Abstract
Rationale
Increased activity of the hypothalamic–pituitary–adrenal (HPA) axis is an important aspect of the pathophysiology of major depression and schizophrenia. Despite the usefulness of atypical antipsychotics in the treatment of depression and their positive influence on cognitive functioning possibly related to their impact on cortisol, little is known about their effect on HPA axis function.
Objective
Therefore, this double-blind, placebo-controlled, randomized cross-over study investigated the influence of the atypical antipsychotics quetiapine and olanzapine in comparison with haloperidol and placebo on plasma adrenocorticotropic hormone (ACTH), cortisol, and prolactin levels. Eleven healthy male volunteers were studied during four sessions one week apart, orally receiving placebo, quetiapine (50 mg), olanzapine (5 mg), or haloperidol (3 mg). Blood samples were taken at hourly intervals from 0900 until 1700 hours. For ACTH, cortisol, and prolactin a significant effect of treatment condition (p≤0.005; p≤0.035; p≤0.0001, respectively) for area under the curve (AUC) was found. In comparison to placebo, quetiapine and olanzapine significantly reduced ACTH (p≤0.002; p≤0.05, respectively) and cortisol (p≤0.005; p≤0.03, respectively). No effect of haloperidol on AUC of ACTH or cortisol levels was observed. In comparison with placebo, haloperidol (p≤0.0001) and olanzapine (p≤0.0001) elevated AUC of prolactin plasma levels, whereas no significant effect was observed for quetiapine as a main effect of treatment condition. The atypical antipsychotics’ strong influence on HPA-function with pronounced ACTH and cortisol lowering is possibly related to the atypicals’ blockade of serotonergic receptors, but blockade of adrenergic or histaminergic receptors may play a role as well. The observed HPA-axis down-regulation may be clinically important for the atypicals’ effects on depressive symptomatology and cognitive functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atypical antipsychotics like quetiapine and olanzapine have been introduced for the treatment of schizophrenia several years ago. Beyond their efficacy in the treatment of positive symptoms such as hallucinations and delusions, they have proved to be a valuable tool in the treatment of negative symptoms. In many of the comparison studies, they showed efficacy in treating negative symptoms superior to that of typical antipsychotics Möller (2003). Additionally, in comparison with classical antipsychotics such as haloperidol drugs like olanzapine and quetiapine, superiority for the treatment of cognitive deficits (Bilder et al. 2002) (Purdon et al. 2000; Velligan et al. 2002) were demonstrated. In addition to successful treatment of psychosis, the atypical antipsychotics are also used as an adjunctive treatment for disorders like post-traumatic stress disorder, mania, obsessive–compulsive disorder, insomnia, and depression (Adityanjee and Schulz 2002). Recently, monotherapy with the atypical antipsychotics olanzapine (Tohen et al. 2003) and quetiapine (Calabrese et al. 2004) has been demonstrated to have antidepressive effects, whereas, there is some indication that typical neuroleptics may induce dysphoria and depression (Harrow et al. 1994).
A wide range of studies investigating the link between hypothalamic–pituitary–adrenal (HPA)-axis dysfunction and psychiatric disorders has demonstrated pathological findings for a variety of disorders including schizophrenia and depression. Most consistently, an overactivity of the HPA axis was found in patients suffering from major depression. Not only have the elevated cortisol levels been linked to the general symptomatology of depression, but they have also been specifically hypothesized to play a role in cognitive deficits in depression (Brown et al. 2004) and negative symptomatology in schizophrenia (Shirayama et al. 2002; Walder et al. 2000).
The differential activity of typical vs atypical antipsychotic compounds on the HPA-axis dysregulation in schizophrenia was hypothesized to contribute to the better clinical outcome achieved in patients treated with atypical neuroleptics compared to those treated with conventional neuroleptics (Altamura et al. 1999). Supporting evidence comes from studies demonstrating that treatment with the atypical antipsychotics olanzapine or clozapine causes a reduction in cortisol levels, usually in association with an improvement in psychopathology (Meltzer 1989; Hatzimanolis et al. 1998; Markianos et al. 1999; Scheepers et al. 2001), although, not all studies have confirmed these findings (Breier et al. 1994). Thus, it remains to be clarified whether other atypical antipsychotics reduce HPA-axis activity, and whether this decrease in cortisol and, possibly, ACTH levels, is independent of changes in psychopathology, and whether it is caused directly by the drugs.
Some studies, therefore, have investigated the effects of antipsychotics on cortisol excretion in healthy subjects. The majority of the studies investigating typical antipsychotics like haloperidol, pimozide, or sulpiride reported unaffected cortisol levels (Jezova Repcekova et al. 1979; Laakmann et al. 1984; Baptista et al. 1997a,b; de Koning and de Vries 1995; von Bahr et al. 1991; Nurnberger et al. 1984; Barbieri et al. 1984; Collu et al. 1975). However, one group found increased cortisol levels after low doses of haloperidol (Murburg et al. 1986, 1993), whereas, in another study, haloperidol antagonized the cortisol increase caused by experimental heat stress (Hennig et al. 1995).
Only little information is available on the influence of atypical antipsychotics on the HPA axis in healthy volunteers. Recently, we were able to demonstrate reduced urinary excretion of cortisol after low doses of quetiapine in healthy volunteers (Cohrs et al. 2004), whereas, the only other atypical antipsychotic investigated in healthy subjects, amisulpride, demonstrated a lack of such a decrease in cortisol (Wetzel et al. 1994). Thus, it remains unclear to what extent olanzapine reduces cortisol excretion in healthy subjects independent of psychopathology, whether the reduced urinary excretion of cortisol observed under quetiapine is due to a down-regulation of the HPA-axis activity including reduced ACTH levels, and whether the typical antipsychotic haloperidol has any effect on the HPA-axis.
Furthermore, treatment with antipsychotic drugs is frequently associated with hyperprolactinemia (Meltzer and Fang 1976; Gruen et al. 1978). Hormonal side-effects associated with hyperprolactinemia are menstrual disturbances, galactorrhoea and impotence (Ghadirian et al. 1982; Weizman et al. 1985; Wieck and Haddad 2003). Haloperidol consistently elevates prolactin levels in patients (Langer et al. 1977) and healthy subjects (Rubin et al. 1976). Conversely, olanzapine may reduce prolactin levels after switching from conventional antipsychotics (Volavka et al. 2004), and its application is associated with less elevation of serum prolactin than haloperidol in schizophrenic patients (Nemeroff 1997). Prolactin levels evaluated in schizophrenic patients treated with quetiapine were comparable to values found under placebo (Wetzel et al. 1995; Borison et al. 1996; Hamner et al. 1996). However, determination of prolactin shortly after application of the drug has been reported to be associated with some increase as well (Alexiadis et al. 2002). To the best of our knowledge, no data comparing the effects of olanzapine and quetiapine, in comparison with haloperidol and placebo, on prolactin secretion in healthy subjects exist.
Therefore, the aim of this study was to determine the influence of comparable doses of the atypical antipsychotics quetiapine and olanzapine in comparison with the classical antipsychotic haloperidol and placebo on plasma ACTH, cortisol, and prolactin levels.
Subjects and methods
Subjects
A total of 12 healthy male subjects (mean age 27.9±4.1 years, range 23–34 years) were included in the study after recording clinical history, physical examination, electrocardiogram (ECG), and routine laboratory examinations (creatinine, urea, liver enzymes, blood cell count, electrolytes, and urine drug screen). Inclusion criteria were: ages 18–65 years and absence of clinically relevant health problems. Exclusion criteria were one of the following disorders: psychiatric disorders including insomnia, major depression, bipolar disorder, schizophrenia, delusions, epilepsy, obsessive–compulsive disorder, social phobia, alcohol-, nicotine- or drug dependence, intolerance to olanzapine, haloperidol, or quetiapine, cardiovascular disease (myocardial infarction, heart insufficiency, ECG-conduction abnormalities), concomitant psychotropic medication, or intake of antipsychotics within the preceding 3 months, serious medical problems requiring treatment, cerebrovascular disease, liver disease, glaucoma, and any condition predisposing to arterial hypotension, e.g., dehydration, hypovolemia, or antihypertensive medication.
Study design
This was a randomized, double-blind, cross-over, placebo-controlled, single-center study. Screening of volunteers preceded randomization and start of the study by a maximum of 14 days. Each subject was studied for a total of 4 days, each separated by a 6-day wash-out period. Order of administration of the drugs was balanced and randomization was undertaken by the hospital’s pharmacy following a pattern so that an equal number of subjects was treated with the four different drugs (olanzapine, quetiapine, haloperidol, placebo) each study day. To achieve blindness medication was filled into identical capsules.
Without having consumed breakfast subjects presented to the laboratory at 0800 hours for insertion of an antecubital vein. They rested in bed during most of the experiment and were only allowed to walk to the restroom. At 0900 hours blood samples were collected for baseline values. Immediately after this procedure, medication (placebo, quetiapine 50 mg, olanzapine 5 mg, or haloperidol 3 mg) was administered orally. Hourly blood samples were taken until 1700 hours. Urine produced from 0900 until 1700 hours was collected in a container. At 1300 hours a light meal was offered, and the volunteers consumed the first food during this day.
The study followed the declaration of Helsinki and was approved by the local ethics committee. Subjects gave written informed consent and were paid an honorarium of Euro 300.
Specimen collection and hormonal analysis
Plasma was separated immediately by centrifugation, frozen at −35°C and stored for the assay of hormone concentration. Plasma concentration of cortisol were determined by radioimmunoassay. Antibodies were purchased from Biogenesis Ltd (Poole BH17 7DA England, UK) and NET-396 Hydrocortisone from Perkin-Elmer Life Sciences, Boston, MA, USA.
ACTH and prolactin were measured using commercially available chemiluminescence immunoassays (Nichols Advantage ACTH assay, Nichols Institute Diagnostics, San Clemente, CA, USA; Roche Diagnostics GmbH, Mannheim, Germany, respectively).
Urine
Subjects were provided with a urine container. For conservation of urine samples, 0.5 g EDTA and 0.5 g of sodium metabisulfite were added to the urine container. Before the start of the collection period (0900 hours), the subjects urinated. During the following 8 h, all the urine produced was collected in the container. The volume of the total amount of urine produced in the 8-h period was measured and an aliquot of 10 ml was frozen at −35°C. Urinary cortisol was measured by radioimmunoassay after steroid extraction in dichlormethane. Total amount of cortisol excretion was calculated for each subject as the product of urine volume and hormone concentration.
Side effects
For determination of general well-being and side effects, the von Zerssen Symptom List (Beschwerdeliste: B-L Zerssen) (Zerssen and Koeller 1976) was applied. This scale is a self-report questionnaire consisting of 24 items including aspects such as restlessness, sleepiness, nausea, irritability, pain, etc. The questionnaire was filled out at 0900, 1200, and 1700 hours to evaluate the time course of possible changes due to medication.
Statistical analysis
Analyses of variance (ANOVA) with repeated measures on two factors (time after drug administration: before and after 1300 hours; treatment condition: placebo vs the different antipsychotic drugs) were used to evaluate the effect of medication on area under the curve (AUC) of plasma ACTH, cortisol, and prolactin levels. Degrees of freedom were adjusted by Mauchly’s W test followed by a Greenhouse Geiser correction of p values. Additionally, ANOVA was calculated for total amount of urinary cortisol excretion. If the F values were significant, post-hoc tests were performed in order to compare the effects of time or treatment on hormone levels. For comparison of the results of the von Zerssen Symptom List, the non-parametric Friedman repeated measures analysis of variance on ranks was chosen. If significant results were obtained, post-hoc Wilcoxon tests were calculated. For all calculations, the level of significance was set at p<0.05. The statistical package STATISTICA 6.1 was used for analysis.
Results
Adverse effects
In general, medication was well-tolerated. One subject withdrew from the study because of personal reasons and restlessness after the first session which turned out to have been the haloperidol condition. Therefore, further analysis is based on 11 subjects. At 900 hours, no significant difference was found between the conditions for the von Zerssen Symptom List scores. At 1200 hours and 1700 hours, conditions significantly differed (both at p<0.01) for self-reported side effects. In comparison with placebo, quetiapine demonstrated significantly higher scores at 1200 hours (p<0.01) and at 1700 hours (p<0.02), which was mainly due to an increase in sleepiness and lassitude, whereas, no significant difference was observed between placebo and olanzapine or haloperidol. Most of the increase in the sum-score was related to an increase in tiredness, sleepiness, and weakness.
Hormone levels
No differences between the treatment conditions were found for baseline values of plasma cortisol, ACTH, and prolactin levels.
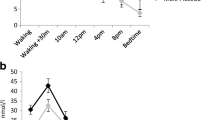
Treatment condition (placebo, quetiapine, olanzapine, and haloperidol) (F=6.19, df=2.5, 24.7; p≤0.005) produced a significant effect on AUC of ACTH plasma levels (Fig. 1 and Table 1). In comparison to placebo, quetiapine (p≤0.002) and olanzapine (p≤0.05) reduced overall AUC of ACTH plasma levels, whereas, no significant effect was observed for haloperidol. No effect of time after application of the drug (before and after 1300 hours) (F=0.03, df=1, 10; p>0.86) was found, and there was only a non-significant trend towards an interaction of treatment condition × time after application (F=3.3, df=1.8, 18.2; p>0.06).
Hourly ACTH, cortisol, and prolactin plasma levels after oral intake of placebo, quetiapine (50 mg), olanzapine (5 mg), or haloperidol (3 mg). In comparison with placebo, area under the curve (AUC) for ACTH was reduced by quetiapine (p≤0.002) and olanzapine (p≤0.05); for cortisol, AUC was reduced by quetiapine (p≤0.005) and olanzapine (p≤0.03) and AUC for prolactin was elevated by haloperidol (p≤0.0001) and olanzapine (p≤0.0001)
ANOVA demonstrated a significant (F=3.72, df=3,30; p≤0.035) effect of treatment condition on AUC of cortisol plasma levels (Fig. 1 and Table 1). In comparison to placebo, quetiapine (p≤0.005) and olanzapine (p≤0.03) reduced AUC of cortisol plasma levels, whereas, no significant effect was observed for haloperidol. Furthermore, time after application of the drug had a significant F=5.6, df=1,10; p≤0.039) influence on AUC of cortisol levels demonstrating a reduction after 1300 hours in comparison to before 1300 hours. No significant interaction of treatment condition × time after application was observed (F=0.21, df=3, 30; p>0.85) for AUC of cortisol plasma levels.
Treatment condition significantly (F=30.9, df=1.4, 13.6; p≤0.0001) influenced AUC of prolactin levels (Fig. 1 and Table 1). In comparison with placebo, haloperidol (p≤0.0001) and olanzapine (p≤0.0001) elevated prolactin, whereas, no significant effect was observed for quetiapine as a main effect of treatment condition. Additionally, post-hoc analysis revealed significantly higher prolactin under haloperidol in comparison with olanzapine (p≤0.05) and quetiapine (p≤0.0001). Furthermore, time after application of the drug had a significant (F=35.7, df=1, 10; p≤0.0005) influence demonstrating an elevation of prolactin levels after 1300 hours. Thirdly, a significant interaction of treatment condition × time after application was observed (F=26.3, df=1.5,15.2; p≤0.0001) for AUC of prolactin plasma levels. Post-hoc analysis demonstrated a significant (p≤0.0001, p≤0.0001, respectively) increase of prolactin after 1300 hours, in comparison with the preceding period for olanzapine and haloperidol, but not for placebo or quetiapine. Additionally, in comparison with placebo, prolactin was elevated already before 1300 hours, under the treatment with haloperidol and olanzapine (p≤0.01, p≤0.05, respectively).
ANOVA of total amount of urinary cortisol excretion showed a strong trend towards differences for treatment condition (F=3.62, df=3, 30; p≤0.055), with significantly lower values under the treatment with quetiapine (p≤0.01) and olanzapine (p≤0.02), in comparison with placebo, and no effect of haloperidol.
Discussion
Both atypical antipsychotics, quetiapine and olanzapine, significantly reduced plasma ACTH and cortisol levels and urinary excretion of cortisol in comparison with placebo, whereas haloperidol lacked those effects on HPA-axis hormones.
These results are in accordance with our findings of an earlier study on the urinary excretion of cortisol during nighttime, after the application of quetiapine 25 and 100 mg (Cohrs et al. 2004), demonstrating that this effect is independent of time of the day. In this study, we were able to demonstrate that a reduction of plasma cortisol levels after the application of quetiapine is due to a reduction in ACTH levels. To the best of our knowledge, no data exist on the influence of olanzapine on cortisol or ACTH in healthy subjects. However, the reduction in ACTH and cortisol found in this study under olanzapine are in line with reduced baseline ACTH and cortisol levels after 6 weeks of treatment with olanzapine in schizophrenic patients (Scheepers et al. 2001), and a comparable reduction of cortisol after the treatment with clozapine (Hatzimanolis et al. 1998; Meltzer 1989; Markianos et al. 1999).
The absence of ACTH, or cortisol changes under the treatment with haloperidol is in accordance with unaffected HPA-axis hormones in most of the studies investigating the influence of typical antipsychotics (Jezova Repcekova et al. 1979; Laakmann et al. 1984; Baptista et al. 1997a, b; de Koning and de Vries 1995; von Bahr et al. 1991; Nurnberger et al. 1984; Barbieri et al. 1984; Collu et al. 1975), and the results found in a study investigating amisulpiride, a relatively selective dopamine D2- and D3-receptor blocker with negligible affinity to serotonergic (5-HT) receptors (Wetzel et al. 1994). Although monoaminergic mechanisms including serotonin and dopamine are known to play an important role in the regulation of ACTH and cortisol secretion (Wilcox et al. 1975; Fuller and Snoddy 1984; Tuomisto and Mannisto 1985; Contesse et al. 2000), the strong inhibitory effect of quetiapine and olanzapine on unchallenged ACTH and cortisol secretion is likely related to their 5-HT2 receptor blocking properties. The attenuation of cortisol secretion, after subchronic administration of olanzapine and clozapine to schizophrenic patients, had already been attributed to 5-HT receptor blockade in earlier studies (Meltzer 1989; Kahn et al. 1993; Hatzimanolis et al. 1998; Markianos et al. 1999; Scheepers et al. 2001). However, a nonspecific reduction of stress-induced activation of the HPA axis as a reflection of the overall improvement under the atypical antipsychotic had been considered an alternative explanation (Meltzer 1989). In line with the hypothesis that 5-HT2 antagonism is an important factor in the down-regulation of the HPA axis are the results of challenge experiments in schizophrenic and non-schizophrenic patient groups with elevated cortisol levels. The 5-HT2 receptor antagonist ritanserin attenuates the effects of the 5-HT agonist, m-chlorophenylpiperazine (mCPP), on positive symptoms and plasma cortisol levels in schizophrenic patients (Abi Saab et al. 2002), and a reduction of cortisol levels after the administration of ritanserin has also been found in patients with Cushing’s disease (Sonino et al. 2000) and healthy subjects (Seibyl et al. 1991). Due to the strong 5-HT2-blocking properties of quetiapine and olanzapine, but not haloperidol (Richelson and Souder 2000), the observed strong reduction in ACTH and cortisol secretion could, therefore, be related to their antiserotonergic activity.
Additionally, other receptors blocked by quetiapine and olanzapine like the adrenergic or histaminergic receptors (Richelson and Souder 2000) might be involved in mediating the suppression of HPA-axis hormone secretion. Animal and human studies demonstrated reduced cortisol levels after administration of α1-adrenergic antagonistic drugs (Laakmann et al. 1986; al Damluji 1993). The antihistaminergic drug cyproheptadine inhibits the corticotropin releasing hormone induced ACTH and cortisol release in normal subjects (Allolio et al. 1987), and the selective histamine 1 (H 1) receptor antagonist meclastine inhibits the hypoglycemia-induced ACTH and cortisol increase (Allolio et al. 1983).
It remains to be clarified whether the observed reduction of ACTH and cortisol levels, after acute application of the atypical antipsychotics quetiapine and olanzapine, is still detectable after intermediate or long-term administration of these drugs, although some evidence for a persistent decrease of HPA-axis activity comes from a 6-week olanzapine treatment study in schizophrenic patients demonstrating an ongoing reduction of ACTH and cortisol (Scheepers et al. 2001). The clinical significance of such a down-regulation of the HPA axis, however, needs further investigation. Despite the decrease in ACTH and cortisol levels, Scheepers et al. (2001) did not find a significant correlation between hormonal changes and clinical improvement. However, Shirayama et al. (2002) demonstrated a clear correlation between plasma ACTH and cortisol levels with negative symptoms in schizophrenic patients.
Furthermore, the HPA axis plays an important role in the pathophysiology of major depression (Holsboer 2000). Antiglucocorticoid treatment strategies have demonstrated antidepressive effects in depressive disorders (Wolkowitz and Reus 1999; Hornig and Amsterdam 2003) and in patients suffering from schizophrenia and schizoaffective disorder (Marco et al. 2002). The antidepressant mirtazapine is a drug which also acutely inhibits cortisol and ACTH secretion in healthy subjects, probably due to its antagonistic effects on 5-HT2- and histamine 1-receptors (Laakmann et al. 1999). The ACTH- and cortisol-lowering effects of quetiapine and olanzapine may also be related to their antidepressive potential (Shelton et al. 2001; Tohen et al. 2003; Calabrese et al. 2004). However, an association between cortisol inhibition and antidepressant efficacy of mirtazepine has not been demonstrated so far (Schule et al. 2003), and data for the atypical antipsychotics are still missing. Additionally, the atypical antipsychotics’ HPA-down-regulation appears to be of relevance, because glucocorticoids play an important role in cognitive functioning and memory formation. Administration of corticoids impairs cognitive functioning (Brown et al. 2004; Belanoff et al. 2001) and a decrease of cortisol levels in patients suffering from Cushing’s disease (Mauri et al. 1993), and in elderly women (Seeman et al. 1997), is associated with an improvement in cognitive functioning. Thus, the cortisol-lowering properties of the atypical antipsychotics quetiapine and olanzapine may be clinically relevant for depression, an amelioration of negative symptomatology and cognitive deficits. Therefore, further clinical studies should investigate the clinical relevance of cortisol-lowering properties of the atypical antipsychotics in different patient populations, including patients suffering from schizophrenia and major depression.
In accordance with earlier findings, prolactin levels increased after the administration of the typical antipsychotic haloperidol and remained unaffected by the administration of a low dose of quetiapine. The missing increase of prolactin under quetiapine has been attributed to its low affinity and fast dissociation from the dopamine d(2) receptor (Kapur et al. 2000). The elevation of prolactin found by Alexiadis et al. (2002) is most likely related to the much higher dose of quetiapine in the range of 700 to 800 mg in that study. The pronounced increase of prolactin levels under olanzapine is somehow discordant to the findings in schizophrenic patients where baseline values under intermediate or long-term treatment with up to 20 mg olanzapine is associated with only negligible changes in prolactin concentrations (Goodnick et al. 2002). However, despite these small changes of baseline prolactin levels in schizophrenic patients under the treatment with olanzapine, an elevation with a peak 290 min after application has been described in this patient group as well (Turrone et al. 2002). Although, in our study, olanzapine elevates prolactin in comparison with placebo, the increase was smaller than under the treatment with haloperidol. This fits to the finding that olanzapine may reduce prolactin levels after switching from conventional antipsychotics (Volavka et al. 2004), and that its application is associated with less elevation of serum prolactin than under haloperidol in schizophrenic patients (Nemeroff 1997).
In conclusion, the tested drugs’ influence on prolactin levels appears to be diverse in that haloperidol elevates this hormone, whereas, there was no significant effect of quetiapine and an intermediate increase of prolactin by olanzapine. Furthermore, the atypical antipsychotics quetiapine and olanzapine reduce plasma ACTH and cortisol levels and urinary excretion of cortisol in comparison with placebo, whereas, haloperidol lacks those hormonal effects. The pronounced down-regulation of the HPA axis observed under the atypical antipsychotics may be related to their serotonin receptor-blocking properties, but their antagonism of adrenergic or histaminergic receptors may play a role as well. The observed HPA-axis down-regulation may be clinically relevant for their positive effects on depression, negative symptomatology, and cognitive functioning.
References
Abi Saab W, Seibyl JP, D’Souza DC, Karper LP, Gueorgueva R, Abi Dargham A, Wong ML, Rajhans S, Erdos JP, Heninger GR, Charney DS, Krystal JH (2002) Ritanserin antagonism of m-chlorophenylpiperazine effects in neuroleptic-free schizophrenics patients: support for serotonin-2 receptor modulation of schizophrenia symptoms. Psychopharmacology (Berl) 162:55–62
Adityanjee, Schulz SC (2002) Clinical use of quetiapine in disease states other than schizophrenia. J Clin Psychiatry 63:32–38
al Damluji S (1993) Adrenergic control of the secretion of anterior pituitary hormones. Baillieres. Clin Endocrinol Metab 7:355–392
Alexiadis M, Whitehorn D, Woodley H, Kopala L (2002) Prolactin elevation with quetiapine. Am J Psychiatry 159:1608–1609
Allolio B, Deuss U, Kaulen D, Winkelmann W (1983) Effect of meclastine, a selective H1 receptor antagonist, upon ACTH release. Clin Endocrinol (Oxf) 19:239–245
Allolio B, Schulte HM, Deuss U, Winkelmann W (1987) Cyproheptadine inhibits the corticotropin releasing hormone (CRH)—induced hormone release in normal subjects. Horm Metab Res Suppl 16:36–38
Altamura AC, Boin F, Maes M (1999) HPA axis and cytokines dysregulation in schizophrenia: potential implications for the antipsychotic treatment. Eur Neuropsychopharmacol 10:1–4
Baptista T, Alastre T, Contreras Q, Martinez JL, Araujo de Baptista E, Paez X, Hernandez L (1997a) Effects of the antipsychotic drug sulpiride on reproductive hormones in healthy men: relationship with body weight regulation. Pharmacopsychiatry 30:250–255
Baptista T, Molina MG, Martinez JL, de Quijada M, Calanche de Cuesta I, Acosta A, Paez X, Martinez JM, Hernandez L (1997b) Effects of the antipsychotic drug sulpiride on reproductive hormones in healthy premenopausal women: relationship with body weight regulation. Pharmacopsychiatry 30:256–262
Barbieri C, Parodi M, Bruno S, Bertassi F, Benaglia D, Moser P, Meroni R, Dubini A (1984) Effects of acute administration of zetidoline, a new antidopaminergic drug, on plasma prolactin and aldosterone levels in man. Eur J Clin Pharmacol 26:29–32
Belanoff JK, Gross K, Yager A, Schatzberg AF (2001) Corticosteroids and cognition. J Psychiatr Res 35:127–145
Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA (2002) Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry 159:1018–1028
Borison RL, Arvanitis LA, Miller BG (1996) ICI 204,636, an atypical antipsychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. US SEROQUEL Study Group J Clin Psychopharmacol 16:158–169
Breier A, Buchanan RW, Waltrip RW 2nd, Listwak S, Holmes C, Goldstein DS (1994) The effect of clozapine on plasma norepinephrine: relationship to clinical efficacy. Neuropsychopharmacology 10:1–7
Brown ES, Varghese FP, McEwen BS (2004) Association of depression with medical illness: does cortisol play a role? Biol Psychiatry 55:1–9
Calabrese JR, Macfadden W, McCoy R, Minkwitz M, Wilson E, Mullen J (2004) Double-blind, placebo-controlled study of quetiapine in bipolar depression. In: Association AP (ed) American psychiatric association 157th annual meeting. American Psychiatric Association, New York, USA, p 284, NR756
Cohrs S, Pohlmann K, Guan Z, Jordan W, Meier A, Huether G, Rüther E, Rodenbeck A (2004) Quetiapine reduces nocturnal urinary cortisol excretion in healthy subjects. Psychopharmacology (Berl) 174:414–420
Collu R, Jequier JC, Leboeuf G, Letarte J, Ducharme JR (1975) Endocrine effects of pimozide, a specific dopaminergic blocker. J Clin Endocrinol Metab 41:981–984
Contesse V, Lefebvre H, Lenglet S, Kuhn JM, Delarue C, Vaudry H (2000) Role of 5-HT in the regulation of the brain–pituitary–adrenal axis: effects of 5-HT on adrenocortical cells. Can J Physiol Pharmacol 78:967–983
de Koning P, de Vries MH (1995) A comparison of the neuro-endocrinological and temperature effects of DU 29894, flesinoxan, sulpiride and haloperidol in normal volunteers. Br J Clin Pharmacol 39:7–14
Fuller RW, Snoddy HD (1984) Central dopamine receptors mediating pergolide-induced elevation of serum corticosterone in rats. Characterization by the use of antagonists. Neuropharmacology 23:1389–1394
Ghadirian AM, Chouinard G, Annable L (1982) Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis 170:463–467
Goodnick PJ, Rodriguez L, Santana O (2002) Antipsychotics: impact on prolactin levels. Expert Opin Pharmacother 3:1381–1391
Gruen PH, Sachar EJ, Langer G, Altman N, Leifer M, Frantz A, Halpern FS (1978) Prolactin responses to neuroleptics in normal and schizophrenic subjects. Arch Gen Psychiatry 35:108–116
Hamner MB, Arvanitis LA, Miller BG, Link CG, Hong WW (1996) Plasma prolactin in schizophrenia subjects treated with Seroquel (ICI 204,636). Psychopharmacol Bull 32:107–110
Harrow M, Yonan CA, Sands JR, Marengo J (1994) Depression in schizophrenia: are neuroleptics, akinesia, or anhedonia involved? Schizophr Bull 20:327–338
Hatzimanolis J, Lykouras L, Markianos M, Oulis P (1998) Neurochemical variables in schizophrenic patients during switching from neuroleptics to clozapine. Prog Neuro-Psychopharmacol Biol Psychiatry 22:1077–1085
Hennig J, Rzepka U, Mai B, Netter P (1995) Suppression of HPA-axis activity by haloperidol after experimentally induced heat stress. Prog Neuro-Psychopharmacol Biol Psychiatry 19:603–614
Holsboer F (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501
Hornig M, Amsterdam JD (2003) Prolactin, growth hormone, insulin, glucagon, and parathyroid hormone. In: Wolkowitz OM, Rothschild AJ (eds) Psychoneuroendocrinology. The Scientific basis of clinical practice. American Psychiatrist Publishing, Arlington, pp 107–136
Jezova Repcekova D, Klimes I, Jurcovicova J, Vigas M (1979) Effect of adrenergic receptor blockade on cortisol and GH response to insulin-induced hypoglycemia in man. Int J Clin Pharmacol Biopharm 17:64–67
Kahn RS, Siever L, Davidson M, Greenwald C, Moore C (1993) Haloperidol and clozapine treatment and their effect on M-chlorophenylpiperazine-mediated responses in schizophrenia: implications for the mechanism of action of clozapine. Psychopharmacology (Berl) 112:S90–S94
Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P (2000) A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry 57:553–559
Laakmann G, Wittmann M, Gugath M, Mueller OA, Treusch J, Wahlster U, Stalla GK (1984) Effects of psychotropic drugs (desimipramine, chlorimipramine, sulpiride and diazepam) on the human HPA axis. Psychopharmacology (Berl) 84:66–70
Laakmann G, Wittmann M, Schoen HW, Zygan K, Weiss A, Meissner R, Mueller OA, Stalla GK (1986) Effects of receptor blockers (methysergide, propranolol, phentolamine, yohimbine and prazosin) on desimipramine-induced pituitary hormone stimulation in humans-III. Hypothalamo–pituitary–adrenocortical axis. Psychoneuroendocrinology 11:475–489
Laakmann G, Schule C, Baghai T, Waldvogel E (1999) Effects of mirtazapine on growth hormone, prolactin, and cortisol secretion in healthy male subjects. Psychoneuroendocrinology 24:769–784
Langer G, Sachar EJ, Gruen PH, Halpern FS (1977) Human prolactin responses to neuroleptic drugs correlate with antischizophrenic potency. Nature 266:639–640
Marco EJ, Wolkowitz OM, Vinogradov S, Poole JH, Lichtmacher J, Reus VI (2002) Double-blind antiglucocorticoid treatment in schizophrenia and schizoaffective disorder: a pilot study. World J Biol Psychiatry 3:156–161
Markianos M, Hatzimanolis J, Lykouras L (1999) Switch from neuroleptics to clozapine does not influence pituitary–gonadal axis hormone levels in male schizophrenic patients. Eur Neuropsychopharmacol 9:533–536
Mauri M, Sinforiani E, Bono G, Vignati F, Berselli ME, Attanasio R, Nappi G (1993) Memory impairment in Cushing’s disease. Acta Neurol Scand 87:52–55
Meltzer HY (1989) Clinical studies on the mechanism of action of clozapine: the dopamine–serotonin hypothesis of schizophrenia. Psychopharmacology (Berl) 99(Suppl):S18–S27
Meltzer HY, Fang VS (1976) The effect of neuroleptics on serum prolactin in schizophrenic patients. Arch Gen Psychiatry 33:279–286
Möller HJ (2003) Management of the negative symptoms of schizophrenia: new treatment options. CNS Drugs 17:793–823
Murburg MM, Paly D, Wilkinson CW, Veith RC, Malas KL, Dorsa DM (1986) Haloperidol increases plasma beta endorphin-like immunoreactivity and cortisol in normal human males. Life Sci 39:373–381
Murburg MM, Wilkinson CW, Raskind MA, Veith RC, Dorsa DM (1993) Evidence for two differentially regulated populations of peripheral beta-endorphin-releasing cells in humans. J Clin Endocrinol Metab 77:1033–1040
Nemeroff CB (1997) Dosing the antipsychotic medication olanzapine. J Clin Psychiatry 58(Suppl 10):45–49
Nurnberger JI Jr, Simmons Alling S, Kessler L, Jimerson S, Schreiber J, Hollander E, Tamminga CA, Nadi NS, Goldstein DS, Gershon ES (1984) Separate mechanisms for behavioral, cardiovascular, and hormonal responses to dextroamphetamine in man. Psychopharmacology (Berl) 84:200–204
Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, Breier A, Tollefson GD (2000) Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Arch Gen Psychiatry 57:249–258
Richelson E, Souder T (2000) Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 68:29–39
Rubin RT, Poland RE, O’Connor D, Gouin PR, Tower BB (1976) Selective neuroendocrine effects of low-dose haloperidol in normal adult men. Psychopharmacologia 47:135–140
Scheepers FE, Gespen de Wied CC, Kahn RS (2001) The effect of olanzapine treatment on m-chlorophenylpiperazine-induced hormone release in schizophrenia. J Clin Psychopharmacol 21:575–582
Schule C, Baghai T, Zwanzger P, Ella R, Eser D, Padberg F, Moller HJ, Rupprecht R (2003) Attenuation of hypothalamic–pituitary–adrenocortical hyperactivity in depressed patients by mirtazapine. Psychopharmacology (Berl) 166:271–275
Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW (1997) Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab 82:2458–2465
Seibyl JP, Krystal JH, Price LH, Woods SW, D’Amico C, Heninger GR, Charney DS (1991) Effects of ritanserin on the behavioral, neuroendocrine, and cardiovascular responses to meta-chlorophenylpiperazine in healthy human subjects. Psychiatry Res 38:227–236
Shelton RC, Tollefson GD, Tohen M, Stahl S, Gannon KS, Jacobs TG, Buras WR, Bymaster FP, Zhang W, Spencer KA, Feldman PD, Meltzer HY (2001) A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 158:131–134
Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T (2002) Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res 58:69–74
Sonino N, Fava GA, Fallo F, Franceschetto A, Belluardo P, Boscaro M (2000) Effect of the serotonin antagonists ritanserin and ketanserin in Cushing’s disease. Pituitary 3:55–59
Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, Mitchell PB, Centorrino F, Risser R, Baker RW, Evans AR, Beymer K, Dube S, Tollefson GD, Breier A (2003) Efficacy of olanzapine and olanzapine–fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 60:1079–1088
Tuomisto J, Mannisto P (1985) Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev 37:249–332
Turrone P, Kapur S, Seeman MV, Flint AJ (2002) Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 159:133–135
Velligan DI, Newcomer J, Pultz J, Csernansky J, Hoff AL, Mahurin R, Miller AL (2002) Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res 53:239–248
Volavka J, Czobor P, Cooper TB, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Lieberman JA (2004) Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychiatry 65:57–61
von Bahr C, Wiesel FA, Movin G, Eneroth P, Jansson P, Nilsson L, Ogenstad S (1991) Neuroendocrine responses to single oral doses of remoxipride and sulpiride in healthy female and male volunteers. Psychopharmacology (Berl) 103:443–448
Walder DJ, Walker EF, Lewine RJ (2000) Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biol Psychiatry 48:1121–1132
Weizman A, Maoz B, Treves I, Asher I, Ben David M (1985) Sulpiride-induced hyperprolactinemia and impotence in male psychiatric outpatients. Prog Neuropsychopharmacol Biol Psychiatry 9:193–198
Wetzel H, Wiesner J, Hiemke C, Benkert O (1994) Acute antagonism of dopamine D2-like receptors by amisulpride: effects on hormone secretion in healthy volunteers. J Psychiatr Res 28:461–473
Wetzel H, Szegedi A, Hain C, Wiesner J, Schlegel S, Benkert O (1995) Seroquel (ICI 204 636), a putative “atypical” antipsychotic, in schizophrenia with positive symptomatology: results of an open clinical trial and changes of neuroendocrinological and EEG parameters. Psychopharmacology (Berl) 119:231–238
Wieck A, Haddad PM (2003) Antipsychotic-induced hyperprolactinaemia in women: pathophysiology, severity and consequences. Selective literature review. Br J Psychiatry 182:199–204
Wilcox CS, Aminoff MJ, Millar JG, Keenan J, Kremer M (1975) Circulating levels of corticotrophin and cortisol after infusions of l-DOPA, dopamine and noradrenaline, in man. Clin Endocrinol (Oxf) 4:191–198
Wolkowitz OM, Reus VI (1999) Treatment of depression with antiglucocorticoid drugs. Psychosom Med 61:698–711
Zerssen von D, Koeller DM (1976) Die Beschwerden-Liste (Manual). Klinische Selbstbeurteilungsskalen aus dem Muenchner Psychiatrischen Informationssystem (PSYCHIS). Beltz-Verlag, Beltz-Verlag
Acknowledgements
We are grateful to the dedicated staff of the Sleep Medicine Center of the Department of Psychiatry and Psychotherapy, University of Göttingen, Germany, including Roswitha Bianco, Iris Bossmann, Brigitte Marxen, and Andreas Müller-Struck.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cohrs, S., Röher, C., Jordan, W. et al. The atypical antipsychotics olanzapine and quetiapine, but not haloperidol, reduce ACTH and cortisol secretion in healthy subjects. Psychopharmacology 185, 11–18 (2006). https://doi.org/10.1007/s00213-005-0279-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0279-x