Abstract
Rationale
Maternal deprivation can result in long-term impairment of neuronal functions and in the development of long-lasting behavioural disorders.
Objectives
This study analysed the effects of a selective cholecystokinin-2 (CCK2) antagonist, 3R-(+)-N-(2,3-dihydro-1methyl-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3yl)-N′-(3-methyl phenyl) urea (L365,260), in anxiety- and stress-related behaviours of adult rats that were deprived (D) from their mother and littermates for 3 h everyday during 14 days after birth.
Methods
The behaviour was studied in actimeter, in open field and after food and water deprivation. Corticosterone plasma levels were quantified after food and water deprivation. The effects of L365,260 were studied in the behavioural changes observed in D rats.
Results
No differences in circadian motor activity between non-deprived (ND) and D rats were observed. D rats showed a 50% decrease in their number of visits to the central (aversive) part of the open field compared to ND rats. This effect was suppressed by L365,260. After 20 h of food and water deprivation, an increase in plasma corticosterone was observed in D and ND rats. However, the raise of corticosterone secretion in D rats was dramatically increased (300%) compared to ND rats, indicating a hypersensitised state revealed by this stressful situation. Consumption of sucrose solution (1%) was higher for D rats than for ND rats after food and water deprivation. Sucrose consumption returned to control values following L365,260 treatment.
Conclusions
These results suggest that maternal deprivation led to an increase in anxiety and stress reactivity in adulthood. We propose that these long-lasting changes are partly dependent on CCKergic transmission involving the activation of CCK2 receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early-life adverse experiences have been shown to increase the risk of developing psychopathology in humans (Holmes and Robbins 1987; Russak and Schwartz 1997; Canetti et al. 1997). It has been demonstrated that postnatal factors such as maternal separation alter both behaviour and brain neurotransmitters in several species (reviewed in Hall 1998; Anisman et al. 1998).
Maternal separation in rat led to an alteration of emotional responses that is differentially impacted by the duration of separation. Brief separations (15 min) of pups appear to protect the animals from age-related deficits and to lead to a decrease in anxiety and enhanced hypothalamic–pituitary–adrenal (HPA) negative feedback (Levine et al. 1967; Meaney et al. 1989; Ogawa et al. 1994; Liu et al. 1997; reviewed in Francis et al. 1999). In contrast, longer periods of maternal separation enhance behavioural and HPA responses to stress- and anxiety-like behaviour (Wigger and Neumann 1999; Meaney et al. 1989; Rosenfeld et al. 1992; Plotsky and Meaney 1993; Ladd et al. 1996; Huot et al. 2001; Kalinichev et al. 2001; Daniels et al. 2004). However, it should be noticed that some studies did not find any increase in anxiety-like behaviour in adult rat after long periods of separation (Ogawa et al. 1994; McIntosh et al. 1999; Pryce and Feldon 2003). There is also no general consensus in the literature regarding studies on the sucrose consumption under stressful conditions. According to the different results published, early maternal separation appears to have no consequences in consummatory behaviour and sucrose preference whatever the nutritional status of the animals (deprived of food and water or not) (Matthews et al. 1996) or appears to induce a decrease or an increase in sucrose intake in separated rats without food and water deprivation (McIntosh et al. 1999; Huot et al. 2001). These discrepancies may be due partly to differences in the maternal separation procedure, in the treatment of control rats, in the rat strain used or the behavioural test studied (Ellenbroek and Cools 2000; reviewed in Pryce and Feldon 2003). Finally, little information is available regarding the neurobiological substrates mediating the alterations of emotional behaviour of the separated rats in adulthood.
Cholecystokinin (CCK) is a brain–gut peptide that interacts with two different receptors, CCK1 and CCK2. CCK is involved in various physiological functions such as digestion in the periphery and centrally mediated actions such as memory processes, nociception, feeding and emotional responses (reviewed in Crawley and Corwin 1994). Most studies performed in adult rats showed a major role for CCK2 receptors in anxiety- and stress-related behaviours in adult rats and panic in humans (reviewed in Crawley and Corwin 1994; Shlik et al. 1997; Daugé and Léna 1998). In addition, this peptide may coordinate the developing infant's digestion, metabolism and growth but may also contribute to non-nutritive aspects of developing infant–mother relations (Uvnas-Moberg 1989; Weller and Feldman 2003). Recent data showed that the feeding suppressant response to CCK was enhanced in separated adult male rats (McIntosh et al. 1999). However, there are no data available on the possible involvement of CCKergic neurotransmission in emotional changes observed in adult separated rats.
This study was performed to examine the long-term effects of early maternal deprivation on the anxiety- and stress-related behaviour and the possible involvement of CCK2 receptor subtype. The manipulations of postnatal environment involved early deprivation (D) of the mother and littermates (3 h/day from the ages of 1 to 14 days). The behaviour of adult male rats submitted to various situations was examined: (1) motor activity was measured in an actimeter and in the open-field test and (2) consummatory behaviour of sucrose solution under food and water deprivation. It has been established that food and water deprivation schedule was stressful for “normal” rodents and produced an increase in corticosterone (CORT) plasma levels (Levine and Coover 1976; Kiss et al. 1994; Adriani et al. 2002). Thus, plasma levels of CORT were measured after 20 h of food and water deprivation to test the reactivity of deprived rats to this stressful situation. The physiological involvement of CCK2 receptors in the behavioural changes observed in deprived rats was studied using the CCK2 antagonist, L365,260. This compound has high affinity and selectivity for CCK2 receptors (CCK2=10 nM; CCK2/CCK1 ratio, 0.009) (Bock et al. 1989) and shows properties to decrease anxiety- and stress-related behaviour in rodents (reviewed in Crawley and Corwin 1994; Shlik et al. 1997; Daugé and Léna 1998).
Experimental procedures
Subjects
Four series of 20 pregnant Long–Evans rats on day 14 of gestation (Janvier, Le Genest St Isle, France) were used. The dams gave birth 1 week ± 12 h after inclusion. Litters were housed in clear plastic cages in a well-ventilated, temperature (24±1°C)- and humidity (50±5%)-controlled environment on a 12-h light, 12-h dark cycle (lights on 0800–2000 hours). Dams received rat chow and water ad libitum, and the cages and all the shavings were changed only once weekly to avoid excessive handling.
“Principles of laboratory animal care” were followed. The experimental procedure and care of the animals were in accordance with local committee guidelines and the European Communities Council Directive of November 24, 1986 (86/609/EEC).
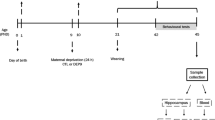
Maternal deprivation
The day of birth was designated day 0. On postnatal day 1, litters were cross-fostered and culled to six to seven male pups. Random redistribution of pups among dams was done to redistribute possible effects of genetic and prenatal factors and to obtain similar litter size. Two investigators collaborated in the determination of each pup's sex, and each pup received similar handling during this procedure. The litters were each assigned to an experimental group. From day 1, mothers were removed from their home cage and put in a new cage for 3 h, the same procedure being applied at each deprivation. Neonates belonging to the maternal deprivation group (D) were individually placed in temperature (30–34°C)- and humidity-controlled cages divided into compartments in a room separated from their mothers. Pups' cages contained 2 cm of fresh shavings covered with absorbing paper. Pups were isolated daily from days 1 to 14, always from 1300 to 1600 hours. At the end of the deprivation period, each litter was replaced in the housing cage, and then the dam was transferred back to the housing cage. To reduce handling to a minimum, pups were transferred from and to their cages quickly and gently. D pups received no other handling except that required to change the bedding in their cages once weekly. Rat pups not subjected to maternal deprivation (ND) remained with their mothers during this period and received no special handling other than that necessary to change the bedding in their cages once weekly. From days 15 to 21, all pups remained with their mother. On day 21 or 22, pups were weaned from their mothers and housed in groups of three or four until 2.5–3 months of age. Each rat was used only once.
Drugs
The selective CCK2 receptor antagonist, 3R-(+)-N-(2,3-dihydro-1methyl-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3yl)-N′-(3-methyl phenyl) urea (L365,260) (Bock et al. 1989), was a generous gift from Rhone-Poulenc Rorer. L365,260 was suspended in 5% carboxymethyl cellulose (CMC) and injected i.p. 45 min before the tests at the selective dose of 0.1 mg/kg (Ladurelle et al. 1997).
Behavioural measures
Motor activity in actimeter
Total spontaneous motor activity was measured in an actimeter (Imetronic, France) (Ladurelle et al. 1997). The metallic grid cages (37×24×22 cm) were provided with two infrared beams (height from the floor, 2.5 cm), allowing horizontal motor activity to be measured. Beam interruptions were counted with a computer. Rats were tested individually and recorded for 24 h. The results are expressed as 24 1-h total counts and as the 24-h cumulative counts.
Exploratory behaviour in the open field
The open field used was a large white open rectangular box (70 cm wide, 90 cm long, 60 cm high) strongly illuminated (500 lx) from above, these conditions being considered as stressful for rats. Black lines on the floor of this field delineated 10×10-cm squares (Daugé et al. 1989). The rats, first placed in the same corner of the open field, were observed for 6 min, and six events were quantified: (1) the latency before moving out from the corner, scored once two squares had been crossed; (2) the locomotor activity, defined as the total number of squares crossed; (3) the number of visits to the middle of the area, and the total number of (4) rearings; (5) groomings; and (6) defecations.
Sucrose solution consumption
The first set of experiment was performed to measure the consumption of sucrose (Sigma) solution (1%) whether under a forced consumption condition with only a bottle of sucrose solution or under an unforced condition with a two-bottle-choice drinking paradigm with ND and D rats. In both cases, the rats were isolated and deprived of food and water for 20 h preceding solution intake test. The rats were trained to consume sucrose solution with or without choice of water in three sessions with 2 days of interval, as previously described (Willner et al. 1987). Sucrose solution with or without choice of water was presented for 60 min in standard drinking bottles with a 5-cm stainless-steel spout. Two days after the last training session, sucrose solution with or without choice of water was given for 60 min, and the sucrose solution and water consumption in milliliters were calculated as the difference between the volume before and after drinking. The preference ratios in the two-bottle-choice drinking paradigm were calculated as the amount of sucrose consumed divided by the total fluid intake. Bottles were reversed at each test session to control for side preference.
A second set of experiment was performed to measure the sucrose solution consumption in a two-bottle-choice paradigm in food and water given ad libitum (ad lib) for 3 days. Because it was not possible to test the sucrose solution consumption for 1 h under ad lib conditions, we measured on the third day the sucrose solution intake for 24 h.
The procedure using food and water deprivation and training to consume forced sucrose solution was used to test the effect of L365,260.
Measurement of plasma corticosterone
ND and D rats fed ad lib or food and water deprived for 20 h (as described above) were each assigned to two groups: ad lib and food and water deprived. The rats were decapitated in the morning, and blood was collected in EDTA-containing tubes and centrifuged (1,000×g, 10 min, 4°C); plasma was frozen and stored at −20°C until use for the determination of CORT. CORT was assayed on plasma samples (10 μl) using a rabbit [125I]-CORT RIA kit from ICN Biomedicals, Inc (USA).
Statistical analysis
The results of behavioural experiments are expressed as means±SEM. Results were compared using a one-way analysis of variance (ANOVA) followed by Fisher's PLSD test for comparisons in motor activity, sucrose solution consumption experiments. Two-way ANOVA (deprivation and treatment factors) was done followed by Fisher's PLSD test for multiple comparison in the pharmacological experiments using L365,260 and in the dosage of plasma CORT. All data were analysed with Statview 5 for Macintosh. The level chosen for statistical significance was α=5%.
Results
Motor activity in actimeter
There were no differences between ND and D rats in between 17 and 16 h the next day. The circadian rhythm of motor activity was observed in two groups, with more motor activity during the dark period and less during the light period (Fig. 1a). For 1-h data using ANOVA: deprivation, F(1,13)=1.50, P=0.24; time, F(22,286)=16.437, P=0.0001; interaction, F(22,286)=0.366, P=0.99. Total motor activity over 24 h was not significantly different between the two groups of rats (Fig. 1b) [ANOVA: F(1,13)=1.60; P=0.22].
Exploratory behaviour in the open-field test
The number of squares crossed, of rearings, of groomings and of defecations and the latency time before moving out of the corner were not different between ND and D rats treated with CMC 5% or L365,260 0.1 mg/kg (Table 1).
The number of squares entered in the middle of the open field (aversive part of the open field) was lower for the D rats than for the ND rats. After injection of the selective CCK2 receptor antagonist, L365,260, the number of visits to the centre of the open field by the D rats was completely restored to control values [deprivation, F(1,35)=6.6, P=0.01; treatment, F(1,35)=4.63, P=0.03; interaction, F(1,35)=5.25, P=0.02] (Table 1, Fig. 2).
Number of visits to the central area of the open field for 6 min in non-deprived (ND) and deprived (D) rats. The selective CCK2 antagonist, L365,260 (L), was injected at the dose of 0.1 mg/kg i.p. 45 min before the test; C controls. The rats were tested at 2.5–3 months old. ND-C, n=9; ND-L, n=10; D-C, n=10; D-L, n=10. The results are means±SEM of the number of visits to the central area of the open field. *P<0.05 vs ND control rats, + P<0.05 vs D control rats, Fisher's PLSD test
Ad lib and food- and water-deprived experiment assessment of plasma CORT levels
Significant differences were showed in the two-way ANOVA [deprivation factor, F(1,22)=22.76, P<0.0001; ad lib/without food and water, F(1,22)=45.06, P<0.0001; interaction, F(1,22)=21.43, P<0.0001]. An increase in CORT level was observed in ND food- and water-deprived group (n=7) compared to ND ad lib group (n=5) [F(1,10)=4.40, P=0.05], as well as in D food- and water-deprived group (n=7) compared to D ad lib group (n=7) [F(1,12)=49.83, P<0.0001]. No differences between ND and D groups ad lib were evident in plasma CORT levels [F(1,10)=0.028, not significant]. However, a significant increase in CORT levels was obtained in D rats with food and water deprivation for 20 h compared to ND rats [F(1,12)=31.09, P<0.0001] (Fig. 3).
Measurement by RIA of plasma CORT levels in non-deprived and deprived rats under food and water ad lib or under food- and water-deprived for 20-h conditions. The results are expressed as means±SEM. ND and D food- and water-deprived groups, n=7 and n=7, respectively; ND and D ad lib, n=5 and n=7, respectively. + P<0.05 vs ND ad lib, ***P<0.001 vs D ad lib and ND food- and water-deprived, Fisher's PLSD test
Sucrose solution consumption
In the forced sucrose solution procedure with food and water deprivation, D (n=9) rats consumed significantly more sucrose solution than ND rats (n=9) [F(1,16)=4.15, P<0.05] (Fig. 4a). In the two-bottle-choice drinking paradigm with food and water deprivation, D rats (n=9) also consumed more sucrose solution than ND rats (n=9) [F(1,16)=7.94, P<0.01]. A significant increase in the preference for sucrose solution was observed in D rats compared to ND rats [F(1,16)=12.33, P<0.003] (Fig. 4b). The total fluid intake was not significantly different between both groups (not shown).
a Sucrose solution (1%) intake (ml) by ND (n=9) and D (n=9) rats over 1 h following 20 h of food and water deprivation in the forced procedure. b Sucrose solution (1%) intake (ml) and sucrose preference (%) by ND (n=9) and D (n=9) over 1 h following 20 h of food and water deprivation in the two-bottle-choice procedure. The rats were tested at 2.5–3 months old. The results are means±SEM of the sucrose solution intake and sucrose preference. *P<0.05, **P<0.01 vs ND control group, Fisher's PLSD test
No difference has been observed in the sucrose solution intake and preference in the two-bottle-choice paradigm with food and water ad lib between D (n=9) and ND (n=9) rats (not shown).
The effect of L365,260 was analysed in the forced sucrose solution procedure. Administration of 0.1 mg/kg i.p. of the selective CCK2 receptor antagonist, L365,260, completely restored the sucrose solution consumption by the D rats to that of untreated ND rats. L365,260 did not affect the sucrose solution intake by the ND group [deprivation, F(1,35)=5.58, P=0.02; treatment, F(1,35)=7.07, P=0.01; interaction, F(1,35)=3.76, P=0.06] (Fig. 5).
Sucrose solution (1%) intake (ml) by non-deprived (ND, n=20) and deprived (D, n=19) rats over 1 h following 20 h of food and water deprivation. The rats were tested at 2.5–3 months old. Rats were injected i.p. with 0.1 mg/kg of the selective CCK2 antagonist, L365,260 (L), 45 min before the sucrose solution consumption test. The results are means±SEM of the sucrose solution intake. *P<0.05 vs ND groups, + P<0.05 vs control D group, Fisher's PLSD test
Discussion
Early deprivation from mother and littermates (3 h/day from the ages of 1–14 days) was chosen instead of early separation from mother [separation of the intact litter from the dam (MS)] because it would appear that D constitutes a more severe postnatal manipulation than MS and might be expected to constitute a postnatal stressor (McCormick et al. 1998). However, it is unclear whether the marked D effects are mediated by human handling, prolonged isolation in a different environment, altered maternal behaviour in the home cage or some combination thereof (reviewed in Pryce and Feldon 2003).
First, the ND and D rats were tested for motor activity in an actimeter. Both groups of adult rats showed a similar motor activity over 24 h, with an activity pattern according to the expected circadian rhythm, motor activity decreasing in light phase and increasing in dark period. On the other hand, in the open-field test, ND and D rats showed a similar locomotor activity at the periphery of the open field, but D rats entered the central area (the most aversive part of the open field) less often than ND rats, suggesting that D rats were more anxious than ND animals (the balance between the self-preservation and the exploration drives being shifted towards the former). This would be consistent with recent data indicating the occurrence of anxiety-like behaviour as a consequence of maternal separation (Wigger and Neumann 1999; Huot et al. 2001; Kalinichev et al. 2001; Daniels et al. 2004). However, other studies did not observe anxiety-like behaviour in the open-field test (Ogawa et al. 1994) or in other tests measuring emotional behaviour (Pryce and Feldon 2003). This discrepancy may be explained by the different strains used and the age of the tested rats [6-week-old Sprague–Dawley rats (Ogawa et al. 1994), whereas we used 10- to 12-week-old Long–Evans rats]; strain and age have been shown to be important factors in stress reactivity (Workel et al. 2001; Faraday 2002) (see also Introduction).
Neurochemical alterations reported in maternal separation models could be related to changes in neurotransmitters and hormone involved in anxiety and stress-related behaviour, such as corticotropin-releasing factor (CRF), serotonin and gamma-aminobutyric acid (GABA) systems (Plotsky and Meaney 1993; Wigger and Neumann 1999; Caldji et al. 2000; Huot et al. 2001; Vazquez et al. 2002). Functional interactions between CCK, CRF, GABA or serotonin have been described in several structures of the brain in relation with anxiety and stress (Becker et al. 2001; reviewed in Shlik et al. 1997; Daugé and Léna 1998). The reversal of anxiety-like behaviour by D rats following treatment with L365,260 in this study suggests that CCK2 receptors may also be acting in the regulation of anxiety-like behaviour, increased in maternally deprived rats. The dose of 0.1 mg/kg of L365,260 was shown to be selective of CCK2 receptor subtype and not to produce an intrinsic effect (Dourish et al. 1990; Ladurelle et al. 1997).
We used food and water deprivation for 20 h to analyse the stress reactivity of D rats. There were no differences in basal CORT levels (ad lib) between ND and D rats, as expected from data of the literature (reviewed in de Kloet et al. 1996; Anisman et al. 1998; Francis et al. 1999). In contrast, an elevation of CORT levels was measured in ND and D rats under food and water deprivation as compared to ad lib conditions. Consistently, an increased activity of the HPA axis has been reported to occur as a consequence of water and/or food deprivation procedures in “normal” rats (Levine and Coover 1976; Kiss et al. 1994; Adriani et al. 2002). Data from the present study showed that the CORT levels' increase is higher in the D rats than in the ND rats under food- and water-deprived conditions, indicating that D rats are more sensitive to this stressful situation. Although CORT measurement with food- and water-deprived condition was not previously studied in maternal separation or deprivation models, this result is consistent with data showing that separated animals are hyperresponsive to stress (generally restraint stress) of the HPA axis (de Kloet et al. 1996; Ladd et al. 1996; Helga et al. 1998). It should be noted that a 20-h food and water deprivation is a strong metabolic challenge. Therefore, it cannot be ruled out that the HPA hypersensitivity observed in D rats can reflect a metabolic disregulation and not a stress hypersensitivity. Metabolic investigations of the ED model are currently performed to test this hypothesis.
The incentive value of sucrose was shown to vary with motivational states. In this context, an increase in sucrose intake was observed following several acute stress (Bertière et al. 1984; Dess 1992; but see also Calvo-Torrent et al. 1999). Voluntary sucrose ingestion was enhanced after injection of corticosterone (Bell et al. 2000). Stress hormones and changes in corticosterone levels have been shown to play a major role in vulnerability to the reinforcing properties of drugs (reviewed in Piazza and Le Moal 1998; Gosnell 2000). Thus, because of the food- and water-deprived schedule, which leads to a strong increase in CORT level, and of the concomitant offer for sucrose consumption, D rats were expected to be particularly vulnerable to sucrose-related reinforcing properties. A similar procedure (water-deprived) was used recently to reveal a clear-cut preference for oral consumption of nicotine (Adriani et al. 2002) and for drug self-administration in “normal rats” (reviewed in Piazza and Le Moal 1998).
An increase in the sucrose solution consumption was observed in D rats compared to ND rats under food- and water-deprived conditions, both in the forced sucrose solution and in the two-bottle-choice drinking paradigms, with an increase in the sucrose solution preference in the latter procedure. The influence of thirst was discarded because total liquid intake did not differ in D and ND rats in the two-bottle-choice paradigm. Because it was not possible to test the sucrose solution consumption for 1 h under ad lib conditions, we measured the sucrose solution intake for 24 h in the two-bottle-choice paradigm. Under these conditions, no difference was measured in sucrose solution consumption in ND and D rats. The increase in sucrose solution consumption could be the consequence of the stressful situation (food and water deprivation), all the more because the blockade of CCK2 receptors by the selective CCK2 antagonist, L365,260, suppressed the increase in sucrose solution consumption in the D rats without modifying those of the ND rats. Although there is no consensus in the literature regarding studies on the sucrose consumption in maternal separation or deprivation models (see Introduction), an increase in sucrose intake was also observed in an experimental procedure similar to the one we are using (McIntosh et al. 1999).
There is evidence that maternal deprivation leads to a hypersensitisation of the HPA axis to stress in separated adult rats (de Kloet et al. 1996; Ladd et al. 1996; Helga et al. 1998). In addition, CCK2 receptors are involved in anxiety- and stress- related behaviours in humans and animals (reviewed in Crawley and Corwin 1994; Shlik et al. 1997; Daugé and Léna 1998). Interestingly, the recruitment of CCKergic pathways innervating the paraventricular thalamic nuclei by acute stress occurred exclusively in animals that underwent previous chronic stress (Bhatnagar et al. 2000). We observed that the behaviour of D rats submitted to open field and food and water deprivation is normalised after administration of the selective CCK2 antagonist, L365,260. These pharmacological results could reveal the existence of a hyperactivity of the CCKergic system through its interaction with CCK2 receptors in D rats submitted to stressful conditions.
In conclusion, these results suggest that maternal deprivation led to an increase in anxiety and in stress reactivity, which is dependent on CCK2 receptors. They stress the importance of further examining the impact of maternal deprivation on the development of CCK functions and its receptors.
References
Adriani W, Macri S, Pacifici R, Laviola G (2002) Restricted daily access to water and voluntary nicotine oral consumption in mice: methodological issues and individual differences. Behav Brain Res 134:21–30
Anisman H, Zaharia MD, Meaney MJ, Meralis Z (1998) Do early-life events permanently alter behavioral and hormonal responses to stressors. Int J Dev Neurosci 16:149–164
Becker C, Thiebot MH, Touitou Y, Hamon M, Cesselin F, Benoliel JJ (2001) Enhanced cortical extracellular levels of cholecystokinin-like material in a model of anticipation of social defeat in the rat. J Neurosci 21:262–269
Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR, Dallman MF (2000) Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. J Neuroendocrinol 12:461–470
Bertière MC, Sy TM, Baigts F, Mandenoff A, Apfelbaum M (1984) Stress and sucrose hyperphagia: role of endogenous opiates. Pharmacol Biochem Behav 20:675–679
Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF (2000) A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic–pituitary–adrenal function. J Neurosci 15:5564–5573
Bock MG, Dipardo RM, Evans BE, Rittle KE, Whitter WL, Veber DF, Anderson PS, Freidinger RM (1989) Benzodiazepine gastrin and brain cholecystokinin receptor ligands: L365,260. J Med Chem 32:13–16
Caldji C, Francis D, Sharma S, Plostsky PM, Meaney MJ (2000) The effects of early rearing environment on the development of GABAA and benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22:219–229
Calvo-Torrent A, Brain PF, Martinez M (1999) Effect of predatory stress on sucrose intake and behavior on the plus-maze in male mice. Physiol Behav 67:189–196
Canetti L, Bachar E, Galili-Weisstub E, De-Nour AK, Shalev AY (1997) Parental bonding and mental health in adolescence. Adolescence 32:381–394
Crawley J, Corwin RL (1994) Biological actions of cholecystokinin. Peptides 15:731–755
Daniels WM, Pietersen CY, Carstens ME, Stein DJ (2004) Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis 19:3–14
Daugé V, Léna I (1998) CCK in anxiety and cognitive processes. Neurosci Biobehav Rev 22:815–825
Daugé V, Rossignol P, Roques BP (1989) Comparison of the behavioural effects induced by administration in rat nucleus accumbens or nucleus caudatus of selective mu and delta opioid peptides or kelatorphan, an inhibitor of enkephalin-metabolism. Psychopharmacology 96:343–352
de Kloet ER, Rots NY, Cools R (1996) Brain-corticosteroid hormone dialogue: slow and persistent. Cell Mol Neurobiol 16:345–356
Dess NK (1992) Divergent responses to saccharin vs. sucrose availability after stress in rats. Physiol Behav 52:115–125
Dourish CT, O'Neill MF, Coughlan J, Kitchener SJ, Hawley D, Iversen SD (1990) The selective CCK-B receptor antagonist L365,260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur J Pharmacol 176:35–44
Ellenbroek BA, Cools AR (2000) The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology 23:99–106
Faraday MM (2002) Rat sex and strain differences in responses to stress. Physiol Behav 75:507–522
Francis DD, Caldji C, Champagne FN, Plotsky PM, Meaney MJ (1999) The role of corticotropin-releasing factor-norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry 46:1153–1166
Gosnell BA (2000) Sucrose intake predicts rate of acquisition of cocaine self-administration. Psychopharmacology 149:286–292
Hall FS (1998) Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol 12:129–162
Helga JJ, van Oers J, de Kloet R, Li C, Levine S (1998) The ontogeny of glucocorticoid negative feedback: influence of maternal deprivation. Endocrinology 439:2838–2846
Holmes RD, Robbins LN (1987) The influence of childhood disciplinary experience on the development of alcoholism and depression. J Child Psychol Psychiatry 28:399–415
Huot RL, Thrivikraman KV, Meaney M, Plotsky PM (2001) Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long–Evans rats and reversal with antidepressant treatment. Psychopharmacology 158:366–373
Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG (2001) Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long–Evans rats. Pharmacol Biochem Behav 73:131–140
Kiss A, Jezova D, Aguilera G (1994) Activity of the hypothalamic–pituitary–adrenal axis and sympathoadrenal system during food and water deprivation in the rat. Brain Res 663:84–92
Ladd CO, Owens MJ, Nemeroff CB (1996) Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology 137:1212–1218
Ladurelle N, Keller G, Blommaert A, Roques BP, Daugé V (1997) The CCK-B agonist, BC264, increases dopamine in the nucleus accumbens and facilitates motivation and attention after intraperitoneal injection in rats. Eur J Neurosci 9:1804–1814
Levine S, Coover GD (1976) Environmental control of suppression of the pituitary–adrenal system. Physiol Behav 17:35–37
Levine S, Haltmeyer GC, Karas GG, Denenberg VH (1967) Physiological and behavioral effects of infantile stimulation. Physiol Behav 2:55–59
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 277:1659–1662
Matthews K, Wilkinson LS, Robbins TW (1996) Repeated maternal separation of preweanling rats attenuates behavioural responses to primary and conditioned incentives in adulthood. Physiol Behav 59:99–107
McCormick CM, Kehoe P, Kovacs S (1998) Corticosterone release in response to repeat, short episodes of neonatal isolation: evidence of sensitization. Int J Dev Neurosci 16:175–185
McIntosh J, Anisman H, Merali Z (1999) Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats: gender-dependent effects. Dev Brain Res 113:97–106
Meaney MJ, Aitken DH, Sharma S, Viau V, Sarrieau A (1989) Postnatal handled increases hippocampal type II, glucocorticoid receptors and enhances adrenocortical negative -feedback efficacy in the rat. Neuroendocrinology 51:597–604
Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K (1994) Periodic maternal deprivation alters stress response in adult offspring; potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacol Biochem Behav 49:961–967
Piazza PV, Le Moal M (1998) The role of stress in drug self-administration. TIPS 19:67–74
Plotsky PM, Meaney MJ (1993) Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res 18:195–200
Pryce CR, Feldon J (2003) Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev 27:57–71
Rosenfeld P, Wetmore JB, Levine S (1992) Effects of repeated maternal separations on the adrenocortical response in preweanling rats. Physiol Behav 52:787–791
Russak LG, Schwartz GE (1997) Feelings of parental care predict health status in midlife: a 35-year follow-up of Harvard Mastery of Stress Study. J Behav Med 20:1–11
Shlik J, Vasar E, Bradwejn J (1997) Cholecystokinin and psychiatric disorders. CNS Drugs 8:134–152
Uvnas-Moberg K (1989) Gastrointestinal hormones in mother and infant. Acta Paediatr Scand 351:88–93
Vazquez DM, Eskandarii R, Zimmer CA, Levine S, Lopez JF (2002) Brain 5-HT receptor system in the stressed infant rat: implications for vulnerability to substance abuse. Psychoneuroendocrinology 27:245–272
Weller A, Feldman R (2003) Emotion regulation and touch in infants: the role of cholecystokinin and opioids. Peptides 24:779–788
Wigger A, Neumann ID (1999) Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav 66:293–302
Willner P, Towell A, Sampson D, Muscat R, Sophokleous S (1987) Reduction of sucrose preference by chronic mild stress and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364
Workel JO, Oitzl MS, Fluttert M, Lesscher H, Karssen A, de Kloet ER (2001) Differential and age-dependent effects of maternal deprivation on the hypothalamic–pituitary–adrenal axis of brown Norway rats from youth to senescence. J Neuroendocrinol 13:569–580
Acknowledgements
The authors are grateful to S. Lamouraux for the technical assistance in pharmacological experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vazquez, V., Farley, S., Giros, B. et al. Maternal deprivation increases behavioural reactivity to stressful situations in adulthood: suppression by the CCK2 antagonist L365,260. Psychopharmacology 181, 706–713 (2005). https://doi.org/10.1007/s00213-005-0029-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0029-0