 min) was calculated and analyzed by Student’s t test. Preference scores (CS+/CS− + CS+) were calculated and used as the indicator of side preference. Student’s t test was used to compare changes in side preference for specific treatments. Phospho-Thr75 DARPP-32 levels were normalized to total DARPP-32 levels in striatal homogenates. Data were expressed as mean ± SEM and analyzed by Student’s t test. In all cases, P < 0.05 was considered statistically significant.
Results
Acute locomotor effects induced by caffeine, SCH58261, and DPCPX
When placed in the test cages, the locomotor activity of mice decreased gradually from the initial high activity to a steady basal level after 2 h habituation (results not shown). Therefore, the 2-h habituation was used for all the experiments. For subsequent experiments, only the activity for the last 10 min prior to the drug administration was presented. Acute caffeine administration (5, 10, 20, and 30 mg/kg) produced a biphasic dose-dependent stimulation of locomotor activity with the peak effect observed at 20 mg/kg, while higher dose of caffeine (30 mg/kg) was less effective, as shown in Fig. 1a. The accumulated activity for the 60-min duration of postdrug treatment is shown in Fig. 1b. Repeated measures ANOVA followed by Tukey’s post hoc test revealed a significant increase in locomotor activity in mice treated with caffeine at the dosage of 5, 10, 20, and 30 mg/kg (P < 0.001). The enhancement of locomotor activity persisted for 60 min at all the doses tested (Fig. 1a).
Dose-dependent effects of caffeine on locomotor activity in habituated C57BL/6 mice. Mice were injected with saline or caffeine (5, 10, 20, and 30 mg/kg, i.p.). The horizontal component of locomotor activity was measured for 60 min. a Time course on locomotor activity measured over 10-min intervals. Data represent means ± SEM (n = 6–11). P < 0.001 versus saline group (by Turkey post hoc test following a one-way ANOVA). b Total locomotor activity counts during the 60-min period following administration of caffeine. Data represent means ± SEM. P < 0.001 versus saline group (by Student’s t test)
Acute SCH58261 administration with lower dosages stimulated the locomotor activity with the peak effect observed at 3.75 mg/kg, whereas 6 mg/kg was less effective (Fig. 2a, b). Repeated measures ANOVA followed by Tukey’s post hoc test revealed a significant increase in locomotor activity with the dose of 2, 3.75 (P < 0.001), and 6 mg/kg (P < 0.01), and the duration lasted over 60 min (Fig. 2a). In contrast to caffeine and SCH58261, acute DPCPX (3 and 6 mg/kg) was without effect on locomotor activity (Fig 3a, b).
Dose-dependent effects of SCH58261 on locomotor activity in habituated C57BL/6 mice. Mice were injected with DMSO or SCH58261 (2, 3.75, and 6 mg/kg, i.p.). The horizontal locomotor activity was measured for 60 min. a Time course on locomotor activity measured over 10-min intervals. Data represent means ± SEM (n = 6–17). P < 0.001 (2 and 3.75 mg/kg) and P < 0.01 (6 mg/kg) versus DMSO group (by Turkey post hoc test following a one-way ANOVA). b Total locomotor activity counts during the 60-min period following administration of caffeine. Data represent means ± SEM. P < 0.001 (2 and 3.75 mg/kg) and P < 0.01 (6 mg/kg) versus DMSO group (by Student’s t test)
Effects of DPCPX on locomotor activity in habituated C57BL/6 mice. Mice were injected with DMSO or DPCPX (3 and 6 mg/kg, i.p.). The horizontal locomotor activity was measured for 60 min. Time course on locomotor activity measured over 10-min intervals (a) and total locomotor activity counts during the 60-min period following administration (b) did not show significant difference (n = 6–21)
Caffeine-induced locomotor sensitization after chronic caffeine treatment
In order to investigate the locomotor sensitization following repeated caffeine administration, the most effective acute dosage of caffeine (20 mg/kg) was selected. Three days after the last injection of saline or caffeine, acute administration of caffeine (20 mg/kg i.p.) resulted in a greater response in locomotor activity in caffeine- as compared with vehicle-pretreated rats (Fig. 4a). The result of two-way ANOVA showed F(1,30) = 6.91 and P < 0.05. In contrast, repeated administration of saline followed by saline challenge did not show any increase in locomotor activity. The total distance traveled was also significantly enhanced following chronic treatment with 20 mg/kg caffeine as assessed by Student’s t test (Fig. 4b).
Locomotor sensitization by repeated administration of caffeine in habituated C57BL/6 mice. Caffeine (20 mg/kg, i.p.) or saline was administered daily for 2 weeks. Three days after the last injection of caffeine or saline, mice were challenged with caffeine (20 mg/kg, i.p.). The horizontal locomotor activity was measured for 60 min. a The time course of locomotor activity measured over 10-min intervals. Data represent means ± SEM (n = 4). P < 0.05 versus saline-pretreated group (by two-way ANOVA). b Total locomotor activity counts during the 60-min period following acute administration of caffeine. Data represent means ± SEM. P < 0.05 versus saline-pretreated group (by Student’s t test)
SCH58261-induced locomotor sensitization after chronic SCH58261 treatment
The most effective acute dosage of SCH58261 (3.75 mg/kg) was selected to investigate the locomotor sensitization. Three days after the last injection of DMSO or SCH58261, acute administration of SCH58261 (3.75 mg/kg i.p.) resulted in a greater response in locomotor activity in SCH58261- as compared with vehicle-pretreated mice (Fig. 5a). The result of two-way ANOVA showed F(1,50) = 5.56 and P < 0.05. Repeated administration of DMSO did not alter the response to acute SCH58261 administration. The total distance traveled was also significantly enhanced following chronic treatment with SCH58261 (3.75 mg/kg) as evaluated by Student’s t test (Fig. 5b).
Locomotor sensitization by repeated administration of SCH58261 in habituated C57BL/6 mice. SCH58261 (3.75 mg/kg) or DMSO was administered daily for 2 weeks. Three days after the last injection of SCH58261 or DMSO, mice were challenged with acute SCH58261 (3.75 mg/kg, i.p.). The horizontal locomotor activity was measured for 60 min. a The time course of locomotor activity measured over 10-min intervals. Data represent means ± SEM (n = 6). P < 0.05 versus DMSO-pretreated group (by two-way ANOVA). b Total locomotor activity counts during the 60-min period following administration of SCH58261. Data represent means ± SEM. P < 0.05 versus DMSO-pretreated group (by Student’s t test)
Conditioned place preference induced by SCH58261 conditioning
Figure 6a–c illustrates the mean ± SEM of time (seconds) spent in the least-preferred compartment during pre- and postconditioning phase. The animals from the eight different experimental groups spent equal time in the least-preferred compartment during the preconditioning phase. The time spent in the postconditioning phase was not altered in the vehicles, DPCPX-paired (3 and 6 mg/kg), and caffeine-paired (20 mg/kg) animals. In contrast, mice receiving caffeine (10 mg/kg) or SCH58261 (2 and 3.75 mg/kg) spent significantly more time in the drug-paired compartment during postconditioning phase. Similar observations were found when percentage preference scores were used (Fig. 6d, e).
Effect of caffeine, SCH58261, and DPCPX in the conditioned place preference paradigm. Mice were administered saline, caffeine (10 and 20 mg/kg), DMSO, SCH58261 (2 and 3.75 mg/kg), or DPCPX (3, 6 mg/kg) on three consecutive conditioning days in a CPP chamber. The amount of time spent by animals in least-preferred compartment is shown. Data represent means ± SEM (n = 3–6) were analyzed using Student’s t test to show differences in time spent in least-preferred compartment during the pre- and postconditioning phase (a–c; *P < 0.05). Similar observations were found when percentage preference scores were used (d, e). White bar means preference score of precondition phase and gray bar means preference score of postcondition phase (*P < 0.05 by Student’s t test)
Chronic treatment with caffeine and SCH58261 increased DARPP-32 phosphorylation at Thr75
Mice were treated with caffeine (20 mg/kg, i.p.) or SCH58261 (3.75 mg/kg, i.p.) for 14 days as described for the locomotor sensitization experiments. Following 3-day washout period, mice were sacrificed 10, 30, or 60 min after acute challenge with caffeine (20 mg/kg) or SCH58261 (3.75 mg/kg i.p.). Striatal membrane was prepared for the Western blotting of total DARPP-32 and phospho-Thr75-DARPP32 expression. Figure 7 showed that Western blotting demonstrated a statistically significant increase in the proportion of DARPP-32 phosphorylation at Thr75 10 and 60 min but not 30 min after caffeine treatment (P < 0.05). Enhanced percentage phosphorylation of DARPP-32 at Thr75 was observed 10, 30, and 60 min after SCH58261 treatment (P < 0.01 at 10 min, P < 0.05 at 30 and 60 min).
Representative Western immunoblots of phospho-Thr75-DARPP-32 in the striatum of the chronic saline- or caffeine-treated groups and the chronic DMSO- or SCH58261-treated groups 10 (a, b), 30 (c, d), and 60 min (e, f) after a challenge with caffeine (20 mg/kg) or SCH58261 (3.75 mg/kg). The experiment was repeated at least three times. The bar graphs indicate quantitative count of phospho-Thr75-DARPP-32, normalized with DARPP-32 signals (n = 3–4; *P < 0.05 and **P < 0.01 by Student’s t test)
Discussion
The present study demonstrates that selective adenosine A2A receptor antagonist SCH58261 but not selective adenosine A1 receptor antagonist DPCPX elicited locomotor stimulation and sensitization and conditioned place preference in C57BL/6 mice. The behavioral sensitization induced by SCH58261, which exhibits 145-fold higher affinity for adenosine A2A than A1 receptor (Weiss et al. 2003), was correlated with the enhanced phosphorylation of DARPP-32 in the striatum. It is important to point out that although phosphorylation of DARPP-32 is essential for mediating the effects of both psychostimulants and antipsychotic drugs, the effects are mediated through different cell-specific pathways (Bateup et al. 2008). Enhanced phosphorylation of DARPP-32 in the striatum is positioned to play an important role in either mediating or modulating the short-term—and perhaps long-term—actions of reinforcer by virtue of its regulation by dopamine and other neurotransmitters linked to the action of reinforcers (Nairn et al. 2004). The present results indicate that the behavioral reinforcing and sensitization effect of caffeine is probably mediated by the adenosine A2A receptor in C57BL/6 mice.
In contrast to drugs like cocaine and amphetamine which demonstrate strong abuse potential and reinforcing properties across species, caffeine is only mildly reinforcing in humans and there is little evidence of its compulsive use (see review by Fredholm et al. 1999). Important variations in individual sensitivity to the reinforcing and physical dependence producing effects of caffeine have been observed (Griffiths et al. 1986). Furthermore, Griffiths and Mumford (1995) reported that caffeine reinforcement occurred in about 45% of moderate or heavy caffeine drinkers. Thus, whether our finding that caffeine and in particular selective adenosine A2A antagonist SCH58261 exerting positive conditioned pace preference and hence reward property may be extended to human caffeine consumption requires additional studies.
The present findings are consistent with previous reports showing that the motor activity and conditioned place preference in mice is increased in a biphasic fashion following the administration of caffeine (Steigerwald et al. 1988; Brockwell et al. 1991; Svenningsson et al. 1995; Bedingfield et al. 1998; Patkina and Zvartau 1998; El Yacoubi et al. 2000). Furthermore, our results also showed that the selective adenosine A2A receptor antagonist SCH58261 exhibited similar effect. Selective adenosine A1 receptor antagonist, DPCPX, did not alter the behavioral response in the present and other studies (Griebel et al. 1991b). Our study support previous findings in rodents suggesting that A2A but not A1 adenosine receptor was mainly involved in the motor-activating effects of acutely administered caffeine (Griebel et al. 1991b; Svenningsson et al. 1997; El Yacoubi et al. 2000; Lindskog et al. 2002; Halldner et al. 2004). Our results, however, cannot explain the motor activating effect of other A1 receptor antagonists, such as CPT (Snyder et al. 1981; Goldberg et al. 1985; Karcz-Kubicha et al. 2003; Antoniou et al. 2005). However, mice lacking one or both copies of A1 adenosine receptor gene did not produce any significant changes in either basal or caffeine-induced locomotion (Halldner et al. 2004). Although A1 adenosine receptor was not primarily involved in the caffeine-mediated locomotor activation, some studies indicated that A1 receptors can modulate the locomotor responses of A2A receptor (Halldner et al. 2004; Kuzmin et al. 2006). Ferré (2008) has proposed a “striatal spine module (SSM)” as an integrative unit to explain the psychostimulant effects of caffeine and other drugs of abuse. The complex anatomical and functional interactions among the components of SSM, which may further vary among different animal species and strains, may allow activation of A1 or A2A receptors alone or in different degree of combination, leading to the observed involvement of A1 or A2A receptor alone or combination of A1 and A2A receptors following administration of caffeine, selective A1, or A2A antagonists.
Many studies have demonstrated that subchronic intermittent treatment with caffeine induces locomotor sensitization in rats (Cauli et al. 2003; Simola et al. 2006; Tronci et al. 2006). Most of these studies compared the locomotor activity after intermittent treatment with that of the same animals on the first administration. Only Tronci et al. (2006) demonstrated locomotor sensitization effect of caffeine comparing activity in caffeine-pretreated (15 mg/kg) with that of vehicle-pretreated rats. Our results were consistent with those of Tronci et al. (2006) indicating that repeated intraperitoneal administration of caffeine (20 mg/kg i.p.) can induce locomotor sensitization. It should be noted that repeated chronic intraperitoneal injection per se may exert a short-lived chronic stress which might increase glucocorticoids and dopamine levels resulting in sensitization of the reward system. This sensitized state would render the subject more responsive to drugs of abuse and consequently more vulnerable to the development of addiction (Sinha 2001; Marinelli and Piazza 2002). In our study, repeated intraperitoneal caffeine but not saline caused behavioral sensitization suggesting that stress might facilitate sensitization but alone could not cause sensitization. In addition, previous reports also observed a tendency to sensitization of motor behavior in either rats or mice after spaced administration of caffeine (Kuribara 1994; Cauli and Morelli, 2002). In the present study, animals were habituated to the test cage for 2 h before receiving caffeine administration. Because caffeine can induce anxiety (Fredholm et al. 1999) and a novel environment itself can also induce anxiety in rodents (Merali et al. 2003), the combined effects may exacerbate the anxiety of experimental animals which could interfere with the development of sensitization. Therefore, 2 h of habituation period is expected to reduce the anxiogenic effect of caffeine and environment.
Locomotor sensitization, proposed to reflect the increase of the wanting for drug reward, would be the result of increasing the basic responsiveness of dopaminergic neurons to stimuli (Robinson and Berridge 2000). Adenosine A2A receptors colocalized with dopamine D2 receptors in the medium-sized spiny GABAergic neurons are highly and selectively expressed in areas receiving a rich dopamine innervation, i.e., the dorsal and ventral striatum and tuberculum olfactorium (Jarvis and Williams 1989; Schiffmann et al. 1991; Fink et al. 1992). Previous studies have demonstrated the existence of a complex antagonistic relationship between adenosine A2A and dopamine D2 receptors. Activation of A2A receptors results in cAMP formation, whereas activation of dopamine D2 receptors decreases the cAMP formation (Dasgupta et al. 1996; Ferré et al. 1997). Increased responsiveness to dopamine D1 and D2 receptor agonists in rats subchronically treated with caffeine has been observed (Cauli and Morelli 2002). In addition, a decrease in A2A receptor level in the striatum and nucleus accumbens of caffeine-sensitized rats was demonstrated indicating that A2A receptors were involved in the adaptive changes for producing behavioral sensitization (Tronci et al. 2006). Thus, it is reasonable to assume that chronic treatment with selective A2A receptor antagonist, analogous to the chronic treatment with caffeine, can result in behavioral sensitization. Our data showing the sensitization effect of SCH58261 on locomotor activity supported the hypothesis that prolonged blockade of A2A receptors by repeated caffeine treatment or selective A2A receptor antagonist may produce persistent modifications in dopamine and adenosine receptor interaction which, in turn, results in the facilitation of dopamine transmission (Simola et al. 2006).
Our and previous studies (Bedingfield et al. 1998; Patkina and Zvartau 1998) have demonstrated that caffeine can produce conditioned place preference, an experimental design considered to facilitate the expression of the reinforcing effects of a drugs. Our study also demonstrated that selective A2A receptor antagonist SCH58261, like chronic treatment with caffeine, induced conditioned place preference in mice. The present result is consistent with a previous study using another A2A receptor antagonist, KW6002 (Harper et al. 2006).
Systemic administration of caffeine or SCH58261 causes an increase of phosphorylation of DARPP-32 at Thr75 in wild-type mice (Lindskog et al. 2002). The stimulatory effects of caffeine and SCH58261 on locomotor activity were greatly reduced in DARPP-32 knockout mice. DARPP-32, like adenosine A2A receptor, is abundantly found in the striatum. Within the striatum, DARPP-32 is expressed at high levels in both strinatonigral and striatopallidal neurons and is regulated by dopamine, as well as other neurotransmitters, which are though to contribute to the etiology of several common neuropsychiatric disorders, including schizophrenia, bipolar disorder, and attention-deficit/hyperactivity disorder, and to play an important role in the actions of drugs with reinforcing efficacy (Svenningsson et al. 2005). In this connection, reduction of cocaine place preference in mice lacking DARPP-32 has been shown (Zachariou et al. 2002). Several studies have also shown that DARPP-32 modulates the effect of behavioral sensitization to psychostimulants (Nairn et al. 2004).
In summary, our study demonstrated that caffeine and A2A adenosine antagonist SCH58261 induced conditioned place preference and sensitization of locomotor activity and is associated with increased DARPP-32 phosphorylation at Thr75 in the striatum. Blockade of A2A adenosine receptor may play a crucial role in the hyperactivity, behavioral sensitization, and reinforcing behavior induced by caffeine.
References
Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, Panlilio L, Muller CE, Goldberg SR, Ferre S (2005) A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology (Berl) 183:154–162
Bastia E, Xu YH, Scibelli AC, Day YJ, Linden J, Chen JF, Schwarzschild MA (2005) A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology 30:891–900
Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P (2008) Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 11:932–939
Bedingfield JB, King DA, Holloway FA (1998) Cocaine and caffeine: conditioned place preference, locomotor activity, and additivity. Pharmacol Biochem Behav 61:291–296
Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P (2001) Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410:376–380
Brockwell NT, Eikelboom R, Beninger RJ (1991) Caffeine-induced place and taste conditioning: production of dose-dependent preference and aversion. Pharmacol Biochem Behav 38:513–517
Casas M, Prat G, Robledo P, Barbanoj M, Kulisevsky J, Jane F (1999) Repeated co-administration of caffeine and bromocriptine prevents tolerance to the effects of caffeine in the turning behavior animal model. Eur Neuropsychopharmacol 9:515–521
Castane A, Soria G, Ledent C, Maldonado R, Valverde O (2006) Attenuation of nicotine-induced rewarding effects in A2A knockout mice. Neuropharmacology 51:631–640
Cauli O, Morelli M (2002) Subchronic caffeine administration sensitizes rats to the motor-activating effects of dopamine D(1) and D(2) receptor agonists. Psychopharmacology (Berl) 162:246–254
Cauli O, Pinna A, Valentini V, Morelli M (2003) Subchronic caffeine exposure induces sensitization to caffeine and cross-sensitization to amphetamine ipsilateral turning behavior independent from dopamine release. Neuropsychopharmacology 28:1752–1759
Celik E, Uzbay IT, Karakas S (2006) Caffeine and amphetamine produce cross-sensitization to nicotine-induced locomotor activity in mice. Prog Neuropsychopharmacol Biol Psychiatry 30:50–55
Chen PC, Chen JC (2005) Enhanced Cdk5 activity and p35 translocation in the ventral striatum of acute and chronic methamphetamine-treated rats. Neuropsychopharmacology 30:538–549
Chen JF, Moratalla R, Yu L, Martin AB, Xu K, Bastia E, Hackett E, Alberti I, Schwarzschild MA (2003) Inactivation of adenosine A2A receptors selectively attenuates amphetamine-induced behavioral sensitization. Neuropsychopharmacology 28:1086–1095
Dasgupta S, Ferre S, Kull B, Hedlund PB, Finnman UB, Ahlberg S, Arenas E, Fredholm BB, Fuxe K (1996) Adenosine A2A receptors modulate the binding characteristics of dopamine D2 receptors in stably cotransfected fibroblast cells. Eur J Pharmacol 316:325–331
De Luca MA, Bassareo V, Bauer A, Di Chiara G (2007) Caffeine and accumbens shell dopamine. J Neurochem 103:157–163
El Yacoubi M, Ledent C, Menard JF, Parmentier M, Costentin J, Vaugeois JM (2000) The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol 129:1465–1473
Fenu S, Morelli M (1998) Motor stimulant effects of caffeine in 6-hydroxydopamine-lesioned rats are dependent on previous stimulation of dopamine receptors: a different role of D1 and D2 receptors. Eur J Neurosci 10:1878–1884
Fenu S, Cauli O, Morelli M (2000) Cross-sensitization between the motor activating effects of bromocriptine and caffeine: role of adenosine A(2A) receptors. Behav Brain Res 114:97–105
Ferré S (2008) An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem 105:1067–1079
Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K (1997) Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20:482–487
Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM (1992) Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res 14:186–195
Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51:83–133
Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM (2005) Adenosine and brain function. Int Rev Neurobiol 63:191–270
Garrett BE, Holtzman SG (1994) D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats. Pharmacol Biochem Behav 47:89–94
Goldberg SR, Prada JA, Katz JL (1985) Stereoselective behavioral effects of N6-phenylisopropyl-adenosine and antagonism by caffeine. Psychopharmacology (Berl) 87:272–277
Goodman RR, Synder SH (1982) Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci 2:1230–1241
Gould TD, Manji HK (2005) DARPP-32: a molecular switch at the nexus of reward pathway plasticity. Proc Natl Acad Sci U S A 102:253–254
Griebel G, Misslin R, Vogel E (1991a) Behavioural effects of selective A2 adenosine receptor antagonists, CGS 21197 and CGS 22706, in mice. Neuroreport 2:139–140
Griebel G, Saffroy-Spittler M, Misslin R, Remmy D, Vogel E, Bourguignon JJ (1991b) Comparison of the behavioural effects of an adenosine A1/A2-receptor antagonist, CGS 15943A, and an A1-selective antagonist, DPCPX. Psychopharmacology (Berl) 103:541–544
Griffiths RR, Mumford (1995) Caffeine—a drug of abuse? In: Bloom FE, Kupfer DJ (eds) Psychopharmacology: the fourth generation of progress. Raven, New York, pp 1699–1713
Griffiths RR, Bigelow GE, Liebson IA (1986) Human coffee drinking: reinforcing and physical dependence producing effects of caffeine. J Pharmacol Exp Ther 239:416–425
Halldner L, Aden U, Dahlberg V, Johansson B, Ledent C, Fredholm BB (2004) The adenosine A1 receptor contributes to the stimulatory, but not the inhibitory effect of caffeine on locomotion: a study in mice lacking adenosine A1 and/or A2A receptors. Neuropharmacology 46:1008–1017
Harper LK, Beckett SR, Marsden CA, McCreary AC, Alexander SP (2006) Effects of the A 2A adenosine receptor antagonist KW6002 in the nucleus accumbens in vitro and in vivo. Pharmacol Biochem Behav 83:114–121
Jarvis MF, Williams M (1989) Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol 168:243–246
Juliano LM, Griffiths RR (2004) A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology (Berl) 176:1–29
Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Muller CE, Fuxe K, Goldberg SR, Popoli P, Ferre S (2003) Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology 28:1281–1291
Kuribara H (1994) Caffeine enhances the stimulant effect of methamphetamine, but may not affect induction of methamphetamine sensitization of ambulation in mice. Psychopharmacology (Berl) 116:125–129
Kuzmin A, Johansson B, Gimenez L, Ogren SO, Fredholm BB (2006) Combination of adenosine A1 and A2A receptor blocking agents induces caffeine-like locomotor stimulation in mice. Eur Neuropsychopharmacol 16:129–136
Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388:674–678
Lin XH, Hashimoto T, Kitamura N, Murakami N, Shirakawa O, Maeda K (2002) Decreased calcineurin and increased phosphothreonine-DARPP-32 in the striatum of rats behaviorally sensitized to methamphetamine. Synapse 44:181–187
Lindskog M, Svenningsson P, Pozzi L, Kim Y, Fienberg AA, Bibb JA, Fredholm BB, Nairn AC, Greengard P, Fisone G (2002) Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature 418:774–778
Marinelli M, Piazza PV (2002) Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci 16:387–394
Merali Z, Levac C, Anisman H (2003) Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry 54:552–565
Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P (2004) The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology 47(Suppl 1):14–23
Ongini E, Fredholm BB (1996) Pharmacology of adenosine A2A receptors. Trends Pharmacol Sci 17:364–372
Parkinson FE, Fredholm BB (1990) Autoradiographic evidence for G-protein coupled A2-receptors in rat neostriatum using [3H]-CGS 21680 as a ligand. Naunyn Schmiedebergs Arch Pharmacol 342:85–89
Patkina NA, Zvartau EE (1998) Caffeine place conditioning in rats: comparison with cocaine and ethanol. Eur Neuropsychopharmacol 8:287–291
Pontieri FE, Tanda G, Di Chiara G (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 92:12304–12308
Rivkees SA, Price SL, Zhou FC (1995) Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum, and basal ganglia. Brain Res 677:193–203
Robinson TE, Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95(Suppl 2):S91–S117
Scheggi S, Rauggi R, Gambarana C, Tagliamonte A, De Montis MG (2004) Dopamine and cyclic AMP-regulated phosphoprotein-32 phosphorylation pattern in cocaine and morphine-sensitized rats. J Neurochem 90:792–799
Schiffmann SN, Jacobs O, Vanderhaeghen JJ (1991) Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem 57:1062–1067
Simola N, Cauli O, Morelli M (2006) Sensitization to caffeine and cross-sensitization to amphetamine: influence of individual response to caffeine. Behav Brain Res 172:72–79
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359
Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW (1981) Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A 78:3260–3264
Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR (2002) Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci 22:6321–6324
Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O (2006) The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology 31:978–987
Steigerwald ES, Rusiniak KW, Eckel DL, O'Regan MH (1988) Aversive conditioning properties of caffeine in rats. Pharmacol Biochem Behav 31:579–584
Svenningsson P, Nomikos GG, Fredholm BB (1995) Biphasic changes in locomotor behavior and in expression of mRNA for NGFI-A and NGFI-B in rat striatum following acute caffeine administration. J Neurosci 15:7612–7624
Svenningsson P, Nomikos GG, Ongini E, Fredholm BB (1997) Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience 79:753–764
Svenningsson P, Nairn AC, Greengard P (2005) DARPP-32 mediates the actions of multiple drugs of abuse. Aaps J 7:E353–E360
Tronci E, Simola N, Carta AR, De Luca MA, Morelli M (2006) Potentiation of amphetamine-mediated responses in caffeine-sensitized rats involves modifications in A2A receptors and zif-268 mRNAs in striatal neurons. J Neurochem 98:1078–1089
Weiss SM, Benwell K, Cliffe IA, Gillespie RJ, Knight AR, Lerpiniere J, Misra A, Pratt RM, Revell D, Upton R, Dourish CT (2003) Discovery of nonxanthine adenosine A2A receptor antagonists for the treatment of Parkinson’s disease. Neurology 61:101–106
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Wooten GF (2001) Anatomy and function of dopamine receptors: understanding the pathophysiology of fluctuations in Parkinson's disease. Parkinsonism Relat Disord 8:79–83
Zachariou V, Benoit-Marand M, Allen PB, Ingrassia P, Fienberg AA, Gonon F, Greengard P, Picciotto MR (2002) Reduction of cocaine place preference in mice lacking the protein phosphatase 1 inhibitors DARPP 32 or Inhibitor 1. Biol Psychiatry 51:612–620
Acknowledgment
This study was supported partly by grants from National Science Council, Taiwan (NSC952745B-320-002-URD-02) and Tzu Chi University. The authors would like to thank the technical assistance of Ms. K. J. Chen.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hsu, C.W., Chen, C.Y., Wang, CS. et al. Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75-DARPP-32 in mice. Psychopharmacology 204, 313–325 (2009). https://doi.org/10.1007/s00213-009-1461-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1461-3