Abstract
The aim of this study was to investigate whether neonatal maternal separation (MS) – chronic stress experience in early life – affects the anorectic efficacy of leptin in the offspring at adolescence. Sprague–Dawley pups were separated from the dam daily for 3 h during postnatal day 1–14 or left undisturbed as non-handled controls (NH). NH and MS male pups received an intraperitoneal leptin (100 μg/kg) or saline on postnatal day (PND) 28, and then food intake and body weight gain were recorded. The hypothalamic levels of leptin-signalling-related genes, phosphorylated signal transducer and activator of transcription-3 (pSTAT3) and protein-tyrosine phosphatase 1B (PTP1B) were examined at 40 min after a single injection of leptin on PND 39 by immunohistochemistry and Western blot analysis. Leptin-induced suppressions in food intake and weight gain was observed in NH pups, but not in MS. Leptin increased pSTAT3 in the hypothalamic arcuate nucleus of NH pups, but not of MS. Interestingly, basal levels of the hypothalamic PTP1B and pSTAT3 were increased in MS pups compared with NH controls. The results suggest that neonatal MS experience may blunt the anorectic efficacy of leptin later in life, possibly in relation with increased expressions of PTP1B and/or pSTAT3 in the hypothalamus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Neonatal maternal separation is considered an animal model of stressful experience early in life. Many of studies have demonstrated that neonatal maternal separation may lead to permanent alterations in the characteristics of the hypothalamic–pituitary–adrenal (HPA) axis responding to stress and the development of depression- and anxiety-like behaviours later in life (see Jahng 2011 for review). We have previously demonstrated that rats that experienced 3 h of daily maternal separation during the first 2 weeks of birth (MS) exhibited depression- and anxiety-like behaviours (Lee et al. 2007; Ryu et al. 2009; Yoo et al. 2013). Interestingly, MS rats showed binge-like eating with increased HPA axis activity when they were challenged with repeated fasting/refeeding cycles during the adolescent period (Ryu et al. 2008). Not only the corticosterone increase but also the hypothalamic feeding peptides expressions responding to food deprivation was exaggerated by MS experience (Ryu et al. 2008; Yoo et al. 2011). Also, stress-induced weight gain was observed in MS rats (Yoo et al. 2013). These findings led us to hypothesize that MS experience may blunt the hypothalamic anorexic signalling system responding to physiologic stresses.
Adipose hormone leptin is known to suppress feeding and increase energy expenditure resulting in body weight loss in rodents (Pelleymounter et al. 1995; Schwartz et al. 1996). Blood leptin levels are proportional to the body fat mass and are normally elevated in human obesity (Considine et al. 1996). Leptin is synthesized in adipocytes and released into the blood stream, and acts on its receptors in the hypothalamic arcuate nucleus (ARC) to regulate energy expenditure and weight gain. Anorexic efficacy of leptin; i.e. reducing food intake and weight gain, is known to be mediated by the effector neurons in the ARC, such as the neurons expressing either the orexic peptides, increasing food intake and weight gain, or the anorexic peptides, suppressing food intake and weight gain. It has been reported that leptin inhibits gene expression of the orexic peptide neuropeptide Y and induces gene expression of the anorexic peptides proopiomelanocortin (POMC) or cocaine- and amphetamine-regulated transcript (CART) in the ARC (Schwartz et al. 2000; Sahu 2004).
Leptin binding to its receptor causes the autophosphorylation and activation of Janus kinase 2 (JAK2), and in turn phosphorylation of downstream signalling molecules signal transducer and activator of transcription-3 (STAT3) and STAT5. And then pSTAT3/pSTAT5 dimers are translocated into the nucleus to induce the transcription of target genes (Ghilardi and Skoda 1997; Sahu 2011). The JAK2-STAT3 pathway in the hypothalamus is crucial in maintaining energy homeostasis, evidenced by the morbid obesity of mice lacking STAT3 in brain (Gao et al. 2004). The growing interest in obesity control has brought into focus the role of protein-tyrosine phosphatase 1B (PTP1B) as a deregulator of leptin signalling (Morris and Rui 2009). Studies have reported that chronic stress or postnatal caloric restriction increases PTP1B level in brain regions (Shin et al. 2012; Qin et al. 2015).
In this study, we examined first the anorectic efficacy of leptin injection on food intake and weight gain of MS rats at adolescence, and then the hypothalamic levels of leptin signalling molecules pSTAT3 and PTP1B with immunohistochemistry and Western blot analysis.
2 Materials and methods
2.1 Animals
Sprague–Dawley rats were purchased (Samtako Bio, Osan,Republic of Korea), and cared for in a pathogen-free barrier area with constant control of temperature (22±1°C), humidity (55%) and a 12/12 h light/dark cycle (lights-on at 07:00 h). Standard laboratory food (Purina Rodent Chow; Purina Co., Seoul, Republic of Korea) and membrane-filtered purified water were available ad libitum. Animals were cared for according to the Guideline for Animal Experiments 2000, edited by the Korean Academy of Medical Sciences, which is consistent with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals, revised 1996. All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals at Seoul National University.
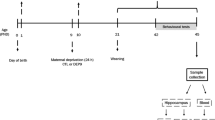
Nulliparous females and proven breeder males were used for breeding in the laboratory of the animal facility, and the pups were reared in a controlled manner to minimize and standardize unwanted environmental stimulation from in utero life. On the next morning of delivery [postnatal day (PND) 1], pups were culled to 5 males and 5 females per litter. Each litter was assigned either to the MS group or the non-handled (NH) group. MS was performed as previously described (Lee et al. 2007; Ryu et al. 2009; Yoo et al. 2013). In brief, MS pups were removed from their dams and home cage and placed closely together in a new cage bedded with woodchips (Aspen shaving, Animal JS Bedding, Cheongyang, Korea) for 180 min, and then returned to their home cage and dams. No additional treatment to keep the pups warm during the separation period other than placing them closely together, was offered, and thus, pup cooling during MS was expected. MS was performed during 09:00 h –12:00 h daily from PND 1–14, and then the pups were left with their dams undisturbed until weaning on PND 22. The NH group remained undisturbed until weaning except for routine cage cleaning. For cage cleaning, all rats were moved to a clean cage twice a week. On the weaning day, male pups in each litter were randomly selected and placed in each cage in a group of 2 or 3, and female pups were excluded from this study.
2.2 Drug treatments
Rats in each cage were assigned together either for leptin (NH/leptin or MS/leptin) or for vehicle (NH/saline or MS/saline) randomly (n=9–10 in each group, total 38 rats from 8 different litters), and received an intraperitoneal injection of leptin (Peprotech, Rocky Hill, NJ, USA) at a dose of 100 μg/2 mL saline/kg or the same injection volume of saline at 30 min before lights-off on PND 28, at early adolescence (Lukkes et al. 2009). Food intake and weight gain during 24 h after the leptin or saline injections were recorded. Total amounts of food consumed by the pups in each cage were divided by the number of pups in each cage and each calculated value was considered as n=1 for the evaluation of 24 h food intake of each rat.
For the analyses of immunostaining and Western blot, rats in each group received an intraperitoneal injection of leptin (100 μg/2 mL saline/kg) or the same injection volume of saline again at 30 min before lights-off on PND 39, and were sacrificed at 40 min after the injections. The injection schedule was decided on in order to minimize any effects of the previous injections done for behavioural assessment, and rats were at mid-adolescence (Lukkes et al. 2009). The hypothalamic brain samples were processed for pSTAT3 and STAT3 immunohistochemistry or PTP1B Western blot analysis.
2.3 Immunohistochemistry
Forty minutes after the leptin or saline injections, rats were anesthetized with overdoses of sodium pentobarbital (Hallym Pharmaceutical Co., Seoul, Korea) and transcardially perfused first with heparinized isotonic saline and then with 4% paraformaldehyde (Merck Co., Damstadt, Germany) in 0.1 M sodium phosphate buffer. Brains were rapidly dissected out, blocked, post-fixed for 2 h, and then transferred into 30% sucrose (Sigma Co., MO, USA) overnight for cryoprotection. Forty-micron coronal sections were cut on a freezing, sliding microtome (HM440E, Microm Co., Germany). Alternate sections were collected throughout the rostro-caudal extent of the hypothalamic arcuate nucleus (ARC). Immunohistochemistry was performed with standard DAB reaction using commercial ABC kit (Vectastain Elite Kit, Vector Laboratories, CA, USA) as previously described (Jahng et al. 1998). Monoclonal mouse anti-pSTAT3 antibodies (1:500 dilution, Cell Signaling Tech Inc., Danvers, MA, USA) were used as primary antibodies, and biotinylated anti-mouse IgG (1:200 dilution, Vector Laboratories, CA, USA) as secondary. The remaining alternate sections were processed for STAT3 immunohistochemistry [Polyclonal rabbit anti-STAT3 antibodies (1:1000 dilution, Calbiochem, Darmstadt, Germany) as primary antibodies, and biotinylated anti-rabbit IgG (1:200 dilution, Vector Laboratories, CA, USA) as secondary]. Immunostained sections were mounted in an anatomical order onto gelatin-coated slides from 0.05 M phosphate buffer, air-dried, dehydrated through a graded ethanol to xylene, and cover-slipped with Permount.
The number of pSTAT3 immunopositive cells in each section was blind-counted by hand, and STAT3 auto-counted, after digitizing the immunostained sections using an Olympus BX-51 microscope (Olympus Co., Tokyo, Japan) attached to a Leica image analysis system (DFC290, Leica Microsystems Gmbh, Wetzlar, Germany). STAT3 immunopositive cells in 720×540 micron images were quantified with Multi Gauge V3.0 software (FUJIFILM, Japan) and the mean relative optical density of pixels with densities of at least 2 S.D. above the mean density of the image background was analysed. pSTAT3 manual count was done and double-confirmed. The number of cells in three sections from the ARC region (closest sections to bregma – 1.88 mm; Paxions and Watson 1986) from each brain was averaged per section, and the individual mean counts were averaged across rats within experimental groups.
2.4 Western blot analysis
Rats were briefly anesthetized in a carbon dioxide chamber at 40 min after the leptin or saline injections, and once unresponsive, brains were removed immediately after decapitation, and the hypothalamic tissues were rapidly dissected on ice, transferred to liquid nitrogen and stored at –80°C. The tissues were homogenized in a single detergent lysis buffer (50 mM Tris, pH 8.0; 150 mM NaCl; 1% Triton X-100; protease and phosphatase inhibitor cocktail 0.5%) and then centrifuged at 13000g for 20 min at 4°C. The supernatants transferred into new tubes were measured for protein contents using a protein assay kit (Biorad DC, Biorad, Inc., Hercules, CA), aliquoted at a 40 μg/20 μL concentration in lysis buffer. The aliquoted samples were then mixed with loading buffer for the next step or stored at –80°C.
The samples were mixed with loading buffer (100 mM Tris, pH 6.8; 200 mM dithiothreitol; 4% SDS; 20% glycerol; 0.2% bromophenol blue) at 1:1 dilution, boiled for 5 min, quickly chilled on ice, and then electrophoresed on 12% SDS-polyacrylamide Tris-glycine gels. The proteins transferred onto nitrocellulose membranes (Hybond-C, Amersham, Bucks, UK) were treated with 5% nonfat dry milk in 1X phosphate buffered saline-Tween (1.46 mM NaH2PO4H2O; 8.05 mM Na2HPO4; 144.72 mM NaCl; 5% Tween 20) overnight at 4°C. The membranes were reacted with monoclonal mouse anti-PTP1B antibodies (Calbiochem, Darmstadt, Germany) at 1:1000 dilution for 1 h, and then reacted with HRP-conjugated goat anti-mouse antibodies (Vector laboratories, CA, USA) at 1:1000 dilution for 1 h at room temperature. The bound antibodies were detected with chemiluminescence according to the manufacturer’s instructions (SUPEX, Neuronex, Korea), and quantified using a digital image analysis system (LAS-1000, Fujifilm, Japan).
2.5 Statistical analysis
Data were analysed by one- and two-way analysis of variance (ANOVA) and preplanned comparisons with the control performed by post hoc Fisher’s PLSD test using StatView software (Abacus, Berkeley, CA). The level of significance was set at P<0.05, and all values were presented as means ± S.E.M.
3 Results
Food intake was measured for 24 h following an intraperitoneal injection of leptin or saline (figure 1A). Leptin administration at a dose of 100 μg/kg suppressed food intake of NH pups (P=0.0499, NH/saline vs. NH/leptin), but not of MS pups. Analysis of food intake with two-way ANOVA revealed no significant effect of MS or leptin and no interaction between separation and drug. Weight gain of leptin-injected NH pups during the 24 h of period following the injection was significantly reduced (P=0.0016) compared with saline-injected controls; however, leptin-induced weight loss was not observed in the MS group (figure 1B). Analysis of weight gain with two-way ANOVA revealed no significant effect of MS, but a main effect of leptin [F(1,33)=5.036, P=0.0317] and interaction between MS and leptin [F(1,33)=7.354, P=0.0105].
Food intake (A) and body weight gain (B) during 24 h after leptin or saline injection. NH and MS pups received an intraperitoneal injection of leptin at a dose of 100 μg/2 mL saline/kg or the same injection volume of saline at 30 min before lights-off on PND 28. NH, non-handled; MS, maternal separation; *P<0.05 vs. NH/saline. Data are presented by means ± S.E.M.
Tissue sections of the hypothalamic ARC were prepared at 40 min after the injections and processed for pSTAT3 or STAT3 immunohistochemistry (figure 2A). Leptin significantly increased the number of pSTAT3 immunopositive cells in the ARC of NH pups (P=0.0046, NH/saline vs. NH/leptin), but this increase was not observed in MS pups (figure 2B). Interestingly, the number of pSTAT3 immunopositive cells in the ARC of saline-injected MS pups were increased (P=0.0199) compared with saline-injected NH pups. Analysis of pSTAT3 levels in the ARC with two-way ANOVA revealed significant effects of MS [F(1,15)=6.331, P=0.0237] and leptin [F(1,15)=5.299, P=0.0361], and interaction between MS and leptin [F(1,15)=5.393, P=0.0347]. Neither leptin nor MS experience altered STAT3 levels in the ARC.
Representative microscopic photos (A) and quantification (B) of pSTAT3/STAT3 immuno-histochemistry in the arcuate nucleus. NH and MS pups received an intraperitoneal injection of leptin at a dose of 100 μg/2 mL saline/kg or the same injection volume of saline at 30 min before lights-off on PND 39, and were sacrificed at 40 min after the injections. NH, non-handled; MS, maternal separation; 3v, third ventricle; ARC, arcuate nucleus; R.O.D., relative optical density; *P<0.05 vs. NH/saline. Data are presented by means ± S.E.M.
In the Western blot analysis of the hypothalamic PTP1B, leptin did not affect PTP1B levels in both groups. However, the hypothalamic PTP1B levels of saline-injected MS pups was markedly increased (P=0.0493) compared with saline-injected NH pups (figure 3).
Western blot analysis of PTP1B. NH and MS pups received an intraperitoneal injection of leptin at a dose of 100 μg/2 mL saline/kg or the same injection volume of saline at 30 min before lights-off on PND 39, and were sacrificed at 40 min after the injections. NH, non-handled; MS, maternal separation; R.O.D., relative optical density; *P<0.05 vs. NH/saline. Data are presented as means ± S.E.M.
4 Discussion
Exogenic leptin as a potent anorectic molecule has been reported to suppress food intake and weight gain in rodent models (Pelleymounter et al. 1995; Schwartz et al. 1996). In the present study, intraperitoneal leptin at a dose of 100 μg/kg suppressed food intake and weight gain of NH control rats at early adolescence, proving its anorectic efficacy. The effect of leptin administration in NH rats seemed to be more obvious in weight gain than in food intake. It has been reported that leptin not only suppresses food intake but also increases locomotor activity and energy expenditure (Pelleymounter et al. 1995; Morton et al. 2011; Ribeiro et al. 2011). Although we did not measure the leptin-induced increases in locomotor activity and energy expenditure in the present study, it is plausible that leptin-induced weight loss in NH rats might have been contributed by increased locomotor activity and energy expenditure, in addition to reduced food intake. Interestingly, leptin-induced anorexia was not observed in rats that experienced neonatal maternal separation in the present study, suggesting that neonatal MS experience may blunt the hypothalamic leptin signalling system. To the extent of our knowledge, this is the first report demonstrating that early life stressful experiences may affect the development of the leptin signalling system in brain. Many studies have reported that leptin is a stress-response hormone and its action may be related with the HPA axis activity (Heiman et al. 1997; Konishi et al. 2006; Tasker 2006; Malendowicz et al. 2007; Jahng et al. 2008). In accordance with many other studies done with various types of MS models, our MS model showed a permanent alteration in the HPA axis activity later in life (Jahng 2011). Thus, it is suggested that alterations in the HPA axis activity by neonatal MS experience may affect, at least partly, the development of the leptin signalling system in MS brain.
Leptin binding to its receptor activates the JAK-STAT3 pathway (Ghilardi and Skoda 1997; Sahu 2011), and the leptin signalling via the JAK-STAT3 pathway in the hypothalamus appeared to be crucial in maintaining energy homeostasis and preventing extra weight gain (Gao et al. 2004). In this study, intraperitoneal leptin markedly increased the pSTAT3 level, but not STAT3, in the hypothalamic arcuate nucleus of NH control rats, showing the activation of leptin signalling system in the arcuate nucleus where neurons containing the anorectic neuropeptides, such as POMC or CART, are located. However, leptin-induced phosphorylation of STAT3 was not observed in the adolescent MS rats, suggesting that leptin signalling in the hypothalamic arcuate nucleus is blunted by neonatal MS experience. Circulating leptin level, which was decreased during food deprivation (Ahima et al. 1996; Schwartz et al. 1995; Makimura et al. 2003), is increased with refeeding (Wronska et al. 2014) and exerts its anorectic efficacy partly by increased expression of POMC and CART in the arcuate nucleus (Korner et al. 1999; Schwartz et al. 2000). We have previously reported that the adolescent MS pups, but not NH controls, show sustained hyperphagia during repeated fasting/refeeding cycles (Ryu et al. 2008). Taken together, it is concluded that a blunted leptin action in the arcuate nucleus of MS rats during the fasting/refeeding cycles might have contributed, at least partly, to the development of sustained hyperphagia.
In this study, the hypothalamic PTP1B level was markedly increased in MS rats compared with NH controls, suggesting a tonic increase of the hypothalamic PTP1B by neonatal MS experience. PTP1B induces dephosphorylation of JAK2, terminating leptin signal transduction (Kaszubska et al. 2002; Zabolotny et al. 2002). Thus, blunted leptin signalling in MS rats was plausibly due to the increased PTP1B level in the hypothalamus. A high level of PTP1B protein in the hypothalamus is associated with leptin resistance, hyperphagia and obesity (Picardi et al. 2008; Chiarreotto-Ropelle et al. 2013). Deregulation of the leptin signalling by PTP1B is related to diabetes and obesity (Cheng et al. 2002; Zabolotny et al. 2002; Asante-Appiah and Kennedy 2003). PTP1B ablation in POMC neurons resulted in leptin hypersensitivity and increased the energy expenditure in mice (Banno et al. 2010). Together with our previous report (Ryu et al. 2008), it is concluded that adolescent MS rats showed the sustained hyperphagia during fasting/refeeding cycles, at least partly, due to an increased PTP1B level in the hypothalamus, possibly blunting the leptin action. In most studies, increased PTP1B expression in obese states correlates with increased PTP1B activity (Dadke et al. 2000; Taghibiglou et al. 2002; Wu et al. 2005), implicating regulation of PTP1B protein expression as a major mechanism mediating increased PTP1B activity. However, it has not been clear how PTP1B expression is regulated in vivo. Chronic stress increased PTP1B activity in the amygdala and induced anxiety in mice, and the behavioural adversity was restored by PTP1B inhibition or by glucocorticoid receptor antagonism (Qin et al. 2015). Also, postnatal caloric restriction increased the hypothalamic PTP1B level (Shin et al. 2012). Our MS rats were separated from dams for 3 h daily during the first 2 weeks of birth; i.e. the MS pups experienced not only maternal deprivation but also food deprivation for 3 h daily. Although we did not examine the amygdala PTP1B level in this study, and Qin et al. (2015) did not report the hypothalamic PTP1B level in their stress model, it is likely that the increased PTP1B level in the hypothalamus of our MS rats is a consequence of repeated (chronic) separation and metabolic stresses in their early lives.
Lastly, pSTAT3 immunopositive cells were increased in the arcuate nucleus of MS rats compared with NH controls in this study. When STAT3 is phosphorylated as a part of leptin signalling cascade, pSTAT3 dimers are translocated into the nucleus and initiate the leptin-induced genes expression, such as POMC or CART (Sahu 2004). However, basal expression levels of POMC and CART in the arcuate nucleus of MS females did not differ from their NH controls (Yoo et al. 2011), and the effect of postnatal caloric restriction on the leptin signalling did not show gender differences (Shin et al. 2012). Thus, it is not likely that the increased pSTAT3 in the arcuate nucleus of MS rats is associated with increased expression of the anorectic genes POMC and CART. In fact, basal food intake and weight gain of MS pups during adolescent period did not differ from the age-matched NH controls (Ryu et al. 2009). Constitutive activation of STAT3 was reported in a cancer cell line, and the STAT3 target genes included ones that were involved in stress response (Xiong et al. 2012). Thus, it is likely that neonatal maternal separation, i.e. early life stressful experiences per se, is implicated in the regulatory mechanism underlying a tonic increase of the arcuate pSTAT3 level in MS rats. Further studies are warranted to define the molecular mechanisms involved in the MS-induced increases of pSTAT3 and PTP1B.
Abbreviations
- ARC:
-
arcuate nucleus
- CART:
-
cocaine- and amphetamine-regulated transcript
- HPA:
-
hypothalamic–pituitary–adrenal
- JAK2:
-
Janus kinase 2
- MS:
-
maternal separation
- NH:
-
non-handled controls
- PND:
-
postnatal day
- POMC:
-
proopiomelanocortin
- pSTAT3:
-
phosphorylated signal transducer and activator of transcription-3
- PTP1B:
-
protein-tyrosine phosphatase 1B
References
Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E and Flier JS 1996 Role of leptin in the neuroendocrine response to fasting. Nature 382 250–252
Asante-Appiah E and Kennedy BP 2003 Protein tyrosine phosphatases: the quest for negative regulators of insulin action. Am. J. Physiol. Endocrinol. Metab. 284 E663–E670
Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W and Bence KK 2010 PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Invest. 120 720–734
Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP and Tremblay ML 2002 Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell. 2 497–503
Chiarreotto-Ropelle EC, Pauli LSS, Katashima CK, Pimentel GD, Picardi PK, Silva VRR, de Souza CT, Prada PO, et al. 2013 Acute exercise suppresses hypothalamic PTP1B protein level and improves insulin and leptin signaling in obese rats. Am. J. Physiol. Endocrinol. Metab. 305 E649–E659
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, et al. 1996 Serum immunoreactive-leptin concentrations in normal weight and obese humans. N. Engl. J. Med. 334 292–295
Dadke SS, Li HC, Kusari AB, Begum N and Kusari J 2000 Elevated expression and activity of protein-tyrosine phosphatase 1B in skeletal muscle of insulin-resistant type II diabetic Goto-Kakizaki rats. Biochem. Biophys. Res. Commun. 274 583–589
Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI and Fu XY 2004 Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. PNAS 101 4661–4666
Ghilardi N and Skoda RC 1997 The leptin receptor activates Janus Kinase 2 and signals for proliforation in a factor-dependent cell line. Mol. Endocrinol. 11 393–399
Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW and Flier JS 1997 Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138 3859–3863
Jahng JW 2011 An animal model of eating disorders associated with stressful experience in early life. Horm. Behav. 59 213–220
Jahng JW, Houpt TA, Kim SJ, Joh TH and Son JH 1998 Neuropeptide Y mRNA and serotonin innervation in the arcuate nucleus of anorexia mutant mice. Brain Res. 790 67–73
Jahng JW, Kim NY, Ryu V, Yoo SB, Kim BT, Kang DW and Lee JH 2008 Dexamethasone reduces food intake, weight gain and the hypothalamic 5-HT concentration and increases plasma leptin in rats. Eur. J. Pharmacol. 581 64–70
Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, Kroeger PE, White DW, et al. 2002 Protein tyrosine phosphatase 1B regatively regulates leptin signaling in a hypothalamic cell line. Mol. Cell. Endocrinol. 195 109–118
Konishi N, Otaka M, Odashima M, Jin M, Wada I, Komatsu K, Sato T, Kato S, et al. 2006 Systemic stress increases serum leptin level. J. Gastroenterol. Hepatol. 21 1099–1102
Korner J, Chus SC Jr, Williams JA, Leibel RL and Wardlaw SL 1999 Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology 70 377–383
Lee JH, Kim HJ, Kim JG, Ryu V, Kim BT, Kang DW and Jahng JW 2007 Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci. Res. 58 32–39
Lukkes JL, Watt MJ, Lowry CA and Foster GL 2009 Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front. Behav. Neurosci. 3 18
Makimura H, Mizno TM, Isoda F, Beasley J, Silverstein JH and Mobbs CV 2003 Role of glucocorticoids in mediating effects of fasting and diabetes on hypothalamic gene expression. BMC Physiol. 3 5
Malendowicz LK, Rucinski M, Belloni AS, Ziolkowska A and Nussdorfer GG 2007 Leptin and the regulation of the hypothalamic-pituitary-adrenal axis. Int. Rev. Cytol. 263 63–102
Morris DL and Rui L 2009 Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 279 E1247–E1259
Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW and Wisse BE 2011 Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am. J. Physiol. Endocrinol. Metab. 300 E392–E401
Paxions G and Watson C 1986 The rat brain in stereotaxic coordinate (New York: Academic Press)
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T and Collins F 1995 Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 269 540–543
Picardi PK, Calegari VC, Prada PO, Moraes JC, Araújo E, Marcondes MC, Ueno M, Carvalheira JB, et al. 2008 Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology 149 3870–3880
Qin Z, Zhou X, Pandey NR, Vecchiarelli HA, Stewart CA, Zhang X, Lagace DC, Brunel JM, et al. 2015 Chronic stress induces anxiety via an amygdalar intracellular cascade that impairs endocannabinoid signaling. Neuron 85 1319–1331
Ribeiro AC, Ceccarini G, Dupre C, Friedman JM, Pfaff DW and Mark AL 2011 Contrasting effects of leptin on food anticipatory and total locomotor activity. PLoS One 6, e23364
Ryu V, Lee JH, Yoo SB, Gu XF, Moon YW and Jahng JW 2008 Sustained hyperphagia in adolescent rats that experienced neonatal maternal separation. Int. J. Obes. 32 1355–1362
Ryu V, Yoo SB, Kang DW, Lee JH and Jahng JW 2009 Post-weaning isolation promotes food intake and body weight gain in rats that experienced neonatal maternal separation. Brain Res. 1295 127–134
Sahu A 2004 Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front. Neuroendocrinol. 24 225–253
Sahu A 2011 Intracellular leptin signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology 93 201–210
Schwartz MW, Dallman MF and Woods SC 1995 Hypothalamic response to starvation: implications for the study of wasting disorders. Am. J. Physiol. 269 R949–957
Schwartz MW, Seeley RJ, Campfield LA, Burn P and Baskin DG 1996 Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 98 1101–1106
Schwartz MW, Woods SC, Porte D Jr, Seeley RJ and Baskin DG 2000 Central nervous system control of food intake. Nature 404 661–671
Shin BC, Dai Y, Thamotharan M, Gibson LC and Devaskar SU 2012 Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J. Neurosci. Res. 90 1169–1182
Taghibiglou C, Rashid-Kolvear F, VanIderstine SC, Le-Tien H, Fantus IG, Lewis GF and Adeli K 2002 Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J. Biol. Chem. 277 793–803
Tasker JG 2006 Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic intergration. Obesity Suppl 5 259S–265S
Wronska A, Sledzinski T, Goyke E, Lawniczak A, Wierzbicki P and Kmiec Z 2014 Short-term calorie restriction and refeeding differently affect lipogenic enzymes in major white adipose tissue depots of young and old rats. J. Physiol. Pharmacol. 65 117–126
Wu Y, Ouyang JP, Wu K, Wang SS, Wen CY and Xia ZY 2005 Rosiglitazone ameliorates abnormal expression and activity of protein tyrosine phosphatase 1B in the skeletal muscle of fat-fed, streptozotocin-treated diabetic rats. Br. J. Pharmacol. 146 234–243
Xiong H, Du W, Wang JL, Wang YC, Tang JT, Hong J and Fang JY 2012 Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J. Mol. Med. 90 1037–1046
Yoo SB, Ryu V, Park E, Kim BT, Kang DW, Lee JH and Jahng JW 2011 The arcuate NPY, POMC, and CART expressions responding to food deprivation areexaggerated inyoung female ratsthat experienced neonatal maternal separation. Neuropeptides 45 343–349
Yoo SB, Kim BT, Kim JY, Ryu V, Kang DW, Lee JH and Jahng JW 2013 Adolescence fluoxetine increases serotonergic activity in the raphe-hippocampus axis and improves depression-like behaviors in female rats that experienced neonatal maternal separation. Psychoneuroendocrinology 38 777–788
Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, et al. 2002 PTP1B regulates leptin signal transduction in vivo. Dev. Cell. 2 489–495
Acknowledgements
This study was supported by a grant from the National Research Foundation of Korea through the Oromaxillofacial Dysfunction Research Center for the Elderly (2015048003) at Seoul National University in Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Neeraj Jain
[Lee JH, Yoo SB, Kim JY, Lee JY, Kim BT, Park K and Jahng JW 2017 Early life stress experience may blunt hypothalamic leptin signalling. J. Biosci. 42]
Rights and permissions
About this article
Cite this article
Lee, J.H., Yoo, S.B., Kim, J.Y. et al. Early life stress experience may blunt hypothalamic leptin signalling. J Biosci 42, 131–138 (2017). https://doi.org/10.1007/s12038-016-9656-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-016-9656-3