Abstract
Gastric ulcer is one of the most frequent gastrointestinal ailments worldwide. Indomethacin, one of the most potent NSAIDs, suffers undesirable ulcerogenic activity. Caffeic acid phenethyl ester (CAPE) has known health benefits. The current study examined the potential of CAPE to combat indomethacin-induced gastric ulcers in rats. Animals were randomized into 5 groups: control, Indomethacin (50 mg/kg) mg/kg), Indomethacin + CAPE (5 mg/kg/day), Indomethacin + CAPE (10 mg/kg), and Indomethacin + Omeprazole (30 mg/kg). CAPE prevented the rise in ulcer index, attenuated histopathological changes and preserved gastric mucin concentration. CAPE efficiently significantly prevented accumulation of malondialdehude (MDA) and prevented exhaustion of the enzymatic activities of catalase (CAT) and superoxide dismutase (SOD). Further, CAPE prevented the rise in the expression of tumor necrosis factor-α (TNF-α), cyclo-oxygenase-2 (COX-2) and nuclear factor kapp-B (NFκB). This was associated with down-regulation of Bax and up-regulation of Bcl-2 mRNA. Finally, CAPE prevented induced indomethacin-induced decrease in heat shock protein 70 (HSP70) in gastric tissues. In conclusion, CAPE possesses the ability to prevent indomethacin-induced gastric ulcer in rats. This involves, at least partially, antioxidation, anti-inflammation, anti-apoptosis and enhancement of HSP70 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peptic ulcer is one of the most frequent gastrointestinal ailments with serious complications and tendency to recur (Li et al. 2022). The main risk factors that contribute to developing gastric ulcer include the use of non-steroidal anti-inflammatory drugs (NSAIDs), age, and Helicobacter pylori (Abraham et al. 2005; Yuan et al. 2006). NSAIDs are often used because of their importance as strong antipyretics and analgesics in a variety of illnesses and disorders, from the common cold to rheumatoid arthritis (Day and Graham 2013). However, NSAIDs often increase the risk of developing stomach mucosal ulcers (Musumba et al. 2009; García-Rayado et al. 2018). Indomethacin is one of the most potent NSAIDs with ulcer-inducing activity (Henry and Robertson 1993). This includes inhibition of the gastroprotective enzyme cyclooxygenase-1 (COX-1) (Yadav et al. 2012). This is in addition to induction of oxidative stress, production of inflammatory mediators and inhibition of angiogenesis (Pai et al. 2000; Suleyman et al. 2010). According to earlier studies, agents with antioxidant, anti-inflammatory and angiogenic activities are helpful in preventing indomethacin stomach ulcers (Alkreathy et al. 2020; Shaik and Eid 2022).
Numerous medications are available to prevent and treat stomach ulcers. However, these medications suffer unwanted adverse effects and fail to inhibit ulcers recurrence (Sivri 2004). The rising incidence of gastric ulcers has driven the need for new gastroprotective compounds. In this regard, natural products with appealing pharmacological activities have been proposed due to their effectiveness, safety, wide acceptance by the public (Liu et al. 2015; Athaydes et al. 2019; Ahmed et al. 2021). Caffeic acid phenethyl ester (CAPE) is a component of bee propolis (Olgierd et al. 2021) with a plethora of pharmacological effects. These include neuroprotective (Bak et al. 2016; Zhang et al. 2021; Pérez et al. 2023), hepatoprotective, (Li et al. 2015), renoprotective (Ogeturk et al. 2005; Nur et al. 2023), cardioprotective (Huang et al. 2005; Khoshandam et al. 2023) and antidiabetic activities (Nie et al. 2017). Its protective effects have been suggested to involve antioxidant (Tolba et al. 2016), anti-inflammatory (Armutcu et al. 2015) and angioprotective properties (Abduljawad et al. 2013). Also, it has been suggested to alleviate drug adverse effects (Bakir et al. 2013; Şahin et al. 2013). However, there is a scanty of information about its potential to combat indomethacin-induced stomach injury. Therefore, the current study aimed to evaluate the ability of CAPE to attenuate indomethacin-induced gastric ulcer in rats.
Materials and methods
Drugs and chemicals
CAPE (> 99%) was obtained from Xi’An, Julong Bio-Teck Company (Xi’An, China). Indomethacin was purchased from Merck KGaA (Darmstadt, Germany). All other chemicals were of the highest purity.
Animals
Male Wistar rats weighing between 150 and 170 g were housed under controlled conditions, including a 12-h light/12-h dark cycle, at a room temperature of 23 ± 2 °C and a humidity level of 60%. The rats had unrestricted access to water and were fed standardized food pellets. All experimental procedures involving animal handling were conducted in accordance with the guidelines and regulations approved by the Research Ethics Committee of the Faculty of Pharmacy, King Abdulaziz University (Reference # PH-1444–38).
Induction of gastric ulcer
To prevent coprophagy, all rats were housed in wire mesh floor cages for 24 h prior to the induction of gastric ulcers. They were provided with free access to water only, which was also withheld for 1 h before the gastric ulcer induction. Afterwards, ulcers were induced by administering a single dose of indomethacin at 50 mg/kg via an oral tube. The indomethacin was suspended in 1% Carboxymethyl Cellulose Sodium Salt (CMC) solution for administration (Shaik and Eid 2022).
Experimental design
Thirty male rats were divided into five groups (six rats each). Animals in each group received the following treatments as represented in Table 1.
The selected doses for the treatments administered to the rats were determined based on a pilot experiment and were chosen to be consistent with those reported in the literature (Eser et al. 2022). In groups III & IV, CAPE was administered orally, 5 mg/kg and 10 mg/kg respectively, for seven days prior the day of ulcer induction with indomethacin. All treatments were performed between 8 Am and 10 AM. The given two doses were separated by a period of 1 h. Dosing volume was 10 ml/kg). Six hours after the indomethacin administration, animals were sacrificed by decapitation and the stomach of each rat was rapidly dissected out. Then, stomachs were opened along their greater curvature, and gently rinsed with saline to get rid of any contents or blood clots. The gastric mucosal surface of the stomachs was then turned upward as they were clamped to a wax plate covered with filter paper. Digital pictures were acquired to assess the gross pathology of the stomach. Then, stomachs were cut with a sharp blade. Some parts were kept in 10% neutral formalin for histological and immunohistochemical examinations. Other parts were flash frozen in liquid nitrogen and kept at -80 °C for biochemical evaluations.
Morphological examination
Macroscopic examination was conducted on the glandular mucosa of the stomachs to assess the presence of hemorrhagic lesions. The length of each individual lesion was measured in millimeters (mm). The ulcer index was determined using the following methodology (Sathish et al. 2011):
Histological examinations
Paraffin blocks were prepared by fixing the tissue samples in a 10% neutral formalin solution. Subsequently, sections with a thickness of 4 µm were obtained using a rotatory microtome. These sections underwent histological examination by staining with Hematoxylin and Eosin stain. The examination was conducted using light microscopy. A histopathologist, who was unaware of the experimental design; [1] epithelial cell loss (score: 0–3), [2] hemorrhage (score: 0–4), [3] inflammatory cell infiltration (score: 0–2) and [4] mucosal erosions (score: 0–4) (Shah et al. 1997).
Assessment of gastric mucin content
The gastric sections were subjected to staining using Alcian blue and Periodic Acid-Schiff (PAS) following established protocols (Bancroft and Gamble 2008).
Following hydration, the sections were immersed in a 3% acetic acid solution for 3 min, followed by staining with Alcian Blue Stain (1%, pH 2.5) for 15 min. Subsequently, the sections were gently rinsed with tap water. The slides were then placed in a solution of periodic acid (0.5%) for 5 min, followed by incubation in Schiff Reagent for 10 min. Afterward, the slides were washed with tap water for 5–10 min. Hematoxylin stain was applied to the slides, and optical density (OD) was assessed in six different areas of each tissue section using image analysis software (ImageJ, 1.48a, NIH, USA).
Assessment of gastric oxidative stress biomarkers
Stomach tissue homogenization was done in 10% of ice-cooled phosphate-buffered saline, pH 7.4. Homogenates were subjected to centrifugation at 10, 000 g for 20 min at 4 °C. The supernatant was then subjected to centrifugation at 10,000 g for 20 min. at 4 °C in order to examine oxidative stress biomarkers. Malondialdehyde (MDA) content and superoxide dismutase (SOD) and catalase (CAT) activities were determined using a commercial kit (Cat. no MD-2529, SD-2521, and CA-25 17 respectively, Bio diagnostic, Giza, Egypt).
Immunohistochemical examinations
Tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2), nuclear factor- κB p65 (NF-κB), and heat shock protein 70 (Hsp 70) were detected immunohistochemically using the avidin–biotin-peroxidase method. In order to inhibit endogenous peroxidase activity, slices were briefly mounted on positively charged glass slides, deparaffinized, hydrated, and then submerged in 10% H2O2. Sections were transferred into boiling sodium citrate buffer (10 mM, pH 6.0) for 10 min for antigen retrieval. The slides were cleaned in phosphate buffered saline (PBS) and then incubated in a blocking solution (5 percent normal goat serum and 0.2 percent triton in PBS). The primary antibody specific to each immunostain (ab307164, ab179800, ab16502 and ab181606 respectively) was then diluted in PBS and applied to the sections for overnight incubation. The sections were then exposed for 1 h to the appropriate secondary antibodies. The antibody-peroxidase complex was detected using 3,3´-diaminobenzidine tetra-hydrochloride chromogen after two PBS washings. The sections were then mounted, dehydrated, counterstained with Mayer’s hematoxylin, and washed with PBS.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Gastric tissues were subjected to RNA extraction using the TRIzol method. The purity of the extracted RNA was confirmed by measuring the A260/A280 ratio. For cDNA synthesis, the Omniscript RT kit (Cat. No. 205113, Qiagen, MD, USA) was employed. Quantification of mRNA was performed using qRT-PCR with a SYBR Green Master Mix (Cat. No. 180830, Qiagen, MD, USA). Primer nucleotide sequences are shown in Table 2. Data were analyzed by the ΔΔCT method, and β-actin was used for normalization (Livak and Schmittgen 2001).
Statistical analysis
Means and standard deviations (SD) were used to present obtained data. One-way analysis of variance (ANOVA) and Tukey’s post hoc test were used to establish to compare between different means. For nonparametric data of the histopathological scoring, Kruskal–Wallis test followed by Dunn test. P values < 0.05 were considered significant.
Results
CAPE ameliorates Indo-induced stomach gross morphological & histopathological changes and mucus secretion
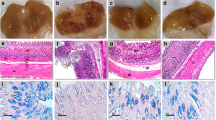
Stomachs from control group exhibited normal mucosa with no observable injuries (Fig. 1A). Nevertheless, challenging animals with indomethacin obviously injured gastric mucosa and showed bloody streaks (Fig. 1B). Rats pretreated with CAP (5 and 10 mg/kg) showed scattered bloody streaks but to a much lesser extent than in indomethacin-exposed animals (Fig. 1C & D; respectively). Pretreatment of animals with omeprazole (positive control) successfully protected the mucosal layer against any ulceration (Fig. 1E). Data in Fig. 1F indicate that CAPE at the doses 5 & 10 mg/kg significantly reduced ulcer formation by 42.6 and 56.2% as compared to Indo group.
Macroscopic photographs of the rats stomachs; A Control group displaying normal mucosa; B Indomethacin-exposed group exhibiting a hemorrhagic and ulcerated mucosal layer; C Indo + CAPE (5 mg/kg); D Indo + CAPE (10 mg/kg); E Omeprazole-pretreated group; F Graphical presentation of ulcer index. Ulcer index = 10/x, where ‘‘x” is total mucosal area / total ulcerated area. The obtained data were subjected to statistical analysis using one-way ANOVA followed by Tukey's test (p < 0.05) for multiple comparisons. a: significant difference from the control group, b: significant difference from the indomethacin (Indo) group, c: significant difference from Indo + CAPE (5 mg/kg), d: significant difference from Indo + CAPE (10 mg/kg)
Histological examination of stomach sections obtained from control animals revealed a normal gastric architecture (Fig. 2A). In contrast, rats exposed to indomethacin alone exhibited notable alterations, including reduced mucosal integrity and disruption of the lining epithelium of the mucosal layer. Additionally, focal ulceration, congested blood vessels, severe hemorrhage, and necrosis were observed, as shown in Fig. 2B. Stomach sections from CAPE-pretreated animals showed observable protection against such pathological changes in a dose-related manner (Fig. 2C & D). Omeprazole pre-treated animals showed almost normal histological structure of gastric tissue (Fig. 2E). Scoring of the histopathological changes showed that CAPE at doses of 5 and 10 mg/kg significantly prevented the rise in stomach histopathological changes induced by indomethacin (Fig. 2F).
Effects of CAPE on indomethacin-induced histopathological changes in gastric tissue. A Control group demonstrating normal histological architecture; B ndomethacin-exposed group, exhibiting reduced mucosal integrity, disrupted lining epithelium of the mucosal layer, and congested blood vessels; C Indo + CAPE (5 mg/kg); D Indo + CAPE (10 mg/kg); E Omeprazole-pretreated group; F Graphical presentation of histologopathological score. The obtained data were subjected to statistical analysis using Kruskal–Wallis test followed by Dunn test (p < 0.05). a: significant difference from the control group, b: significant difference from the indomethacin (Indo) group, c: significant difference from Indo + CAPE (5 mg/kg)
In Fig. 3A, gastric sections from control animals exhibit strong Alcian blue/PAS staining, which is evident as a dark blue or purple coloration of the mucosal layer. Conversely, gastric tissue from indomethacin-exposed rats displayed minimal or no reaction to the staining, particularly in the ulcerative areas, as illustrated in Fig. 3B. However, in the CAPE pretreated group (5 mg/kg), a slight improvement in mucus content was observed, as depicted in Fig. 3C. In addition, pretreatment of animals with CAPE (10 mg/kg) demonstrated marked preservation of mucus content (Fig. 3D). Omeprazole group showed strong blue coloration comparable to that in the control animals (Fig. 3E). Quantification of blue color intensity as a marker of gastric mucin concentration confirmed that both doses of CAPE (5 & 10 mg/kg) significantly prevented the decline in gastric mucin induced by indomethacin (Fig. 3F).
Effects of CAPE pretreatment on changes of mucus content as indicated by Alcian blue/PAS staining. A Control group showing strong positive staining; B Indomethacin-exposed group showing negative reaction indicating diminished mucin content; C Indo + CAPE (5 mg/kg) and D Indo + CAPE (10 mg/kg) showing moderate staining; E Omeprazole-pretreated group; F Graphical presentation of OD as a quantitative marker of mucin content. Data were analyzed using one-way ANOVA followed by Tukey’s test (p < 0.05). a: significant difference from the control group, b: significant difference from the indomethacin (Indo) group, c: significant difference from Indo + CAPE (5 mg/kg), d: significant difference from Indo + CAPE (10 mg/kg)
CAPE attenuates Indo-induced gastric oxidative stress in rats
The data in Table 3 indicate that indomethacin significantly enhanced lipid peroxidation as indicated by elevation in gastric MDA concentration. This was accompanied by depletion of CAT and SOD activities. However, pretreatment of animals with CAPE (5 & 10 mg/kg) significantly prevented MDA accumulation by 37.2 & 61.0%, CAT exhaustion by 45.4 & 100%, and SOD decreased activity by 36.0 & 68.7% respectively; as compared to corresponding indomethacin-alone treated group. Also, omeprazole almost inhibited indomethacin-induced oxidative stress with MDA, CAT and SOD values comparable to those observed in control animals.
CAPE suppresses gastric inflammation in Indo-treated rats
As shown in Fig. 4, indomethacin markedly up-regulated gastric expression of TNF-α, COX-2 and NFκB (upper, middle and lower panels respectively). On the other side, CAPE (5 and 10 mg/kg) significantly attenuated expression of TNF-α by 42.3 & 52,5%, COX-2 by 34.5 & 48.4%, and NFκB by 35.1 & 49.8% respectively; as compared to corresponding indomethacin-alone treated group.
Effect of CAPE pretreatment on expression of TNF-α (upper panel), COX-2 (middle panel) and NFκB (Lower panel) by immunohistochemical staining. Data are mean ± SD (n = 6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s test (p < 0.05). a: significant difference from the control group, b: significant difference from the indomethacin (Indo) group, c: significant difference from Indo + CAPE (5 mg/kg), d: significant difference from Indo + CAPE (10 mg/kg)
Effects of CAPE on Bax and Bcl-2, mRNA Expression
As illustrated in Fig. 5A, the administration of indomethacin resulted in a significant increase of 310% in the mRNA expression of the proapoptotic regulator Bax compared to the control group. However, treatment with CAPE at doses of 5 and 10 mg/kg significantly mitigated this elevation by 26.0% and 44.5%, respectively. In terms of the antiapoptotic regulator Bcl-2, indomethacin caused a significant reduction in Bcl-2 mRNA expression. However, treatment with CAPE at doses of 5 and 10 mg/kg significantly attenuated this change, leading to an enhancement in Bcl-2 mRNA expression by 36.2% and 44.3%, respectively, as shown in Fig. 5B.
Effects of CAPE on gastric mRNA expression of Bax A and Bcl-2 B in indomethacin-challenged rats. Statistical analysis was performed using one-way ANOVA followed by Tukey’s test (p < 0.05). a: significant difference from the control group, b: significant difference from the indomethacin (Indo) group, c: significant difference from Indo + CAPE (5 mg/kg), d: significant difference from Indo + CAPE (10 mg/kg)
CAPE downregulates HSP70 in Indo-treated rats
The data in Fig. 6 indicate that challenging rats with indomethacin resulted in a significant decline of HSP70 expression in gastric tissues by 61.3% as compared to control animals. However, CAPE at doses of 5 and 10 mg/kg significantly prevented the decrease in HSP70 expression by 100 and 90% respectively, as compared to indomethacin alone group. Similarly, omeprazole pretreatment prevented the decrease in HSP70 expression.
(A) Control group; (B) Indomethacin-exposed group; (C) Indo + CAPE (5 mg/kg); (D) Indo + CAPE (10 mg/kg); (E) Omeprazole-pretreated group; (F) Graphical presentation of HSP70 expression by immunohistochemical staining. Data are mean ± SD (n = 6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s test (p < 0.05). a: significant difference from the control group, b: significant difference from the indomethacin (Indo) group, c: significant difference from Indo + CAPE (5 mg/kg), d: significant difference from Indo + CAPE (10 mg/kg)
Discussion
Gastric ulcers remain a health challenge as its worldwide prevalence has been reported to be 8.4% (Salari et al. 2022). The use of non-steroidal anti-inflammatory drugs (NSAIDs) is a major contributor of the disease (Yadav et al. 2022). Due to their efficacy and safety, natural products have been proposed as new approach for the prevention and treatment of gastric ulcer (Singh and Easwari 2022). CAPE is a naturally occurring phenolic compound found in propolis, cereals, fruits, and vegetables. It has been demonstrated to provide a number of health benefits including healing properties (Tolba et al. 2016; Nasrullah 2022). In the current study, pretreatment of animals with CAPE significantly attenuated indomethacin-induced gastric ulcer. This was evidenced by reduced ulcer index. Further, histopathological examinations confirmed the protective effects of CAPE. This was associated with preservation of mucin concentrations in gastric tissues as indicated by Alcian blue staining. This is consistent with the previously reported ability of propolis to maintain the intestinal mucosal barrier in diabetic rats (Xue et al. 2019). Further, CAPE was shown to prevent colon ulcerations in rats induced by dextran sulphate sodium (Metwaly et al. 2022).
Oxidative stress plays a significant role in the pathogenesis of gastric ulcer (Bhattacharyya et al. 2014). In particular, experimental investigations indicated that indomethacin-induced gastric ulcer is associated with disturbed oxidative status (Dengiz et al. 2007; Eraslan et al. 2020). These reports give support to the observed lipid peroxidation and antioxidant enzyme exhaustion induced by indomethacin in the current study. On the other hand, CAPE gastroprotective effects were accompanied by preventing MDA accumulation and enhancing CAT and SOD enzymatic activities. These findings are in line with the reported potent antioxidant activities of CAPE in several experimental studies (Ramazan Yilmaz et al. 2004; Eser et al. 2022; Ren et al. 2023; Wang et al. 2023).
The pathogenesis and recurrence of gastric ulcer is strongly linked to inflammation (Watanabe et al. 2002). This is based on the enhanced release of the mucosal pro-inflammatory mediators following NSAIDs administration (Watanabe et al. 2004). In the current study, indomethacin challenge resulted in increased expression of TNF-α, COX-2 and NFκB. This is in line with previous studies highlighting the role of inflammatory mediators in indomethacin-induced gastric ulcer (Alp Yildirim et al. 2015; Fu et al. 2018). However, the data on COX-2 are controversial. Several reports indicated that indomethacin, with duration of 1 h, inhibits COX-2 (Liu et al. 2023). In contrast, other experimental studies illustrated the ability of indomethacin, with duration of 6 h, to enhance COX-2 (Mahmoud et al. 2023a). Our data are consistent with the latter studies. It seems that the effect of indomethacin is time-dependent. It is noteworthy to report that the effects of CAPE on COX-2 expression were not dose-dependent. This can indicate that CAPE (5 mg/kg) with enough to attain the maximum of this particular effect (Holford and Sheiner 1981). In other words, higher doses are not associated with higher effects (). In addition, the observed inflammation can be linked to oxidative stress induced by indomethacin (Martin and Wallace 2006). Herein, administration of CAPE at both doses prevented the increased expression of the inflammatory markers TNF-α, COX-2 and NF-κB in comparison to the ulcer control animals. These findings are aligned with previous data on CAPE ability to prevent activation of NF-κB and consequently down-regulation of expression of inflammatory mediators (Tambuwala et al. 2019; Sun et al. 2022). This is in addition to the reported anti-inflammatory effects of CAPE (Dos Santos and Monte-Alto-Costa 2013; Li et al. 2019).
Apoptotic mucosal death has been given a central role in the development of gastric ulcer induced by NSAIDs (Szabó and Tarnawski 2000). In line, the obtained data in this study indicated that indomethacin-induced ulcer was associated with up-regulation of Bax and down-regulation of Bcl-2. This is in line with previous studies (Nagano et al. 2005; Küçükler et al. 2022) and may be largely linked to the ability of indomethacin to induce oxidative stress (Orrenius 2007). CAPE pre-treatment could attenuate of mucosal cell apoptosis. Our findings are supported by the documented anti-apoptotic effects of CAPE (Abdallah and El-Refaei 2022; Owumi et al. 2022; Eser et al. 2022). The anti-apoptotic activities of CAPE and their role in the its protective effects have been recently reviewed (Ehtiati et al. 2023). In this regard, HSP70 is a molecular chaperone that is responsible for cellular healing (Mayer and Bukau 2005), protects against gastric mucosal damage under ulcerogenic conditions (Tanaka and Mizushima 2009) and thus inhibits apoptosis of mucoal cells (Moghadamtousi et al. 2014). Our data indicated that indomethacin suppressed expression of HSP70. This is in line with several experimental studies (Liu et al. 2015; El Badawy et al. 2021; Mahmoud et al. 2023b). On the contrary, oral gavage of CAPE to rats in this study enhanced expression of HSP70. This is supported by the ability of propolis and CAPE analogs to enhance expression of this chaperone (Tandean et al. 2019; Kinger et al. 2023). Taken together, these findings lend further support to the observed anti-inflammatory and apoptotic actions of CAPE (Borges et al. 2012; Zhai et al. 2019). In conclusion, CAPE protects against indomethacin-induced gastric ulcer in rats. This is attributed, at least partly, to its antioxidant, anti-inflammatory, anti-apoptotic and HSP70-enhancing activities.
Data availability
Data are available within the article or from the corresponding author upon reasonable request.
References
Abdallah EAA, El-Refaei MF (2022) Caffeic acid phenethyl ester mitigates infertility: A crucial role of metalloproteinase and angiogenic factor in cadmium-induced testicular damage. J Biochem Mol Toxicol 36. https://doi.org/10.1002/JBT.22960
Abduljawad SH, El-Refaei MF, El-Nashar NN (2013) Protective and anti-angiopathy effects of caffeic acid phenethyl ester against induced type 1 diabetes in vivo. Int Immunopharmacol 17:408–414. https://doi.org/10.1016/J.INTIMP.2013.06.019
Abraham NS, El-Serag HB, Johnson ML et al (2005) National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterology 129:1171–1178. https://doi.org/10.1053/J.GASTRO.2005.08.003
Ahmed MAE, Mohanad M, Ahmed AAE, et al (2021) Mechanistic insights into the protective effects of chlorogenic acid against indomethacin-induced gastric ulcer in rats: Modulation of the cross talk between autophagy and apoptosis signaling. Life Sci 275. https://doi.org/10.1016/J.LFS.2021.119370
Alkreathy HM, Alghamdi MK, Esmat A (2020) Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: Impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm J SPJ off Publ Saudi Pharm Soc 28:916–926. https://doi.org/10.1016/J.JSPS.2020.06.012
Alp Yildirim FI, Uyanik Ö, Özyoʇurtçu H et al (2015) Aggravating effect of atorvastatin on indomethacin-induced gastric injury: Focus on PGE2, TNF-α, neutrophils and iNOS. Prostaglandins Other Lipid Mediat 121:53–62. https://doi.org/10.1016/J.PROSTAGLANDINS.2015.07.002
Armutcu F, Akyol S, Ustunsoy S, Turan FF (2015) Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Exp Ther Med 9:1582–1588. https://doi.org/10.3892/ETM.2015.2346
Athaydes BR, Alves GM, de Assis ALEM et al (2019) Avocado seeds (Persea americana Mill.) prevents indomethacin-induced gastric ulcer in mice. Food Res Int 119:751–760. https://doi.org/10.1016/J.FOODRES.2018.10.057
Bak J, Kim HJ, Kim SY, Choi YS (2016) Neuroprotective effect of caffeic acid phenethyl ester in 3-nitropropionic acid-induced striatal neurotoxicity. Korean J Physiol Pharmacol 20:279–286. https://doi.org/10.4196/KJPP.2016.20.3.279
Bakir S, Özbay M, Gün R et al (2013) The protective role of caffeic acid phenethyl ester against streptomycin ototoxicity. Am J Otolaryngol 34:16–21. https://doi.org/10.1016/J.AMJOTO.2012.07.003
Bancroft JD, Gamble M (2008) Theory and Practice of Histological Techniques, 6th edn. Churchill Livingstone Elsevier, China
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol Rev 94:329–354. https://doi.org/10.1152/physrev.00040.2012
Borges TJ, Wieten L, Van Herwijnen MJC, et al (2012) The anti-inflammatory mechanisms of Hsp70. Front Immunol 3. https://doi.org/10.3389/FIMMU.2012.00095
Day R, Graham G (2013) Non-steroidal anti-inflammatory drugs (NSAIDs). BMJ 346:1396–1396. https://doi.org/10.1136/BMJ.F3195
Dengiz GO, Odabasoglu F, Halici Z et al (2007) Gastroprotective and antioxidant effects of montelukast on indomethacin-induced gastric ulcer in rats. J Pharmacol Sci 105:94–102. https://doi.org/10.1254/JPHS.FP0070122
Dos Santos JS, Monte-Alto-Costa A (2013) Caffeic acid phenethyl ester improves burn healing in rats through anti-inflammatory and antioxidant effects. J Burn Care Res 34:682–688. https://doi.org/10.1097/BCR.0B013E3182839B1C
Ehtiati S, Alizadeh M, Farhadi F et al (2023) Promising influences of caffeic acid and caffeic acid phenethyl ester against natural and chemical toxins: A comprehensive and mechanistic review. J Funct Foods 107:105637. https://doi.org/10.1016/j.jff.2023.105637
El Badawy SA, Ogaly HA, Abd-Elsalam RM, Azouz AA (2021) Benzyl isothiocyanates modulate inflammation, oxidative stress, and apoptosis via Nrf2/HO-1 and NF-κB signaling pathways on indomethacin-induced gastric injury in rats. Food Funct 12:6001–6013. https://doi.org/10.1039/D1FO00645B
Eraslan E, Tanyeli A, Güler MC et al (2020) Agomelatine prevents indomethacin-induced gastric ulcer in rats. Pharmacol Rep 72:984–991. https://doi.org/10.1007/S43440-019-00049-2
Eser N, Cicek M, Yoldas A, et al (2022) Caffeic acid phenethyl ester ameliorates imidacloprid-induced acute toxicity in the rat cerebral cortex. Environ Toxicol Pharmacol 96. https://doi.org/10.1016/J.ETAP.2022.103980
Fu Y, Wu HQ, Cui HL et al (2018) Gastroprotective and anti-ulcer effects of oxymatrine against several gastric ulcer models in rats: Possible roles of antioxidant, antiinflammatory, and prosurvival mechanisms. Phytother Res 32:2047–2058. https://doi.org/10.1002/PTR.6148
García-Rayado G, Navarro M, Lanas A (2018) NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert Rev Clin Pharmacol 11:1031–1043. https://doi.org/10.1080/17512433.2018.1516143
Henry D, Robertson J (1993) Nonsteroidal anti-inflammatory drugs and peptic ulcer hospitalization rates in New South Wales. Gastroenterology 104:1083–1091. https://doi.org/10.1016/0016-5085(93)90277-J
Holford NHG, Sheiner LB (1981) Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet 6:429–453. https://doi.org/10.2165/00003088-198106060-00002
Huang SS, Liu SM, Lin SM et al (2005) Antiarrhythmic effect of caffeic acid phenethyl ester (CAPE) on myocardial ischemia/reperfusion injury in rats. Clin Biochem 38:943–947. https://doi.org/10.1016/J.CLINBIOCHEM.2005.07.003
Khoshandam A, Hedayatian AH, Mollazadeh AR, et al (2023) Propolis and its constituents against cardiovascular risk factors including obesity, hypertension, atherosclerosis, diabetes, and dyslipidemia: A comprehensive review. Iran J Basic Med Sci 26:853–871. https://doi.org/10.22038/IJBMS.2023.67793.14835
Kinger S, Dubey AR, Kumar P, et al (2023) Molecular Chaperones’ Potential against Defective Proteostasis of Amyotrophic Lateral Sclerosis. Cells 12. https://doi.org/10.3390/CELLS12091302
Küçükler S, Kandemir FM, Yıldırım S (2022) Protective effect of chrysin on indomethacin induced gastric ulcer in rats: role of multi-pathway regulation. Biotech Histochem 97:490–503. https://doi.org/10.1080/10520295.2021.2014569
Li M, Wang XF, Shi JJ et al (2015) Caffeic acid phenethyl ester inhibits liver fibrosis in rats. World J Gastroenterol 21:3893–3903. https://doi.org/10.3748/WJG.V21.I13.3893
Li Y, Liu LH, Yu XQ et al (2019) Transglycosylation Improved Caffeic Acid Phenethyl Ester Anti-Inflammatory Activity and Water Solubility by Leuconostoc mesenteroides Dextransucrase. J Agric Food Chem 67:4505–4512. https://doi.org/10.1021/ACS.JAFC.9B01143
Li H, Zhao A, Xu R, Ding H (2022) A meta-analysis of the prevalence of peptic ulcer in Chinese adults. MEDS Public Heal Prev Med 2. https://doi.org/10.23977/phpm.2022.020401
Liu YH, Zhang ZB, Zheng YF et al (2015) Gastroprotective effect of andrographolide sodium bisulfite against indomethacin-induced gastric ulceration in rats. Int Immunopharmacol 26:384–391. https://doi.org/10.1016/J.INTIMP.2015.04.025
Liu R, Zhu N, Hao Y, et al (2023) The Protective Effect of Walnut Oligopeptides against Indomethacin-Induced Gastric Ulcer in Rats. Nutrients 15. https://doi.org/10.3390/NU15071675
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/METH.2001.1262
Mahmoud MF, Abdo W, Nabil M, et al (2023a) Apple (Malus domestica Borkh) leaves attenuate indomethacin-induced gastric ulcer in rats. Biomed Pharmacother 160. https://doi.org/10.1016/J.BIOPHA.2023.114331
Mahmoud MF, Abdo W, Nabil M, et al (2023b) Apple (Malus domestica Borkh) leaves attenuate indomethacin-induced gastric ulcer in rats. Biomed Pharmacother 160. https://doi.org/10.1016/J.BIOPHA.2023.114331
Martin GR, Wallace JL (2006) Gastrointestinal inflammation: a central component of mucosal defense and repair. Exp Biol Med (Maywood) 231:130–137. https://doi.org/10.1177/153537020623100202
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684. https://doi.org/10.1007/S00018-004-4464-6
Metwaly A, Elmoghazy H, Hussein M, et al. (2022) CAPE Improves Vanin-1/AKT/miRNA-203 Signaling Pathways in DSS-induced Ulcerative Colitis. Biomed Res Ther 9:5313–5325. https://doi.org/10.15419/bmrat.v9i9.769
Moghadamtousi SZ, Rouhollahi E, Karimian H et al (2014) Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther 8:2099–2111. https://doi.org/10.2147/DDDT.S70096
Musumba C, Pritchard DM, Pirmohamed M (2009) Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther 30:517–531. https://doi.org/10.1111/J.1365-2036.2009.04086.X
Nagano Y, Matsui H, Muramatsu M, et al (2005) Rebamipide significantly inhibits indomethacin-induced mitochondrial damage, lipid peroxidation, and apoptosis in gastric epithelial RGM-1 cells. Dig Dis Sci 50 Suppl 1. https://doi.org/10.1007/S10620-005-2810-7
Nasrullah MZ (2022) Caffeic Acid Phenethyl Ester Loaded PEG-PLGA Nanoparticles Enhance Wound Healing in Diabetic Rats. Antioxidants (Basel, Switzerland) 12.. https://doi.org/10.3390/ANTIOX12010060
Nie J, Chang Y, Li Y et al (2017) Caffeic Acid Phenethyl Ester (Propolis Extract) Ameliorates Insulin Resistance by Inhibiting JNK and NF-κB Inflammatory Pathways in Diabetic Mice and HepG2 Cell Models. J Agric Food Chem 65:9041–9053. https://doi.org/10.1021/ACS.JAFC.7B02880
Nur G, Caylak E, Deveci HA, et al (2023) The protective effect of caffeic acid phenethyl ester in the nephrotoxicity induced by α-cypermethrin. Open Med (Warsaw, Poland) 18. https://doi.org/10.1515/MED-2023-0781
Ogeturk M, Kus I, Colakoglu N et al (2005) Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J Ethnopharmacol 97:273–280. https://doi.org/10.1016/J.JEP.2004.11.019
Olgierd B, Kamila Ż, Anna B, Emilia M (2021) The Pluripotent Activities of Caffeic Acid Phenethyl Ester. Molecules 26. https://doi.org/10.3390/MOLECULES26051335
Orrenius S (2007) Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev 39:443–455. https://doi.org/10.1080/03602530701468516
Owumi SE, Irozuru CE, Arunsi UO, Oyelere AK (2022) Caffeic acid protects against DNA damage, oxidative and inflammatory mediated toxicities, and upregulated caspases activation in the hepatorenal system of rats treated with aflatoxin B1. Toxicon 207:1–12. https://doi.org/10.1016/J.TOXICON.2021.12.021
Pai R, Szabo IL, Kawanaka H et al (2000) Indomethacin inhibits endothelial cell proliferation by suppressing cell cycle proteins and pRb phosphorylation: A key to its antiangiogenic action? Mol Cell Biol Res Commun 4:111–116. https://doi.org/10.1006/mcbr.2000.0260
Pérez R, Burgos V, Marín V, et al (2023) Caffeic Acid Phenethyl Ester (CAPE): Biosynthesis, Derivatives and Formulations with Neuroprotective Activities. Antioxidants (Basel, Switzerland) 12. https://doi.org/10.3390/ANTIOX12081500
Ramazan Yilmaz H, Uz E, Yucel N et al (2004) Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J Biochem Mol Toxicol 18:234–238. https://doi.org/10.1002/JBT.20028
Ren C, Zhou P, Zhang M, et al (2023) Molecular Mechanisms of Oxidative Stress Relief by CAPE in ARPE-19 Cells. Int J Mol Sci 24. https://doi.org/10.3390/IJMS24043565
Şahin A, Kürşat Cingü A, Kaya S et al (2013) The protective effects of caffeic acid phenethyl ester in isoniazid and ethambutol-induced ocular toxicity of rats. Cutan Ocul Toxicol 32:228–233. https://doi.org/10.3109/15569527.2012.759958
Salari N, Darvishi N, Shohaimi S et al (2022) The Global Prevalence of Peptic Ulcer in the World: a Systematic Review and Meta-analysis. Indian J Surg 84:913–921. https://doi.org/10.1007/s12262-021-03189-z
Sathish R, Sahu A, Natarajan K (2011) Antiulcer and antioxidant activity of ethanolic extract of Passiflora foetida L. Indian J Pharmacol 43:336–339. https://doi.org/10.4103/0253-7613.81501
Shah AH, Khan ZA, Baig MZ et al (1997) Gastroprotective effects of pretreatment with Zizyphus sativa fruits against toxic damage in rats. Fitoterapia 68:226–234
Shaik RA, Eid BG (2022) Piceatannol Affects Gastric Ulcers Induced by Indomethacin: Association of Antioxidant, Anti-Inflammatory, and Angiogenesis Mechanisms in Rats. Life (Basel, Switzerland) 12. https://doi.org/10.3390/LIFE12030356
Singh KP, Easwari ST (2022) Natural Medicines as Gastro-protective Therapy in the Treatment of Peptic Ulcer: A Multifaceted Approach. Curr Nutr Food Sci 18:559–573
Sivri B (2004) Trends in peptic ulcer pharmacotherapy. Fundam Clin Pharmacol 18:23–31. https://doi.org/10.1111/J.1472-8206.2004.00203.X
Suleyman H, Albayrak A, Bilici M et al (2010) Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 33:224–234. https://doi.org/10.1007/S10753-009-9176-5
Sun W, Xie W, Huang D, et al (2022) Caffeic acid phenethyl ester attenuates osteoarthritis progression by activating NRF2/HO‑1 and inhibiting the NF‑κB signaling pathway. Int J Mol Med 50. https://doi.org/10.3892/IJMM.2022.5190
Szabó I, Tarnawski AS (2000) Apoptosis in the gastric mucosa: molecular mechanisms, basic and clinical implications - PubMed. https://pubmed.ncbi.nlm.nih.gov/10768847/. Accessed 5 Jul 2023
Tambuwala MM, Khan MN, Thompson P, McCarron PA (2019) Albumin nano-encapsulation of caffeic acid phenethyl ester and piceatannol potentiated its ability to modulate HIF and NF-kB pathways and improves therapeutic outcome in experimental colitis. Drug Deliv Transl Res 9:14–24. https://doi.org/10.1007/S13346-018-00597-9
Tanaka K-I, Mizushima T (2009) Protective role of HSF1 and HSP70 against gastrointestinal diseases. Int J Hyperth 25:668–676. https://doi.org/10.3109/02656730903213366
Tandean S, Japardi I, Loe ML et al (2019) Protective Effects of Propolis Extract in a Rat Model of Traumatic Brain Injury via Hsp70 Induction. Open Access Maced J Med Sci 7:2763–2766. https://doi.org/10.3889/OAMJMS.2019.736
Tolba MF, Omar HA, Azab SS et al (2016) Caffeic Acid Phenethyl Ester: A Review of Its Antioxidant Activity, Protective Effects against Ischemia-reperfusion Injury and Drug Adverse Reactions. Crit Rev Food Sci Nutr 56:2183–2190. https://doi.org/10.1080/10408398.2013.821967
Wang Y, Cai Z, Zhan G et al (2023) Caffeic Acid Phenethyl Ester Suppresses Oxidative Stress and Regulates M1/M2 Microglia Polarization via Sirt6/Nrf2 Pathway to Mitigate Cognitive Impairment in Aged Mice following Anesthesia and Surgery. Antioxidants (basel, Switzerland) 12:714. https://doi.org/10.3390/ANTIOX12030714
Watanabe T, Higuchi K, Tanigawa T et al (2002) Mechanisms of peptic ulcer recurrence: role of inflammation. Inflammopharmacology 10:291–302. https://doi.org/10.1163/156856002321544765
Watanabe T, Higuchi K, Hamaguchi M, et al (2004) Monocyte chemotactic protein-1 regulates leukocyte recruitment during gastric ulcer recurrence induced by tumor necrosis factor-alpha. Am J Physiol Gastrointest Liver Physiol 287. https://doi.org/10.1152/AJPGI.00372.2003
Xue M, Liu Y, Xu H, et al (2019) Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed Pharmacother 118. https://doi.org/10.1016/J.BIOPHA.2019.109393
Yadav SK, Adhikary B, Chand S et al (2012) Molecular mechanism of indomethacin-induced gastropathy. Free Radic Biol Med 52:1175–1187. https://doi.org/10.1016/J.FREERADBIOMED.2011.12.023
Yadav R, Kumar J, Kumar Singh A, et al (2022) A Review on the Pathogenesis, Treatment and Prevention of Peptic Ulcer Disease. Asian J Med Princ Clin Pract
Yuan Y, Padol IT, Hunt RH (2006) Peptic ulcer disease today. Nat Clin Pract Gastroenterol Hepatol 3:80–89. https://doi.org/10.1038/NCPGASTHEP0393
Zhai C, Lv J, Wang K, et al (2019) HSP70 silencing aggravates apoptosis induced by hypoxia/reoxygenation in vitro. Exp Ther Med 18. https://doi.org/10.3892/ETM.2019.7697
Zhang M, Wang L, Wen D, et al (2021) Neuroprotection of retinal cells by Caffeic Acid Phenylethyl Ester (CAPE) is mediated by mitochondrial uncoupling protein UCP2. Neurochem Int 151. https://doi.org/10.1016/J.NEUINT.2021.105214
Acknowledgements
This project was funded by the Institutional Fund Projects under grant no. (IFPRC-150-166-2020). Therefore, the author gratefully acknowledges the technical and financial support from the Ministry of Education and King Abdulaziz University, Jeddah, Saudi Arabia. In addition, I am grateful to Gamal Abdel Aziz of the Department of Anatomy and Histology, Faculty of Medicine, King Abdulaziz University for his help in the histological studies.
Funding
This project was funded by the Institutional Fund Projects under grant no. (IFPRC-150–166-2020). Therefore, the author gratefully acknowledges the technical and financial support from the Ministry of Education and King Abdulaziz University, Jeddah, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Thikryat Neamatallah is responsible for the conceptualization, methodology, formal analysis, data curation, writing (original draft preparation), writing (review, editing), and project administration. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the Faculty of Pharmacy’s Research Ethics Committee, King Abdulaziz University (Reference # PH-1444–38).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neamatallah, T. Caffeic acid phenethyl ester attenuates indomethacin-induced gastric ulcer in rats. Naunyn-Schmiedeberg's Arch Pharmacol 397, 1791–1801 (2024). https://doi.org/10.1007/s00210-023-02730-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02730-z