Abstract
Estrone (E1) constitutes the primary component in oral conjugated equine estrogens (CEEs) and serves as the principal estrogen precursor in the female circulation in the post-menopause. E1 induces endothelium-dependent vasodilation and activate PI3K/NO/cGMP signaling. To assess whether E1 mitigates vascular dysfunction associated with postmenopause and explore the underlying mechanisms, we examined the vascular effects of E1 in ovariectomized (OVX) rats, a postmenopausal experimental model. Blood pressure was measured using tail-cuff plethysmography, and aortic rings were isolated to assess responses to phenylephrine, acetylcholine (ACh), and sodium nitroprusside. Responses to ACh in rings pre-incubated with superoxide dismutase (SOD), catalase (CAT), or apocynin were also evaluated. Protein expression of SOD, CAT, NOX1, NOX2, and NOX4 was determined by Western blotting. E1 treatment resulted in decreased body weight and retroperitoneal fat, increased uterine weight, and prevented elevated blood pressure in the OVX group. Furthermore, E1 improved endothelium-dependent ACh vasodilation, activated compensatory antioxidant mechanisms – i.e. increased SOD and CAT antioxidant enzymes activity, and decreased NOX4 expression. This, in turn, helped prevent oxidative stress and endothelial dysfunction in OVX rats. Additionally, E1 treatment reversed the increased total LDL cholesterol observed in the OVX group. The findings underscore protective effects of E1 on the cardiovascular system, counteracting OVX-related oxidative stress and endothelial dysfunction in Wistar rats. E1 exhibits promising therapeutic benefits for managing cardiovascular health, particularly in postmenopausal conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) affects both men and women, but postmenopausal women have a particularly increased cardiovascular risk, partially attributed to structural changes in the vasculature and injuries to endothelial function (Samargandy et al. 2020). The regulation of vascular homeostasis and tone involves vasoactive components such as nitric oxide (NO), prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor (EDHF), and decreased synthesis or release of these mediators disrupts homeostasis balance, culminating in endothelial dysfunction, an important risk factor for CVD (Cohen et al. 1983; Furchgott and Vanhoutte 1989; Darley-Usmar et al. 1997; Leung et al. 2006; Qiao et al. 2008; Feletou and Vanhoutte 2009).
Postmenopausal women show an increased rate of arterial hypertension, compared to men at the same age. On the other hand, fertile women show similar or smaller rates of arterial hypertension in relation to men. The variation is attributed to decreased estrogen levels that culminate in increased blood pressure, and higher prevalence of CVD in postmenopausal women (Hernandez et al. 2000; Lobo et al. 2018; Brahmbhatt et al. 2019). Hormone replacement therapy (HRT) for the prevention and management of CVD in postmenopausal women has been a subject of debate (Hulley et al. 1998; Virdis et al. 2000; Rossouw et al. 2002; Mercuro et al. 2004; Prentice et al. 2009). However, recent studies showed that HRT for younger or early postmenopausal women has beneficial effects on the cardiovascular system, reducing coronary heart disease and all-cause mortality (Langer 2017; Cagnacci and Venier 2019; Langer et al. 2021). Furthermore, among HRT formulations, oral conjugated equine estrogens (CEEs) have been commonly used to manage menopausal symptoms, preventing osteoporosis, reducing CVD risk and Alzheimer’s disease (Bhavnani and Stanczyk 2014; Saleh et al. 2023).
CEEs consist of several biologically active estrogenic molecules, with estrone (E1), derived from the urine of pregnant mares, being the main component. Although E1 predominates in the female circulation following menopause, limited information on the vascular effects of E1 is available, and studies focusing on the biological actions of this hormone, including its action in the cardiovascular system, are warranted (Ruggiero and Likis 2002). A previous study on rat thoracic aorta demonstrated that E1, through non-estrogen receptor and genomic effects, induces endothelium-dependent vasodilation (Oliveira et al. 2018). Here, our objective was to examine the vascular effects of E1 in ovariectomized rats - a postmenopausal experimental model. We hypothesized that E1 alleviates vascular dysfunction related to postmenopause. In light of the significant impact of CVD in postmenopausal women, elucidating the mechanisms underlying the impact of E1 on vascular function may contribute to a better understanding of the potential benefits of estrogen-based therapies in the management of CVD in postmenopausal women.
Methods
Chemicals
Acetylcholine (ACh), apocynin, aprotinin, bestatin, bovine serum albumin (BSA), calcium chloride (CaCl2), catalase (CAT), dithiothreitol, epinephrine bitartrate, estrone (E1), 17β−estradiol (E2), ethylenediaminetetraacetic acid (EDTA), glucose, ketamine, leupeptin, magnesium sulfate heptahydrate (MgSO4•7H2O), pepstatin A, phenylephrine (Phe), phenylmethylsulphonyl fluoride (PMSF), potassium chloride (KCl), potassium dihydrogen phosphate (KH2PO4), sodium orthovanadate sodium chloride (NaCl), sodium hydrogen carbonate (NaHCO3), sodium orthovanadate sodium nitroprusside, superoxide dismutase (SOD), xylazine, and dithiothreitol were purchased from Sigma (St. Louis, MO, USA). All other chemicals were obtained in an analytical grade or from standard commercial suppliers.
Animals
Female Wistar rats at 12 weeks of age, weighing 180–250 g, obtained from the colony of the Federal University of Goias were used in the present study. The animals were housed under 12 h (h) light/dark cycles, controlled temperature (22–23 ºC) and humidity (40–60%), with ad libitum access to food and water. All procedures were carried out in accordance with the Regulations of the National Council for the Control of Animal Experimentation (CONCEA) for the Production or Use of Animals in Teaching Activities or Scientific Research, complying with the ARRIVE guidelines and in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Ethics Committee on Animal Use of the Federal University of Goiás (UFG) (protocol number CEUA/UFG 20/2013).
Ovariectomy
Since rodents do not naturally undergo menopause within a feasible timeframe, ovariectomy was performed to induce a menopausal state in Wistar rats. The ovariectomy procedure was carried out under anesthesia with 70 mg.kg− 1 of ketamine and 10 mg.kg− 1 of xylazine administered intraperitoneally (i.p.). An abdominal incision was made through the skin, and the underlying muscle was carefully dissected to locate the ovary and fallopian tube. Subsequently, a suture line was ligated around the fallopian tube, and the ovary was removed. The muscle and skin were then sutured to close the incision and, as a precaution against infection, the rats received an intramuscular injection of 2.5% enrofloxacin antibiotic (0.1 mL). For the sham group, the same surgical procedure was performed, but the ovary was left intact during the surgery.
Hormone treatment
The Wistar female rats were divided into four groups: Sham (physiological estrous), ovariectomized (OVX), OVX treated with E1, and OVX treated with E2. Following a 60-day period post-ovariectomy, hormonal treatment commenced with daily subcutaneous injections of 0.1 mL, containing 825 µg.kg− 1 of E1 or 15 µg.kg− 1 of E2, both diluted in corn oil (vehicle), while the Sham and OVX groups received subcutaneous injections of the vehicle. The treatments had a duration of 30 days.
The OVX procedure and treatment protocols were based on studies by El-Swefy et al. (2002) and Silva et al. (2020). Subsequent to the surgery, the animals underwent a 60-day recovery period to closely replicate the effects of prolonged ovarian dysfunction, akin to that observed in postmenopausal women. The efficacy of ovariectomy and experimental hormone replacement therapy was evaluated by measuring uterine weight and plasma estrogen levels, determined by enzyme radioimmunoassay, following the manufacturer’s instructions (Monobind Inc., Lake Forest, CA 92,630, USA).
The selected doses of E1 and E2 were chosen to replicate the individual doses used in HRT for postmenopausal women. The usual dose of E2 in HRT is 1 mg (Lobo et al. 2018) and considering a woman weighing 70 kg, the equivalent dose would be 15 µg.kg− 1. On the other hand, the dose of E1 was based on the ratio of this hormone present in the preparation of CEE’s compared to E2 (E1/E2), which is 55 times higher (55/1) (Berrodin et al. 2009). Therefore, we chose the dose of 825 µg.kg− 1 of E1 for this study.
Hemodynamic measurements
At the end of the treatment (E1, E2 or vehicle for 30 days), arterial blood pressure was assessed in unanesthetized animals using an indirect tail-cuff method with a pneumatic transducer (Insight® Ribeirão Preto, Brazil). The measurements were performed by placing a tail-cuff device around the tail of the rats, and the blood pressure was recorded using the transducer. Three consecutive measurements were taken for each rat, and the average of these measurements was calculated to obtain the final blood pressure reading.
Blood collection and lipid profile
Following the blood pressure measurement, blood samples were collected from the tail artery and transferred to EDTA-coated tubes. Subsequently, blood samples were centrifuged at 4,000 rpm for 15 min to separate the serum, which was then stored at -20 °C for further analysis. Serum levels of total cholesterol (TC) (Gold Analisa®, kit ref number 460 lot 5012), triglycerides (TG) (Gold Analisa®, kit ref number 459 lot 2008), high-density lipoprotein cholesterol (HDL) (Gold Analisa®, kit ref number 400 lot 2005), and low-density lipoprotein cholesterol (LDL) (Gold Analisa®, kit ref number 401 lot 6104) were measured according to the manufacturer’s instructions (Gold Analisa®, Belo Horizonte, MG, Brazil).
Vascular reactivity
Vascular reactivity was determined following the methodology outlined by Oliveira et al. (2016). After blood collection, the animals were anesthetized using a combination of ketamine and xylazine hydrochloride. Subsequently, they were euthanized by decapitation using a small guillotine. The aortic rings were then rapidly excised and cleaned of adhering fat and connective tissue to evaluate vascular reactivity. Following that, the 4 mm-length rings were placed in an ice-cold (4 °C) modified Krebs-Henseleit solution with the following composition (in mM): 130 NaCl, 14.9 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4•7H2O, 5.5 glucose, 1.56 CaCl2•2H2O, and 0.026 EDTA. The segments were then mounted on stainless steel hooks in organ baths containing 10.0 mL of warmed (37ºC) and oxygenated (95% O2 and 5% CO2) modified Krebs-Henseleit solution. Changes in basal tension were recorded using isometric transducers connected to a data acquisition system AQCAD (AVS Projetos, São Carlos, SP, Brazil).
Endothelial function was evaluated by assessing the vasorelaxant effect of ACh (10 µM) on vessels pre-contracted with Phe (1 µM). Rings with intact endothelium (E+) were deemed suitable for the study if the relaxation induced by ACh, following pre-contraction with Phe, was at least 90%. Rings were classified as endothelium-denuded (E-) if the maximum relaxation induced by ACh was less than 10%, relatively to the contraction induced by Phe. In experiments involving E- vessels, the endothelium of the aortic rings was mechanically removed by gently rolling a thin cotton-coated wire along the vessel lumen. After 30 min of equilibration, cumulative-concentration response curves to Phe (0.1 nM to 30 µM), ACh (10 pM to 10 µM), and sodium nitroprusside (SNP, 1 pM to 30 µM) were performed. Phe is an α1-adrenoreceptor agonist, ACh is an endothelium-dependent vasodilator, and SNP is a nitric oxide donor.
After 30 min of equilibration, cumulative-concentration response curves to Phe (ranging from 0.1 nM to 30 µM), ACh (10 pM to 10 µM), and sodium nitroprusside (SNP, 1 pM to 30 µM) were performed. Phe is an α1-adrenoreceptor agonist, ACh is an endothelium-dependent vasodilator, and SNP is a nitric oxide donor. To evaluate the role of reactive oxygen species (ROS) in ACh-induced vasodilation, aortic rings from Sham, OVX, E1, and E2 rats were incubated with either vehicle, apocynin (10 mM) - an NADPH oxidase inhibitor, or antioxidant enzymes such as SOD (150 units (U)/mL) or CAT (100 U/mL) during a 30-minute period.
Non-linear regression analysis using Graph-Pad Prism software (USA) was employed to calculate the maximal effect (Emax) and the logarithm of the agonist concentration resulting in 50% of the Emax (pCE50) for each concentration–response curve to ACh.
Western blotting analysis
Experimental protocols were performed as described by Costa et al. (2022). The endothelium-intact aortic segments were dissected out, cleaned of connective tissue, frozen in liquid nitrogen, and kept at -70ºC until the day of analysis. The samples were homogenized in a buffer containing 4-{2-aminoetil} benzene sulfonyl fluoride, pepstatin, E-64, bestatin, leupeptin, aprotinin, sodium orthovanadate, PMSF, and sodium fluoride. Homogenates were centrifuged at 4,000 rpm for 20 min. The proteins were extracted (70 µg) and separated by electrophoresis on 10% polyacrylamide gels and transferred onto nitrocellulose membranes. Non-specific binding sites were blocked with 5% BSA in tris-buffered saline containing 0.1% Tween 20 (for 1 h at 24 ºC). Membranes were incubated with antibodies overnight at 4 ºC. Antibodies used included: anti-CAT (1:1000 dilution; Cell Signaling, #D5N7V), anti-Cu/Zn SOD (1:1000 dilution; Abcam, #ab13498), anti-Mn SOD (1:500 dilution; Millipore, #06-984), anti-NOX-1 (1:500 dilution; Abcam, #ab55831), anti-gp91phox (1:1000 dilution; Abcam, #43,801), and anti-NOX-4 (1:500 dilution; Abcam, #ab133303). After incubation with secondary antibodies, signals were obtained by chemiluminescence, visualized by autoradiography, and quantified densitometrically (ImageJ, National Institute of Mental Health, Bethesda, Maryland). Beta-actin expression was used as the housekeeping protein, and results were expressed as arbitrary units (AU) relatively to the control.
Enzymatic activities of CAT and SOD
Samples of endothelium-intact aortic segments were homogenized (100 mg/mL) in a phosphate buffer (50 mM, pH 7.4) and then centrifuged at 4,000 rpm for 20 min. The supernatant obtained from the centrifugation was used for the experimental procedures. Catalase (CAT) activity was measured spectrophotometrically at room temperature by monitoring the decrease in absorbance at 240 nm, resulting from the decomposition of H2O2 in the supernatant samples. The adapted method of Chance et al. (1979) was used for this measurement, and the results are expressed in pmoles of CAT per mg of protein. Superoxide dismutase (SOD) activity was determined based on the superoxide dismutase-sensitive rate of adrenochrome formation, measured by the absorption change at 480 nm. The adapted method of McCord and Fridovich (1969) was used for this determination, and the results were expressed in U of SOD per mg of protein.
Lucigenin-enhanced chemiluminescence
This experiment was performed following the protocol outlined by Costa et al. (2022). The aortic segments were washed and harvested in a lysis buffer containing: KH2PO4 (20 mM), EGTA (1 mM), aprotinin (1 µg/mL), leupeptin (1 µg/mL), pepstatin (1 µg/mL), and PMSF (1 mM). Next, 50 µL of the sample were mixed with a suspension containing 175 µL of assay buffer composed of: KH2PO4 (50 mM), EGTA (1 mM), sucrose (150 mM), and lucigenin (5 µM) (Sigma-Aldrich, #M8010) at pH 7.4. To this mixture, NADPH (1 µM) was added (300 µL of suspension containing lucigenin). Luminescence was measured every 18 s for 3 min using a luminometer (AutoLumat LB 953, Berthold), both before and after stimulation with NADPH. A buffer blank was subtracted from each reading to correct the measurements. The results were expressed as counts per milligram of protein and as a percentage of the control.
Statistical analysis
The relaxation effect was expressed as a percentage of contraction to Phe. Individual concentration-response curves were analyzed by non-linear regression analysis to fit the curves and two-way ANOVA followed by Tukey’s post-hoc test. The pEC50 (defined as the negative logarithm of the EC50 values) and Emax were compared using t-tests or ANOVA followed by Tukey’s post-hoc test. Prism software, version 8.0 (GraphPad Software Inc., San Diego, CA, USA), was utilized for data analysis and to fit the sigmoid curves. The results are presented as mean ± SEM of 5–6 experiments, with N representing the number of animals used. P values less than 0.05 were considered statistically significant.
Results
Hemodynamic and biometric parameters
Table 1 presents the hemodynamic and biometric parameters of the study. The OVX group showed a significant increase in body weight (~ 22%) compared to the Sham group (p < 0.05), while treatments with E1 and E2 decreased body weight (~ 14% and ~ 12%, respectively) compared to the OVX group (p < 0.05). Similarly, retroperitoneal fat was significantly increased in the OVX group (~ 94%) compared to the Sham group (p < 0.05), and both E1 and E2 treatments led to a decrease in retroperitoneal fat (~ 64% and ~ 56%, respectively) compared to the OVX group (p < 0.05). The OVX group also exhibited a significant decrease in uterus weight (~ 70%) compared to the Sham group (p < 0.05), while treatments with E1 and E2 increased uterus weight (~ 264% and ~ 241%, respectively) compared to the OVX group (p < 0.05).
Furthermore, the OVX group showed increased systolic (~ 12%) and diastolic (~ 7%) blood pressure compared to the Sham group (p < 0.05). E1 and E2 treatments were able to reduce the systolic (~ 15% and ~ 8%, respectively) and diastolic (~ 9% and ~ 8%, respectively) blood pressure, compared to the OVX group (p < 0.05). On the other hand, there was no significant difference in heart weight between the groups (p > 0.05).
Plasma hormone levels
In the OVX group, estrogen levels were significantly decreased (~ 61%) compared to the Sham group (p < 0.05). However, treatments with E1 and E2 significantly increased serum levels of estrogen (~ 355% and ~ 315%, respectively), compared to the OVX group (p < 0.05) (Table 1).
Cholesterol profiles
In the OVX group, total cholesterol levels increased significantly by approximately ~ 64%, LDL cholesterol by ~ 80%, and TG by ~ 159%, compared to the Sham group (p < 0.05). However, hormonal treatment with E1 resulted in a favorable modification of the lipid profile in OVX rats, leading to a decrease in total cholesterol by ~ 40%, LDL cholesterol by ~ 31%, and TG by ~ 83% (p < 0.05). Similarly, E2 treatment also decreased total cholesterol by ~ 11%, LDL cholesterol by ~ 10%, and TG by ~ 28%, compared to the OVX group (p < 0.05) (Table 1). Additionally, HDL cholesterol levels were not significantly different between the groups (p > 0.05) (Table 1).
Vascular reactivity
The precontraction levels in E + vessels, before any vasodilator drugs, were as follows: Sham (1.14 ± 0.06 g), OVX (1.81 ± 0.13 g), E1 (1.31 ± 0.13 g), and E2 (1.20 ± 0.07 g) groups. Considering the increased precontraction in E + rings of the OVX group (p < 0.05), the relaxation responses were evaluated in rings exposed to the concentration of Phe that induces 80% of the maximum response in each group.
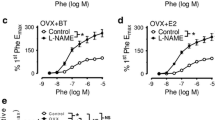
In E + and E- aortic rings isolated from Sham, OVX, E1, and E2 groups, Phe evoked concentration-dependent contractions. In E + preparations, the maximum contraction induced by Phe was increased in aortas from the OVX group (~ 57%) compared to the Sham group (p < 0.05). However, hormonal treatment with E1 (~ 23%) and E2 (~ 32%) decreased Phe-induced contractions compared to the OVX group (p < 0.05) (Fig. 1A). In contrast, no significant differences in Phe contractions were observed in E- aortic ring preparations among all groups (p > 0.05) (Fig. 1B).
Hormonal treatment with E1 and E2 effectively reverses the heightened vascular reactivity to phenylephrine (Phe) in ovariectomized (OVX) Wistar rats. Concentration-response curves to Phe (0.1 nM – 30 µM) in E+ (A) or E- (B) aortic rings isolated from Sham (square), OVX (triangle), E1 (rhombus), and E2 (circle) groups. Each data point represents the mean ± SEM (n = 5–6 animals per group). *p < 0.05 difference between OVX and Sham groups; #p < 0.05 difference between OVX and E1 groups; +p < 0.05 difference between OVX and E2 groups, by two-way ANOVA followed by Tukey’s post hoc test
Endothelium-dependent relaxation induced by ACh was decreased in aortas from the OVX group (~ 37%) compared to the Sham group (p < 0.05). Hormonal treatment with E1 (~ 55%) and E2 (~ 56%) restored the relaxation induced by ACh compared to the OVX group (p < 0.05) (Fig. 2). There were no significant changes in the relaxation response to SNP among the groups (p > 0.05) (Fig. 3).
Hormonal treatment with E1 and E2 effectively restores the diminished endothelium-dependent relaxation caused by ovariectomy. Concentration-response curves to ACh (10 pM – 10 µM) were obtained in E + aortic ring preparations from Sham, OVX, E1, and E2 groups. Each data point represents the mean ± SEM (n = 5–6 animals per group). *p < 0.05 OVX versus Sham groups; #p < 0.05 OVX versus E1 groups; +p < 0.05 OVX versus E2 groups; two-way ANOVA followed by Tukey’s post hoc
Vascular reactivity to SNP is not altered by OVX or E1 and E2 treatments. Concentration-response curves (10 pM – 10 µM) in E + aortic ring preparations from Sham, OVX, E1, and E2 groups. Each data point represents the mean ± SEM (n = 5–6 animals per group). Two-way ANOVA followed by Tukey’s post hoc revealed no significant differences (p ˃ 0.05)
Acute treatment of aortic rings from the OVX group with SOD (150 U/mL) restored (~ 60%) the impaired relaxation to ACh compared to the OVX group without SOD treatment (Fig. 4B). Similarly, acute treatment of aortas of the OVX group with CAT (100 U/mL) restored (~ 59%) the impaired relaxation to ACh compared to the OVX group without CAT treatment (Fig. 5B). Likewise, acute treatment with apocynin (10 µM) restored (~ 56%) the impaired relaxation to ACh in the OVX group compared to the OVX group without apocynin treatment (Fig. 6B). Sham group did not demonstrate impaired relaxation to ACh in SOD, CAT and apocynin treatment (Figs. 4A, 5A and 6A, respectively).
Involvement of SOD in the impaired relaxation response to ACh in endothelium-intact preparations. Concentration-response curves to ACh (10 pM – 10 µM) performed in E+ aortic ring preparations from Sham (A), OVX (B), E1 (C), and E2 (D) groups. Representative Immunoblots (top) and quantitative analysis (bottom) were conducted for Cu/Zn-SOD (E) and Mn-SOD (F) protein expressions in E + aortic rings isolated from Sham, OVX, E1, and E2 groups. Each data point represents the mean ± SEM (n = 5–6 animals per group). Figures A, B, C, D = #p < 0.05 by two-way ANOVA followed by Tukey’s post hoc; Figure E and F = #p < 0.05 when compared to OVX group by one-way ANOVA followed by Tukey’s post hoc
Involvement of CAT in the impaired relaxation response to ACh in endothelium-intact preparations. Concentration-response curves to ACh (10 pM – 10 µM) was conducted in E + aortic ring preparations from Sham (A), OVX (B), E1 (C), and E2 (D) groups. Representative Immunoblots (top) and quantitative analysis (bottom) were performed for CAT (E) protein expression in E + aortic rings isolated from Sham, OVX, E1, and E2 groups. Each data point represents the mean ± SEM (n = 5–6 animals per group). Figures A, B, C, D = #p < 0.05 by two-way ANOVA followed by Tukey’s post hoc; Figure E = #p < 0.05 when compared to OVX group by one-way ANOVA followed by Tukey’s post hoc
Evaluation of NADPH oxidase activity in the impaired relaxation response to ACh in endothelium-intact preparations. Concentration-response curves to ACh (10 pM – 10 µM) in the presence of apocynin (10 µM) was conducted in E + aortic ring preparations from Sham (A), OVX (B), E1 (C), and E2 (D) groups. Representative immunoblots (top) and quantitative analysis (bottom) were performed for NOX-1 (E), gp91phox (F), and NOX-4 (G) protein expression in E + aortic rings isolated from Sham, OVX, E1, and E2. Investigation of ROS generation (H) was also conducted in E + aortic rings isolated from Sham, OVX, E1, and E2 groups. Each data point represents the mean ± SEM (n = 5–6 animals per group). Figures A, B, C, D = #p < 0.05 by two-way ANOVA followed by Tukey’s post hoc; Figures E, F, G, H = #p < 0.05 when compared with OVX groups; *p < 0.05 difference between E2 and Sham groups; +p < 0.05 difference between E2 and E1 groups, by one-way ANOVA followed by Tukey’s post hoc
Hormonal treatment with E1 demonstrated a beneficial effect to the impaired relaxation to ACh showed in OVX group, since the curves to ACh with SOD (Fig. 4C), CAT (Fig. 5C) or apocynin (Fig. 6C) of rats submitted to E1 treatment did not demonstrate any difference with the control curves in absence of SOD, CAT or apocynin. E2 treatment also demonstrated effect with SOD, CAT and apocynin treatment (Figs. 4D, 5D and 6D, respectively).
Effect of hormonal treatments in CAT, SOD and NADPH oxidase expression
In the OVX group, the expression of Cu/Zn-SOD was increased (~ 70%) compared to the Sham group (p < 0.05). E1 (~ 36%) and E2 (~ 32%) treatments lowered the Cu/Zn-SOD expression when compared to the OVX group (p < 0.05) (Fig. 4E). There was no difference in Mn-SOD expression between the groups (p > 0.05) (Fig. 4F).
CAT expression was increased in the OVX group (~ 210%), and E1 treatment restored CAT (~ 39%) to levels observed in the Sham and E2 groups (p > 0.05) (Fig. 5E).
Aortas from Sham, OVX, E1, and E2 presented similar expression of total NOX-1 and gp91phox (p > 0.05) (Fig. 6E and F). On the other hand, NOX-4 expression was increased in OVX aortas (~ 81%) compared to the Sham group. E1 and E2 treatments decreased NOX-4 expression by approximately 46% and 31%, respectively, indicating that both hormones normalize NADPH activity (p < 0.05) (Fig. 6G).
Effect of E1 treatment in CAT and SOD activity
Figure 7A shows the effect of E1 and E2 treatments on CAT activity. E1 at doses of 15 µg.kg− 1 (~ 37%), 150 µg.kg− 1 (~ 51%), and 825 µg.kg− 1 (~ 60%), as well as E2 at 15 µg.kg− 1 (~ 44%), decreased CAT activity compared to the OVX group (p < 0.05) (Fig. 7A).
CAT (A) and SOD (B) enzymatic activities evaluated in E+ aortic ring preparations from Sham, OVX, E1 (15/150 and 825 µg.kg− 1), and E2 (15 µg.kg− 1) groups. Each data point represents the mean ± SEM (n = 5–6 animals per group). The statistical analysis using one-way ANOVA followed by Tukey’s post hoc test revealed significant differences: *p < 0.05 compared to Sham group; #p < 0.05 compared to OVX group
Figure 7B presents the effect of E1 and E2 treatments on SOD activity. All treatments with E1 at doses of 15 µg.kg− 1 (~ 19%), 150 µg.kg− 1 (~ 27%), and 825 µg.kg− 1 (~ 34%), as well as E2 at 15 µg.kg− 1 (~ 29%), decreased SOD activity compared to the OVX group (47.80 ± 0.95) (p < 0.05).
Effect of E1 treatment on ROS generation
Figure 6H displays the results of lucigenin-enhanced chemiluminescence assays. The OVX group exhibited a significant increase in ROS production (~ 150%) compared to the Sham group (p < 0.05). E1 (~ 57%) and E2 (~ 51%) treatments successfully lowered vascular ROS production in aortas from OVX rats (p < 0.05).
Discussion
Ovariectomy (OVX) in rats promoted significant body weight gain, abnormal lipid profiles, increased blood pressure, endothelial dysfunction, and dysregulation of enzyme profiles. This menopausal-like state in the rats allowed us to investigate the effects of E1. E1 treatment for 30 days demonstrated vascular protective effects in OVX rats, with an important decrease in arterial blood pressure and improvement of endothelial function. This positive outcome was attributed to the decreased expression of SOD and CAT, as well as NOX-4 expression, leading to normal ROS generation. E1 effects were similar to the effects of E2, known by its protective vascular actions.
E1 is an equine estrogen present in commonly prescribed hormonal replacement therapies, such as Premarin®. E1 is the main compound of these preparations, accounting for approximately 48% of its composition (Berrodin et al. 2009; Barha and Galea, 2013). Our research group showed that stimulation of isolated rat thoracic aorta with E1 promotes endothelium-dependent vasodilation and activation of PI3K/NO/cGMP signaling, via estrogen receptor and non-genomic effects (Oliveira et al. 2018).
Endothelial dysfunction is a pathological condition commonly observed in postmenopausal women, and it is directly associated with the decrease in hormonal plasma estrogen levels (Virdis et al. 2000). This condition is characterized by decreased levels of nitric oxide (NO), decreased endothelium-dependent relaxation or increased vasoconstriction, all linked to CVD (Vanhoutte 1997). The decrease in NO levels is associated with an increase in reactive oxygen species (ROS), as ROS leads to the premature breakdown of endothelium-derived NO to ONOO−, thereby promoting endothelial dysfunction (Adams et al. 2005; Rush and Aultman, 2007; Yao and Abdel-Rahman 2018; Moreau et al. 2020). In this context, the OVX model and the menopausal-like state are associated with an increase in both ROS formation and blood pressure (Reckelhoff and Fortepiani 2004; Li et al. 2020). In addition, treatment with compounds exhibiting antioxidant profiles normalizes endothelial function and blood pressure in OVX rats (Fabricio et al. 2017; Moreau et al. 2020; Hou et al., 2021; da Silva et al., 2022). Thus, the restoration of endothelial function by E1 as well as E2 showed in this study corroborate that estrogens protect against menopause-associated endothelial dysfunction, an effect that involves antioxidant properties of these molecules (Lamas et al. 2015).
OVX also decreased the vasorelaxant effect of ACh and increased Phe contractions. Furthermore, endothelial dysfunction was associated with the overexpression of NOX-4, consistent with other studies demonstrating endothelial dysfunction in OVX rats (Paredes-Carbajal et al. 1995; Claudio et al. 2017; Shih et al. 2017; Pocs et al. 2019). In this study, both E1 and E2 treatments effectively inhibited NOX-4 expression and corrected the endothelial dysfunction, corroborating previous studies (Zhang et al. 2017; Liu et al. 2019; Feng et al. 2021). In addition, antioxidant enzymes such as SOD and CAT, as well as a NADPH oxidase inhibitor, successfully reversed the impaired ACh relaxation observed in the OVX group. Furthermore, E1 treatment normalized the altered expression and activity of the antioxidant enzymes SOD and CAT. These results demonstrate that E1 treatment effectively restored the redox balance, which was impaired by OVX.
Oxidized low-density lipoprotein (LDL) induces intracellular oxidative stress and impairs endothelial function (Chan et al. 2003). In this study, OVX increased LDL levels, while E1 treatment effectively decreased LDL levels and improved the antioxidant defense system, supporting the association between LDL and oxidative susceptibility in menopause (Wakatsuki et al. 2004). Similar effects were also observed with E2 treatment, consistent with previous studies (Yung et al. 2013). Overall, these findings suggest that E1 contributes to the maintenance of endothelial function and vascular health in the context of menopause.
The decrease in hormonal levels following OVX can lead to changes in vascular response and to increased blood pressure (Dantas et al. 1999). In this study, we demonstrated that OVX increases systolic blood pressure, further supporting that the decline in hormone levels contributes to the development of hypertension. These data are reinforced by the Sham group, i.e. animals in their physiological estrous cycle, which exhibited significantly lower blood pressure values, further supporting protective effects of estrogens.
Supporting the hypothesis that conjugated equine estrogens (CEEs) reduce blood pressure, the present study demonstrates that treatment with E1 alone decreased systolic blood pressure. suggest that this estrogen is able to counteract the increased blood pressure induced by OVX by restoring the antioxidant system, improving endothelial function, and normalizing NOX-4 expression. Additionally, while the involvement of endothelium-derived hyperpolarizing factor (EDHF) in the beneficial effects of E1 on vascular function was not directly analyzed in this study, previous research has indicated that estrogen replacement restores EDHF response in OVX rats (Liu et al. 2002; Sakuma et al. 2002; Nawate et al. 2005; Caliman et al. 2013), and in postmenopausal women. Therefore, estrogen treatment improves both NO-dependent and EDHF-mediated vasodilatations (Félétou, 2011), indicating that E1’s effect on vascular tone may also involve EDHF-mediated responses.
Altogether, our data reinforce the protective effects of E1 on endothelial function and blood pressure regulation, and highlight the importance of further research to explore its potential clinical applications. Moreover, the fact that E1 is a major component of CEEs used in hormone replacement therapy (HRT) adds further significance to our findings.
Conclusion
The results provide valuable insights into the vascular effects of estrone (E1). Using a postmenopausal rat model, we demonstrated that E1 treatment exerts beneficial effects on vascular function, reducing arterial blood pressure through the improvement of endothelial function, along with decreased expression of oxidative stress-related markers, such as NOX-4, and decreased reactive oxygen species (ROS) generation. In conclusion, this study provides evidence on beneficial vascular effects of E1 and establishes a foundation for future investigations into the potential therapeutic role of E1 in managing CVD in postmenopausal women.
Data availability
No datasets were generated or analysed during the current study.
References
Adams V, Linke A, Kränkel N (2005) Impact of Regular Physical Activity on the NAD(P)H Oxidase and Angiotensin Receptor System in Patients With Coronary Artery Disease. Circulation 111(5): 555–562. https://doi.org/10.1161/01.CIR.0000154560.88933.7E.
Barha CK, Galea LA (2013) The hormone therapy, Premarin, impairs hippocampus-dependent spatial learning and memory and reduces activation of new granule neurons in response to memory in female rats. Neurobiol Aging 34(3):986–1004. https://doi.org/10.1016/j.neurobiolaging.2012.07.009.
Berrodin TJ, Chang KC, Komm BS et al (2009) Differential biochemical and cellular actions of Premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination. Mol Endocrinol 23(1):74–85. https://doi.org/10.1210/me.2008-0366
Bhavnani BR, Stanczyk FZ (2014) Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action. J Steroid Biochem Mol Biol 142:16–29. https://doi.org/10.1016/j.jsbmb.2013.10.011
Brahmbhatt Y, Gupta M, Hamrahian S (2019) Hypertension in Premenopausal and Postmenopausal women. Curr Hypertens Rep 21(10):74. https://doi.org/10.1007/s11906-019-0979-y
Cagnacci A, Venier M (2019) The controversial history of hormone replacement therapy. Med (Kaunas) 55(9):602. https://doi.org/10.3390/medicina55090602
Caliman IF, Lamas AZ, Dalpiaz PLM, Medeiros ARS, Abreu GR et al (2013) Endothelial relaxation mechanisms and oxidative stress are restored by atorvastatin therapy in Ovariectomized rats. PLoS ONE 8(11):e80892. https://doi.org/10.1371/journal.pone.0080892
Chan H, Lougheed M, Laher I et al (2003) Oxidized low-density lipoprotein inhibits endothelium-dependent vasodilation by an antioxidant-sensitive, lysophosphatidylcholine-independent mechanism. J Cardiovasc Pharmacol 41(6):856–865. https://doi.org/10.1097/00005344-200306000-00005
Claudio ER, Almeida SA, Mengal V et al (2017) Swimming training prevents coronary endothelial dysfunction in ovariectomized spontaneously hypertensive rats. Braz J Med Biol Res 50(1):e5495. https://doi.org/10.1590/1414-431X20165495
Cohen RA, Shepherd JT, Vanhoutte PM (1983) Inhibitory role of the endothelium in the response of isolated coronary arteries to platelets. Science 221(4607):273–274. https://doi.org/10.1126/science.6574604
Costa RM, Alves-Lopes R, Alves JV et al (2022) Testosterone contributes to Vascular Dysfunction in Young Mice Fed a high Fat Diet by promoting nuclear factor E2–Related factor 2 downregulation and oxidative stress. Front Physiol 13:837603. https://doi.org/10.3389/fphys.2022.837603
Dantas AP, Scivoletto R, Fortes ZB (1999) Influence of female sex hormones on endothelium-derived vasoconstrictor prostanoid generation in microvessels of spontaneously hypertensive rats. Hypertension 34(4 Pt 2):914–919. https://doi.org/10.1161/01.hyp.34.4.914
Darley-Usmar VM, McAndrew J, Patel R et al (1997) Nitric oxide, free radicals and cell signalling in cardiovascular disease. Biochem Soc Trans 25(3):925–929. https://doi.org/10.1042/bst0250925
de Oliveira TS, de Oliveira LP, Costa RMD et al (2018) Activation of PI3K/Akt pathway mediated by estrogen receptors accounts for estrone-induced vascular activation of cGMP signaling. Vascul Pharmacol 110:42–48. https://doi.org/10.1016/j.vph.2018.07.003
El-Swefy SE, Asker ME, Ali SI et al (2002) A Novel Concept to preserve the Beneficial effects of hormone replacement therapy in bilaterally female ovariectomized rats: role of Lovastatin Therapy. Pharmacol Res 45(3). https://doi.org/10.1006/phrs.2001.0876
Fabricio V, Oishi JC, Biffe BG, Ruffoni LD, Silva KA, Nonaka KO, Rodrigues GJ (2017) Resveratrol Treatment normalizes the endothelial function and blood pressure in Ovariectomized rats. Arq Bras Cardiol 108(2):116–121. https://doi.org/10.5935/abc.20170012
Feletou M, Vanhoutte PM (2009) EDHF: an update. Clin Sci (Lond) 117(4):139–155. https://doi.org/10.1042/CS20090096
Félétou M (2011) The Endothelium: Part 2: EDHF-Mediated Responses “The Classical Pathway”. Morgan & Claypool Life Sciences Publisher, San Rafael (CA). PMID: 21850764.
Feng D-d, Zheng B, Yu J et al (2021) 17β-Estradiol inhibits proliferation and oxidative stress in vascular smooth muscle cells by upregulating BHLHE40 expression. Front Cardiovasc Med 8:768662. https://doi.org/10.3389/fcvm.2021.768662
Furchgott RF, Vanhoutte PM (1989) Endothelium-derived relaxing and contracting factors. FASEB J 3(9):2007–2018 PMID: 2545495
Hernandez I, Delgado JL, Diaz J et al (2000) 17beta-estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 279(5):R1599–R1605. https://doi.org/10.1152/ajpregu.2000.279.5.R1599
Hulley S, Grady D, Bush T et al (1998) Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin replacement study (HERS) Research Group. JAMA 280(7):605–613. https://doi.org/10.1001/jama.280.7.605
Lamas AZ, Caliman IF, Dalpiaz PL et al (2015) Comparative effects of estrogen, raloxifene and tamoxifen on endothelial dysfunction, inflammatory markers and oxidative stress in ovariectomized rats. Life Sci 124:101–109. https://doi.org/10.1016/j.lfs.2015.01.004
Langer RD (2017) The evidence base for HRT: what can we believe? Climacteric 20(2):91–96. https://doi.org/10.1080/13697137.2017.1280251
Langer RD, Hodis HN, Lobo RA et al (2021) Hormone replacement therapy - where are we. now? Climacteric 24(1):3–10. https://doi.org/10.1080/13697137.2020.1851183
Leung HS, Leung FP, Yao X et al (2006) Endothelial mediators of the acetylcholine-induced relaxation of the rat femoral artery. Vascul Pharmacol 44(5):299–308. https://doi.org/10.1016/j.vph.2006.01.010
Li M, Hao L, Li L et al (2020) Cinnamtannin B-1 prevents Ovariectomy-Induced osteoporosis via attenuating Osteoclastogenesis and ROS Generation. Front Pharmacol 11:1023. https://doi.org/10.3389/fphar.2020.01023
Liu MY, Hattori Y, Sato A, Ichikawa R et al (2002) Ovariectomy attenuates hyperpolarization and relaxation mediated by endothelium-derived hyperpolarizing factor in female rat mesenteric artery: a concomitant decrease in Connexin-43 expression. J Cardiovasc Pharmacology™ 40(6):938–948. https://doi.org/10.1097/00005344-200212000-00016
Liu Z, Duan Y-L, Ge S-L et al (2019) Effect of estrogen on right ventricular remodeling of monocrotaline-induced pulmonary arterial hypertension in rats and its mechanism. Eur Rev Med Pharmacol Sci 23:1742–1750. https://doi.org/10.26355/eurrev_201902_17136
Lobo RA, Archer DF, Kagan R et al (2018) A 17beta-Estradiol-progesterone oral Capsule for Vasomotor symptoms in Postmenopausal women: a Randomized Controlled Trial. Obstet Gynecol 132(1):161–170. https://doi.org/10.1097/AOG.0000000000002645
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055 PMID: 5389100
Mercuro G, Zoncu S, Saiu F et al (2004) Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas 47(2):131–138. https://doi.org/10.1016/s0378-5122(03)00252-4
Moreau KL, Hildreth KL, Klawitter J, Blatchford P, Kohrt WM (2020) Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience 42(6):1699–1714. https://doi.org/10.1007/s11357-020-00236-7
Nawate S, Fukao M, Sakuma I et al (2005) Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br J Pharmacol 144(2):178–189. https://doi.org/10.1038/sj.bjp.0706091
Oliveira LM, Oliveira TS, Costa RM, Gil ES, Costa EA, Passaglia RCAT, Filgueira FP, Ghedini PC (2016) The vasorelaxant effect of gallic acid involves endothelium dependent and –independent mechanisms. Vascul Pharmacol 81:69–74. https://doi.org/10.1016/j.vph.2015.10.010
Paredes-Carbajal MC, Juarez-Oropeza MA, Ortiz-Mendoza CM et al (1995) Effects of acute and chronic estrogenic treatment on vasomotor responses of aortic rings from ovariectomized rats. Life Sci 57(5):473–486. https://doi.org/10.1016/0024-3205(95)00281-a
Pocs L, Janovszky A, Ocsovszki I et al (2019) Microcirculatory consequences of limb ischemia/reperfusion in ovariectomized rats treated with zoledronic acid. J Orthop Surg Res 14(1):95. https://doi.org/10.1186/s13018-019-1117-x
Prentice RL, Manson JE, Langer RD et al (2009) Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol 170(1):12–23. https://doi.org/10.1093/aje/kwp115
Qiao X, McConnell KR, Khalil RA (2008) Sex steroids and vascular responses in hypertension and aging. Gend Med 5 suppl A. https://doi.org/10.1016/j.genm.2008.03.006. S46-S64
Reckelhoff JF, Fortepiani LA (2004) Novel mechanisms responsible for postmenopausal hypertension. Hypertension 43:918–923. https://doi.org/10.1161/01.HYP.0000124670.03674.15
Rossouw JE, Anderson GL, Prentice RL et al (2002) Writing Group for the women’s Health Initiative, Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s Health Initiative randomized controlled trial. JAMA 288(3):321–333. https://doi.org/10.1001/jama.288.3.321
Ruggiero RJ, Likis FE (2002) Estrogen: physiology, pharmacology, and formulations for replacement therapy. J Midwifery Womens Health 47(3):130–138. https://doi.org/10.1016/S1526-9523(02)00233-7
Rush JWE, Aultman CD (2008) Vascular biology of angiotensin and the impact of physical activity. Appl Physiol Nutr Metab 33:162–172. https://doi.org/10.1139/H07-147
Sakuma I, Liu M-Y, Sato A et al (2002) Endothelium-dependent hyperpolarization and relaxation in mesenteric arteries of middle-aged rats: influence of oestrogen. Br J Pharmacol 135(1):48–54. https://doi.org/10.1038/sj.bjp.0704465
Saleh RNM, Hornberger M, Ritchie CW et al (2023) Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimers Res Ther 15(1):10. https://doi.org/10.1186/s13195-022-01121-5
Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E et al (2020) Arterial stiffness accelerates within 1 year of the final menstrual period: the SWAN Heart Study. Arterioscler Thromb Vasc Biol 40(4):1001–1008. https://doi.org/10.1161/ATVBAHA.119.313622
Shih YN, Chen YT, Shih CJ et al (2017) Association of weekend effect with early mortality in severe sepsis patients over time. J Infect 74(4):345–351. https://doi.org/10.1016/j.jinf.2016.12.009
Silva LD, Veridiano JM, Oliveira JCC et al (2020) The Effect of Testosterone replacement on Intramedullary Inguinal and Visceral Fat in Ovariectomized rats. Rev Bras Ginecol Obstet 42(1):43–50. https://doi.org/10.1055/s-0040-1701460
Vanhoutte PM (1997) Endothelial dysfunction and atherosclerosis. Eur Heart J. https://doi.org/10.1016/s0195-668x(97)90005-1. 18 Suppl E: E19-E29
Virdis A, Ghiadoni L, Pinto S et al (2000) Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 101(19):2258–2263. https://doi.org/10.1161/01.cir.101.19.2258
Wakatsuki A, Ikenoue N, Shinohara K et al (2004) Small low-density lipoprotein particles and endothelium-dependent vasodilation in postmenopausal women. Atherosclerosis 177(2):329–336. https://doi.org/10.1016/j.atherosclerosis.2004.07.005
Yao F, Abdel-Rahman AA (2018) Estrogen receptor α and β play major roles in ethanol-evoked myocardial oxidative stress and dysfunction in conscious ovariectomized rats. Alcohol Clin Exp Res 41(2):279–290. https://doi.org/10.1111/acer.13290
Yung LM, Tian XY, Wong WT et al (2013) Chronic cranberry juice consumption restores cholesterol profiles and improves endothelial function in ovariectomized rats. Eur J Nutr 52(3):1145–1155. https://doi.org/10.1007/s00394-012-0425-2
Zhang XJ, Cao XQ, Zhang CS, Zhao Z et al (2017) 17β-estradiol protects against doxorubicin-induced cardiotoxicity in male Sprague-Dawley rats by regulating NADPH oxidase and apoptosis genes. Mol Med Rep 15(5):2695–2702. https://doi.org/10.3892/mmr.2017.6332
Acknowledgements
The authors express their gratitude to Dra. Taís Andrade and Lucas Breseghelo for their assistance in animal management. Additionally, de Oliveira T.S. acknowledges the fellowship provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Funding
This declaration is not applicable.
Author information
Authors and Affiliations
Contributions
TSO, HMC and RCM conducted experiments. FCAS, EAC, RCG and JAL contributed new reagents or analytical tools. RCT, NSL contributed to writing, review & editing. TSO, FPF and PCG conceived and designed research. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was carried out in accordance with the Regulations of the National Council for the Control of Animal Experimentation (CONCEA) for the Production or Use of Animals in Teaching Activities or Scientific Research, complying with the ARRIVE guidelines and in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Ethics Committee on Animal Use of the Federal University of Goiás (UFG) (protocol number CEUA/UFG 20/2013).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliveira, T.S., Campos, H.M., Costa, R.M. et al. Estrone-mediated lowering of ROS and NOX4 improves endothelial function in ovariectomized wistar rats. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03106-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03106-7