Abstract
Recently, we have isolated and identified several bioactive flavonoids and stilbenoids with potential anticancer activity from Thai orchids. In this study, we further investigated the cytotoxic and chemosensitizing activities of these phytochemicals (namely, pinocembrin, cardamonin, isalpinin, galangin, pinosylvin monomethyl ether, 2,3′-dihydroxy-5′-methoxystilbene, (E)-2,5′-dihydroxy-2′-(4-hydroxybenzyl)-3′-methoxystilbene, 2,3-dihydroxy-3′,5′-dimethoxystilbene, 2,3′-dihydroxy-5,5′-dimethoxystilbene, 3,4′-dihydroxy-5-methoxystilbene and batatasin III) against breast cancer MCF7 cells and its two multidrug resistant (MDR) sublines (MCF7/DOX and MCF7/MX). Cytotoxicity was determined with MTT assay for the estimation of the half maximal cytotoxic concentrations (IC50). Effects of the test compounds on activities of efflux transporters (BCRP, P-gp, MRP1, and MRP2) were evaluated with substrate accumulation assays using fluorometry and flow cytometry analysis. Out of these 11 test compounds, the stilbene pinosylvin monomethyl ether displayed its cytotoxicity specifically toward MCF7 cells (IC50 = 6.2 ± 1.2 μM, 72-h incubation) with 4.96 folds higher than normal fibroblast. Its potency decreased in MCF7/DOX and MCF7/MX cells by 3.94 and 7.38 folds, respectively. Our transporter assay indicated that this stilbene significantly reduced the activities of P-gp, MRP1, and MRP2, but not BCRP. After 48-h co-incubation, this stilbene (at 2 μM) synergistically increased doxorubicin- and mitoxantrone-mediated cytotoxicity in MCF7, MCF7/DOX, and MCF7/MX cells potentially by increasing the intracellular level of cytotoxic drug. Pinosylvin monomethyl ether could sensitize breast cancer cells to chemotherapy and overcome MDR, in part, via the inhibition of drug efflux transporters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the leading causes of cancer mortality in women worldwide. Chemotherapy drugs such as anthracyclines (e.g., doxorubicin (DOX), epirubucin, mitoxantrone (MX)) and paclitaxel have been widely used for its treatment (Waks and Winer 2019). Unfortunately, cancer cells can develop several adaptive mechanisms in order to survive the toxic effect of these drugs, leading to chemoresistance and therapeutic failure (Wang et al. 2019). Moreover, chemotherapy-resistant cancer can withstand various structurally unrelated cytotoxic agents, namely, called multi-drug resistance (MDR) phenomenon (Bukowski et al. 2020). Increasing the drug dosage may not effectively boost up therapeutic success due to patients’ intolerability to serious adverse effects. Alternatively, combination of cytotoxic drugs with MDR modulators may provide better therapeutic outcome.
Overexpression of the ATP-binding cassette (ABC) efflux transporters largely contributes to development of MDR in cancer cells (Bukowski et al. 2020). These transporters limit the intracellular accumulation of their cytotoxic drug substrates such as DOX and MX, resulting in the loss of drug effectiveness (Chaisit et al. 2017). The most extensively studied ABC transporters in MDR cancer are the P-glycoprotein (P-gp/ABCB1), multidrug resistance associated protein 1 (MRP1/ABCC1), multidrug resistance associated protein 2 (MRP2/ABCC2), and breast cancer resistance protein (BCRP/ABCG2). In this regard, inhibiting these efflux transporters may overcome MDR and restore the sensitivity of cancer cells to anticancer drugs.
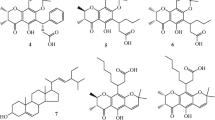
Various types of phytochemicals such as flavonoids (e.g., baicalein, quercetin), stilbenoids (e.g., resveratrol), naphthoquinone (e.g., rhinacanthin-C), coumarins (e.g., phenylfurocoumarin derivative), and alkaloids (e.g., piperine) have been reported as potential chemosensitizers that can suppress the function of MDR transporters (Chaisit et al. 2017; Li et al. 2018a, b, c; Li et al. 2019; Kokubo et al. 2021). Orchidaceae, which is the second largest family of flowering plants, has been shown to contain a variety of bioactive compounds, particularly flavonoids and stilbenoids, which display broad spectrum of pharmacological activities including anticancer, anti-inflammatory, antimicrobial, antioxidant, and antiplatelet activities (Śliwiński et al. 2022). In our continuing studies of chemical constituents of Thai orchids and their anticancer activity (Lertnitikul et al. 2016, 2020, 2022), we have isolated and identified several flavonoids and stilbenoids from these plants. Preliminary investigation suggested that a number of these phytochemicals exhibited interesting inhibitory effects against cancer cells. We have selected eleven flavonoids and stilbenoids (Fig. 1) which were found in sizable amounts, namely, pinocembrin (F1), cardamonin (F2), isalpinin (F3), galangin (F4), pinosylvin monomethyl ether (S5), 2,3′-dihydroxy-5′-methoxystilbene (S6), and (E)-2,5′-dihydroxy-2′-(4-hydroxybenzyl)-3′-methoxystilbene (S7) from Paphiopedilum dianthum, 2,3-dihydroxy-3′,5′-dimethoxystilbene (S1), 2,3′-dihydroxy-5,5′-dimethoxystilbene (S2) and 3,4′-dihydroxy-5-methoxystilbene (S3) from Paphiopedilum godefroyae, and batatasin III (S4) from Cymbidium finlaysonianum for this study. It is of great interest to determine whether these compounds are able to exert their anticancer action in breast cancer MCF7 cells, and also to sensitize MDR cancer cells toward standard cytotoxic drugs.
This study aimed to investigate anticancer activity and potential MDR reversal property of eleven flavonoids and stilbenoids from orchids. Their chemo-sensitizing effects toward DOX- and MX-resistant MCF7 cells were also assessed in MCF7/DOX and MCF7/MX cells, respectively.
Materials and methods
Pinocembrin (F1), cardamonin (F2), isalpinin (F3), galangin (F4), pinosylvin monomethyl ether (S5), 2,3′-dihydroxy-5′-methoxystilbene (S6), and (E)-2,5′-dihydroxy-2′-(4-hydroxybenzyl)-3′-methoxystilbene (S7) were isolated from Paphiopedilum dianthum Tang & F.T.Wang (Lertnitikul et al. 2022), 2,3-dihydroxy-3′,5′-dimethoxystilbene (S1), 2,3′-dihydroxy-5,5′-dimethoxystilbene (S2) and 3,4′-dihydroxy-5-methoxystilbene (S3) from Paphiopedilum godefroyae (God.-Leb.) Stein (Lertnitikul et al. 2016), and batatasin III (S4) from Cymbidium finlaysonianum Lindl. (Lertnitikul et al. 2020).
Calcein acetoxymethyl ester (calcein AM), carboxy-dichlorofluorescein (CDCF), 5(6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDCF-DA), pheophorbide A (PPA), verapamil (VER), indomethacin (INDO), KO143, DOX, and MX were purchased from Sigma Chemical Co. (St Louis, MO, USA). RPMI-1640 medium Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), and methylimidazole tetrazolium (MTT) were purchased from Gibco Life Technologies (Grand Island, NY, USA). Other chemicals and solvents were commercially available in reagent grade.
Cell cultures
Human breast cancer MCF7 cells were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA), and maintained in RPMI-1640 culture medium supplemented with 10% FBS and 1% penicillin/streptomycin. The DOX-resistant and MX-resistant sublines of MCF7 cells (MCF7/DOX and MCF7/MX) were developed and characterized in our laboratory, using a stepwise selection method as previously described (Chaisit et al. 2017). The resistant cells were maintained in the RPMI-1640 complete medium containing 1.5 μM DOX (for MCF7/DOX) or 0.7 μM MX (for MCF7/MX). In addition, normal fibroblast NIH/3T3 cells (ATCC, Rockville, MD, USA) were grown in DMEM medium supplemented with 10% FBS, 2.5 mM L-glutamine, and 1% penicillin/streptomycin. All cell lines were maintained at 37 °C in a humidified atmosphere of 5% CO2.
Cytotoxicity assessment
Cytotoxicity study was performed using MTT assay. Cells were seeded at the density of 5 × 103 cells/well in 96-well plates overnight prior to treatment. They were incubated with various concentrations of test compounds, ranging from 0 to 100 μM, or vehicle (0.5% DMSO) at 37 °C for 72 h. Then, the cells were washed and further incubated with MTT solution (0.5 mg/ml) for 4 h. Intracellular formazan crystals were solubilized with DMSO and quantified spectrophotometrically at 570 nm with a microplate reader.
MTT assay was also employed in the study of the effect of selected test compound to enhance DOX- and MX-mediated cytotoxicity toward cancer cells. The cells were treated with various concentrations of DOX or MX in the presence of the test compound at its non-cytotoxic concentration for 48 h prior to an assay.
Determination of inhibitory activity on ABC transporter functions
Uptake assay
Effects of test compounds on functions of the ABC transporters P-gp, MRP1, and MRP2 in MCF7 and MCF7/MX cells were assessed using uptake assay, as previously described (Wongsakul et al. 2022). Cells were seeded at a density of 1 × 105 cells/well in 48-well plates overnight prior to incubation with test compounds (at 100 μM) or known inhibitors (i.e., 100 μM VER and 500 μM INDO) at 37 °C for 30 min. Then, a specific substrate of P-gp (0.4 μM calcein AM), MRP1 (5.2 μM CDCF), or MRP2 (5 μM CDCF-DA) was added for another 30-min incubation. At the end of incubation period, the cells were lysed with 0.1% Triton X-100. The fluorescence intensity of each substrate was measured at excitation/emission wavelengths of 485/535 nm with a microplate reader.
Effects of test compounds on BCRP function in MCF7/MX cells were analyzed by flow cytometry (Kokubo et al. 2021). Cells were seeded at a density of 1 × 106 cells/well in 6-well plates overnight prior to incubation with test compounds (at 100 μM) or 10 μM KO143 at 37 °C for 30 min. Then, 10 μM PPA, a BCRP substrate, was added for a further 30-min incubation. The cells were then trypsinized and fixed with 4% formaldehyde. The intensity of intracellular fluorescence was measured at 635/670 nm (excitation/emission), using a BD FACSCalibur flow cytometer (BD Biosciences, CA, USA).
Studies on the accumulation of DOX and MX in cancer cells
Accumulation of DOX and MX in MCF7, MCF7/DOX, and MCF7/MX cells was determined by flow cytometry (Mirzaei et al. 2018). Cells were seeded at 1 × 106 cells/well in 6-well plates overnight prior to incubation with either 1 μM DOX or 5 μM MX in the presence of selected test compound at 37 °C for 3 h. Then, the cells were collected, fixed with 4% formaldehyde, and the intracellular fluorescence intensity was determined at excitation wavelength of 488 nm, an emission wavelength of 550 nm for DOX and 670 nm for MX, using a BD FACSCalibur flow cytometer.
Data analysis
Data were expressed as the mean ± SEM of data from three separated experiments. The percentage of cell viability was calculated using the following equation: \(\mathrm{\% Viability}= \frac{\mathrm{OD}(\mathrm{test})}{\mathrm{OD}(\mathrm{control})} \times 100\), where OD(test) and OD(control) were the optical density of cells treated with test compound and that of untreated cells, respectively. The concentration-cell viability curves were plotted and used for estimation of half maximal inhibitory concentration (IC50) values with non-linear regression analysis, using GraphPad Prism version 9.0.1 (GraphPad Software, San Diego, CA, USA).
The combination index (CI) and cytotoxicity enhancement ratio (CER) were calculated as follows: \({CI=}\frac{\mathrm{IC}50(\mathrm{DOX\; or\; MX\; in\; combination})}{\mathrm{IC}50(\mathrm{DOX\; or\; MX\; alone})}+\frac{\mathrm{IC}50(\mathrm{test\; in\; combination})}{\mathrm{IC}50(\mathrm{test\; alone})}\) and \(\mathrm{CER}= \frac{\mathrm{IC}50(\mathrm{DOX\; or\; MX\; alone})}{\mathrm{IC}50(\mathrm{DOX\; or\; MX\; in\; combination})}\) where IC50(DOX or MX in combination) and IC50(test in combination) values were the concentrations of either DOX or MX and the test compound that produced 50% cell death when used in combination treatment. The IC50(DOX or MX alone) and IC50(test alone) values were the concentrations of either DOX or MX and the test compound that generated 50% cell death when given alone. The CI values of lesser than, equal to, and greater than 1 were considered indicative of synergistic, additive, and antagonistic interaction, respectively, between DOX or MX and the test compound (Chou 2010). In addition, the ability of test compound to increase cancer cell sensitivity toward DOX or MX was demonstrated by the CER value (> 1). Higher CER values indicated greater enhancing effect of test compound on the cytotoxicity of anticancer drugs.
Statistical analysis was carried out by one-way analysis of variance (ANOVA), followed by post hoc Dunnett’s test and Student’s t-test, where appropriate. P < 0.05 indicated statistical significance.
Results
Cytotoxicity assessment of flavonoids and stilbenoids on breast cancer cells
Cytotoxicity of four flavonoids and seven stilbenoids from three orchid species was determined in breast cancer MCF7, MCF7/DOX, and MCF7/MX cells, as well as normal fibroblast NIH/3T3 cells, using MTT assay. The IC50 value of each compound after 72-h exposure is shown in Table 1. At our maximal test concentration of 100 μM, three flavonoids (F1, F2, and F4) and a stilbene (S3) were considered non-cytotoxic against breast cancer cells and normal fibroblast cells. A flavonoid, isalpinin (F3), was moderately cytotoxic to MCF7 and MCF7/DOX cells, but not to MCF7/MX cells. MCF7/MX cells were more resistant to the cytotoxicity of F3 than their parental MCF7 cells by at least 1.9 folds (resistance index, RI > 1.9). It should be noted that, at 100 μM, F3 was not cytotoxic to NIH/3T3 cells. Its selectivity toward wild-type MCF7 cells over normal cells, as shown by selective toxicity index (SI) in Table 1, was at least 1.9 folds.
Among the seven stilbenoids, S7 was the most potently cytotoxic compound against all cells tested, whereas S3 was the least potent one. The cytotoxicity of these stilbenoids against MCF7 cells, in descending order, was S7 > S5 > S1 ~ S6 > S2 > S4 > S3. Batatasin III (S4) displayed cytotoxicity with comparable IC50 values in each cell type (with SI and RI values of about 1), suggesting its non-selectivity toward breast cancer cells. Four stilbenoids (S1, S2, S5, and S6) were moderately toxic to normal fibroblast NIH/3T3 cells, with IC50 values ranging from 30 to 45 μM. These stilbenes, except S2, were more toxic against wild-type MCF7 cells than normal cells. The cytotoxicity of pinosylvin monomethyl ether (S5) was the most selective toward MCF7 cells, with the highest SI value of ~ 5.
According to the estimated RI values (Table 1), MCF7/DOX cells were less sensitive to S5- and S7-induced cytotoxicity than MCF7 cells by about 3–4 folds. MCF7/MX cells were more resistant than their parental MCF7 cells to four stilbenes (S1, S5, S6, and S7) and one flavonoid (F3) by 1.9–7.4 folds. The stilbene S5 exhibited the highest RI values in both MCF7/DOX (RI = 3.94) and MCF7/MX (RI = 7.38), suggesting its potential as a substrate of the MDR transporters.
Effects of flavonoids and stilbenoids on activities of the ABC efflux transporters
Activities of the key ABC efflux transporters (namely, P-gp, MRP1, MRP2, and BCRP) in wild-type MCF7 cells and its drug-resistant sublines were demonstrated by substrate accumulation assay employing specific substrate and inhibitor of each transporter. As shown in Fig. 2A, the presence of active P-gp and BCRP functions was confirmed in MCF7/DOX and MCF7/MX cells, respectively. The activity of MRP1 was observed in the wild type MCF7 cells, while the activity of MRP2 was detected in both parental MCF7 cells and their two resistant sublines.
Activities of the ABC efflux transporters as indicated by an accumulation of specific substrate in the presence of an inhibitor. Cells were pre-treated with known inhibitors (P-gp, VER 100 μM; MRP1 and MRP2, INDO 500 μM; BCRP, KO143 10 μM) or test compounds (100 μM) for 30 min, followed by addition of specific substrate (P-gp, calcein AM 0.4 μM; MRP1, CDCF 5.2 μM; MRP2, CDCF-DA 5 μM; BCRP, PPA 10 μM) for another 30 min; then fluorescent intensity of a specific substrate was measured. a Baseline activities of MRP1, MRP2, P-gp, and BCRP in MCF7, MCF7/DOX, and MCF7/MX cells. b Effects of the test compounds (100 μM) on the activities of MRP1 and MRP2 in MCF7 cells, P-gp in MCF7/DOX cells, and BCRP in MCF7/MX cells. Data are expressed as the percentage of the substrate alone group (control). Each value represents the mean ± S.E.M. (n = 3). *P < 0.05 compared with control (a Student’s t-test; b, one-way ANOVA with post hoc Dunnett’s test)
Effects of the test compounds on activities of MRP1, MRP2, P-gp, and BCRP transporters are displayed in Fig. 2B. Equal concentration (100 μM) of all test compounds was used. Among these compounds, only two stilbenes (S5 and S6) were able to significantly increase MRP1-related fluorescence in MCF7 cells, suggesting potential inhibitory action of both compounds against MRP1 function. On the other hand, two flavonoids (F1 and F2) and four stilbenes (S1, S2, S5, and S6) significantly increased the intracellular accumulation of CDCF in the CFCF-DA uptake assay, indicating their ability to interfere with MRP-2 function. Furthermore, one flavonoid and three stilbenes were able to interfere with P-gp activity, in decreasing order of potency S7 > S5 > F1 > S2, as evidenced by increased retention of calcein in the MCF7/DOX cells. Nearly all test compounds, except S3 and S5, were able to significantly increase the amount of PPA within MCF7/MX cells, suggesting their interference on BCRP function. The stilbene S7 was evidently the most potent BCRP modulator.
Effect of combining pinosylvin monomethyl ether (S5) with cytotoxic drugs on the viability of breast cancer cells
A stilbene, pinosylvin monomethyl ether (S5), was further investigated for its potentiation effect on DOX and MX toxicity to breast cancer cells. Combinations of S5 at a non-cytotoxic concentration (2 μM) either with DOX in MCF7 and MCF7/DOX cells or with MX in MCF7 and MCF7/MX cells were tested for their effect on the viability of cancer cells after 48-h treatment, using MTT assay. The concentration of 2 μM S5 was selected because, after 72-h treatment, it did not produce cytotoxicity of greater than 20% (i.e., IC20) in all cells tested. The enhancement effects of S5 on DOX and MX toxicity to breast cancer cells are illustrated as concentration-cytotoxicity curves in Fig. 3. The IC50 values of DOX and MX in the presence and absence of S5 for each cell line are documented in Table 2.
According to the CER values in Table 2, combination of S5 and DOX significantly reduced the viability of MCF7 cells by 8.5 folds and MCF7/DOX cells by 19.9 folds, when compared to DOX alone. The corresponding CI values were less than 1, indicating synergistic interaction between S5 and DOX in these cancer cells (Table 2). S5 also enhanced the toxicity of MX to both MCF7 and MCF7/MX cells in a synergistic fashion. When used in combination with S5, the IC50 values of MX markedly decreased by 3.7 folds (CI = 0.36) in MCF7 cells, and 5.1 folds (CI = 0.21) in MCF7/MX cells, respectively (Table 2).
Effects of pinosylvin monomethyl ether (S5) on intracellular accumulation of cytotoxic drugs in breast cancer cells
To investigate the potential enhancing mechanism of S5 on DOX and MX cytotoxicity, we measured intracellular fluorescence signals of both cytotoxic drugs in the presence of S5 in MCF7, MCF7/DOX, and MCF7/MX cells after 3-h incubation. It was worth noting that each treatment had no effect on cell viability. As shown in Fig. 4A, the fluorescence intensity of DOX in both MCF7 and MCF7/DOX cells was higher in the combination-treated groups than when DOX was used alone. Similarly, the fluorescence intensity of DOX and MX markedly increased in both MCF7 and MCF7/MX cells in the presence of S5 (Fig. 4B).
Effect of pinosylvin monomethyl ether (S5) at 2 μM on intracellular accumulation of a DOX in MCF7 and MCF7/DOX cells, and b MX in MCF7 and MCF7/MX cells after 3-h incubation. Data are expressed as the ratio of anticancer drug in combination with S5 to drug alone group (control). Each value represents the mean ± S.E.M. (n = 3). *P < 0.05 compared with control (Student’s t-test)
Discussion
Diverse types of natural compounds (e.g. flavonoids, stilbenoids, lignans, coumarins, curcuminoids, alkaloids) have been reported to increase the sensitivity of cancer cells to chemotherapy through the suppression of activities of the ABC efflux transporters (Costea et al. 2020; Feyzizadeh et al. 2022). Therefore, these natural chemosensitizers are able to increase intracellular amount of cytotoxic drugs and, hence, exhibit their MDR reversal effect (Feyzizadeh et al. 2022). In our search for potential chemotherapeutic sensitizers with ability to overcome MDR in breast cancer cells, we have selected four flavonoids and seven stilbenoids from three orchid species to be assayed for their cytotoxicity and modulating effect on four major ABC drug efflux transporters (namely, MRP1, MRP2, P-gp, and BCRP). Ideal anticancer drug should specifically kill cancer cells with very low toxicity toward normal cells. As such, we applied the selective toxicity index (calculated from the IC50 ratio between the normal fibroblast NIH/3T3 and the cancerous MCF7 cells) of not less than 3 as our suggestion criteria (Indrayanto et al. 2021).
According to their IC50 values, the stilbene S7, which is different from other stilbenoids tested in that it possesses an additional 4-hydroxybenzyl substitution on the stilbene scaffold, elicited the highest cytotoxicity. Nevertheless, when selectivity was considered, S5 was the best candidate for anticancer activity against MCF7 cells due to its high SI value of 4.96. Apparently, S6, which differs from S5 by possessing one additional hydroxy group at position 2 of the second benzene ring, was 2.7 folds less toxic against MCF7 cells than S5, whereas their cytotoxicity against normal fibroblast cells was comparable. Moreover, S1 and S2, both of which have one more methoxy group than S6, displayed comparable toxicity against MCF7 cells to S6. Modification of S5 by either the addition of a hydroxy group at para position of the second benzene ring (as in S3) or changing the central double bond to single bond (as in the dihydrostilbene S4) markedly attenuated the cytotoxicity and selectivity of the molecule. These findings suggested that, for the stilbene backbone with hydroxyl and methoxy substitutions at positions 3 and 5 on one aromatic ring, the presence of an additional hydroxyl group on the second aromatic ring might diminish their cytotoxic activity toward cancer cells. However, the presence of 4-hydroxybenzyl substitution at position 2 of the first ring might enhance the activity of these stilbenoids, as evidenced by the lowest IC50 values observed with S7. All flavonoids tested were non-toxic to either MCF7 or NIH/3T3 cells, except F3 which was cytotoxic to MCF7 cells with an SI value of 1.92. This observed cytotoxicity might be related to the presence of a methoxy substituent at position 7 of the flavonol backbone, rendering a part of this flavonoid molecule to be similar to S5 molecule. Further studies on the mechanisms of cell death in relation to the molecular structure of these natural compounds might be of great interest.
It has been established that reduction of cancer cell responses to various cytotoxic agents can be largely attributed to high expression levels of the ABC transporters such as P-gp, MRPs, and BCRP (Aires et al. 2019). In this regard, we also determined the inhibitory effect of these phenolic compounds from orchids on each transporter (i.e., MRP1, MRP2, P-gp, and BCRP) using substrate accumulation assays. Interestingly, S5 could significantly interfere with P-gp, MRP1 and MRP2 activities, but not BCRP activity. Thus, in comparison to the parental MCF7 cells, the decreased sensitivity of both resistant sublines toward this stilbene was related to the fact that S5 was a substrate of P-gp (in MCF7/DOX cells) and MRP-2 (in MCF7/DOX and MCF7/MX cells). The stilbene S7 could also significantly interfere with both P-gp and BCRP, but not MRP1 and MRP2 activities. The reduction of its cytotoxicity in the drug-resistant MCF7 cells might therefore be due to its being a substrate of both P-gp and BCRP. Furthermore, our results indicated that F3 was a substrate of BCRP, S1 and S6 were substrates of BCRP and MRP2, while the latter might also be a substrate of MRP1. Thus, their toxicity toward the DOX-resistant MCF7 was relatively similar to the parental MCF7 cells.
Several flavonoids (e.g., kaempferol, quercetin, baicalein) and stilbenoids (e.g., resveratrol and its methoxy derivatives) are substrates or modulators of the ABC efflux transporters, depending on their aromaticity, hydrophobicity, type, number, and position of substitutions on the molecular structure (Kitagawa et al. 2005; Valdameri et al. 2011; Wissel et al. 2017). Among the eleven compounds tested, the highly hydrophobic stilbene S7, with a para-hydroxybenzyl substituent to its core structure, was the most potent inhibitor on the activities of BCRP and P-gp, but without any effect on MRP1 and MRP2. Although none of the ABC transporters were affected by the stilbene S3, the absence of 4-hydroxyl group from one aromatic ring of this chemical structure, as seen in S5, apparently enabled this stilbene (S5) to significantly decrease activities of MRP1, MRP2 and P-gp. However, the stilbene S6, which is an isomer of S3 but possess a 2-hydroxyl instead of 4-hydroxyl unit, elicited its inhibitory effect on the activities of BCRP, MRP1, and MRP2 activities, but not P-gp. These findings suggested that the presence and position of a hydroxy group on this aromatic ring of the stilbene structure could be a key determinant to differentiate between P-gp and BCRP modulation. Addition of a methoxy group para to this 2-hydroxyl substitution, as seen in S2, restored the inhibitory effect on P-gp, but the effect on MRP1 was lost. The selective interference of flavonoids on the transporter activities was also demonstrated in this study. All four flavonoids tested could decrease BCRP function, but none were able to modulate the activity of MRP1. In addition, F1 and F2 were able to interact with MRP2 binding site, whereas F3 and F4 could not. Out of these four flavonoids, only F1 showed significant inhibitory effect against P-gp function. These findings suggested that the less planar structure of the flavanone F1, compared to flavonols F3 and F4 which have fixed length between aromatic rings on both sides of the molecule, was more suitable for interacting with P-gp.
Combinatorial approach using cytotoxic drugs and sensitizing agents has been an effective method to increase chemotherapeutic success and overcome MDR. Based on SI value, the stilbene pinosylvin monomethyl ether (S5), at its non-cytotoxic concentration (IC20 at 72-h incubation), was chosen for further investigation on its potential uses in combination with cytotoxic anticancer drugs. Although S5 might be a substrate of P-gp, MRP1, and MRP2, it was able to markedly enhance cytotoxicity mediated by DOX and MX against MCF7 cell line and its two resistant sublines (MCF7/DOX and MCF7/MX). The nature of the interaction of S5 with these two cytotoxic compounds was synergism. Moreover, the increased toxicity in both wild type and resistant cells could stem from a significant increase of intracellular cytotoxic drugs in the presence of S5. The cytotoxic drugs DOX and MX are known substrates of P-gp, MRP1, MRP2, and BCRP (Saraswathy and Gong 2013). It was very likely that S5 and DOX or MX competitively interacted with P-gp and both MRPs, and, thus, retarding efflux transport of these anticancer drugs.
In conclusion, pinosylvin monomethyl ether (S5), a stilbene from P. dianthum, exhibited its anticancer and chemo-sensitizing effects with good selective toxicity index. At its non-cytotoxic concentration, this stilbene could effectively increase DOX- and MX-mediated cell death, and overcome MDR through modulation of P-gp, MRP1 and MRP2 drug efflux transporters.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aires V, Colin DJ, Doreau A et al (2019) P-glycoprotein 1 affects chemoactivities of resveratrol against human colorectal cancer cells. Nutrients 11:2098. https://doi.org/10.3390/nu11092098
Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21:3233. https://doi.org/10.3390/ijms21093233
Chaisit T, Siripong P, Jianmongkol S (2017) Rhinacanthin-C enhances doxorubicin cytotoxicity via inhibiting the functions of P-glycoprotein and MRP2 in breast cancer cells. Eur J Pharmacol 795:50–57. https://doi.org/10.1016/j.ejphar.2016.12.002
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70:440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Costea T, Vlad OC, Miclea LC et al (2020) Alleviation of multidrug resistance by flavonoid and non-flavonoid compounds in breast, lung, colorectal and prostate cancer. Int J Mol Sci 21:401. https://doi.org/10.3390/ijms21020401
Feyzizadeh M, Barfar A, Nouri Z et al (2022) Overcoming multidrug resistance through targeting ABC transporters: lessons for drug discovery. Expert Opin Drug Discov 22:1–15. https://doi.org/10.1080/17460441.2022.2112666
Indrayanto G, Putra GS, Suhud F (2021) Validation of in-vitro bioassay methods: application in herbal drug research. Profiles Drug Subst Excip Relat Methodol 46:273–307. https://doi.org/10.1016/bs.podrm.2020.07.005
Kitagawa S, Nabekura T, Takahashi T et al (2005) Structure-activity relationships of the inhibitory effects of flavonoids on P-glycoprotein-mediated transport in KB-C2 cells. Biol Pharm Bull 28:2274–2278. https://doi.org/10.1248/bpb.28.2274
Kokubo S, Ohnuma S, Murakami M et al (2021) A phenylfurocoumarin derivative reverses ABCG2-mediated multidrug resistance in vitro and in vivo. Int J Mol Sci 22:12502. https://doi.org/10.3390/ijms222212502
Lertnitikul N, Jittham P, Khankhampoch L et al (2016) Cytotoxic stilbenes from the roots of Paphiopedilum godefroyae. J Asian Nat Prod Res 18:1143–1150. https://doi.org/10.1080/10286020.2016.1183
Lertnitikul N, Pattamadilok C, Chansriniyom C, Suttisri R (2020) A new dihydrophenanthrene from Cymbidium finlaysonianum and structure revision of cymbinodin-A. J Asian Nat Prod Res 22:83–90. https://doi.org/10.1080/10286020.2018.1540605
Lertnitikul N, Liangsakul J, Jianmongkol S, Suttisri R (2022) Three new cytotoxic stilbene dimers from Paphiopedilum dianthum. Nat Prod Res 18:1–9. https://doi.org/10.1080/14786419.2022.2101049
Li H, Krstin S, Wang S, Wink M (2018a) Capsaicin and piperine can overcome multidrug resistance in cancer cells to doxorubicin. Molecules 23:557. https://doi.org/10.3390/molecules23030557
Li J, Duan B, Guo Y et al (2018b) Baicalein sensitizes hepatocellular carcinoma cells to 5-FU and Epirubicin by activating apoptosis and ameliorating P-glycoprotein activity. Biomed Pharmacother 98:806–812. https://doi.org/10.1016/j.biopha.2018.01.002
Li S, Zhao Q, Wang B et al (2018c) Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother Res 32:1530–1536. https://doi.org/10.1002/ptr.6081
Li Y, Guo Y, Feng Z et al (2019) Involvement of the PI3K/Akt/Nrf2 signaling pathway in resveratrol-mediated reversal of drug resistance in HL-60/ADR cells. Nutr Cancer 71:1007–1018. https://doi.org/10.1080/01635581.2019.1578387
Mirzaei SA, Reiisi S, Tabari PG et al (2018) Broad blocking of MDR efflux pumps by acetylshikonin and acetoxyisovalerylshikonin to generate hypersensitive phenotype of malignant carcinoma cells. Sci Rep 8:3446. https://doi.org/10.1038/s41598-018-21710-5
Saraswathy M, Gong S (2013) Different strategies to overcome multidrug resistance in cancer. Biotechnol Adv 31:1397–1407. https://doi.org/10.1016/j.biotechadv.2013.06.004
Śliwiński T, Kowalczyk T, Sitarek P, Kolanowska M (2022) Orchidaceae-derived anticancer agents: a review. Cancers 14:754. https://doi.org/10.3390/cancers14030754
Valdameri G, Rangel LP, Spatafora C et al (2011) Methoxy stilbenes as potent, specific, untransported, and noncytotoxic inhibitors of breast cancer resistance protein. ACS Chem Biol 7:322–330. https://doi.org/10.1021/cb200435y
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321:288–300. https://doi.org/10.1001/jama.2018.19323
Wang X, Zhang H, Chen X (2019) Drug resistance and combating drug resistance in cancer. Cancer Drug Resist 2:141–160. https://doi.org/10.20517/cdr.2019.10
Wissel G, Deng F, Kudryavtsev P et al (2017) A structure-activity relationship study of ABCC2 inhibitors. Eur J Pharm Sci 103:60–69. https://doi.org/10.1016/j.ejps.2017.02.005
Wongsakul A, Lertnitikul N, Suttisri R, Jianmongkol S (2022) N-trans-p-coumaroyltyramine enhances indomethacin-and diclofenac-induced apoptosis through endoplasmic reticulum stress-dependent mechanism in MCF-7 cells. Anticancer Res 42:1833–1844. https://doi.org/10.21873/anticanres.15659
Funding
This work was supported by a grant (Fundamental Fund No. CUFRB65 hea(53)-062–33-06) from Thailand Science Research and Innovation (TSRI), Thailand. We also received graduate scholarship from Chulalongkorn University for ASEAN and Non-ASEAN countries to KL Sein.
Author information
Authors and Affiliations
Contributions
Khin Lay Sein and Suree Jianmongkol conceived and designed research. Khin Lay Sein conducted experiments. Khin Lay Sein and Suree Jianmongkol conducted data analysis and interpretation. Nonthalert Lertnitikul and Rutt Suttisri contributed the test materials. Khin Lay Sein wrote the original draft of manuscript. Rutt Suttisri and Suree Jianmongkol reviewed and edited the final draft of manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sein, K.L., Lertnitikul, N., Suttisri, R. et al. Anticancer and chemosensitizing activities of stilbenoids from three orchid species. Naunyn-Schmiedeberg's Arch Pharmacol 396, 749–758 (2023). https://doi.org/10.1007/s00210-022-02352-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02352-x