Abstract
Viaminate, a retinoic acid derivative developed in China, has been clinically used for acne treatment to regulate and control keratinocyte cell differentiation and proliferation, inhibit keratinization, reduce sebum secretion, and regulate immune and anti-inflammatory functions; however, its potential molecular mechanism has not yet been elucidated. Therefore, we induced ear acne in rats using Propionibacterium acnes and sebum application. Symptoms of ear redness, epidermal thickening, inflammatory reaction, keratin overproduction, subcutaneous oil, and triglyceride (TG) accumulation improved significantly in acne model rats treated with viaminate for 30 days. Transcriptome analysis of rat skin tissues suggested that viaminate had significant regulatory effects on fatty acid metabolism and cellular keratinization pathways. Molecular target prediction suggested that toll-like receptor 2 (TLR2) may be a key target of viaminate’s therapeutic mechanism. Western blotting results confirmed that viaminate inhibited the TLR2 and its downstream pathways, nuclear factor-kappa B (NF-κB) [NF-κB inhibitor alpha (IκBα)/NF-κB-p65] and mitogen-activated protein kinases (MAPKs) [MAPK p38/c-Jun N-terminal kinase (JNK)/extracellular regulated kinase 1/2 (ERK1/2)] in acne vulgaris rats. In vitro studies revealed that viaminate treatment attenuated P. acnes proliferation and P. acnes-induced inflammatory response in human keratinocytes and has an inhibitory effect on the activation of NF-κB and MAPKs, while overexpression of TLR2 attenuated these effects. In conclusion, viaminate ameliorates P. acnes-induced acne by inhibiting the proliferation and inflammatory response of keratinocytes, ascribed to the deactivation of the TLR2-mediated NF-κB and MAPK pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acne is a chronic skin condition, which usually occurs frequently in adolescence can also be observed in adults. The pathophysiology of acne involves the following three factors: excessive secretion of sebaceous glands, abnormal keratinization of hair follicles, and abnormal proliferation of Propionibacterium acnes. Current treatments for acne vulgaris include pharmacotherapy, optical therapy, and physical therapy. Drugs are the preferred mode of treatment for acne, and different drugs are prescribed according to the symptoms and severity. However, long-term application of drugs is limited due to their toxicity and side effects (Leung et al. 2021). Therefore, further clarification of the targets and mechanism of these drugs in acne treatment may facilitate further drug discovery.
Retinoids (such as tretinoin, tazarotene, adapalene, and trifarotene) are a class of vitamin A derivatives, which are preferred for the treatment and maintenance of mild or moderate acne vulgaris (Leung et al. 2021). These drugs inhibit the proliferation of keratin-forming cells, thereby reducing follicle obstruction and preventing microcomedones formation. Viaminate, structurally classified as a retinoid, was developed in China in the 1980s, and it is synthesized from all-trans retinoic acid (ATRA) and ethyl para-aminobenzoate (Cao et al. 2008). Viaminate inhibits the activity of ornithine decarboxylase, diminishing its potential to induce tumorigenesis in the skin. Additionally, viaminate enhances the self-repair ability of keratin-forming cells, preferentially allowing the differentiation of normal keratin-forming cells while inhibiting the proliferation and differentiation of abnormal keratin-forming cells, restoring keratinization abnormalities, and exfoliating keratin (Cao et al. 2008; Yin et al. 2007). However, its specific mechanism of function in the healing of acne has not yet been clarified.
Toll-like receptors (TLRs) are a group of recognition receptors that identify pathogen-associated molecular patterns (PAMPs), shared by a large number of microorganisms, as well as endogenous alarm signals, such as apoptosis-associated membrane fragments and inflammatory ligands. Upon detecting these potentially harmful signals, TLRs initiate the body’s immune response by triggering the production of inflammatory cytokines (El-Zayat et al. 2019). Ten TLRs have been identified in humans, among which keratin-forming cells can express TLRs 1–6. Studies have shown that acne is primarily caused by P. acnes (recently renamed Cutibacterium acnes), which triggers inflammatory skin response and can cause the deterioration of inflammatory acne lesions by activating TLRs to release chemokines (Hwang et al. 2020). TLR activation leads to the increase of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, which exacerbate acne development (Kim et al. 2002). Fragments of enzymes, such as lipase, protease, and hyaluronidase, secreted by P. acnes can also be recognized by TLR2 in keratin-forming cells near sebaceous glands. The recognition signal is then transmitted from TLR2 and TLR1/6 to the downstream bridging protein, MyD88, to further stimulate the activation of TRAF6, which in turn promotes the phosphorylation of IKK and the increase of nuclear factor-kappa B (NF-κB) inhibitor alpha (IκBα). NF-κB is separated from IκB and transferred to the nucleus and transcribes cytokines. Inflammatory acne was induced by cytokines in sebum-clogged pores, and persistently high levels of cytokines may cause the acute disease to evolve into chronic disease. In addition, P. acnes can also upregulate the expression of TLRs, including TLR2 and TLR4, and play a considerable role in acne-associated inflammation (Jugeau et al. 2005). Notably, ATRA inhibits acne progression by downregulating TLR2 and its coreceptor, CD14 (P. T. Liu et al. 2005). Therefore, the TLR system is a significant target in the treatment of acne, and viaminate, the structural analog of ATRA, plays a role in acne treatment via the TLR.
At present, the underlying mechanism of viaminate in acne treatment is not clear, and previous studies are based on the analysis of clinical samples, without further animal or cell experiments. This research investigated the therapeutic effects of viaminate on acne treatment by establishing a rat acne model. The potential mechanism of action of viaminate was analyzed by transcriptome analysis and target prediction and verified and confirmed by an in vitro keratinocyte model, which will lay the foundation to find anti-acne drugs.
Materials and methods
Bacterial culture
Propionibacterium acnes cultures (ATCC11827; Guangzhou Microbial Strain Conservation Center, China) were grown in herpes flesh medium (Basebio, China) at 37 °C under anaerobic conditions. Log phase bacterial cultures (OD 600 = 1.0) were obtained and centrifuged at 5000 g for 15 min at 4 °C. The culture was resuspended in phosphate-buffered saline (PBS) and the concentration was 8.0 × 107 CFU/20 μL.
Viaminate preparation
Viaminate (> 98% purity) was purchased from Psaitong Reagent Co., Ltd. Viaminate dosage was based on the tissue metabolism level of rats (Cao et al. 2008), and 1/3 × and 3 × of this standard dose were used in the low and high dose groups, respectively. Viaminate was dissolved in PBS containing 5% carboxypropyl cyclodextrin to obtain 1.7 (low), 5 (standard), and 15 (high) mg/mL solutions at 4 °C in the dark.
Animal models and administration
The rat acne model was constructed according to Stacey et al. (Kolar et al. 2019). Fifty male SD rats (250 ± 20 g) were divided into 5 groups on average. Group 1 (normal control; NC) was injected with 20 μL PBS and groups 2–5 were intradermally injected in the left auricle with 20 μL P. acnes (dissolved in PBS, 8.0 × 107 CFU per mouse) for 4 consecutive days to induce the acne model. After the induction of the acne model (i.e., day 5), rats in groups 3, 4, and 5 were given a daily gavage of viaminate at low (1.7 mg/kg), medium (5 mg/kg), and high (15 mg/kg) dose, respectively, for 30 days. Additionally, to accelerate the induction of acne formation, the left ears of the rats in groups 2–5 were smeared daily with artificial mixed sebum [17% oleic acid, 45% triglyceride (TG), 25% jojoba oil, and 13% squalene; 100 μL/ rat; Shanghai Maclin Reagent Company]. The rats were euthanized using CO2 asphyxiation method after 30 days of treatment.
Enzyme-linked immunosorbent assay (ELISA)
Blood was drawn from the main abdominal vein of the rats and centrifuged to separate the serum. Thereafter, the serum levels of IL-6, TNF-α, and IL-1β (RK00020, RK00029, and RK00009, respectively; Abclonal, China) were measured by an enzyme marker (CMax Plus, Molecular Devices, USA) following the manufacturer’s instructions.
Histopathological analysis
The left ear tissues of the rats were paraffin-embedded, sectioned, dewaxed, and stained with hematoxylin and eosin (HE) to detect histopathological conditions. Additionally, the slides were stained with Masson staining using the modified Masson trichrome staining kit (G1346, Solarbio, China) following the manufacturer’s instructions. A light microscope was used to observe and photograph.
Tissue immunofluorescence
Dewaxed rat ear tissue sections were placed in 0.01 M citrate buffer (121 °C, 20 min) then were put in 1% bovine serum albumin (BSA) for 20 min. Thereafter, the sections were incubated with rabbit anti-rat Ki67 (ab16667, Abcam) antibodies for 16 h at 4 °C and Alexa Fluor 488-coupled secondary antibodies (ab150077, Abcam) for 1 h at room temperature. Slides were then restained with 1 μg/mL Hoechst 33,342 dye and observed and photographed with the fluorescence microscope (DM500, Leica). The fluorescence intensity was quantified by Image-Pro Plus 6.0 (IPP 6.0) software, and the Ki67 positivity rate was counted in three different fields of view.
Immunohistochemistry (IHC)
Dewaxed rat ear tissue sections were placed in 0.01 M citrate buffer at 121 °C for 20 min, and 1% BSA for 20 min. Thereafter, the sections were combined with rabbit anti-rat keratin 7 (KRT7; ab181598, Abcam) and vimentin (ab92547, Abcam) antibodies separately for 16 h at 4 °C. After that, the sections were combined with goat anti-rabbit IgG-HRP (ab6721, Abcam) secondary antibody for 1 h. DAB and hematoxylin was used to stain the cells. The sections were photographed by optical microscope, and the positive chromogenic optical density values were quantified by IPP 6.0 software in three different fields of view.
Western blot
Rat ear tissue was homogenized by incubating with RIPA buffer for 5–10 min on ice. The protein lysates were boiled and denatured, separated by SDS–PAGE, and transferred to PVDF membranes, then incubated with 5% nonfat dry milk. Thereafter, the membranes were combined with primary antibodies (1:1000) at room temperature. Different rabbit anti-rat primary antibodies purchased from Abcam were used for western blotting: extracellular regulated protein kinases (ERK, ab184699); phosphorylated (p)-ERK (ab76299); c-Jun N-terminal kinase (JNK, ab179461); p-JNK (ab76572); p38 alpha/MAPK14 (p38, ab170099); p-p38 (ab4822); vimentin (ab92547); KRT7 (ab181598); patatin-like phospholipase domain containing 2 (PNPLA2, ab109251); lipoprotein lipase (LPL, ab91606); TLR2 (ab209217); nuclear factor kappa-B p65 subunit (NF-κB-p65, ab16502); p-NF-κB-p65 (ab76302); NF-kappa-B inhibitor alpha (IκBα, ab32518); and p-IκBα (ab92700). Thereafter, the membranes were combined with goat anti-rabbit IgG-HRP antibody (1:2000) for 2 h. ECL chemiluminescence substrate was added to emit light, and imaging was performed using the ChemiDoc-It Imaging System. The β-actin was used as the internal reference protein. The optical density values of protein bands were quantified and statistically analyzed using Image J software.
Transcriptome analysis
The left ear tissues of control, model, and ViaHi rats were subjected to transcriptome sequencing analysis. GO, KEGG, and Reactome enrichment analyses were performed for differentially expressed genes (DEGs) associated with TLR gene expression.
Molecular simulation docking
Viaminate structure was from Pubchem (https://pubchem.ncbi.nlm.nih.gov/), and the 3D structural model of TLR2 was from PDB (https://www.rcsb.org/). AutoDockTools-1.5.6 software was used to calculate the potential molecular force of viaminate and TLR2 and determine the binding site, which was visualized using PyMOL V.2.3 software.
qPCR detection
Total RNA was extracted from 50 mg rat left ear tissue by the TRIzol, and the mRNA was reverse transcribed into cDNA with cDNA Synthesis Kit (Novoprotein, China). And the qPCR methods were according to SYBR qPCR SuperMix Plus kit (Novoprotein) and 7500 Fast Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, Inc.): 95 °C, 2 min, 95 °C for 10 s, annealing at 60 °C for 10 s; and extension at 72 °C for 30 s (40 cycles). The relative gene expression was calculated using the 2−ΔΔCt method by using β-actin as an internal reference (Rao et al. 2013). The sequences of the primers are as follows: Human-TLR2 Forward: 5′-CTT CAC TCA GGA GCA GCA AGC A-3′, Human-TLR2 Reverse: 5′-GCT TGG TGG TTT GCT ACG AC-3′; Human-β-actin Forward: 5′-GAG GGC AAG ATG GTG TCG GAT A-3′, Human-β-actin Reverse: 5′-CTT CTC AGC CAG GTG TTC CAA G-3′; Rat-TLR2 Forward: 5′-TGG AGG TCT CCA GGT CAA ATC-3′, Rat-TLR2 Reverse: 5′-ACC AGC AGC ATC ACA TGA CA-3′; and Rat-β-actin Forward: 5′-CAC CCG CGA GTA CAA CCT T-3′; Rat-β-actin Reverse: 5′-ATA CCC ACC ATC ACA CCC TGG-3′.
TG analysis
Approximately 0.1 g of left ear tissue was mixed with normal saline (1:9, m/v), homogenized in a tissue grinder, and centrifugation at 3000 g for 10 min in a refrigerated centrifuge. Subsequently, tissue TG levels were detected according to the kit’s instructions (BC0625, Solarbio, China).
Detection of cell proliferation activity
Propionibacterium acnes (8.0 × 107 CFU/20 μL) was heated at 80 °C for 30 min to get heat-killed P. acnes (HKP), which was then freeze-dried and stored at 4 °C. The human keratinocyte cell line, HaCaT (Zhejiang Ruyao Biotechnology Co., Ltd.), was grown in DMEM containing 10% FBS, 1% penicillin–streptomycin, and L-glutamine respectively cultured in a constant temperature incubator (37 °C, 5% CO2). HaCaT cells were transferred to the 96-well plates (8000 cells/well) and treated with HKP (0, 50, 100, 200, 400, and 800 μg/mL) or viaminate (0, 62.5, 125, 250, 500, 1000, 2000, and 4000 nmol/L) for 48 h. The proliferation activity of HaCaT cells was detected by MTT assay (Wang et al. 2011). Additionally, 200 μg/mL HKP was combined with 0.1, 0.5, and 1 μmol/L viaminate, and the influence of viaminate on the HKP-induced proliferation of HaCaT cells was performed to observe with MTT assay.
In addition, the protein of Ki67 was detected by western blot after incubation of 200 μg/mL HKP with viaminate (0.1, 0.5, and 1 μmol/L) for 48 h. The optical density values of the protein bands were quantified with Image J software.
Cell immunofluorescence
HaCat cells were added into 24-well plates. After treatment, the coverslips were obtained and the cells were treated with 4% paraformaldehyde for about 15 min. After that, HaCat cells were treated with 0.5% Triton X-100 for 10 min and blocked with 1% BSA for 30 min. Thereafter, the HaCat cells were incubated overnight with the primary antibodies (vimentin and KRT7) at 4 °C then incubated with FITC-conjugated goat anti-rabbit secondary antibody for 1 h in dark condition. HaCat cells were combined with DAPI for 10 min. A fluorescence microscope (Leica, Germany) was used to get images, and fluorescence staining intensity was quantified by IPP 6.0.
Cell transfection
The pCMV6-TLR2 (NM_003264; RC207597) and pCMV6-Entry control (PS100001) plasmids were from Origene. The plasmids were transiently transfected into HaCaT cells (60–80% cell confluency before transfection) with the cationic lipid (X-TremeGene HP DNA transfection reagent, Roche). About 48 h of incubation, some cells were collected for qPCR detection of transfection efficiency.
Responsive experiment
The cells were divided into 4 groups: group 1 (control group); group 2 (model group): HaCaT cells were incubated with 200 μg/mL HKP for 48 h; group 3 (Via group): HaCaT cells were incubated with 200 μg/mL HKP and 1 μM viaminate for 48 h; and group 4 (TLR2 group): HaCaT cells (transfected with pCMV6-TLR2 plasmid) were incubated with 200 μg/mL HKP and 1 μM viaminate for 48 h. After 48 h of incubation, cell proliferation and keratinization levels were tested by MTT, immunofluorescence, and western blotting.
Statistical analysis
All data are presented as means ± standard deviation based on three replicates. The results were calculated using GraphPad Prism 8 (GraphPad Software, Inc.). T-tests were used for comparisons between two groups, and P < 0.05 was considered statistically significant.
Results
Viaminate can protect against P. acnes-induced inflammatory damage of ear skin in rat acne model
The skin surface of the rats was observed to examine the therapeutic effect of viaminate on P. acnes-induced acne in rats. Figure 1A shows the flow chart of animal experimental modeling and treatment. The ear edema was prominent and the skin surface was rough in the rats of the acne model group; however, the ear edema improved post-treatment, with the ViaHi group showing the best results (Fig. 1B). Subsequently, the inflammatory factors in the tissues showed that IL-1β, IL-6, and TNF-α in the ViaMed and ViaHi groups were evidently lower than the model group (P < 0.01; Fig. 1C–E). Pathological HE staining (Fig. 1F) revealed that in the model group, the structure of the skin epidermis (shown in the red box) and the thickness of the entire ear were increased, accompanied by much inflammatory cell infiltration (shown in the blue box). In contrast, the structure of the skin epidermis and the thickness of the entire ear were reduced and the structure of the sebaceous glands was normal in the viaminate-treated group. Additionally, Masson staining results (Fig. 1G, H) showed the area of positive collagen staining in the ear post-viaminate treatment was evidently higher than that in the model group, and a large area of collagen was lost in the model group.
Viaminate reduces Propionibacterium acnes-induced inflammatory damage of ear skin in rat acne model. A Flow chart of rat acne model treatment experiments; B changes in the skin surface of the rat ear; C–E enzyme-linked immunosorbent assay to detect the secretion levels of IL-6, TNF-α, and IL-1β in rat ear skin tissue; F HE staining to detect the pathological changes in rat ear skin tissue (red and blue boxes represent the enlarged epidermis and enlarged inflammatory cells, respectively); G Masson staining to detect changes in the tissue collagen levels; and H the proportion of Masson-stained collagen area (represented by blue). **P < 0.01, compared with the control group and ##P < 0.01 and #P < 0.05, compared with the model group. Scale bar is 50 or 200 μm
Viaminate can inhibit the abnormal proliferation of epidermal tissue in rat acne model
Acne is usually accompanied by abnormal proliferation of the epidermal cells. To determine the influence of viaminate on epidermal cell proliferation, Ki67, a cell proliferation-associated protein was detected by tissue immunofluorescence (Fig. 2A). It showed that the Ki67-positive rate was 36 ± 2.3% in the model group, while that in the Via groups were significantly lower (22 ± 3.6%, 26 ± 5.4%, and 15 ± 4.9% in the ViaLow, ViaMed, and ViaHi groups, respectively; P < 0.05; Fig. 2B). Additionally, western blotting (Fig. 2C–F) results indicate that compared with the model group, gradiantly doses of viaminate markedly inhibited the phosphorylation level of p38/JNK/ERK proteins (P < 0.05).
Viaminate can inhibit abnormal proliferation of epidermal tissue in rat acne model. A, B Tissue immunofluorescence detection of Ki67 expression and statistical quantification of Ki67-positive rate; C western blot detection of the expression levels of p38/extracellular regulated kinase (ERK)/c-Jun N-terminal kinase (JNK) and its phosphorylated proteins; and D–F statistical quantification of western blot band optical density values. **P < 0.01, compared with the control group, and ##P < 0.01 and #P < 0.05, compared with the model group. Scale bar is 100 μm
Viaminate inhibits the epidermal keratinization in rat acne model
Excessive keratinization of the skin epidermis is also an important factor in acne progression; therefore, analysis of skin epidermis keratinization will facilitate in evaluating acne progression. Immunohistochemistry (Fig. 3A–D) and western blotting results (Fig. 3E–G) showed that the cell keratin-associated proteins, vimentin and KRT7, were substantially upregulated in the model group (P < 0.05) compared with the control group. However, vimentin and KRT7 levels were evidently lower in the epidermal tissues of the acne-prone rats in the ViaMed and ViaHi groups (P < 0.05) compared with the model group.
Viaminate inhibits the level of epidermal keratinization in rat acne model. A, B Immunohistochemical (IHC) detection of vimentin and keratin 7 (KRT7) protein expression levels; C, D statistical quantification of IHC results; E western blot detection of vimentin and keratin 7 (KRT7) protein expression levels; and F, G statistical quantification of western blot band optical density values. **P < 0.01, compared with the control group and ##P < 0.01 and #P < 0.05, compared with the model group. Scale bar is 200 μm
Transcriptome analysis of the viaminate-treated rat acne model
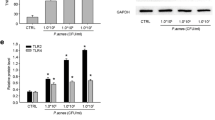
Although viaminate is effective in the treatment of acne, its specific therapeutic mechanism remains to be elucidated. Therefore, we analyzed the ear tissues of the normal, model, and viaminate-treated rats to explain the therapeutic mechanism of viaminate with transcriptome analysis. As seen in Fig. 4A, B, compared with the control group, 3581 and 1813 genes were evidently downregulated in the skin tissue of the model group. Additionally, compared with the model group, ViaHi upregulated 2401 genes and downregulated 862 genes. Figure 4C is a heatmap of gene expression. KEGG (Fig. 4D) and Reactome (Fig. 4E) enrichment analyses were performed on the genes that were evidently inhibited in the viaminate group and significantly upregulated in the model group. The results suggested that post-viaminate treatment, the genes were significantly enriched during the regulation of B lymphocyte activation, fatty acid metabolism, and cell keratin pathways. Transcriptome analysis revealed that the TLR2 gene was most significantly upregulated in the model group (Fig. 4F); however, TLR2 expression decreased after viaminate treatment. Furthermore, molecular docking simulations suggest a possible binding between viaminate and TLR2, with the binding energy of − 9.6 kcal/mol (Fig. 4G).
Transcriptome analysis of the viaminate-treated rat acne model. A The left image shows the volcano plot of differentially expressed genes (DEGs) compared with the model group. The right image shows the volcano plot of DEGs compared with the viaminate-treated (via) group; B histograms of the DEGs in the volcano plot; C heatmap comparing the DEGs of the three groups; D KEGG enrichment analysis results; E Reactome enrichment analysis results; F differential expression levels of genes associated with the toll-like receptor (TLR) family in the transcriptome data; the results are presented as heatmap; and G docking of viaminate and TLR2 protein structures visualized by PyMOL software
Viaminate reduces oil accumulation in the ear skin tissue of rat acne model
Transcriptome data showed that viaminate affected sebum synthesis in the ears of acne model rats. Transcriptome results indicated (Fig. 5A) that, in comparison with the control group, the TG levels degradation-associated genes, PNPLA2 and LPL, were decreased in the model group but increased in the ViaHi group. These results were also confirmed by western blotting assay (Fig. 5B–D). Analysis of TG content in rat ear tissues (Fig. 5E) showed that the TG content increased significantly in the model group, while it was in a dose-dependent decline in the viaminate-treated group (P < 0.05).
Viaminate reduces oil accumulation in the ear skin tissue of rat acne model. A relative expression levels of the TG degradation-related genes, patatin-like phospholipase domain containing 2 (PNPLA2) and lipoprotein lipase (LPL), in the transcriptome data; the results are presented as heatmap; B western blot detection of PNPLA2 and COX-2 protein expression levels; C-D statistical quantification of western blot band optical density values; E Triglyceride (TG) content in the rat ear skin tissue; **P < 0.01, compared with the control group, and #P < 0.05, compared with the model group
Viaminate inhibits P. acnes-induced abnormal proliferation and keratinization of HaCaT cells
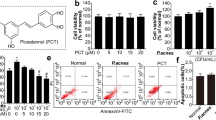
We conducted MTT assays to consider the influence of viaminate on the proliferation and keratinization of human immortalized keratinocytes. MTT assays (Fig. 6A–C) revealed that 200 μg/mL HKP had a significant promoting effect on HaCat cell viability (P < 0.05), compared with the 0 μg/mL group, while viaminate from 0 to 1000 nM did not have any significant cytotoxic effect on HaCat cells (P > 0.05). However, treatment with 0.5 and 1 μM viaminate significantly inhibited the promoting effect of HKP on HaCaT cells (P < 0.05), in comparison with the model group. The western blotting results of Ki67 showed that (Fig. 6D) different doses of viaminate prominently inhibited the HKP-induced proliferation of HaCaT cells (P < 0.05), compared with the model group. Additionally, immunofluorescence assay of vimentin and KRT7 indicated (Fig. 6E, F) that viaminate inhibited the HKP-induced expression of keratinization-associated proteins of HaCaT cells (P < 0.05).
Viaminate inhibits Propionibacterium acnes (P. acnes)-induced abnormal proliferation and keratinization of HaCaT cells. A–C MTT assay was used to screen the concentrations of heat-killed P. acnes (HKP) and viaminate and to determine the regulatory effect of viaminate on the HKP-stimulated activity of HaCat cells; D western blotting to detect Ki67 protein expression and quantification of the optical density values; and E, F immunofluorescence assay to detect the expression and localization of vimentin and keratin 7 (KRT7) proteins in the cells. **P < 0.01, compared with the control group, and ##P < 0.01 and #P < 0.05, compared with the model group. Scale bar is 50 μm
TLR2 overexpression attenuates the inhibitory effect of viaminate on HaCaT cell proliferation and keratinization
qPCR (Fig. 7A) confirmed that 0.1, 0.5, and 1 μM viaminate had a prominent inhibitory influence on TLR2 gene expression (P < 0.01), compared with the model group. To prove that viaminate exerts inhibitory effects on HaCaT cell hyperproliferation and keratinization via TLR2, the TLR2 expression plasmid was transfected into the HaCaT cells. qPCR results indicated that the TLR2 gene level was prominently increased in the pCMV6-TLR2-transfected HaCaT cells (P < 0.01; Fig. 7B), and TLR2 transfection reversed the inhibitory effect of viaminate on the TLR2 gene significantly (P < 0.01; Fig. 7C, compared with the Via group). The cell growth curve was tested by MTT assay, and the results suggested that viaminate could attenuate HKP-induced HaCaT proliferation, while overexpression of TLR2 could effectively reverse the effect of viaminate (Fig. 7D). Western blotting method was used to verify the IκB/NF-κB p65 and MAPK p38/JNK/ERK pathways downstream of TLR2, and the results showed that overexpression of TLR2 could significantly reverse the inhibitory effect of viaminate on IκB/NF-κB p65 and MAPK p38/JNK/ERK pathway protein phosphorylation (P < 0.05; Fig. 7E, F), compared with the Via group. Immunofluorescence results indicated (Fig. 7G, H) that with the inhibition of the MAPK p38/JNK/ERK pathway, the secretion levels of vimentin and KRT7 proteins in HaCaT cells were inhibited post-viaminate treatment; however, vimentin and KRT7 were expressed after TLR2 overexpression. Figure 8 shows the therapeutic mechanism of vistaminic acid on a rat model of acne.
Toll-like receptor 2 (TLR2) overexpression attenuates the inhibitory effect of viaminate on HaCaT cell proliferation and keratinization. A–C qPCR detection of TLR2 gene expression level; D MTT detection of cell viability; E western blot detection of nuclear factor-kappa B (NF-κB) inhibitor alpha IκBα/NF-κB p65 and mitogen-activated protein kinases (MAPKs) p38/JNK/ERK pathway protein expression levels; F statistical quantification of western blot band optical density values; and G, H immunofluorescence detection of vimentin and KRT7 protein expression and subcellular localization. **P < 0.01 and *P < 0.05, compared with the control group, and ##P < 0.05 and #P < 0.05, compared with the model group. Scale bar is 50 μm
Acne treatment mechanism of viaminate in rat acne model. In acne models, viaminate exerts an anti-inflammatory effect by inhibiting the toll-like receptor 2 (TLR2)/NF-κB pathway. Additionally, viaminate inhibits keratinocyte proliferation and hyperkeratinization via the TLR2/mitogen-activated protein kinases (MAPKs) [MAPK p38/JNK/ERK 1/2] pathway
Discussion
To study the specific treatment mechanism of viaminate in vivo, we established a rat model of acne, similar to human acne lesions, using P. acnes. Within 3 days of P. acnes inoculation, the ears of the acne model rats were swollen and red (Kolar et al. 2019). However, viaminate intervention treatment for 30 days significantly relieved the inflammatory symptoms in the ears of the acne model rats, reduced the proliferation of keratinocytes, the symptoms of hyperkeratosis, and the number of infiltrating inflammatory cells in skin lesions. Although research on the applications of viaminate in animal acne models is lacking, the anti-hyperkeratotic and anti-inflammatory functions of viaminate have been confirmed in clinical acne treatments (Ma et al. 2021; Nabatanzi et al. 2020); however, its mechanism of action remains unclear. Therefore, we determined a specific regulatory mechanism of viaminate using the transcriptome data of acne model rats. Considering the importance of the TLR receptor family in acne disease progression, we focused on the regulation of TLR members in transcriptome data. Although many TLRs are significantly regulated by viaminate, the TLR2 gene had the highest background expression level in the model group. Based on this, it was speculated that TLR2 may be associated with acne progression and thus, targeted by viaminate. In addition, viaminate targets were also predicted by PyMOL molecular simulation docking software, and the results suggested a potential binding between TLR2 and viaminate.
During acne progression, activation of TLR2 in skin cells can trigger innate immunity that promotes inflammatory lesions of acne (Köllisch et al. 2005). In vitro, P. acnes stimulates keratinocytes and monocytes to activate TLR2, resulting in improving the secretion of proinflammatory cytokines (IL-1β, IL-6, TNF-α, etc.) (Graham et al. 2004; Grange et al. 2009). In animal experiments, TLR2 gene was upregulated together with NF-κB in acne lesions, suggesting that P. acnes activates TLR2 (Hwang et al. 2020). Coralie et al. revealed that TLR-2 could recognize P. acnes CAMP factor 1 (Lheure et al. 2016), and in this study, TLR2/NF-κB pathway proteins were also observed to be activated in the ear skin tissue of acne model rats. The TLR2/NF-κB pathway is primarily responsible for the regulation of inflammatory factors, such as IL-6, TNF-α, and IL-1β (Balkrishna et al. 2020; Franz et al. 2022). ELISA and western blotting results indicated that viaminate prominently inhibited the phosphorylation levels of the inflammatory factors, IκBα and NF-κB p65. However, TLR2 overexpression could restore the effect of viaminate, indicating that viaminate could exert an anti-inflammatory effect through the TLR2/NF-κB pathway.
Notably, the results of KEGG and Reactome enrichment analyses of DEGs indicate that viaminate may act a major role to regulate abnormal cellular keratinization and fatty acid metabolism in acne treatment. In keratinocytes, bacterial lipopeptide-mediated activation of TLR2 can trigger the production of proinflammatory cytokines and enhance the tight junction barrier function of the epidermis upon pathogen invasion simultaneously, even enhances the level of cytokeratinization (Meisgen et al. 2014; Yuki et al. 2011). Moreover, a positive correlation between keratinocyte keratinization levels and TLR2 is often observed in acne disorders (Zhu et al. 2019).
Propionibacterium acnes-activated TLR2 further activates the MAPK p38 pathway in addition to affecting the NF-κB pathway (Huang et al. 2015). MAPK family members, such as JNK and MAPK p38, activate the MAPK p38/JNK/ERK pathway to promote keratinocyte proliferation (Kim et al. 2018). Simultaneously, the above pathway can also promote the formation of cytokeratin and reduce the decomposition of the keratin filament network (Wöll et al. 2007). Inhibition of MAPK p38 reduces the secretion of keratinization-related proteins from the epidermal keratinocytes (Cursons et al. 2015; Jonak et al. 2011; Meng et al. 2018; Usuki et al. 2022). Therefore, it is speculated that the inhibition of epidermal hyperkeratosis by viaminate via TLR2 under acne conditions is caused by MAPK p38. The in vitro and in vivo data of our study indicated that P. acnes activated the MAPK p38/JNK/ERK pathway, while overexpression of TLR2 prominently reversed the active repressions of viaminate on the MAPK p38/JNK/ERK pathway and promoted HaCaT cell proliferation. Secreted keratinization-related proteins, vimentin and KRT7. Vimentin stabilizes the keratin filaments and prevents keratin degradation (Keeling and Gavara 2020). Additionally, western blotting and Ki67 tissue immunofluorescence assays revealed that viaminate inhibits MAPK p38/JNK/ERK1/2 activation and Ki67 expression. These results demonstrate that viaminate can simultaneously inhibit the NF-κB and MAPK p38 pathways via TLR2, exert anti-inflammatory effects, and inhibit keratinocytes proliferation and keratinization.
In addition to the abnormal proliferation and hyperkeratinization of keratinocytes, acne formation is also associated with skin oil secretion disorders. P. acnes can activate the TLR2 pathway in sebocytes, thereby mediating inflammation and simultaneously upregulating the level of adipokines (Kovács et al. 2020; Törőcsik et al. 2018). Combined with the transcriptome data in our research results, it was suggested that viaminate treatment significantly inhibited the activity of the fatty acid metabolism pathway. The results also confirmed that viaminate can reduce oil accumulation and TG synthesis and simultaneously upregulate the PNPLA2 and LPL levels, which are associated with TG degradation (Jha et al. 2014; C. Liu et al. 2018). Therefore, we hypothesized that inhibition of TLR2 by viaminate can reduce the level of fatty acid metabolism. MAPK p38 is reported to be an adipogenesis signaling molecule, and specific p38 inhibitors block adipogenesis and the expression of fatty acid synthases (Ji et al. 2015). Blockade of MAPK p38 reduces the induction of lipid accumulation in human monocytes by TRL agonists (Lopez et al. 2013). Simultaneous activation of p38 MAPK/JNK signaling and NF-κB signaling in human sebocytes can be induced by thermal radiation, thereby upregulating inflammation and adipogenesis (Kwon et al. 2019). Accordingly, it is believed that viaminate may inhibit excessive sebum secretion in acne by inhibiting the TLR2/MAPK p38 pathway. However, since there is no suitable source of sebocytes in China, verification studies have not been performed at the sebocyte level. Therefore, the regulatory influence of viaminate on the MAPK p38 pathway been confirmed only in animal experiments and HaCaT cells. There are limited experimental studies on fatty acid metabolism. In this study, the observed phenomenon is explained only by the characteristics of the TLR2/MAPK p38 pathway. Further research on fatty acid metabolism in sebaceous gland cells is required to deepen our cognition of the therapeutic effect of viaminate on acne treatment.
This study has the following limitations. Firstly, the data in this study are based on transcriptome analysis of rat ear tissues, and the regulatory mechanisms analyzed based on this may lack specificity. This is due to the presence of many complex cell populations in the ear tissue, such as epidermal keratinocytes, fibroblasts, sebaceous gland cells, and various lymphocytes. However, this study primarily focuses on the mechanism of epidermal keratinocytes. Thus, the mechanistic studies of viaminate may have only uncovered the tip of the iceberg. In addition, transcriptome data revealed that viaminate has a regulatory effect on B lymphocyte recruitment, indicating that viaminate has a definite function on the chemotaxis of B lymphocytes. However, our lab is currently not equipped to carry out further research in this regard, and this will be pursued in the future research.
Summarily, this research verified the therapeutic ability of viaminate on P. acnes-induced epidermal inflammation, abnormal fatty acid metabolism, and abnormal proliferation and keratinization of epidermal cells in acne model rats. Additionally, transcriptome and experimental data proved that viaminate may play a role in regulating TLR2 and its downstream pathways, NF-κB and MAPK p38. Furthermore, in vitro experiments using HaCaT cells proved that viaminate inhibits the excessive proliferation and keratinization of HaCaT cells via the TLR2/MAPK p38/JNK/ERK pathway. This study conducted a specific mechanism of viaminate in the treatment of acne and effectively filled the gap in the understanding its therapeutic mechanism in acne treatment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Balkrishna A, Nain P, Chauhan A, Sharma N, Gupta A, Ranjan R, Varshney A (2020) Super critical fluid extracted fatty acids from Withania somnifera seeds repair psoriasis-like skin lesions and attenuate pro-inflammatory cytokines (TNF-α and IL-6) release. Biomolecules 10(2). https://doi.org/10.3390/biom10020185
Cao L, Zheng F, Ma P, Liu W, Sun D, Chen X, ... Gou, M (2008) LC-APCI-MS-MS method for the tissue distribution of viaminate after oral administrations to rats. J Chromatogr Sci 46(8): 701-706. https://doi.org/10.1093/chromsci/46.8.701
Cursons J, Gao J, Hurley DG, Print CG, Dunbar PR, Jacobs MD, Crampin EJ (2015) Regulation of ERK-MAPK signaling in human epidermis. BMC Syst Biol 9:41. https://doi.org/10.1186/s12918-015-0187-6
El-Zayat SR, Sibaii H, Mannaa FA (2019) Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl Res Cent 43(1):187. https://doi.org/10.1186/s42269-019-0227-2
Franz S, Ertel A, Engel KM, Simon JC, Saalbach A (2022) Overexpression of S100A9 in obesity impairs macrophage differentiation via TLR4-NFkB-signaling worsening inflammation and wound healing. Theranostics 12(4):1659–1682. https://doi.org/10.7150/thno.67174
Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E (2004) Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol 150(3): 421–428. https://doi.org/10.1046/j.1365-2133.2004.05762.x
Grange PA, Raingeaud J, Calvez V, Dupin N (2009) Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J Dermatol Sci 56(2):106–112. https://doi.org/10.1016/j.jdermsci.2009.08.001
Huang YC, Yang CH, Li TT, Zouboulis CC, Hsu HC (2015) Cell-free extracts of Propionibacterium acnes stimulate cytokine production through activation of p38 MAPK and toll-like receptor in SZ95 sebocytes. Life Sci 139:123–131. https://doi.org/10.1016/j.lfs.2015.07.028
Hwang DH, Lee DY, Koh PO, Yang HR, Kang C, Kim E (2020) Rosa davurica Pall. Improves Propionibacterium acnes-induced inflammatory responses in mouse ear edema model and suppresses pro-inflammatory chemokine production via MAPK and NF-κB pathways in HaCaT cells. Int J Mol Sci 21(5). https://doi.org/10.3390/ijms21051717
Jha P, Claudel T, Baghdasaryan A, Mueller M, Halilbasic E, Das SK, ... Trauner M (2014) Role of adipose triglyceride lipase (PNPLA2) in protection from hepatic inflammation in mouse models of steatohepatitis and endotoxemia. Hepatology 59(3): 858-869. https://doi.org/10.1002/hep.26732
Ji J, Zhu J, Hu X, Wang T, Zhang X, Hou AJ, Wang H (2015) (2S)-7,4′-dihydroxy-8-prenylflavan stimulates adipogenesis and glucose uptake through p38MAPK pathway in 3T3-L1 cells. Biochem Biophys Res Commun 460(3):578–582. https://doi.org/10.1016/j.bbrc.2015.03.072
Jonak C, Mildner M, Klosner G, Paulitschke V, Kunstfeld R, Pehamberger H, ... Trautinger F (2011) The hsp27kD heat shock protein and p38-MAPK signaling are required for regular epidermal differentiation. J Dermatol Sci 61(1): 32-37. https://doi.org/10.1016/j.jdermsci.2010.10.009
Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, Dreno B (2005) Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol 153(6):1105–1113. https://doi.org/10.1111/j.1365-2133.2005.06933.x
Keeling MC, Gavara N (2020) Withaferin-A can be used to modulate the keratin network of intermediate filaments in human epidermal keratinocytes. Int J Mol Sci 21(12). https://doi.org/10.3390/ijms21124450
Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, ... Modlin RL (2002) Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol 169(3): 1535-1541. https://doi.org/10.4049/jimmunol.169.3.1535
Kim J, Shin YK, Kim KY (2018) Promotion of keratinocyte proliferation by tracheloside through ERK1/2 stimulation. Evid Based Complement Alternat Med 2018:4580627. https://doi.org/10.1155/2018/4580627
Kolar SL, Tsai CM, Torres J, Fan X, Li H, Liu GY (2019) Propionibacterium acnes-induced immunopathology correlates with health and disease association. JCI Insight 4(5). https://doi.org/10.1172/jci.insight.124687
Köllisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J ... Ollert M (2005) Various members of the toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 114(4): 531-541. https://doi.org/10.1111/j.1365-2567.2005.02122.x
Kovács D, Fazekas F, Oláh A, Törőcsik D (2020) Adipokines in the skin and in dermatological diseases. Int J Mol Sci 21(23). https://doi.org/10.3390/ijms21239048
Kwon HC, Kim TY, Lee CM, Lee KS, Lee KK (2019) Active compound chrysophanol of Cassia tora seeds suppresses heat-induced lipogenesis via inactivation of JNK/p38 MAPK signaling in human sebocytes. Lipids Health Dis 18(1):135. https://doi.org/10.1186/s12944-019-1072-x
Leung AK, Barankin B, Lam JM, Leong KF, Hon KL (2021) Dermatology: how to manage acne vulgaris. Drugs Context 10. https://doi.org/10.7573/dic.2021-8-6
Lheure C, Grange PA, Ollagnier G, Morand P, Désiré N, Sayon S ... Dupin N (2016) TLR-2 recognizes Propionibacterium acnes CAMP factor 1 from highly inflammatory strains. PLoS ONE 11(11): e0167237. https://doi.org/10.1371/journal.pone.0167237
Liu C, Li L, Guo D, Lv Y, Zheng X, Mo Z, Xie W (2018) Lipoprotein lipase transporter GPIHBP1 and triglyceride-rich lipoprotein metabolism. Clin Chim Acta 487:33–40. https://doi.org/10.1016/j.cca.2018.09.020
Liu PT, Krutzik SR, Kim J, Modlin RL (2005) Cutting edge: all-trans retinoic acid down-regulates TLR2 expression and function. J Immunol 174(5):2467–2470. https://doi.org/10.4049/jimmunol.174.5.2467
Lopez S, Jaramillo S, Varela LM, Ortega A, Bermudez B, Abia R, Muriana FJ (2013) p38 MAPK protects human monocytes from postprandial triglyceride-rich lipoprotein-induced toxicity. J Nutr 143(5):620–626. https://doi.org/10.3945/jn.113.174656
Ma Y, Cao X, Zhang L, Zhang JY, Qiao ZS, Feng WL (2021) Neuropathy and chloracne induced by 3,5,6-trichloropyridin-2-ol sodium: report of three cases. World J Clin Cases 9(5): 1079–1086. https://doi.org/10.12998/wjcc.v9.i5.1079
Meisgen F, Xu Landén N, Wang A, Réthi B, Bouez C, Zuccolo M ... Pivarcsi A (2014) MiR-146a negatively regulates TLR2-induced inflammatory responses in keratinocytes. J Invest Dermatol 134(7): 1931-1940. https://doi.org/10.1038/jid.2014.89
Meng X, Qiu L, Song H, Dang N (2018) MAPK pathway involved in epidermal terminal differentiation of normal human epidermal keratinocytes. Open Med (wars) 13:189–195. https://doi.org/10.1515/med-2018-0029
Nabatanzi A, Mafuru M, Male M, Tian C, Zhang L, Wu T ... Huang C (2020) Feasibility study for the long-term management of refractory hyperkeratotic eczema with calcipotriol and betamethasone dipropionate (Daivobet(®)), viaminate and concomitant conventional therapies: a retrospective study. Clin Cosmet Investig Dermatol 13: 789-794. https://doi.org/10.2147/ccid.S276148
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 3(3):71–85
Törőcsik D, Kovács D, Póliska S, Szentkereszty-Kovács Z, Lovászi M, Hegyi K ... Ståhle M (2018) Genome wide analysis of TLR1/2- and TLR4-activated SZ95 sebocytes reveals a complex immune-competence and identifies serum amyloid A as a marker for activated sebaceous glands. PLoS ONE 13(6): e0198323. https://doi.org/10.1371/journal.pone.0198323
Usuki S, Tamura N, Tamura T, Yuyama K, Mikami D, Mukai K, Igarashi Y (2022) Konjac ceramide (kCer)-mediated signal transduction of the Sema3A pathway promotes HaCaT keratinocyte differentiation. Biology (Basel) 11(1). https://doi.org/10.3390/biology11010121
Wang Y, Zhang Z, Chen L, Guang H, Li Z, Yang H ... Lai R (2011) Cathelicidin-BF, a snake cathelicidin-derived antimicrobial peptide, could be an excellent therapeutic agent for acne vulgaris. PLoS ONE 6(7): e22120. https://doi.org/10.1371/journal.pone.0022120
Wöll S, Windoffer R, Leube RE (2007) p38 MAPK-dependent shaping of the keratin cytoskeleton in cultured cells. J Cell Biol 177(5):795–807. https://doi.org/10.1083/jcb.200703174
Yin J, Ni KY, Shen Y, Ma PC, Cao L, Wang WP, Wang Y (2007) Development and validation of a LC-MS/MS method for the determination of viaminate in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 856(1–2):376–380. https://doi.org/10.1016/j.jchromb.2007.06.033
Yuki T, Yoshida H, Akazawa Y, Komiya A, Sugiyama Y, Inoue S (2011) Activation of TLR2 enhances tight junction barrier in epidermal keratinocytes. J Immunol 187(6):3230–3237. https://doi.org/10.4049/jimmunol.1100058
Zhu T, Wu W, Yang S, Li D, Sun D, He L (2019) Polyphyllin I inhibits Propionibacterium acnes-induced inflammation in vitro. Inflammation 42(1):35–44. https://doi.org/10.1007/s10753-018-0870-z
Author information
Authors and Affiliations
Contributions
JC and SX contributed to the study conception and design. All authors collected the data and performed the data analysis. All authors contributed to the interpretation of the data and the completion of figures and tables. All authors contributed to the drafting of the article and final approval of the submitted version. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval was given by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (No.2022–1469).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, J., Xu, M., Zhu, L. et al. Viaminate ameliorates Propionibacterium acnes-induced acne via inhibition of the TLR2/NF-κB and MAPK pathways in rats. Naunyn-Schmiedeberg's Arch Pharmacol 396, 1487–1500 (2023). https://doi.org/10.1007/s00210-022-02379-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02379-0