Abstract
Rationale
Previous studies have implicated platelet amyloid precursor protein (APP) as a candidate biomarker for Alzheimer’s disease (AD). Platelets contain more than 95% of the circulating APP and enclose the enzymatic machinery for the APP metabolism yielding both soluble APP and amyloid-β peptides.
Objectives
The objective of this study is to compare the ratio of 130- to 110-kDa fragments of APP in platelets from patients with AD, mild cognitive impairment (MCI), and elderly controls.
Materials and methods
After subjects were grouped according to diagnosis, APP ratio in platelets was evaluated by means of Western blot analysis.
Results
The APP ratio was significantly lower in AD patients (1.01 ± 0.21) as compared to controls (1.24 ± 0.21, p = 0.001) and MCI patients (1.18 ± 0.21, p = 0.027), but no significant differences were found between MCI and controls (p = 0.904). In addition, we found positive correlations between the APP ratio and 1,6-diphenyl-1,3,5-hexatriene anisotropy (r = 0.3, p = 0.01), as well as with certain parameters of cognitive decline, namely, the mini-mental state examination score (r = 0.33, p = 0.003), the total Cambridge cognitive test (CAMCOG) score (r = 0.37, p = 0.001), and the score on the memory subscale of the CAMCOG (r = 0.38, p = 0.001).
Conclusions
The pattern of platelet APP fragments was altered in patients with AD but not in patients with MCI. The alteration of APP fragments was correlated with membrane fluidity and the cognitive decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most important pathophysiological characteristics of Alzheimer’s disease (AD) are the senile plaques and the neurofibrillary tangles. The major component of plaques is the amyloid-β peptide (Aβ). The Aβ is a highly hydrophobic peptide of 39–43 amino acids, released by the proteolitic cleavage of amyloid precursor protein (APP), and aggregates to form oligomers. These oligomers further aggregate into fibers, which deposit in the cerebral parenchyma, and originate the senile plaques through the amyloid cascade (Haass 2004). The neurotoxic effects of Aβ also involves changes in the neuronal membrane composition and structure, affecting its physicochemical properties.
APP is an integral membrane protein expressed in various cell types. Three isoforms (APP695, APP751, and APP770) are derived from the alternative splicing of same mRNA. It is a transmembrane protein of type I, with a long N-terminal extracellular portion, one single passage through the membrane, and a small intracellular C-terminal portion (Daly et al. 1998). It is one of the most abundant proteins in the central nervous system and is also expressed in peripheral tissues, such as muscular, epithelial, and blood cells (Di Luca et al. 2000; Skovronsky et al. 2001; Racchi and Govoni 2003).
Among the different peripheral cells that express APP, platelets represent an important source, as they contain more than 95% of the circulating APP. Both the secreted (soluble) fragment (sAPP) and the Aβ peptide are stored in the α-granules and released through platelet activation. Accordingly, Davies et al. (1997) demonstrated that platelets release the content of their granules under physiological stimuli secreting fragments of sAPP. Li et al. (1998) proposed that platelets contain all the enzymatic machinery to produce the by-products of the APP breakdown by the activity of the α-secretase (ADAM10) and β-secretase (BACE1). More recently, Tang et al. (2006) found in platelets of AD increased levels of Aβ, increased immunoreactivity of BACE1, and decreased immunoreactivity of ADAM10, indicating that the amyloidogenic pathway of the APP metabolism is activated in platelets of AD patients, paralleling the intracerebral APP processing in AD.
The first attempts to determine APP changes in platelets of AD patients, as a peripheral correlate of the biochemical abnormalities of the disease process, have addressed the ratio between two carboxyl-truncated fragments of sAPP (Di Luca et al. 1996). Western blots with the monoclonal antibody 22C11, which is raised to bind to the N-terminal portion of APP, have shown that the molecular weights of these platelet APP fragments are 120–130 and 110 kDa. Rosenberg et al. (1997) found that the ratio between 120-to-130- and 110-kDa APP was reduced in patients with AD as compared to controls. These findings were in accordance with those by Di Luca et al. (1996, 1998). Both groups have further implicated the reduced APP ratio with other biological and clinical features of the disease, namely, a greater effect in the presence of the apolipoprotein E4 allele (Rosenberg et al. 1997) and positive correlations between APP ratio and cognitive performance both in clinical (Baskin et al. 2000) and preclinical AD (Padovani et al. 2002). These data support the putative role of platelet APP as peripheral marker for AD.
Among other neurotoxic effects, Aβ disturbs several aspects of membrane metabolism. Aβ impacts on cholesterol homeostasis, modifying its distribution within the lipid bilayer and, thus, affecting membrane fluidity (reviewed by Wood et al. 2003). Such abnormality has been estimated by 1,6-diphenyl-1,3,5-hexatriene (DPH) anisotropy (see “Materials and methods” for details), which is inversely correlated with membrane fluidity. A reduction in membrane fluidity has been consistently described in several regions of the AD brain (Müller et al. 1997), including hippocampal membranes (Eckert et al. 2000), and in animal models of AD (Müller et al. 1995). Conversely, anisotropy data in platelets of AD patients have so far proven inconsistent, with reports of increased membrane fluidity in familial (Zubenko et al. 1987a,b, 1999) but not in sporadic AD (Kukull et al. 1992; Fernandes et al. 1999).
The objective of the present study is to determine the proportion of 130- and 110-kDa fragments of APP in platelets from subjects with AD, with mild cognitive impairment (MCI), and healthy elderly individuals and to investigate the association of this abnormality with membrane anisotropy, which has been consistently described in platelet membranes of AD patients as a consequence of abnormal membrane metabolism.
Materials and methods
Characteristics of the subjects
There were 23 patients with DA, 25 subjects with MCI, and 30 age-matched healthy controls enrolled to the study. All patients were recruited at the Memory Clinic of the Laboratory of Neuroscience (LIM 27), Department and Institute of Psychiatry, Faculty of Medicine, University of São Paulo, Brazil. Written informed consent was obtained after the procedures had been fully explained to all patients. The study was approved by the local ethics committee. Exclusion criteria were illiteracy, visual and hearing disabilities, and other relevant health conditions that could either affect cognition or limit the administration of neuropsychological tests. Patients with severe dementia, concomitant psychiatric diseases, and clinical evidence of cerebrovascular disease (as the main cause of cognitive decline) were also excluded.
Mental state examination was performed using the Cambridge examination for mental disorders in the elderly semi-structured interview (Roth et al. 1986), which comprises the following subtests in the Cambridge cognitive test (CAMCOG) schedule, the Blessed dementia scale (Blessed et al. 1968), the ten-item information, memory and concentration test (abbreviated mental test; Roth and Hopkins 1953), the mini-mental state examination (MMSE; Folstein et al. 1975), and the Hachinski ischemic score (Hachinski et al. 1975). The clock drawing test according to Sunderland et al. (1989) and the informant questionnaire on cognitive decline in the elderly (Jorm and Jacomb 1989) were also administered as screening test for cognitive impairment, and the 21-item Hamilton depression scale (Hamilton 1960) was used to evaluate depressive symptoms. Laboratorial exams were carried out for every patient, encompassing thyroid function, complete blood count, blood chemistry serum levels, folic acid and vitamin B12, blood lipid profile, and syphilis tests to rule out potentially reversible causes of cognitive impairment. Neuroimaging studies (computed tomography scans or magnetic resonance imaging) were completed in patients with suspected dementing disorders.
The neuropsychological battery included the Rivermead behavioral memory test (Wilson et al. 1985; Oliveira and Schmidt 1999), the Fuld object-memory evaluation (Fuld 1980), the trail making test A and B (Army Individual Test Battery 1944), the short cognitive test (Erzigkeit 1991; Flaks et al. 2006), and the Wechsler adult intelligence scale—revised vocabulary and block design tests (Wechsler 1981).
Diagnoses of dementia and AD were, respectively, made according to the Diagnostic and Statistical Manual of Mental Disorders—IV (American Psychiatric Association 1994) and the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorder Association criteria (Mc Khann et al. 1984). Diagnosis of MCI was established according to Petersen et al. (1999) criteria.
Demographic and cognitive data of AD, MCI, and control subjects are presented in Table 1. In the AD group, 56.5% had mild or very mild dementia and 45.5% had moderate dementia, most of which (87%) were currently medicated with cholinesterase inhibitors. The MCI group was further subdivided into amnestic MCI (seven individuals, 28%), non-amnestic MCI (seven individuals, 28%), and multiple domain MCI (11 individuals, 44%).
Platelet collection and preparation
Peripheral blood (15 ml) was collected in 0.1 M sodium-citrate-coated tubes (S-Monovett, Sarsted). Blood samples were homogenized with 300 μl of 0.09 M ethylenediamine tetraacetic acid and centrifuged at 200 × g (1,300 rpm) for 10 min and at room temperature. Platelet-rich plasma was separated from blood cells, and platelets were collected by centrifugation at 1,159 × g (2,400 rpm) for 10 min at room temperature. Pellets were washed with 5 ml of 10 mM Tris, pH = 7.4, and resuspended in lysis buffer containing 10 mM Tris–HCl, pH = 7.4, 1 mM ethylne glycol tetraacetic acid, 100 mM phenylmethanesulphonyl fluoride, and protease inhibitors (M222, Amresco). Platelet aliquots were stored at −70°C. Protein concentrations were determined in each sample by a modified Lowry method (Bio-Rad DC Protein Assay) before the Western blot assay.
APP Western blotting
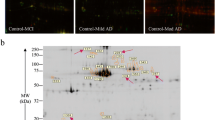
For each sample, 24 μg of protein (prepared in Laemmli sample buffer) was separated by electrophoresis in 8% polyacrylamide gels (120 V, 2 h) and transferred to nitrocellulose membranes (200 mA, 90 min). Unspecific bindings was blocked with nonfat milk, and membranes were subsequently incubated for 2 h with the primary antibody m22C11 (Chemicon International, dilution 1:1,500). Membranes were then incubated for 1 h with goat anti-mouse secondary antibodies labeled with horseradish peroxidase (Sigma, dilution 1:10,000), after which the enhanced chemoluminescence (ECL; GE) reagent was poured. Imaging was performed in a Chemiimager™ 4,000 equipment (Alpha Innotech), which captures the chemiluminescent light emission from the reaction of the peroxidase-conjugated antibody with the ECL reagent (GE). Light emission was continuously captured for 15 min, and the densitometry of distinct bands was performed with specific software tools. All measurements were registered as the proportion of the 130- to 110-kDa band densities (130- to 110-kDa ratio). An internal control (IC) consisting of a standard suspension of platelets (prepared in-house from a pool of platelets from young healthy volunteers) was added to each gel to control for analytical differences between blots performed in different days (inter-assay variation). The IC reading in proportion to the 130- to 110-kDa ratio was then calculated, yielding the normalized estimate of the 130- to 110-kDa ratio (APPr). Each sample was analyzed in triplicates, and additional blots of the same samples were performed whenever the reading between replicates displayed a variation >15%.
Membrane fluidity measurements
For anisotropy measurements, platelet isolation and platelet membrane homogenate preparation were performed according to a minor modification of the method of Zubenko et al. (1987a). Membrane fluidity of the individual platelet homogenates was determined using DPH as a fluorescence probe incorporated in the inner lipid bilayer. Samples were diluted with 5 mM Tris–HCl buffer, pH 7.4, to attain approximately 300 μg protein/ml. Anisotropy measurements were then performed in 5× replicate aliquots comprising 100 μl of working membrane samples incubated with 900 μl of Tris–HCl buffer and 1,000 μl of 33 μM DPH solution (prepared from a stock solution of 5 mM DPH in tetrahydrofuran) for 60 min at 37°C. The steady-state anisotropy was measured in a PTI spectrofluorometer (Photon Technology International, Monmouth Junction, NJ, USA), using excitation and emission wavelengths of 360 and 450 nm, respectively. The maximum and minimum readings for each sample were systematically discarded, so that the statistical analysis was based on triplicates of each sample.
Statistical analysis
Data analysis was performed with the aid of the Statistical Package for the Social Sciences (SPSS 13.0 for Windows). Analysis of variance with Bonferroni correction for post hoc multiple comparisons was used to compare group means. Parametric correlations (Pearson correlations coefficients) were obtained for normal/small subsample data.
Results
The mean APPr was significantly reduced in the AD group as compared to controls (AD, 1.01 ± 0.21; controls, 1.24 ± 0.21, p = 0.001). In the MCI group, the mean APPr was 1.18 ± 0.21, with no significant difference as compared to controls (p = 0.904), but significantly reduced as compared to AD patients (p = 0.027; Table 2; Fig. 1). The mean DPH anisotropy values in the three diagnostic groups are presented in Table 2, but no significant differences were observed between the three groups. On the other hand, DPH anisotropy values were significantly correlated with 130- to 110-kDa APP ratio (r = 0.30, p = 0.01). Figure 2 illustrates the 130- and 110-kDa bands in a representative blot.
As the mean values of the APPr in each group were distributed in a normal curve, parametric tests were performed to assess the correlations between mean APPr values and the respective cognitive scores for each diagnostic group. We found positive correlations between APPr and the MMSE score (r = 0.33, p = 0.003), APPr and total CAMCOG (r = 0.37, p = 0.001), and APPr and the score on the memory of the CAMCOG (r = 0.38, p = 0.001; Fig. 3).
Pearson’s correlations between APP ratio and the raw numeric values of three cognitive measurements, irrespective of diagnostic classification. The ratio between 130- and 110-kDa APP fragments in platelets was positively correlated with total MMSE scores (r = 0.33, p = 0.003), total CAMCOG scores (r = 0.37, p = 0.001), and the memory sub-score of the CAMCOG schedule (r = 0.38, p = 0.001)
Discussion
Platelets contain more than 95% of the total circulating APP, 10% of which corresponds to the full-length protein. The remaining 90% of platelet APP corresponds to carboxyl-truncated secreted (soluble) APP residues (APPs) of 751 or 770 amino acids, which bear the Kunitz proteinase inhibitor domain, and are stored in the α-granules. These granules also contain amounts of Aβ. The simultaneous presence of both APPs and Aβ suggests that platelets contain the enzymatic machinery necessary for APP processing (Li et al. 1998). Thus, the abnormal regulation of APP processing, which leads to the accumulation of Aβ in the AD brain, may be also represented in platelets. Should this hypothesis prove correct, then the determination of APP metabolites in platelets might yield an insight into this seminal process in the pathophysiology of AD, reinforcing its role in the search of peripheral markers of the disease. The measurement of 130- to 110-kDa APP ratio in platelets fulfills some critical requirements for the selection of a candidate biomarker of AD, as platelets can be easily obtained in samples of peripheral blood, albeit requiring careful manipulation and processing.
Our results are in agreement with the previous finding of Di Luca et al. (1998) and Rosenberg et al. (1997). With regard to the ratio between 130- to 110-kDa APP in platelets (APPr), we found significant differences between AD patients and elderly controls (p = 0.001) and, to a lesser extent, between AD and MCI (p = 0.027). However, no significant differences were detected between controls and MCI (p = 0.904), although the latter group displayed intermediate values of APPr as compared to AD patients and controls. We further addressed the correlations between three cognitive variables (MMSE, total CAMCOG score, and memory subscore of the CAMCOG) and the APP ratios of patients (AD and MCI) and controls irrespective of their diagnostic categories. The same procedure was repeated for each diagnostic group separately, but no significant correlations were detected (data not shown). We understand that this may be due to the small intragroup variance of both measurements (i.e., APP ratios and cognitive scores) in addition to the reduced statistical power as a consequence of the numeric limitation of the subsamples. Thus, we argue that, in addition to the significant intergroup differences of mean APP ratio values (AD vs controls and AD vs MCI), this abnormality of APP metabolism is also positively correlated with the cognitive performance itself.
Other studies of platelet APP in patients with MCI and different stages of AD have shown statistically significant differences between controls and cognitively impaired subjects (MCI included), with a trend of decline of the mean APPr values along the progression of the disease. Nevertheless, no significant differences were found along the earlier stages of cognitive decline, i.e., between MCI and very mild/mild AD (Padovani et al. 2002). In the study by Di Luca et al. (1998), although platelet APPr was significantly decreased in severe AD as compared to mild AD, only marginally significant differences were detected between mild and moderate forms of the disease. It must be argued that MCI has not yet been established as a distinct clinical entity, given its biological and neuropsychological heterogeneity, which support important prognostic implications. In our sample, the MCI group was composed by amnestic, non-amnestic, and multiple-domain MCI subtypes. The association between MCI and AD is better defined for single- and multiple-domain amnestic types (Petersen 2005). With regard to the scores on cognitive screening tests, but not in the neuropsychological battery used for the diagnosis of MCI (data not shown), the performance of the MCI group was quite similar to the control group. Such result is expected, as the individuals with MCI generally perform well in the evaluation by MMSE and the CAMCOG, due to ceiling effects. Hence, these tests have low discriminatory power, rendering a careful neurophysiologic evaluation necessary. As the APPr values apparently depend on the stage of the underlying pathology in AD, one possible speculation is that our MCI group comprised cases of more subtle cognitive decline (given the mean total CAMCOG scores of 92.7), therefore, with putative, a longer time lag for the conversions to AD. A follow-up study of this MCI cohort is in course in our group, and the prospective evaluation of APPr values will help determine its prognostic value.
Although the numeric magnitude of the 130- to 110-kDa platelet APP ratio widely varied across the studies from Rosenberg et al. (1997), Di Luca et al. (1998), and our study, which results from methodological differences, the same tendencies were detected toward a reduction of the 130- to 110-kDa platelets APP ratio in AD as compared to controls. The analytical differences are probably due to preparation of platelet samples, determination of total protein levels, Western blotting, and radiographic development. Each of these steps yields variations (intra- and inter-testing) and, for this reason, the determination must have an IC (standard sample). In other words, the method is not quantitative.
The monoclonal antibody 22C11 recognizes the N-terminal portion of APP at the 66 and 81 amino acid residues. As this region corresponds to the extracellular portion of APP, it has been suggested that the antibody detects soluble APP (isoforms 770 and 751). The precise meaning of the reduction in platelet APP ratio in AD is not yet clear. If the amount of platelet APP corresponds to the soluble APP generated by the ADAM10 (non-amyloidogenic pathway) and this amount is diminished in AD, it could be hypothesized that the secretory, non-amyloidogenic pathway is reduced in platelets of AD patients, maybe due to a decrement in ADAM10 activity, an increase in BACE1 activity, or both. This speculation is in line with the recent findings of Tang et al. (2006), who determined by Western blot the simultaneous expression of APP specimens, Aβ peptide, BACE1, and ADAM10. Should these hypotheses prove correct, platelet levels of APP would reflect in a peripheral compartment the abnormalities of intracerebral APP metabolism that occur in AD patients.
Our anisotropy data further suggest that abnormal APP processing is associated with membrane fluidity abnormalities. However, this issue requires cautious interpretation. Although there are consistent reports of reduced membrane fluidity within the AD brain, as well as in laboratory models of membrane function after treatment with Aβ specimens, the DPH anisotropy of platelet membranes, as a putative peripheral correlate of such abnormality, is highly controversial. Zubenko et al. (1987a,b, 1999) have demonstrated a decrease in platelet DPH anisotropy (suggestive of increased membrane fluidity) in platelets in familial AD. Nevertheless, Kukull et al. (1992) and Fernandes et al. (1999) failed to demonstrate such differences in platelets of sporadic AD as compared to controls. This discrepancy in platelet anisotropy as compared to neuronal membranes may be attributed to the short life span of platelets, rendering the membranes less vulnerable to long-term modifications.
Longitudinal, neuropathologic, and biological marker studies suggest that diagnosis of MCI is associated with greater risks to the development of dementia. The current most consistent works to establish biological markers for AD or MCI are performed in cerebrospinal fluid (Hansson et al. 2006; Hampel et al. 2004; Maruyama et al. 2001), in which total and phosphorylated protein tau and Aβ42 peptide are measured by different analytical methods. However, this measurement requires a lumbar puncture, which is a more invasive method and not yet approved in many countries for this purpose. Therefore, the identification of an alternative or adjunctive peripheral marker, easily obtainable as in blood samples, would consist in valuable information for the diagnostic workout of AD in the clinical practice.
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington
Army Individual Test Battery (1944) Manual of directions and scoring war department, Adjunt General’s Office Trail, Washington
Baskin F, Rosenberg RN, Iyer L, Hynan L, Cullum CM (2000) Platelet APP isoform ratios correlated with declining cognition in AD. Neurology 54:1907–1909
Blessed G, Tomlinson BE, Roth M (1968) The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 114:797–811
Daly IVJ, Lahiri DK, Justus DE, Kotwal GJ (1998) Detection of the membrane-retained carboxy-terminal tail containing polypeptides of the amyloid precursor protein in tissue from Alzheimer’s disease brain. Life Sci 63:2121–2131
Davies TA, Long HJ, Sgro K, Rathbun WH, Mcmenamin ME, Seetoo K, Tibbles H, Billingslea AM, Fine RE, Fishman JB, Levesque CA, Smith SJ, Wells JM, Simons ER (1997) Activated Alzheimer disease platelets retain more beta amyloid precursor protein. Neurobiol Aging 18:147–153
Di Luca M, Pastorino L, Cattabeni F, Zanardi R, Scarone S, Racagni G, Smeraldi E, Perez J (1996) Abnormal pattern of platelet APP isoforms in Alzheimer disease and Down syndrome. Arch Neurol 53:1162–1166
Di Luca M, Pastorino L, Bianchetti A, Perez J, Viagnolo LA, Lenzi GL, Trabucchi M, Cattebeni F, Padovani A (1998) Differential level of platelet amyloid β precursor protein isoforms—an early marker for Alzheimer disease. Arch Neurol 55:1195–1200
Di Luca M, Colciaghi F, Pastorino L, Borroni B, Padovani A, Cattabeni F (2000) Platelets as a peripheral district were to study pathogenetic mechanisms of Alzheimer disease: the case of amyloid precursor protein. Eur J Pharmacol 405:277–283
Eckert GP, Cairns NJ, Maras A, Gattaz WF, Muller WE (2000) Cholesterol modulates the membrane-disordering effects of beta-amyloid peptides in the hippocampus: specific changes in Alzheimer’s disease. Dement Geriatr Cogn Disord 11(4):181–186
Erzigkeit H (1991) The development of the SKT project. In: Hindmarch I, Hippius H, Wilcock GK (eds) Dementia: molecules, methods and measures. Wiley, Chichester, England, pp 101–108
Fernandes MAS, Proença MT, Nogueira AJA, Oliveira LMV, Santiago B, Santana I, Oliveira CR (1999) Effects of apolipoprotein E genotype on blood lipid composition and membrane platelet fluidity in Alzheimer’s disease. Biochim Biophys Acta 1454:89–96
Flaks MK, Yassuda MS, Regina ACB, Cid AG, Camargo CHP, Gattaz WF, Forlenza OV (2006) The short test (SKT)—a transcultural test for early detection and discrimination of dementia: a preliminary study in Brazil. Int Psychogeriatr 18:121–133
Folstein MF, Folstein SE, Mchugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Fuld P (1980) Guaranteed stimulus processing in the evaluation of memory and learning. Cortex 16:255–271
Haass C (2004) Take five-Bace and the γ-secretase quartet conduct Alzheimer’s amyloid β-peptide generation. EMBO J 23:483–488
Hachinski VC, Iliff ID, Zihkla E, Du Boulay GH, McAllister VL, Marshall J, Russel RW, Symon L (1975) Cerebral blood flow in dementia. Arch Neurol 32:632–637
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hampel H, Teipel SJ, Fuchsberger T, Andreasen N, Wiltfang J, Otto M, Shen Y, Dodel R, Du Y, Farlow M, Möller H-J, Blennow K, Buerger K (2004) Value of CSF β-amyloid1–42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol Psychiatry 9:705–710
Hansson O, Zetterberg H, Buchhave P, Londos L, Blennow K, Minthon L (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5:228–234
Jorm AF, Jacomb PA (1989) The informant questionnaire on cognitive decline in the elderly: sociodemographics correlates, reliability, validity and some norms. Psychol Med 19:1015–1022
Kukull WA, Hinds TR, Schellenberg GD, Belle Gv, Larson EB (1992) Increased platelet membrane fluidity as a diagnostic marker for Alzheimer’s disease. Neurology 42:607–614
Li QX, Whyte S, Tanner JE, Evin G, Beyreuther K, Masters CL (1998) Secretion of Alzheimer’s disease Aβ amyloid peptide by activated human platelets. Lab Invest 78:461–469
Maruyama M, Arai H, Sugita M, Tanji H, Higuchi M, Okamura N, Matsui T, Higuchi S, Matsushita S, Yoshida H, Sasaki H (2001) Cerebrospinal fluid amyloid β1–42 levels in the mild cognitive impairment stage of Alzheimer’s disease. Exp Neurol 172:433–439
Mc Khann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer disease: report of NINCDS–ADRDA work group under the auspices of Department of Health and Human Service Task Force on Alzheimer disease. Neurology 34:939–944
Müller WE, Kock S, Eckert A, Hartmann H, Scheuer K (1995) β-amyloid peptide decreases membrane fluidity. Brain Res 674:133–136
Müller WE, Koch S, Scheur K, Rostock A, Bartsch R (1997) Effects of piracetam on membrane fluidity in the aged mouse, rat, and human brain. Biochem Pharmacol 53(2):135–140
Oliveira R, Schmidt SL (1999) Teste Comportamental de Memória de Rivermead. Cognição, Rio de Janeiro
Padovani A, Borroni B, Colciaghi F, Pettenati C, Cottini E, Agosti C, Lenzi GL, Caltagirone C, Trabucchi M, Cattabeni F, Di Luca M (2002) Abnormalities in the pattern of platelet amyloid precursor protein forms in patients with mild cognitive impairment and Alzheimer disease. Arch Neurol 59:71–75
Petersen RC (2005) Mild cognitive impairment: useful or not? Alzheimer’s Dement 1:5–10
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308
Racchi M, Govoni S (2003) The pharmacology of amyloid precursor protein processing. Exp Gerontol 38:145–157
Rosenberg RN, Baskin F, Fosmire JA, Risser R, Adams P, Svetlik D, Honig LS, Cullun MC, Weiner MF (1997) Altered amyloid protein processing in platelets of patients with Alzheimer disease. Arch Neurol 54:139–144
Roth M, Hopkins B (1953) Psychological test performance in patients over 60. I. Senile psychosis and affective disorders of old age. J Ment Sci 99:439–450
Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goddard R (1986) CAMDEX: a standardized instrument for the diagnosis of mental disorders in the elderly with special reference to early detection of dementia. Br J Psychiatry 149:698–709
Skovronsky DM, Lee VMY, Praticò D (2001) Amyloid precursor protein and amyloid β peptide in human platelet. J Biol Chem 276:17036–17043
Sunderland T, Hill Jl, Mellow AM, Lawlor BA, Gundersheimer J, Newhouse PA, Grafman JH (1989) Clock drawing in Alzheimer’s disease. A novel measure of disease severity. J Am Geriatr Soc 37:725–729
Tang K, Hynan LS, Baskin F, Rosenberg RN (2006) Platelet amyloid precursor protein processing: a bio-marker for Alzheimer’s disease. J Neurol Sci 240:53–58
Wechsler DI (1981) Examiner’s manual: Wechsler adult intelligence scale-revised. Psychological Corporation, New York
Wilson B, Cockburn J, Baddeley AD (1985) Rivermead behavioural memory test. Thames Valley, Suffolk
Wood WG, Eckert GP, Igbavboa U, Müller WE (2003) Amyloid beta-protein interactions with membranes and cholesterol: causes or casualties of Alzheimer’s disease. Biochimica et Biophysica Acta 1610(2):281–290
Zubenko GS, Malinakova I, Chojnacki B (1987a) Proliferation of internal membranes in platelets from patients with Alzheimer’s disease. J Neuropathol Exp Neurol 46:407–418
Zubenko GS, Wusylko M, Cohen BM, Boller F, Teply I (1987b) Family study of platelet membrane fluidity in Alzheimer disease. Science 238:539–542
Zubenko GS, Kopp U, Seto T, Firestone LL (1999) Platelet membrane fluidity individuals at risk for Alzheimer’s disease: a comparison of results from fluorescence spectroscopy and electron spin resonance spectroscopy. Psychopharmacology 145:175–180
Acknowledgments
The present work was supported by FAPESP, Fundação de Amparo à Pesquisa do Estado de São Paulo (Project 02/13633-7). The authors are grateful to Dr. Breno S. O. Diniz and Carolina A. Torres. The Laboratory of Neurosciences receives financial support from Associação Beneficente Alzira Denise Hertzog da Silva.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zainaghi, I.A., Forlenza, O.V. & Gattaz, W.F. Abnormal APP processing in platelets of patients with Alzheimer’s disease: correlations with membrane fluidity and cognitive decline. Psychopharmacology 192, 547–553 (2007). https://doi.org/10.1007/s00213-007-0748-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0748-5