Abstract
Cyclophosphamide (CP) is a popular cancer treatment; however, despite its efficacy, it is known to cause harm to the testicles. To mitigate the reproductive damage caused by CP in male rats, we examined the protective effect of azilsartan (AZ) on CP-induced testicular damage. Thirty Sprague–Dawley male rats were equally divided into three groups: normal control group: received 0.5% CMC suspension for 13 days; induction group: received a single dose of 200 mg/kg of CP on day 6 by intraperitoneal (IP) injection, azilsartan group: received azilsartan (4 mg/kg) orally for 5 days followed by a single dose of 200 mg/kg of (CP) on day 6 by IP injection, then azilsartan administered again for 7 days. Animals were sacrificed on day 14, and sperm characteristics, testosterone levels, and testicular histopathology were evaluated. Induction with CP caused a significant reduction in median value compared to normal control in sperm count (12.0 vs. 22.0 × 106/mm3), sperm motility (30 vs. 90%), abnormal sperm (30.32 vs. 14.43%), dead sperm count (32.43 vs. 10.49 × 106/mm3), DNA fragmentation (21.57 vs. 5.49%); meanwhile, azilsartan prevent these effects on median sperm count (17.0 × 106/mm3), sperm motility (70.0%), abnormal sperm (23.19%), dead sperm count (26.17 × 106/mm3), DNA fragmentation (13.81%), and improved plasmatic testosterone levels compared to the CP group and prevented histopathological alterations of the testes. Azilsartan’s mitigation of CP’s effects suggests it can prevent male rats’ reproductive damage caused by CP. One possible explanation for AZ’s protective effects is that it inhibits lipid peroxidation and has antioxidant properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility is a multifaceted disorder that affects 15% of couples. Infertility is defined as the inability to conceive a pregnancy through regular unprotected sexual intercourse for 12 months or more (Vander Borght and Wyns 2018). The male component is accountable for around 50% of infertility instances, making an equivalent contribution to the female factor (Kaya et al. 2019). Diagnosing male infertility often involves analyzing standard semen criteria, as outlined by the World Health Organization (WHO) guidelines (Organization 2021). However, a significant number of infertile males do not acquire a definitive diagnosis and are classified as idiopathic or unexplained cases (Schlegel et al. 2021; Al-Hamadani et al. 2019).
The imbalance between reactive oxygen species (ROS) formation and the body’s ability to defend against them with antioxidants is called oxidative stress (Vona et al. 2021). Several studies have indicated that oxidative stress is a recognized cause of unexplained male infertility (Mannucci et al. 2021; Takalani et al. 2023).
Cyclophosphamide (CP) is a cytostatic alkylating agent used in animals and humans (Kaya et al. 2019). Its use is associated with male infertility in both human (Yang et al. 2019; Ledingham et al. 2020) and animals (Al-Niwehee and Alrudaini 2019). Evidence from studies on cell proliferation shows that CP has harmful effects, mainly on rapidly dividing cells, in particular, the effect of CP on testes (Joseph et al. 2020). The interactions between CP and tissues with rapid turnover constitute the basis for the drug’s therapeutic and harmful effects (van den Boogaard et al. 2022). Testicular tissues are more susceptible to CP effects for several reasons, such as increased absorption of polyunsaturated fatty acids, essential in protecting against oxidative damage, and because CP works on rapidly dividing cells (Alkhalaf et al. 2020).
Management of the reproductive toxicity of CP still requires innovative therapeutic approaches (Alkhalaf et al. 2020). Due to the risk of drug exposure during the manufacturing and transportation process, it is crucial to address CP hazards for both cancer patients and healthcare professionals, including chemists. Therefore, effective management of the reproductive toxicity of CP requires the implementation of innovative therapeutic techniques (Anan et al. 2018).
The renin-angiotensin system (RAS) plays a crucial role in maintaining the body’s balance of fluids and electrolytes and regulating the resistance of blood vessels throughout the body (Kalupahana and Moustaid-Moussa 2024). The renin–angiotensin–aldosterone system (RAAS) and its crucial element, angiotensin II (Ang II), are notably potent inducers of inflammation, reactive oxygen species (ROS), and oxidative stress in several diseases (Ramalingam et al. 2017). According to reports, different components of this system are found in different areas of the male reproductive tract, including the epididymis, vas deferens, prostate, seminal fluid, testes, and spermatozoa (Pascolo et al. 2020). Given that Leydig cells are responsible for producing their transcripts, it can be inferred that the local RAS regulates testosterone synthesis (Pascolo et al. 2020).
Due to the ability of angiotensin II to generate reactive oxygen species, which can trigger inflammatory responses and harm cells, blocking the RAAS has emerged as a logical therapeutic strategy for addressing oxidative stress and inflammation, which are the underlying causes of the disease. Mucosal vasoconstriction is the primary route by which these effects are communicated (Sachse and Wolf 2007; Dikalov and Nazarewicz 2012). Azilsartan possesses lower acidity and higher lipophilicity than other angiotensin receptor blockers (ARBs) due to its close structural similarity to candesartan. This structural similarity contributes to its increased potency and extended duration of action. Furthermore, it exhibits a strong affinity for AT1 receptors and has a prolonged dissociation rate, distinguishing it from other ARBs currently approved for clinical use (Kurtz and Kajiya 2012). Due to the potential of azilsartan as an antioxidant, among other possible mechanisms, and the close relationship between the damage of ROS against testicular dysfunction, we examined the potential use of azilsartan as a novel agent to ameliorate male infertility. The main objective of this study is to investigate azilsartan’s effectiveness as an innovative approach for treating male infertility using the CP-induced rat model.
Methods
Chemicals
Cyclophosphamide was supplied as powder preparation by Hangzhou Royal pharm®/China. Azilsartan was supplied as powder preparation by Hangzhou Royal pharm®/China.
Animals and study settings
Thirty Sprague–Dawley Male rats (weighing 280–350 g, ranging in age between 8 and 9 weeks). The rats were purchased from the Iraqi Center for Cancer Research and Medical Genetics. Rats were acclimatized for 10 days before experiments. They were housed individually in plastic cages covered by stainless-steel nets. The study was carried out et al.-Nahrain University/College of Medicine between the October 1, 2023 and April 2024.
Wood shavings were autoclaved and used as bedding material. Water and standard food pellets (Elazig Food Company, Turkey) were provided ad libitum in the ventilated room at 24 ± 2 °C with a 12-h-light/dark cycle and 40–60% humidity.
Preparation of azilsartan suspension (Lan et al. 2018)
The azilsartan suspension was produced in a sterile colloidal suspension. Azilsartan was dissolved to make a 0.5% w/v suspension of carboxy methyl cellulose (CMC), as follows: first, 500 mg of Na-CMC was dissolved in 7 ml of distilled water using a flame alcohol burner (60℃). While stirring, distilled water was slowly added until a colloidal suspension of 100 ml in a volumetric flask was formed, and 0.5% NaCMC was formed (Lan et al. 2018). Then, the 12 mg of azilsartan powder is added gradually with continuous stirring in 10 ml CMC suspension until a homogeneous suspension is formed, with the resultant azilsartan 0.5%-CMC suspension (Hussain et al. 2017).
Study design
Testicular toxicity was induced by intraperitoneal (IP) administration of CP (200mg/kg as a single dose) on day 6 of the experiment (Chabra et al. 2014). Rats in the normal control group (n = 10) received 1.0 ml of the vehicle (0.5% CMC suspension) for 13 days, while rats in the CP group (n = 10) received no intervention for 5 days, followed by a single dose of 200 mg/kg of CP on day 6 by IP injection and were left untreated for the following 7 days. In the azilsartan group, the animals received 4mg/kg of freshly prepared azilsartan suspension and were given orally once daily using gastric gavage (Gupta et al. 2020; Alaaeldin et al. 2023) for 5 days. On day 6, the animals received a single dose of 200 mg/kg of CP by IP injection. On day 7 (after 24 h of CP induction), azilsartan was continued orally at 4 mg/kg once daily until day 13, as illustrated by Fig. 1.
Preparation and sampling of animals
On day 14 of the experiment, the animals were euthanized by cervical dislocation under anesthesia with ketamine-xylazine (all rats were anesthetized intraperitoneally (IP) with 80 mg/kg of ketamine (ketamine 10%, Alfasan Nederland BV, Holand) and 10 mg/kg of xylazine (XYL-M2, VMD® Livestock Pharma, Belgium)) (Dodelet-Devillers et al. 2016; Underwood and Anthony 2020; Pierozan et al. 2017) (The animals were selected randomly by an observer who was unaware of the grouping), only animals with confirmed infertility status in CP-treated rats were included in the study (which was evaluated by the sperm characteristics and histopathological examination).
All animals were weighed on the 14th day, and blood samples were collected from cardiac puncture with 5-mL syringes for the hormonal assay. Immediately after blood collection, testes and epididymis were dissected and weighed by sensitive balance. The blood samples were transferred to gel tubes, and their serum samples were obtained using a centrifuge unit with 3000 RPM for 5 min. The serum samples were transferred to Eppendorf tubes and stored at − 20 °C until hormonal and biochemical measurements were taken. At the same time, the weight of the testis and epididymis was expressed as a percentage of body weight. A single testis was taken for histopathologic assessment (maintained in formalin 10%), and the other was collected for quantitative measurement of testosterone, oxidative, and antioxidant effects by using enzyme-linked immunosorbent assay (ELISA) manufactured by (Cloud-clone crop® /England).
Testicular homogenates
Each testis sample, weighing 100 mg, was homogenized in 900 µL of 0.1 M phosphate-buffered saline (PBS) with a pH of 7.4. Subsequently, the sample was centrifuged at 4500 revolutions per minute for 15 min at a temperature of 4 °C. The resulting liquid portion was then collected (Mesbahzadeh et al. 2021). The homogenates were used to measure the concentrations of malondialdehyde (MDA), glutathione peroxidase (GPx), and activated oxidized protein product (AOPP) spectrophotometrically using the ELISA technique (Cloud-clone crop® /England) and the ELISA Reader (ELISA reader, Diagnostic Automation / Cortez Diagnostics®, California, USA).
Serum testosterone
Serum level of testosterone was assessed using ELISA according to the manufacturer’s instructions.
Sperm analysis
Motility
The cauda epididymis of each rat was extracted and purified from the epididymal fat pad. It was then finely chopped in a Petri dish with 1 ml of a phosphate buffer saline solution and left to incubate for 2 min at a temperature of 35 °C. Sperm motility was assessed by depositing a ten-microliter sample of sperm suspension on a clean slide, which was then covered with a 24 × 24 coverslip. The sample was viewed under a microscope using × 400 lenses. A sperm was deemed motile when it displayed discernible flagella movement. Sperm motility was evaluated by examining at least five microscopic areas and calculating the mean value (Akinwande et al. 2019).
Viability
Sperm viability was evaluated under a microscope by creating smears; this involved combining 10 μL of eosin-nigrosine stain with 10 μL of epididymal suspensions, incubating the mixture for 30 s, spreading the stained suspensions on glass slides, and allowing them to dry in the air. After 30 s, a small amount of the combination was deposited onto a glass slide and evenly distributed to create a thin layer, left to dry in the air. The smear was analyzed using a light microscope with × 100 oil immersion. Unstained sperm that were alive were observed, while dead sperm were stained with a pink or red color. A minimum of 200 spermatozoa were assessed, and the vitality of the sperm was measured as a ratio of dead-to-live sperm (Okolo et al. 2017).
Sperm count
The semen samples were extracted from the posterior region of the epididymis. First, a portion of the epididymis was coarsely chopped into small pieces and placed in 1 mL of a saline solution. It was then kept at 37℃ for approximately 2 min to allow the sperms to escape from the epididymis. An aqueous solution containing 25 mg of eosin per 100 mL of distilled water, 1 mL of formalin (35%), and 5 g of sodium bicarbonate (NaHCO3) was used to dilute the supernatant. A 10-μL droplet of this solution was introduced into the sperm-counting chamber and observed using a light microscope at a magnification of 400 × (Ijaz et al. 2023).
DNA fragmentation using acridine orange (AO) assay
The AO assay quantifies the capacity of sperm nuclear DNA to undergo acid-induced denaturation, resulting in a metachromatic shift of AO fluorescence from green (indicating native DNA) to red (indicating denatured DNA). AO, a fluorochrome, attaches to double-stranded DNA as a monomer and binds to single-stranded DNA. When the AO molecule is attached to intact DNA, it emits a green fluorescence. However, when the AO molecules are clumped together on unfolded DNA, they emit a red fluorescence (Mohammed et al. 2015).
To conduct this fluorescence microscopy assay, dense semen layers are treated with a fixative solution (methanol: glacial acetic acid 3:1) for 2 h. The slides are immersed in a staining solution for 5 min and washed with water. The slides were rinsed with distilled water, placed beneath a glass cover, and seen using a fluorescence microscope with an excitation wavelength of 450–490 nm. The same examiner examined an average of 200 sperm cells on each slide. Spermatozoa exhibiting green fluorescence possessed intact DNA, while those displaying a yellow-orange to red fluorescence spectrum were deemed to have DNA damage (Hoshi et al. 1996).
Histopathology assay
The testicle tissues were fixed and dehydrated in a 10% formalin solution and subsequently covered with paraffin. Subsequently, the tissues were sliced into sections: 5 μm in thickness and stained with Hematoxylin and eosin (H & E) stain.
Johnsen’s scoring (JS) system
The Johnsen’s score (JS) method was utilized to classify the effectiveness of spermatogenesis. The extent of testicular injury was assessed using a scale from 1 to 10, with 1 indicating the absence of seminiferous epithelium and 10 signifying full spermatogenesis and intact tubules (Tang et al. 2018). All the evaluations were done blindly.
Gonadosomatic index (GSI) evaluation
The testis and cauda that were removed after dissection were weighted accurately using electrical balance, and then the animal organ index was calculated according to the following equation (Chen et al. 2021):
Statistical analysis
Due to the data’s non-normal distribution, the Kruskal–Wallis and post hoc Dunn tests were employed to compare groups. The data are displayed as the median and interquartile range (IQR). The data underwent analysis using GraphPad Prism 10.2, which generated graphs and figures. The animal sample size was determined using the G.Power 3.1 software (post hoc sample size was done with an effect size of 0.5 and an alpha level of 0.05, F-family tests with a total sample size of 30 for each group of ten animals).
Results
Effect of azilsartan on seminal fluid analysis
The analysis results revealed a statistically significant reduction in sperm count and motility in animals that received CP induction compared to normal control. In contrast, there was a significant elevation in the dead sperms, abnormal cells, and DNA fragmentation of the seminal fluid analysis in the CP induction group compared to the normal control group, as illustrated by Figs. 2 and 3.
Sperm analysis of the animal groups showing the percentages of A dead sperm, B sperm motility, C abnormal cells, D DNA fragmentation, and E total sperm count. (n = 10 for each group, a single asterisk (*) indicates p-value ≤ 0.05, quadruple asterisks (****) indicate p-value ≤ 0.0001). CP, cyclophosphamide (the induction agent); DNA, deoxyribonucleic acid
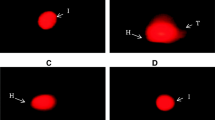
A representative image of some of the parameters of the seminal fluid analysis of the animal groups. A represents the life and dead sperms where the dead sperms were stained bright pink with (eosin-nigrosin stain), B represents the DNA fragmentation analysis of the sperms where sperms showing bright yellow-orange heads were those with fragmented DNA. CPM, cyclophosphamide (the induction agent); DNA, deoxyribonucleic acid
In azilsartan-treated animals, the results revealed a marked statistical improvement in all the parameters of the seminal fluid analysis (except for dead sperm count, which showed non-significant improvement) compared to the CP induction group (Table S1), as illustrated by Figs. 2 and 3.
Effect of azilsartan on gonadosomatic index
Results of the study revealed that the induced CP-treated animals expressed a marked and statistically significant reduction (≤ 0.0001) in the GSI (testicular and cauda) as compared to the normal (healthy) animals. Compared to the induced CP group, azilsartan-treated animals statistically improved the GSI (P ≤ 0.05), as seen in Fig. 3C and D.
Effects of azilsartan on histopathological features and score
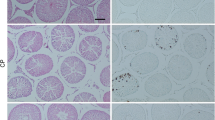
The histopathological examination of the normal control group revealed the presence of intact testicular structure, characterized by well-defined seminiferous tubules at various stages of spermatogonial cell development (including normal spermatogonia, primary spermatocytes, spermatids, and Sertoli cells). Furthermore, the slide displayed a normal spermatogenesis process, with a visible lumen containing spermatozoa and interstitial cells (Fig. 4A). Rats subjected to CP treatment (induction group) exhibited a deficiency in primary spermatocytes, spermatids, and Sertoli cells. Additionally, there was disorganization of spermatogenic cells with a decreased number of spermatids and damage and disruption of the epithelial walls of the seminiferous tubules (Fig. 4B). The azilsartan group showed well-preserved testicular tissue with a significant presence of spermatogonia, primary spermatocytes, spermatids, and Sertoli cells. The epithelial walls of the seminiferous tubules were shown to be intact in Fig. 4C.
Changes in the testis histology and index among animal groups that represent A histopathological features in the control group, B histopathological features in the induction group, C histopathological features in the azilsartan group, D testicular index, E cauda index, and F Johnsen’s score. H and E stain 10 × and 40 × power. (n = 10 for each group, a single asterisk (*) indicates p-value ≤ 0.05, double asterisks (**) indicate p-value ≤ 0.01, triple asterisks (***) indicate p-value ≤ 0.001, quadruple asterisks (****) indicate p-value ≤ 0.0001). CP, cyclophosphamide (the induction agent)
The testicular index, Cauda index, and Johnsen’s score were significantly lower in the induction group compared to the normal control group; the azilsartan group showed significantly higher testicular index, Cauda index, and Johnsen’s score compared to the induction group, as seen in Fig. 4D, E, and F.
Effect of azilsartan on oxidative stress markers
The results revealed that there was a statistically significant (P ≤ 0.0001) elevation in the tissue level of the oxidative markers (MDA and AOPP) in the induction group compared to normal control, as seen in Fig. 5A and B. In contrast, there was an evident and significant reduction (P ≤ 0.0001) in the tissue level of the antioxidant marker (GPx) in the induction group compared to the normal control, as seen in Fig. 5C.
Changes in testicular tissue level of oxidative markers among animal groups. A MDA levels, B AOPP levels, and C GPx levels. (n = 10 for each group, a single asterisk (*) indicates p-value ≤ 0.05, triple asterisks (***) indicate p-value ≤ 0.001, quadruple asterisks (****) indicate p-value ≤ 0.0001). CP, cyclophosphamide (the induction agent); MDA, malondialdehyde; AOPP, advanced oxidation protein product; GPx, glutathione peroxidase
In azilsartan-treated animals, the analysis of the results shows a remarkable reduction in the tissue level of (MDA and AOPP), an elevated level of the antioxidant GPx with very high (P ≤ 0.0001), which was statistically significant compared to the induction group, as illustrated in Fig. 5.
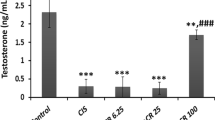
Effect of azilsartan on the testosterone level
The results revealed a drastic and significant reduction in the serum level of testosterone in the induction group compared to the normal control group. In azilsartan-treated animals, the analysis of the results shows a remarkable elevation in the serum level of testosterone with statistical significance compared to the induction group, as seen in Fig. 6.
Discussion
The advancement of therapeutic protocol to treat cancer patients has led to improvement in the survival rates of such patients; this increase in survival rates necessitates managing adverse effects associated with using an anticancer medication; among these adverse effects is infertility. CP is a common component of several anticancer medication protocols like breast cancer (Lambertini and Partridge 2021; Shihab et al. 2024), Hodgkin lymphoma (Nayak et al. 2021), and acute lymphoblastic leukemia (Zhao et al. 2019); these cancers are associated with a high rate of survival; in these patients, CP-associated infertility is a major burden (Abu-Risha et al. 2022). Assessments of testicle injury rely on evaluations of sperm and hormonal levels. CP causes significant testicular damage, especially during the first stages of sperm production (Ghafouri-Fard et al. 2021).
This led to searching for safe options to prevent initially on one hand while maintaining its therapeutic potential as an anticancer agent; this concept was the major drive in this study’s hypothesis (Moss et al. 2016; Nurgali et al. 2018). Testicular toxicity is a significant problem in cancer treatment, which has led to the development of a new field of medicine known as oncofertility (Ntemou et al. 2019). The goal of oncofertility is to preserve the reproductive capacity of people who have undergone chemotherapy or radiotherapy (Ahmed et al. 2022).
The present investigation found that CP treatment resulted in testicular toxicity, characterized by a notable decrease in sperm motility, sperm viability, and sperm count. Additionally, there was an increase in the proportion of sperm with DNA fragmentation and various sperm abnormalities (tail defects, neck and middle piece defects, and head defects, along with changes in the histological structure of the testes and a significant reduction in primary spermatocytes, spermatids, and Sertoli cells.
In line with our research, Kaya et al. provided evidence that CP treatment caused DNA damage in sperm and decreased sperm count and motility in rats following a single dose of exposure (Kaya et al. 2019). Previous reports have indicated that rats treated with CP showed a reduction in the number of normal testicular germ cells (hypo-spermatocytes), increased abnormal sperms, and abnormal testicular histology reflecting infertility (Delessard et al. 2020; Chabra et al. 2014; Abarikwu et al. 2012; Khamis et al. 2023; Hussein et al. 2024b). These findings provide more evidence supporting the harmful impact of CP on male reproductive function.
Oxidative stress is a well-defined factor in male infertility (Asadi et al. 2021), since testicular tissue can be damaged by peroxidative stress owing to its high mitotic activity (Alkhalaf et al. 2020; Khamees et al. 2018). Sujayraj et al. discovered a correlation between an increase in germ cell apoptosis and the harmful effects of CP on testicular weight and GSI parameters (Sujayraj et al. 2016). Similarly, our investigation found that CP treatment significantly lower testicular and cauda weights than the control group.
In the current study, CP treatment was associated with an elevation in oxidative stress markers (AOPP and MDA) and a reduction in GPx levels compared to the control group. On the other hand, pretreatment with azilsartan showed a reduction in the negative effects induced by CP, with significant elevation in GPx levels and reduction in MDA and AOPP levels in the testicular tissue compared to the CP-treated group. These findings indicate azilsartan offered partial protection against CP-treatment-associated infertility.
In addition to changes in body and sexual organ weights, testosterone levels are also influenced by CP treatment. Testosterone has a crucial role in controlling the process of spermatogenesis through its effect on androgen receptors (Smith and Walker 2014). The injection of CP leads to a decrease in testosterone secretion; this has a detrimental impact on testicular function, resulting in a reduction in sperm count, motility, and viability, as well as an increase in sperm morphological abnormalities (Gajjar et al. 2015). These findings were seen in the current investigation.
Remarkably, in rats subjected to CP treatment, the administration of azilsartan was correlated with the ability to enhance testosterone levels, suggesting the intensity of pituitary positive feedback was less pronounced and functioning Leydig cell–associated testosterone biosynthesis was partially preserved with azilsartan pretreatment. These findings are in agreement with a previous study that showed that treatment with CP increases the levels of HSH and LH and reduces the levels of testosterone (Ibrahim et al. 2023).
Azilsartan is a novel angiotensin II type 1 receptor (AT1) blocker that distinguishes itself from existing approved ARBs by containing a 5-member oxo-oxadiazole ring instead of a tetrazole ring. This structural feature is associated with decreased acidity and increased lipophilicity of azilsartan, making it more capable of binding to the AT1 receptor than other members (Miura et al. 2013).
The Mitogen-activated protein kinases (MAPK) signaling pathway is prominent in cellular signaling pathways. Various external cues, including hormones, cytokines, and cell stress, can trigger the activation of this pathway (Cargnello and Roux 2011). The P38 MAPK signaling pathway is the primary constituent of the MAPK (Li et al. 2022b). The p38 MAPK signaling pathway is significantly involved in the damage caused to the testes due to oxidative stress. Furthermore, it is intricately linked to the control of spermatogenesis (Luo et al. 2022). Since the primary mechanism of CP-induced testicular toxicity is caused by ROS damage (Alkhalaf et al. 2020). Previous studies have shown that increased levels of ROS can trigger the activation of the p38 MAPK signaling pathway in cases of testicular injury in individuals with diabetes (Omolaoye and Du Plessis 2021). The blood–testis barrier integrity is a critical physiological mechanism that protects spermatogenesis from the immune system and harmful chemicals in the blood; this integrity is directly affected by increased p38 MAPK activity (Li et al. 2022a). Concerning this issue, a previous study by Sukumaran et al. showed that olmesartan, an AT1 receptor antagonist, protects against experimental autoimmune myocarditis by reducing the activation of p38MAPK (Sukumaran et al. 2012). Also, Bekhit et al. showed that azilsartan has a hepatoprotective effect against cisplatin-induced liver damage by controlling the MAPK signaling pathway (Bekhit et al. 2023). Additionally, compared to valsartan (another AT1 receptor antagonist), azilsartan significantly increased adipogenesis in rats by causing overexpression of PPAR-γ (Kajiya et al. 2011). Activated PPAR-γ has a crucial role in connecting lipid metabolism and reproduction in a broad sense. Sperm physiology and male fertility depend on the energy derived from glucose and fat metabolism, which the PPAR-signaling pathway regulates (Bekhit et al. 2023, Raheem and Mohammed Ali Mahmood 2023, Hussein et al. 2024a).
Study limitation
While animal models provide valuable insights into human disorders, they may need to depict the complex nature of male infertility in humans fully. The study did not utilize clinical data acquired from human volunteers. Although the results in the rat model indicate promise, further inquiry is necessary to determine the safety and efficacy of azilsartan in humans.
Conclusion
Azilsartan pretreatment showed an ameliorative effect against cyclophosphamide-induced testicular toxicity; this effect was associated with improved testosterone levels, indicating preservation of Leydig cell–associated testosterone biosynthesis. Azilsartan showed a potent antioxidant effect, as indicated by improved glutathione peroxidase activity and reduced oxidative stress markers. These findings indicate azilsartan’s potential as a protective agent in cancer patients receiving cyclophosphamide.
Data availability
Data are available upon request from the corresponding authors.
References
Abarikwu SO, Otuechere CA, Ekor M, Monwuba K, Osobu D (2012) Rutin ameliorates cyclophosphamide-induced reproductive toxicity in male rats. Toxicol Int 19:207–214. https://doi.org/10.4103/0971-6580.97224
Abu-Risha SE, Mousa MA, Elsisi AE (2022) Protective role of irbesartan against cyclophosphamide-induced testicular damage in rats via up-regulating PPAR-γ signaling and ameliorating NF-κB/NLRP3/IL-18 inflammatory axis. Life Sci 289:120218. https://doi.org/10.1016/j.lfs.2021.120218
Ahmed Y, Khan AMH, Rao UJ, Shaukat F, Jamil A, Hasan SM, Abrar S, Qureshi BM, Abbasi AN (2022) Fertility preservation is an imperative goal in the clinical practice of radiation oncology: a narrative review. Ecancermedicalscience 16:1461. https://doi.org/10.3332/ecancer.2022.1461
Akinwande DV, Adeyemi JA, Olawuyi ST, Akinola BK, Adedire CO (2019) Protective effects of Camellia sinensis on Syzygium aromaticum- or chlorpyrifos-induced reproductive toxicity in male Wistar rats. J Basic Appl Zool 80:48. https://doi.org/10.1186/s41936-019-0122-2
Alaaeldin R, Bakkar SM, Mohyeldin RH, Ali FEM, Abdel-Maqsoud NMR, Fathy M (2023) Azilsartan modulates HMGB1/NF-κB/p38/ERK1/2/JNK and apoptosis pathways during renal ischemia reperfusion injury. Cells 12:185
Al-Hamadani IT, Shehab EM, Aziz NA (2019) Comparative changes in sexual dysfunction of married women after colpoperineorrhaphy versus colpoperineorrhaphy with additional platelet rich plasma injection. Indian J Forensic Med Toxicol 13:314–320. https://doi.org/10.5958/0973-9130.2019.00309.8
Alkhalaf MI, Alansari WS, Alshubaily FA, Alnajeebi AM, Eskandrani AA, Tashkandi MA, Babteen NA (2020) Chemoprotective effects of inositol hexaphosphate against cyclophosphamide-induced testicular damage in rats. Sci Rep 10:12599. https://doi.org/10.1038/s41598-020-68608-9
Al-Niwehee NA, Alrudaini AT (2019) Effect of cyclophosphamide treatment during the embryonic period on fertility of adult male Mice. Iraqi J Sci 60:706–714
Anan HH, Zidan RA, Abd El-Baset SA, Ali MM (2018) Ameliorative effect of zinc oxide nanoparticles on cyclophosphamide induced testicular injury in adult rat. Tissue Cell 54:80–93. https://doi.org/10.1016/j.tice.2018.08.006
Asadi A, Ghahremani R, Abdolmaleki A, Rajaei F (2021) Role of sperm apoptosis and oxidative stress in male infertility: a narrative review. Int J Reprod Biomed 19:493–504. https://doi.org/10.18502/ijrm.v19i6.9371
Bekhit AA, Beshay ON, Fawzy MA, Abdel-Hafez SMN, Batiha GE-S, Ataya FS, Fathy M (2023) Curative effect of AD-MSCs against cisplatin-induced hepatotoxicity in rats is potentiated by azilsartan: targeting oxidative stress, MAPK, and apoptosis signaling pathways. Stem Cells International 2023:6767735. https://doi.org/10.1155/2023/6767735
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75:50–83. https://doi.org/10.1128/mmbr.00031-10
Chabra A, Shokrzadeh M, Naghshvar F, Salehi F, Ahmadi A (2014) Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Hum Exp Toxicol 33:185–195. https://doi.org/10.1177/0960327113489052
Chen Z, Shi K, Kuang W, Huang L (2021) Exploration of the optimal strategy for dietary calcium intervention against the toxicity of liver and kidney induced by cadmium in mice: An in vivo diet intervention study. PLoS ONE 16:e0250885. https://doi.org/10.1371/journal.pone.0250885
Delessard M, Saulnier J, Dumont L, Rives-Feraille A, Rives N, Rondanino C (2020) Paradoxical risk of reduced fertility after exposure of prepubertal mice to vincristine or cyclophosphamide at low gonadotoxic doses in humans. Sci Rep 10:17859. https://doi.org/10.1038/s41598-020-74862-8
Dikalov SI, Nazarewicz RR (2012) Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal 19:1085–1094. https://doi.org/10.1089/ars.2012.4604
Dodelet-Devillers A, Zullian C, Beaudry F, Gourdon J, Chevrette J, Hélie P, Vachon P (2016) Physiological and pharmacokinetic effects of multilevel caging on Sprague Dawley rats under ketamine-xylazine anesthesia. Exp Anim 65:383–392. https://doi.org/10.1538/expanim.16-0026
Gajjar R, Miller SD, Meyers KE, Ginsberg JP (2015) Fertility preservation in patients receiving cyclophosphamide therapy for renal disease. Pediatr Nephrol 30:1099–1106. https://doi.org/10.1007/s00467-014-2897-1
Ghafouri-Fard S, Shoorei H, Abak A, Seify M, Mohaqiq M, Keshmir F, Taheri M, Ayatollahi SA (2021) Effects of chemotherapeutic agents on male germ cells and possible ameliorating impact of antioxidants. Biomed Pharmacother 142:112040. https://doi.org/10.1016/j.biopha.2021.112040
Gupta V, Dhull DK, Joshi J, Kaur S, Kumar A (2020) Neuroprotective potential of azilsartan against cerebral ischemic injury: possible involvement of mitochondrial mechanisms. Neurochem Int 132:104604. https://doi.org/10.1016/j.neuint.2019.104604
Hoshi K, Katayose H, Yanagida K, Kimura Y, Sato A (1996) The relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human sperm. Fertil Steril 66:634–639. https://doi.org/10.1016/S0015-0282(16)58581-1
Hussain SA, Utba RM, Assumaidaee AM (2017) Effects of azilsartan, aliskiren or their combination on high fat diet-induced non-alcoholic liver disease model in rats. Med Arch 71:251–255. https://doi.org/10.5455/medarh.2017.71.251-255
Hussein ZA, Abu-Raghif AR, Fawzi HA (2024a) The mitigating effect of para-hydroxycinnamic acid in bleomycin-induced pulmonary fibrosis in mice through targeting oxidative, inflammatory and fibrotic pathways. Basic Clin Pharmacol Toxicol 135:23–42. https://doi.org/10.1111/bcpt.14018
Hussein ZA, Abu-Raghif AR, Tahseen NJ, Rashed KA, Shaker NS, Fawzi HA (2024b) Vinpocetine alleviated alveolar epithelial cells injury in experimental pulmonary fibrosis by targeting PPAR-γ/NLRP3/NF-κB and TGF-β1/Smad2/3 pathways. Sci Rep 14:11131. https://doi.org/10.1038/s41598-024-61269-y
Ibrahim D, Abozied N, Abdel Maboud S, Alzamami A, Alturki NA, Jaremko M, Alanazi MK, Alhuthali HM, Seddek A (2023) Therapeutic potential of bone marrow mesenchymal stem cells in cyclophosphamide-induced infertility. in Pharmacol 14. https://doi.org/10.3389/fphar.2023.1122175
Ijaz MU, Najam S, Hamza A, Azmat R, Ashraf A, Unuofin JO, Lebelo SL, Simal-Gandara J (2023) Pinostrobin alleviates testicular and spermatological damage induced by polystyrene microplastics in adult albino rats. Biomed Pharmacother 162:114686. https://doi.org/10.1016/j.biopha.2023.114686
Joseph KB, Awadallah N, Delay ER, Delay RJ (2020) Transient effects of cyclophosphamide on basal cell proliferation of olfactory epithelia. Chem Senses 45:549–561. https://doi.org/10.1093/chemse/bjaa039
Kajiya T, Ho C, Wang J, Vilardi R, Kurtz TW (2011) Molecular and cellular effects of azilsartan: a new generation angiotensin II receptor blocker. J Hypertens 29:2476–2483. https://doi.org/10.1097/HJH.0b013e32834c46fd
Kalupahana NS, Moustaid-Moussa N (2024) Beyond blood pressure, fluid and electrolyte homeostasis – role of the renin angiotensin aldosterone system in the interplay between metabolic diseases and breast cancer. Acta Physiol 240:e14164. https://doi.org/10.1111/apha.14164
Kaya C, Barbaros Baseskioglu A, Yigitaslan S, Yasemin Ozatik F, Ozatik O, Uslu S (2019) The therapeutic potential of amifostine on cyclophosphamide-induced testicular dysfunction in rats: an experimental study. Int J Reprod Biomed 17:245–52. https://doi.org/10.18502/ijrm.v17i4.4549
Khamees AH, Abdulhussein AJ, Sahib HB, Fawzi HA (2018) Anti-angiogenic and antioxidant activity of Iraqi Cyperus rotundus ethanol extract. Int J Pharmacol 14:546–552. https://doi.org/10.3923/ijp.2018.546.552
Khamis T, Hegazy AA, El-Fatah SSA, Abdelfattah ER, Abdelfattah MMM, Fericean LM, Arisha AH (2023) Hesperidin mitigates cyclophosphamide-induced testicular dysfunction via altering the hypothalamic pituitary gonadal axis and testicular steroidogenesis, inflammation, and apoptosis in male rats. Pharmaceuticals 16:301
Kurtz TW, Kajiya T (2012) Differential pharmacology and benefit/risk of azilsartan compared to other sartans. Vasc Health Risk Manag 8:133–143. https://doi.org/10.2147/VHRM.S22595
Lambertini M, Partridge AH (2021) Cyclophosphamide-free adjuvant chemotherapy for the potential prevention of premature ovarian insufficiency and infertility in young women with breast cancer. J Natl Cancer Inst 113:1274–1276. https://doi.org/10.1093/jnci/djab066
Lan W, He L, Liu Y (2018) Preparation and properties of sodium carboxymethyl cellulose/sodium alginate/chitosan composite film. Coatings 8:291
Ledingham D, Plant M, Mustafa F, Patil B (2020) Preserving fertility: using cyclophosphamide and other cytotoxics in young people. Pract Neurol 20:148–153. https://doi.org/10.1136/practneurol-2019-002247
Li J, You Y, Zhang P, Huang X, Dong L, Yang F, Yu X, Chang D (2022a) Qiangjing tablets repair of blood-testis barrier dysfunction in rats via regulating oxidative stress and p38 MAPK pathway. BMC Complement Med Ther 22:133. https://doi.org/10.1186/s12906-022-03615-z
Li Z, Dai A, Yang M, Chen S, Deng Z, Li L (2022b) p38MAPK signaling pathway in osteoarthritis: pathological and therapeutic aspects. J Inflamm Res 15:723–734. https://doi.org/10.2147/jir.S348491
Luo D, He Z, Yu C, Guan Q (2022) Role of p38 MAPK Signalling in testis development and male fertility. Oxid Med Cell Longev 2022:6891897. https://doi.org/10.1155/2022/6891897
Mannucci A, Argento FR, Fini E, Coccia ME, Taddei N, Becatti M, Fiorillo C (2021) The impact of oxidative stress in male infertility. Front Mol Biosci 8:799294. https://doi.org/10.3389/fmolb.2021.799294
Mesbahzadeh B, Hassanzadeh-Taheri M, Aliparast M-S, Baniasadi P, Hosseini M (2021) The protective effect of crocin on cisplatin-induced testicular impairment in rats. BMC Urol 21:117. https://doi.org/10.1186/s12894-021-00889-2
Miura S-I, Okabe A, Matsuo Y, Karnik SS, Saku K (2013) Unique binding behavior of the recently approved angiotensin II receptor blocker azilsartan compared with that of candesartan. Hypertens Res 36:134–139. https://doi.org/10.1038/hr.2012.147
Mohammed EE, Mosad E, Zahran AM, Hameed DA, Taha EA, Mohamed MA (2015) Acridine orange and flow cytometry: which is better to measure the effect of varicocele on sperm DNA integrity? Adv Urol 2015:814150. https://doi.org/10.1155/2015/814150
Moss JL, Choi AW, Fitzgerald Keeter MK, Brannigan RE (2016) Male adolescent fertility preservation. Fertil Steril 105:267–273. https://doi.org/10.1016/j.fertnstert.2015.12.002
Nayak L, Jain H, Bonda A, Epari S, Laskar S, Gokarn A, Shet T, Gujral S, Khanna N, Bagal B, Punatar S, Goda J, Thorat J, Rengaraj K, Sengar M (2021) Hodgkin lymphoma in adolescent and young adults: real-world data from a single tertiary cancer center in India. J Adolesc Young Adult Oncol 10:581–587. https://doi.org/10.1089/jayao.2020.0061
Ntemou E, Alexandri C, Lybaert P, Goossens E, Demeestere I (2019) Oncofertility: pharmacological protection and immature testicular tissue (ITT)-based strategies for prepubertal and adolescent male cancer patients. Int J Mol Sci 20. https://doi.org/10.3390/ijms20205223
Nurgali K, Jagoe RT, Abalo R (2018) Editorial: Adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front Pharmacol 9:245. https://doi.org/10.3389/fphar.2018.00245
Okolo KO, Orisakwe OE, Siminialayi IM (2017) Pleurotus tuber-regium mushrooms in the diet of rats ameliorates reproductive and testicular injury caused by carbon tetrachloride. Clin Phytoscience 3:14. https://doi.org/10.1186/s40816-017-0051-x
Omolaoye TS, Du Plessis SS (2021) The effect of streptozotocin induced diabetes on sperm function: a closer look at AGEs, RAGEs, MAPKs and activation of the apoptotic pathway. Toxicol Res 37:35–46. https://doi.org/10.1007/s43188-020-00040-7
World Health Organization (2021) WHO laboratory manual for the examination and processing of human semen. World Health Organization. Accessed May 2024
Pascolo L, Zito G, Zupin L, Luppi S, Giolo E, Martinelli M, De Rocco D, Crovella S, Ricci G (2020) Renin angiotensin system, COVID-19 and male fertility: any risk for conceiving? Microorganisms 8:1492
Pierozan P, Jernerén F, Ransome Y, Karlsson O (2017) The choice of euthanasia method affects metabolic serum biomarkers. Basic Clin Pharmacol Toxicol 121:113–118. https://doi.org/10.1111/bcpt.12774
Raheem NM, Mohammed Ali Mahmood N (2023) Azilsartan suppresses the antiapoptotic biomarker and pro-inflammatory cytokines in rat model of cisplatin-induced retinal and optic nerve toxicity. Hum Exp Toxicol 42:09603271231155092. https://doi.org/10.1177/09603271231155092
Ramalingam L, Menikdiwela K, LeMieux M, Dufour JM, Kaur G, Kalupahana N, Moustaid-Moussa N (2017) The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta Mol Basis Dis 1863:1106–1114. https://doi.org/10.1016/j.bbadis.2016.07.019
Sachse A, Wolf G (2007) Angiotensin II–induced reactive oxygen species and the kidney. J Am Soc Nephrol 18:2439–46
Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, Mulhall JP, Niederberger C, Sandlow JI, Sokol RZ, Spandorfer SD, Tanrikut C, Treadwell JR, Oristaglio JT, Zini A (2021) Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. J Urol 205:36–43. https://doi.org/10.1097/ju.0000000000001521
Shihab EM, Kadhim HM, Shahooth SS (2024) Dapagliflozin mitigates oxidative stress, inflammatory, and histopathological markers of aging in mice. J Med Life 17:157–163. https://doi.org/10.25122/jml-2023-0343
Smith LB, Walker WH (2014) The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 30:2–13. https://doi.org/10.1016/j.semcdb.2014.02.012
Sujayraj S, Naik P, Vishma L (2016) Cyclophosphamide induced testicular toxicity – a comparison between acute and subchronic doses. Int J Sci Res 5(3):211–214
Sukumaran V, Veeraveedu PT, Gurusamy N, Lakshmanan AP, Yamaguchi K, Ma M, Suzuki K, Nagata M, Takagi R, Kodama M, Watanabe K (2012) Olmesartan attenuates the development of heart failure after experimental autoimmune myocarditis in rats through the modulation of ANG 1–7 mas receptor. Mol Cell Endocrinol 351:208–219. https://doi.org/10.1016/j.mce.2011.12.010
Takalani NB, Monageng EM, Mohlala K, Monsees TK, Henkel R, Opuwari CS (2023) Role of oxidative stress in male infertility. Reprod Fertil 4. https://doi.org/10.1530/raf-23-0024
Tang WH, Zhou SJ, Song SD, He HY, Wu H, Zhang Z, Yang YZ, Zhang HL, Mao JM, Liu DF, Zhao LM, Lin HC, Hong K, Ma LL, Zhuang XJ, Jiang H (2018) A clinical trial on the consistency of bilateral testicular tissue histopathology and Johnsen score: single side or bilateral side biopsy? Oncotarget 9:23848–23859. https://doi.org/10.18632/oncotarget.24748
Underwood W, Anthony R (2020) AVMA guidelines for the euthanasia of animals: 2020 edition. 1–121. Version 2020.0.1 Accessed May 2024
van den Boogaard WMC, Komninos DSJ, Vermeij WP (2022) Chemotherapy side-effects: not all DNA damage is equal. Cancers 14:627
Vander Borght M, Wyns C (2018) Fertility and infertility: definition and epidemiology. Clin Biochem 62:2–10. https://doi.org/10.1016/j.clinbiochem.2018.03.012
Vona R, Pallotta L, Cappelletti M, Severi C, Matarrese P (2021) The impact of oxidative stress in human pathology: focus on gastrointestinal disorders. Antioxidants 10:201
Yang H, Ramstein J, Smith J (2019) Non-oncologic indications for male fertility preservation. Curr Urol Rep 20:51. https://doi.org/10.1007/s11934-019-0915-3
Zhao YR, Song HM, Ni L (2019) Cyclophosphamide for the treatment of acute lymphoblastic leukemia: a protocol for systematic review. Medicine (Baltimore) 98:e14293. https://doi.org/10.1097/md.0000000000014293
Author information
Authors and Affiliations
Contributions
Conceptualization, investigation, and Manuscript preparation, Ahmed HA, Gatea FK, and Hussein ZA. Supervision, Gatea FK. Statistical analysis and review of final results, Hussein ZA. Manuscript review and editing, Ahmed HA, Gatea FK, and Hussein ZA. All authors have read and agreed to the published version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The animals used in this study were acquired specifically for research purposes, and their use was approved by the Research Ethical Committee of the College of Medicine et al.-Nahrain University (IRB/122, Approval number: UNCOMIRB20240519, on 17th September 2023).
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, H.A., Gatea, F.K. & Hussein, Z.A. Azilsartan as a preventive agent against cyclophosphamide-induced testicular injury in male rats. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03339-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03339-6