Abstract
Drug induced liver injury (DILI) is a relatively rare hepatic condition in response to the use of medications, illegal drugs, herbal products or dietary supplements. It occurs in susceptible individuals through a combination of genetic and environmental risk factors believed to modify drug metabolism and/or excretion leading to a cascade of cellular events, including oxidative stress formation, apoptosis/necrosis, haptenization, immune response activation and a failure to adapt. The resultant liver damage can present with an array of phenotypes, which mimic almost every other liver disorder, and varies in severity from asymptomatic elevation of liver tests to fulminant hepatic failure. Despite recent research efforts specific biomarkers are not still available for routine use in clinical practice, which makes the diagnosis of DILI uncertain and relying on a high degree of awareness of this condition and the exclusion of other causes of liver disease. Diagnostic scales such as the CIOMS/RUCAM can support the causality assessment of a DILI suspicion, but need refinement as some criteria are not evidence-based. Prospective collection of well-vetted DILI cases in established DILI registries has allowed the identification and validation of a number of clinical variables, and to predict a more severe DILI outcome. DILI is also in need of properly designed clinical trials to evaluate the efficacy of new DILI treatments as well as older drugs such as ursodeoxycholic acid traditionally used to ameliorate cholestasis or corticosteroids now widely tried in the oncology field to manage the emergent type of hepatotoxicity related to immune checkpoint inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is central to biotransformation (metabolism) of xenobiotics entering the gastrointestinal tract and, consequently, is susceptible to the harmful effect of many drugs, herbs and dietary supplements that can damage hepatocytes or other liver cells. Drug-induced liver injury (DILI) remains a leading cause of drug development termination and postmarketing warnings and restriction of use. Hepatotoxicity is a major reason for acute liver failure (ALF) in Western countries (Hillman et al. 2016) and accounted for 32% of drug withdrawals in the period 1975–2007 (Stevens and Baker 2009). Main stakeholders became more aware of DILI following the publication of “FDA Guidance for Industry on DILI Premarketing Clinical Evaluation” (FDA et al. 2009) in 2009 and no postmarketing drug withdrawal due to hepatotoxicity has been reported in the USA since then. Nevertheless, a comprehensive understanding of a drug liver safety profile needs the exposure of many thousand patients to the compound in the postmarketing setting.
Although many drugs in common use have been related to DILI (NIDDK 2017) the relative risk varies widely between agents. Categorization of the risk has been proposed based on published reports (Björnsson and Hoofnagle 2016), but this method is not without limitations. For instance, statins, which have been associated with hepatotoxicity in various case reports and case series (Perdices et al. 2014; Russo et al. 2014) have a very low hepatotoxic potential, estimated as < 1 in 50,000 treated patients considering the large number of individuals exposed to these agents (Björnsson et al. 2012).
The pathogenesis of idiosyncratic DILI is complex and scarcely known, and probably differs even among subjects for a given drug, which might explain the range of phenotypic signatures of this adverse hepatic reaction both in clinical expression and severity. Indeed, DILI is one of the most challenging diagnoses that clinicians face, because of the large number of medications, but also herbs and dietary supplements that have been related to hepatotoxicity, its relative rarity, its varied clinical and histopathological presentation and, most importantly, the absence of specific biomarkers. All these factors complicate DILI assessment, which diagnosis still relies on a high degree of suspicion and careful exclusion of alternative etiologies of liver damage. In this review we address current concepts and gaps in DILI epidemiology, pathogenesis, clinical presentation, diagnosis, prognosis and management.
Types of drug-induced liver injury
Drugs or chemicals that cause direct and predictable toxicity in liver cells can produce a variety of hepatotoxicity that is typically dose-dependent occurring shortly once a threshold dose or exposure, which can be different among individuals, is reached. This category of liver injury is called intrinsic and with the exception of paracetamol is nowadays seldom seen in clinical practice.
The bulk of drugs causing DILI, however, induce liver injury very rarely, in an unpredictable fashion. This category is termed idiosyncratic, as it is associated with unique host characteristics. Idiosyncratic DILI refers to a hepatic reaction to drugs that usually occurs in < 1 of every 10,000 exposed individuals, has a longer latency period (from a few days to several weeks) and is unexpected from the pharmacological mechanism of action of the agent. Idiosyncratic DILI is believed to be a multifactorial process, which is precipitated in an individual by the interplay of several critical factors including toxicological drug properties as well as host-related factors and environmental conditions (Chen et al. 2015).
In an attempt to overcome the fact that this hepatotoxicity categorization has been considered overly simplistic (Corsini et al. 2012) a third “indirect” category was recently proposed to distinguish a type of liver damage that is caused by “what the drug does rather than what the drug is” (Hoofnagle and Björnsson 2019). This category encompasses a group of drugs such as immune checkpoint inhibitors, which alter the immune system and can lead to immune-mediated hepatotoxicity, or agents that exacerbate an underlying liver condition (hepatitis B or fatty liver) (Table 1) (Hoofnagle and Björnsson 2019). However, this concept is controversial as it is obvious that the indirect damage is not predictable at the light of the current knowledge. Indeed, drugs that cause DILI by this mechanism do it in generally a small fraction of individual exposed.
Epidemiology
The true incidence of idiosyncratic DILI is difficult to estimate. A major hurdle is that a DILI diagnosis remains challenging and is often delayed or left unrecognized and therefore underreported. Furthermore, differences in DILI case qualification and diagnostic criteria used, variations in use of drugs, herbal and dietary supplements (HDS) and prescription patterns, which may vary across the world, may account for notable differences reported in the literature (Table 2).
Data on DILI epidemiology has mainly been provided by retrospective database studies from the pharmaceutical industry, pharmacovigilance centers and general practice, which typically have collected information on the spectrum of associated drugs as well as the clinical presentation and outcome of hepatotoxicity. The retrospective design of these studies is an important drawback as missing information in many instances precludes an accurate diagnosis of DILI events.
A retrospective study from the General Practice Research database (GPRD) in the UK with data obtained from 1982 to 1986 found differences in DILI incidence for prescribed drugs. Ampicillin, methyldopa and NSAIDs were found responsible for 1 case per 100,000 prescriptions while erythromycin was responsible for 14 cases per 100,000 prescriptions. The use of more than two drugs was found to provide increased risk for DILI (Pérez Gutthann and García Rodríguez 1993). A review of epidemiological research also using the GPRD showed the greatest incidence of DILI in patients who took chlorpromazine and isoniazid (greater than 100/100,000 prescriptions), a moderate incidence for patients taking amoxicillin-clavulanate and cimetidine (greater than 10/100,000) and an incidence less than 10/100,000 for those who were prescribed NSAIDs, omeprazole, amoxicillin and ranitidine (García Rodríguez et al. 1997). A later study from the GRPD found a DILI incidence of 2.4 per 100.000 person-year with the highest incidence rates for chlorpromazine, amoxicillin clavulanic acid and flucloxacillin (approximately 1 per 1000 users) (de Abajo et al. 2004).
A different approach using a database of medical and laboratory records in Massachusetts estimated the incidence of DILI in the general population to be 40.6 cases per 100 000 inhabitants (Duh et al. 1999). Similarly, a Swedish retrospective study using an outpatient database estimated a DILI incidence of 2.3 cases per 100,000 persons and year (De Valle et al. 2006).
In general, population-based studies have reported higher incidences of DILI than those of retrospective studies. Up to date three prospective population-based studies have been published. A study carried out in France over a 3-year period found an annual incidence rate of 13.9 per 100,000 inhabitants. The most frequent implicated drugs were NSAIDs, anti-infectious, psychotropic and hypolipidemic agents (Sgro et al. 2002). In Iceland, a 2-year period study found a DILI incidence of 19.1 per 100,000 inhabitants, with amoxicillin-clavulanate, diclofenac and azathioprine being the most frequent causative agents. In this study the quantitative risk of DILI associated with different causative drugs was evaluated. The risk of DILI due to amoxicillin-clavulanate, the most commonly implicated agent, was found to be 1 in 2,350 users, whereas the highest risk of hepatotoxicity was associated with azathioprine, 1 in 133 users (Bjornsson et al. 2013). More recently, in Delaware (US), a 2-year study found an incidence of 2.7 cases of DILI per 100,000 adult residents, 36% due to antibiotics and 43% to HDS (Vega et al. 2017). In this study a higher cut off for alanine aminotransferase (ALT) values (> 5 × upper limit of normal (ULN) in two separate occasions) was used for case definition than in the French (> 2 × ULN) and Icelandic (> 3 × ULN) studies, and could account at least in part for the lower incidence.
Other epidemiological approaches have explored the frequency of hospitalization due to DILI. As expected, the incidence is higher in hospitalized patients. In a prospective study performed in 1997 among hospitalized patients, using the University Hospital of Toulouse (France) laboratory database), the incidence of DILI was estimated as 6.6 per 1000 inpatients/week. Case qualification criteria were ALT > 2 × ULN or alkaline phosphatase (ALP) > 1.5 × ULN if associated with an increase in ALT, gamma-glutamyl transferase (GGT) or total bilirubin (TBL) (Bagheri et al. 2000). In a nation-based prospective study performed in Korea from 2005 to 2007, the estimated incidence of hospitalization due to DILI was 12 per 100,000 persons/year with herbal medications predominating among the culprit agents (Suk et al. 2012). A study using a Swiss database of hospitalized patients found that 1 in 100 patients developed DILI during hospitalization with the highest incidence for antineoplastic and tuberculostatic agents (Meier et al. 2005). A recent single-center, 1 year, prospective Colombian study reported that among hospitalized patients with elevated liver tests 6% had DILI. Case qualifications were ALT > 3 × ULN and/or ALP > 2 × ULN. Anti-infectives (isoniazid, rifampicin, nitrofurantoin) and anticonvulsants (phenytoin, and valproic acid) were the most frequent implicated drugs (Cano-Paniagua et al. 2019).
The prevalence of DILI among jaundiced patients has been addressed in other studies. In Sweden, 2.3% of patients with severe jaundice were found to have DILI (Björnsson et al. 2003), while in United States the incidence of DILI in patients with new onset jaundice of non-alcoholic aetiologies was 4%. However, most cases were attributed to paracetamol and idiosyncratic DILI represented only 0.7% of the cases (Vuppalanchi et al. 2007).
The prevalence of DILI among patients with ALF has also been reported in a number of studies. In a classic study in USA paracetamol overdose was the cause of ALF in 39% of cases, whereas 13% of cases were attributed to idiosyncratic DILI (Ostapowicz et al. 2002). Similar prevalence was found in a later North American multicentre study with 11% of ALF cases due to idiosyncratic DILI, with antimicrobials being the most frequently implicated agents (Reuben et al. 2010). In Sweden, DILI was overall the most important cause of ALF, with 42% due to paracetamol and 15% due to other drugs (Wei et al. 2007). A retrospective cohort study in California identified DILI as the most frequent cause of ALF, ranking paracetamol as the first etiology followed by herbs and antimicrobials (Goldberg et al. 2015).
Prospective studies provide more reliable data on DILI causative agents, clinical presentation and outcome. A prospective single-center cohort study undertaken in India showed antituberculosis drugs as the first cause of DILI in this country. Prospective national and international DILI Registries have been set-up in Spain, USA, Europe, Latin American, Japan, China among other countries to collect phenotypic data and biological samples to undertake studies on clinical characteristics, risk factors and outcome of DILI as well as to describe the most frequently implicated agents. More detailed description of each registry can be found in the recently launched CIOMS consensus document on DILI (CIOMS 2020). Antimicrobials (mainly amoxicillin-clavulanate) have been the most frequent agents involved in DILI in the Spanish DILI Registry (Andrade et al. 2005) and in the Drug-Induced Liver Injury Network (DILIN) (Chalasani et al. 2008).
The largest cohort of DILI patients analyzed to date comprises 25.927 cases and was retrospectively collected in Mainland China over a 3-year period. In this study, traditional Chinese medicines and HDS were the most frequent causes of DILI (Shen et al. 2019).
Efforts to establish prospective DILI registries are in progress in China and India. This is particularly important as the spectrum of culprit agents and probably also other contributing factors, such as genetics and environmental factors, in Eastern countries are likely to differ from those identified in Western countries. Hence, continuing efforts across the world are crucial for advancing the knowledge of DILI.
Pathogenesis
Idiosyncratic DILI is a complex condition which molecular mechanism is not yet fully elucidated. It is believed that the combination of various host, drug and environmental factors determines DILI susceptibility and phenotypic expression (Chen et al. 2015). The implication of being a multifactorial condition is reflected in the lack of a general animal model to study the underlying mechanism of DILI. Nevertheless, substantial progress has been made in the form of animal models that demonstrate susceptibility to liver injury caused by specific drug treatments. However, the clinical relevance of such models may be compromised as they are typically dependent on pre-treatments or genetic alterations designed to predispose the animal to injury (McGill and Jaeschke 2019). Hepatic cell line models have also been applied to DILI studies (Kuna et al. 2018). Inherent differences in drug metabolism, particularly cytochrome P450 (CYP)-mediated metabolism, between cell lines and humans must be kept in mind when working with these models as drug metabolism is believed to be a vital process in DILI development. Furthermore, the phenotype of cultured cells can differ from hepatic cells in vivo due to variations in microRNA-mediated gene expressions (Lauschke et al. 2016).
The idiosyncratic nature of DILI speaks in favor of an important role for genetic variations, which have been a focal point in DILI studies over several decades. Based on studies performed to date it is unlikely that a single genetic variation would be accountable for DILI. A number of genetic variations are most likely required, resulting in a specific cellular environment that when exposed to the impact of a drug and other environmental factors can lead to DILI development.
Drug-induced cell damage
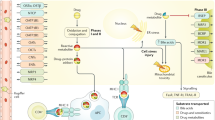
The current understanding of the molecular mechanism of idiosyncratic DILI is to a large extent based on findings from acetaminophen-based studies. Increasing evidence points towards acetaminophen-mediated hepatotoxicity being an active process involving death signaling pathways, rather than a passive process of overwhelming biochemical injury (Han et al. 2013). Idiosyncratic DILI, however, is believed to differ from intrinsic DILI in that both non-immune- and immune-mediated mechanisms are required for hepatic injury to occur (Fig. 1). Hepatocyte exposure to increased cellular stress is assumed to be the initial step in DILI development with drug metabolism being a potential source through the formation of chemically reactive drug metabolites. Drug metabolism is principally undertaken in the liver to produce a more soluble compound through CYP-mediated reactions (Phase I) and conjugation reactions (Phase II) to facilitate drug excretion usually via hepatic transporters (Phase III). This often leads to formation of reactive metabolites, i.e. electrophilic species able to conjugate with cellular proteins and other macromolecules. The extent of reactive metabolite formation and subsequent cellular stress varies depending on drug properties, the host’s drug metabolism capacity as well as the level of protective mechanisms in the liver. Activation of nuclear factor erythroid 2-relatd factor 2 (Nrf2) is a major defense mechanism against oxidative stress. This transcription factor is bound to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm under physiological conditions. Under conditions of increased oxidative stress Nrf2 is released from Keap1, translocates to the nucleus and binds to the antioxidant response element (ARE) in the target gene promoter to induce defense gene expression (Nguyen et al. 2009). Recent findings have demonstrated that various phytochemicals have high binding affinity for Keap1 and subsequently activates Nrf2, suggesting that the diet can influence redox homeostasis (Li et al. 2019).
Mechanistic view of idiosyncratic drug-induced liver injury (DILI). Hepatocyte exposure to increased cellular stress is assumed to be the initial step in DILI development with drug metabolism being a potential source through the formation of chemically reactive drug metabolites. The inability to detoxify reactive drug metabolites can cause oxidative stress and mitochondrial damage and subsequently activate signaling pathways. Reactive metabolites can also function as haptens and form neoantigens, which when presented on HLA molecules, or bind directly in a non-covalent fashion to HLA molecules, may lead to T-cell activation and an adaptive immune response. The liver, however, has a predisposition towards immune tolerance that must be interrupted to produce an adaptive immune response and clinically relevant liver injury. This process is dependent on various factors such as the innate immune system, cytokine environment, recognition of DAMPs from broken cells and/or PAMPs from concurrent infections or microbiota-derived components by PRRs (such as Toll-like receptors). Ability to repair tissue damage and regenerate new tissue will further differentiate between individuals with clinical adaptation (mild liver injury) and defective adaptation (clinically significant DILI). BSEP bile salt export pump, DAMPs damage-associated molecular patterns, GSH glutathione, HMGB1 high-mobility group 1, HSP, heat shock protein, JNK c-Jun N-terminal kinase, NRF2 nuclear factor erythroid 2-related factor 2, PAMPs pathogen-associated molecular pattern, LPS lipopolysaccharide, PRR pattern recognition receptor, ROS reactive oxygen species
Cellular stress reaching a critical threshold can lead to activation of c-Jun N-terminal kinase (JNK) signaling pathways and mitochondrial damage. Some drugs, for example nucleoside reverse transcriptase inhibitors and valproic acid, have also been demonstrated to provide hepatocyte stress by direct mitochondrial disruption (Pessayre et al. 2012).
Drugs can also aggravate cellular stress through secondary functions, such as bile salt export pump (BSEP) transporter inhibition, which can cause accumulation of bile salts, which are toxic due to their detergent-like nature. BSEP inhibition has been hypothesized to play a role in, among others, troglitazone hepatotoxicity (Funk et al. 2001). However, recent findings suggest that troglitazone also inhibits bile acid amidation that leads to accumulation of nonamidated bile acids. This can have a profound effect on bile acid homeostasis and subsequently amplify the level of stress imposed on the hepatocyte (Ogimura et al. 2017). Drug transporters, especially efflux transporters, have also been targeted in DILI studies, however limited evidence are available to date to support that these proteins have a prominent role in DILI development.
The liver is, however, relatively resistant to drug-induced stress and readily adapts even to prolonged drug treatments. Additional factors are therefore likely required to amplify the cellular insult in order for overt liver injury to occur. The immune system is currently believed to be an additional factor implicated in the underlying mechanism of idiosyncratic DILI.
Immune system
In addition to producing cellular stress, reactive metabolites can also function as haptens and bind to endogenous proteins. Such hapten-carrier adducts can form neoantigens that when presented on specific human leukocyte antigen (HLA) molecules potentially elicit an adaptive immune response. Alternatively, certain drugs or reactive metabolites may directly bind non-covalently to HLA alleles and consequently activate the immune system. These theories, in the context of DILI, are supported by findings of specific HLA alleles being associated with DILI ascribed to specific causative agents (Stephens and Andrade 2020). Furthermore, idiosyncratic DILI often have delayed onset of clinical symptoms, which is consistent with the participation of drug-specific T cells. In fact, drug-responsive CD4+ and CD8+ T cells have been identified and characterized using peripheral blood samples from patients with flucloxacillin and amoxicillin-clavulanate hepatotoxicity (Monshi et al. 2013; Kim et al. 2015), which specific HLA risk alleles have been identified (flucloxacillin: HLA-B*57:01; amoxicillin-clavulanate: HLA-A*02:01, DRB1*15:01 and DQB1*06:02) (Daly et al. 2009; Lucena et al. 2011).
Due to its biological role, however, the liver is constantly exposed to foreign antigens and therefore has a predisposition towards immune tolerance to avoid excessive inflammatory reactions and subsequent tissue damage. To activate an adaptive immune response the inherent state of immune tolerance needs to be broken. The liver’s ability to maintain immune tolerance or provide an immune response after drug intake has been put forward in the adaptation hypothesis to explain the rareness of DILI. Most people with HLA alleles favoring an immunogenic response to a specific drug do not develop liver injury or only mild transient liver profile elevations that resolve with continued drug exposure (clinical adaptation). Only a small amount of patients develop persistent liver injury and clinically significant DILI due to defective adaptation (failure to dampen the initiating mechanism of injury because of diminished adaptive responses) (Dara et al. 2016).
Hepatic immune tolerance is to a large extent dependent on autocrine and paracrine effects of cytokines (Kubes and Jenne 2018). In addition, recent findings suggest that constitutively released hepatocyte-derived exosomes could likewise play a role in maintaining normal liver immune tolerance (Holman et al. 2019). Stress due to infection, inflammation or altered redox balance can also alter the cytokine milieu and may co-stimulate the immune response as well as elicit immune-mediated hepatic cell death (Iorga et al. 2017). Concurrent cell damage leading to the release of danger-associated molecular pattern molecules (DAMPs), for example heat-shock proteins and high-mobility group box1 (HMGB1) protein, can initiate and perpetuate a non-infectious inflammatory response. The implication of immune checkpoints in maintaining hepatic immune tolerance is becoming apparent with new cancer immunotherapies, for example ipilimumab, pembrolizumab and nivolumab, which specifically target immune checkpoints, such as programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte associated antigen 4 (CTLA-4), to produce active immune responses towards cancer cells. Several cases of DILI due to immune checkpoint inhibitor-based treatments have been reported (De Martin et al. 2018; Jennings et al. 2019; Miller et al. 2020).
Microbiota
The microbiota is unique to each individual and its composition depends on both genetic and environmental factors including diet, geographical location, toxin exposure and hormones. The microbiota plays an important role in immune system maturation that starts at an early age with continued fine-tuning across the lifespan. Hence, the microbiota educates the immune system to properly distinguish between commensal and pathogenic microbes. Emerging evidence points towards a role for the intestinal microbiota in various hepatic conditions, such as alcoholic liver disease, non-alcoholic fatty liver conditions and cholestatic liver diseases (Adolph et al. 2018). It has also been postulated that the microbiota could be a factor in the underlying mechanism of idiosyncratic DILI (Fontana 2014), but confirmatory evidence is still lacking. The proposed role of the microbiota in DILI is to a large extent based on its effect on immunity and drug metabolism. The gastrointestinal tract and the liver have a close relationship. On one hand, bile produced in the liver flows to the gut and directly influences the resident microbial environment. On the other hand, venous blood carrying products of the microbiota and the host’s immunological responses to these organisms travels from the gut to the liver via the portal vein. These bacterial translocations occur normally in small amounts and are counteracted by liver immune cells that respond with immune tolerance against commensal microbiota components. However, alterations in the number and composition of the intestinal microbiota (dysbiosis) can lead to increased intestinal permeability and consequently increased microbial translocation to the liver as well as exposure of exogenous antigens to hepatic immune cells resulting in protective immune responses. Microbial elements detected by toll-like receptors expressed by hepatic immune cells can affect immune homeostasis indirectly by modulating cytokine profiles or directly by affecting immune cell proliferation. Hence, dysbiosis may subsequently aid the breakdown of hepatic immune tolerance, which in patients on drug treatments that form a drug-related neoantigen, could promote an adaptive immune response and DILI development. Regulation of hepatic immune functions, such as T cell regulation, by intestinal microbiota components has also been proposed as an underlying mechanism in autoimmune hepatitis (AIH) (Czaja 2016). Interestingly, animal studies have demonstrated that the intestinal microbiota composition may also influence gender bias for autoimmune diseases in genetically susceptible subjects, such as females being more prone to develop AIH (Markle et al. 2013). This raises the question of a potential role for the microbiota in gender biased severity in DILI, with women being more likely to develop drug-induce ALF than men. Furthermore, liver regeneration has been demonstrated to cause fluctuating changes in the microbiota (Bao et al. 2020). Thus, the efficiency of hepatic tissue repair and regeneration is at least partly controlled by each individual’s microbiota composition. More efficient tissue repair may be achieved by manipulation of the microbiota leading to enhanced metabolic responses in liver regeneration.
It is now evident that the microbiota also can influence drug metabolism, both directly and indirectly. The most important drug biotransformations performed by intestinal bacteria involve reductive metabolism and hydrolytic reactions, but also demethylations, deaminations, dehydroxylations, deacylations, decarboylations and oxidations are known occur and could have an impact on drug metabolism (Wilson and Nicholson 2017). This may result in altered level of reactive metabolites and subsequently affect DILI susceptibility. The intestinal microbiota may also affect drug metabolism by altering the expression of drug metabolizing genes, as demonstrated for CYP3A in mice liver (Toda et al. 2009). In fact, comparing mRNA transcriptomes from livers of germ-free and conventional mice Selwyn et al. have demonstrated that the intestinal microbiota markedly impacts the ontogeny of various hepatic drug metabolizing genes in a gender-specific manner (Selwyn et al. 2015).
Susceptibility factors
As DILI is assumed to be a multifactorial condition, its susceptibility is likely affected by various risk factors associated with the causative agent, the host and the environment. The impact of these risk factors may vary between individuals and may not have the same influence on all forms of DILI, but could at times be limited to specific causative agents. Drug dose was initially thought to be of little importance in idiosyncratic DILI, however it is now becoming evident that a higher dose is more likely to cause DILI. A higher dose has the potential to induce more cellular stress, although the extent and subsequent level of cell damage may vary from person to person depending on differences in compensatory mechanisms between individuals. The existence of an individual drug threshold dose is supported by reports of DILI appearing first after a dose increase is required for better pharmacological effect in patients that have tolerated the same drug at a lower dose (Carrascosa et al. 2015). It is tempting to conclude that the high dose generally prescribed for many antibiotics could be a reason for that these drugs are commonly seen as DILI causative agents in many large DILI cohorts. However, no mechanistic evidence is currently available to confirm this. Physiochemical properties will also affect a drug’s hepatotoxicity potential in addition to its pharmacological effect and potency. Drug development is therefore often a fine balance between achieving enhanced drug qualities and reduced risk of adverse drug reactions. Lipophilicity is an important drug property that affects drug uptake and metabolism. It has also been proposed as a potential risk factor for DILI together with drug dose, whereby drugs associated with high lipophilicity (LogP ≥ 3) and a recommended daily dose of ≥ 100 mg were found to have a higher risk of DILI (Chen et al. 2013). In addition, high lipophilicity has been found as one of several drug properties more prevalent in drugs with age-biased DILI reporting frequency (George et al. 2018; Hunt et al. 2014). Other drug properties suggested to affect DILI susceptibility include the ability to form reactive metabolites, mitochondrial liability and hepatic transporter inhibition. It should be pointed out that while reactive metabolites appear to play an important role in DILI development, drugs unknown to form reactive metabolites can also cause DILI. For example, ambrisentan, flecainide, maraviroc and bosentan are drugs with black box warnings for hepatotoxicity despite the absence of structural alerts in their chemical structure and no reports on reactive metabolite formation (Stepan et al. 2011). Bosentan, however, is a strong inhibitor of BSEP, which could be a more important risk factor associated with this drug (Rodrigues et al. 2018). Although, the interpretation and predictive value of in vitro BSEP inhibition assay results as a reflection of increased DILI potential for a specific drug is currently debated (Kenna et al. 2018; Chan and Benet 2018).
With regards to host factors, genetic variations have long been considered of crucial importance in DILI. Early candidate gene studies in this area focused mainly on drug metabolizing genes and several variances were found to be associated with different forms of DILI, but none of these have been confirmed in more recent genome-wide association (GWA) studies (Urban et al. 2012). Most genetic risk factors in DILI with genome-wide significance identified to date are located in the HLA region on chromosome 6 and are specific to DILI caused by distinct causative agents (Stephens and Andrade 2020). All of these HLA risk alleles share the fact that they have a low positive predictive value, which limits their use for genetic screening prior to prescription to prevent DILI development. On the other hand, the relatively high negative predictive values associated with these HLA risk alleles can be of diagnostic value when the patient has taken multiple drugs prior to the DILI episode. HLA genotyping in this situation can help determine the most likely causative agent, provided that HLA risk alleles are identified for the suspected medications. Likewise, it has been postulated that HLA screening could be useful in distinguishing DILI from AIH (Kaliyaperumal et al. 2018). In contrast to identified HLA risk alleles that appear to be specific for the causative agents, a variant (rs2476601) in the protein tyrosine phosphatase non-receptor type 22 (PTPN22) gene is the first general genetic risk factor in DILI identified in a GWA study. Carrier of the minor allele resulting in an amino acid change from arginine to tryptophan at codon 620 of this gene were found to have a higher risk of developing DILI (OR = 1.44) caused by multiple drugs (Cirulli et al. 2019). Interestingly, PTPN22 is believed to be involved in T cell response regulation and polymorphisms in this gene have previously been associated with increased risk of various autoimmune conditions, which further supports the implication of immune alterations in the underlying mechanism of DILI (Brownlie et al. 2018). In search for common genetic risk factors across various liver conditions, 13 single nucleotide polymorphisms previously determined as being associated with non-alcoholic fatty liver disease (NAFLD) and other liver conditions were tested in 832 Caucasian DILI cases. However, none of the tested variants were significantly associated with DILI development, severity or outcome, suggesting that the pathogenesis of DILI differs from those of other liver conditions (Bonkovsky et al. 2019).
Lifestyle and comorbidities have also been postulated as potential risk factors for DILI. However, conclusive evidence is still lacking. Obesity, which is an established risk factor for NAFLD, is known to increase the activity of various CYP isoforms and reduce the level of glutathione. This could potentially increase reactive metabolite formation, while reducing the detoxification capacity and subsequently lead to increased risk of DILI development (Fromenty 2013). Nevertheless, this would not increase the risk of all forms of DILI, but be limited to DILI caused by drugs that are metabolized by the affected CYPs. Obesity often entails additional comorbidities with corresponding drug treatments, which could also lead to increased risk of drug-drug interactions. Drug–drug interactions are a reason behind many adverse drug reactions, although the role in DILI is not yet elucidated.
The effect of underlying hepatic conditions on DILI is similarly relatively unidentified. Based on reports from DILI registries, the proportion of DILI patients with underlying hepatic conditions do not exceed that of the general population (Chalasani et al. 2015). This suggests that the effect of having additional liver conditions is limited with regards to risk of DILI development. Specific liver conditions, however, may have a more profound effect on DILI due to distinct causative agents. For example, chronic viral hepatitis B and C have been reported as risk factors for DILI caused by anti-TBC treatments (Lomtadze et al. 2013; Sun et al. 2016), although contradicting findings are also available (Nooredinvand et al. 2015; Saha et al. 2016). Despite the absence of convincing evidence that underlying hepatic conditions increases the risk of DILI development, DILI patients with pre-existing liver diseases seem to have an increased risk of a more severe outcome. In 843 Spanish DILI patients, 7.5% of the patients with underlying hepatic conditions (n = 53) died due to liver-related problems, while only 3.7% of those without underlying conditions suffered the same fate or underwent a liver transplantation (Spanish DILI Registry: unpublished data). Hence, the presence of an underlying hepatic condition may diminish the liver’s capacity to recuperate from a DILI episode. North American DILI patients with underlying hepatic conditions have also been reported to have a higher proportion of deadly outcomes, although the proportion of liver-related deaths was not found to be significantly different from that of DILI patients without pre-existing liver conditions (Chalasani et al. 2015).
Case characterization, clinical features and phenotypes
Drugs, herbs and dietary supplements can induce any type of acute or chronic liver damage, depending on the severity of the injury and the affected liver cell types. Most of these adverse reactions are asymptomatic or produce mild unspecific symptoms that resolve after withdrawal of the culprit drug. Hence, DILI is generally detected by increases in liver enzymes with or without signs or symptoms of liver disease. In 2011, a consensus group proposed for acute DILI case qualification that the subject should exceed a given threshold in serum liver enzyme activities: ALT ≥ 5 × ULN; ALP ≥ 2 × ULN; or ALT ≥ 3 × ULN combined with TBL > 2 × ULN (Aithal et al. 2011).
Occasionally, severe DILI manifestations, progression to acute liver failure or chronic liver disease may occur. The severity of liver injury is not determined by the degree of elevation of liver enzymes, but must take into account clinical features and other analytical values. The International DILI Expert Working Group graded severity as follows (Aithal et al. 2011) (a) Mild DILI cases have elevated ALT or ALP values reaching criteria for DILI, without increases of total bilirubin values (TBL < 2 × ULN); (b) Moderate DILI if elevated ALT/ALP values reaching criteria for DILI are associated with increased bilirubin values (TBL ≥ 2 × ULN), or hepatitis symptoms; (c) Severe DILI when the previous values of ALT/ALP and bilirubin increases are associated with one of the following: International normalized ratio (INR) ≥ 1.5, ascites and/or encephalopathy, disease duration < 26 weeks, and absence of underlying cirrhosis or other organ failure considered to be due to DILI; and finally, fatal/transplantation is defined as death or liver transplantation due to DILI. Similarly, the US DILIN (Fontana et al. 2010) proposed a severity classification with 5 grades (mild, moderate, moderate-severe, severe and fatal). In this classification, moderate-severe grade includes patients who need hospitalization. Hospitalization criteria, however, can be different across hospitals and countries, and this classification is therefore less generalizable.
In those cases in which DILI cause symptoms, the typical presentation is an acute “hepatitis-like” syndrome, with nausea, fatigue, jaundice, and abdominal discomfort (Andrade et al. 2007). In cholestatic cases, pruritus and asthenia can be prominent and may limit the patient's quality of life. Other associated manifestations, classically referred to as immunoallergic features include fever, rash, arthralgia, or lymph node enlargement, and can be present in a quarter of DILI patients (Andrade et al. 2005; Devarbhavi and Raj, 2019). Skin reactions can accompany DILI related to certain drugs, and range from unspecific rashes, drug reaction with eosinophilia and systemic symptoms (DRESS) to severe cutaneous adverse reactions such as Stevens-Johnson syndrome (SJS) or toxic epidermal necrosis (TEN), (Medina-Cáliz et al. 2017; Devarbhavi et al. 2016). DILI with skin reactions seems to have severe outcomes including acute liver failure, multiorgan failure, liver transplant, or death more frequently, with a mortality ranging from 36 to 44% of cases. (Devarbhavi et al. 2016; Chalasani et al. 2015).
Besides, DILI can manifest with a wide variety of clinical presentations such as cholestasis, chronic hepatitis, steatosis, veno-occlusive disease and even neoplasms (Zimmerman 1999; EASL 2019) (Table 3). As liver biopsy is not routinely performed in clinical practice for DILI diagnosis, suspected DILI cases are not generally characterized according to histopathological features. Instead, liver biochemistry is used to define the type of liver damage, which is calculated by the R (ratio) value, where R = (ALT subject/ULN)/(ALP subject/ULN). The resultant pattern is classified as hepatocellular (R ≥ 5), cholestatic (R ≤ 2) and mixed cases (R > 2 and < 5). To calculate the R value, the first blood test available after DILI initiation should be used, because the type of injury could change over time, with a tendency towards lower R values as the episode progresses (Andrade et al. 2006). The international consensus also recommended that aspartate aminotransferase (AST) can substitute ALT when the latter is unavailable (Aithal et al. 2011). This recommendation has been validated by authors from the Spanish DILI Registry who undertook an analysis in a large DILI cohort that demonstrated that values of AST can reliably replace those of ALT, whereas GGT is less reliably as an ALP substitute (Robles-Diaz et al. 2015). The Spanish DILI group also proposed a newR (nR) to define Hy’s Law for ALF prediction. This proposal is based on the finding that the AST level is independently associated with the development of ALF/liver transplantation at all-time points, but is most predictive at DILI recognition compared with the other time points (Robles-Diaz et al. 2014).
There are a number of special phenotypes of DILI with some differential characteristics that do not fit the biochemical definitions stated above (EASL, 2019; Andrade et al. 2019). Drug-induced autoimmune hepatitis (DI-AIH) is characterized by a hepatocellular pattern of liver damage, with serological (detectable titers of autoantibodies, high serum IgG levels) and/or histological features of idiopathic AIH (interface hepatitis), making the differential diagnosis between idiopathic and drug-induced hepatitis troublesome in the majority of cases. Features than can further support the diagnosis of this entity are the absence of cirrhosis in liver biopsy and the low recurrence rate once steroids are tapered (Bjornsson et al. 2010; Björnsson et al. 2017). Drugs that have been associated with this phenotype are methyldopa, minocycline, nitrofurantoin, diclofenac (deLemos et al. 2014), biological agents (Rodrigues et al. 2015) and statins (Perdices et al. 2014).
A new special DILI phenotype is the one related to immune checkpoint inhibitors (ICIs), which can cause immune-mediated hepatitis. These antitumor drugs act by blocking immune checkpoint receptors, specifically cytotoxic-T lymphocyte A-4, CTLA-4 (ipilimumab) and programmed cell death-1, PD-1 (pembrolizumab and nivolumab) as well as programmed cell death ligand 1, PD-L1 (avelumab, atezolizumab and durvalumab). The frequency of liver injury related to ICIs ranges from 2 to 30% of patients, with the hepatocellular pattern being the most frequent (albeit not exclusive) type of liver injury (EASL 2019). Anti-CTLA-4 therapy is more hepatotoxic than Anti-PD-1 and PD-L1 treatments (Darvin et al. 2018) with the ipilimumab and nivolumab combination having the highest hepatotoxicity potential (Larkin et al. 2015). Steroid therapy is indicated in certain cases, however it is recommended to consult hepatologists prior to such treatments (EASL 2019).
Drug-induced fatty liver disease is another special phenotype of DILI characterized by variable degrees of accumulation of lipids in hepatocytes. Two types of histological injury are described: microvesicular and macrovesicular. The first pattern is associated with drugs such as amiodarone, cocaine, glucocorticoids, nucleoside reverse transcriptase inhibitors, tetracycline and valproic acid. A particularly serious liver steatosic syndrome is acute fatty liver characterized by mitochondrial dysfunction that produces lactic acidosis, acute microvesicular steatosis and hepatic dysfunction. A classic example is Reye syndrome linked to aspirin that usually occurs in children after a viral illness. Drugs capable of causing Reye-like syndrome, include amiodarone, valproate, and nucleoside analogs (LiverTox: Clinical and Research Information on Drug-Induced Liver Injury 2012). The drug-related macrovesicular steatosis pattern has been described in association with amiodarone, 5-fluorouracil, glucocorticoids, methotrexate and tamoxifen (Fisher et al. 2015; Ramachandran and Kakar 2009; Dash et al. 2017). Furthermore, steatosis associated with other patterns of injury is frequently found in liver biopsies of DILI patients, reaching up to 65% of cases in a study from the DILIN group (Kleiner et al. 2014).
Drugs can also cause several types of biliary insult and as a consequence the injury can progress to gradual loss of intrahepatic bile ducts that when affects more than 50% of portal tracts is called ductopenia and vanishing bile duct syndrome, which often result in biliary cirrhosis. The drugs most commonly associated are chlorpromazine, amoxicillin, carbamazepine, azathioprine, meropenem and flucloxacillin (Sundaram and Björnsson 2017). Besides, secondary sclerosing cholangitis, characterized by an acute DILI with primary sclerosing cholangitis features on liver biopsy or magnetic resonance imaging has also been described in association with drugs or herbs. Amiodarone, amoxicillin–clavulanate, atorvastatin, infliximab, 6-mercaptopurine and venlafaxine are the agents more frequently related to sclerosing cholangitis (Gudnason et al. 2015).
The endothelial sinusoidal cell is a potential target for reactive metabolites generated by some drugs and herbal products (pyrrolizidine alkaloids) and hepatotoxicity can consequently manifes with a phenotype similar to various vascular liver disorders. These include nodular regenerative hyperplasia, characterized by the development of diffuse nodularity without advanced fibrosis leading to non-cirrhotic portal hypertension and sinusoidal obstruction syndrome (SOS) that occurs when the portal venules are affected resulting in obstruction or dropout of smaller radicles. Typical agents that cause these phenotypes of DILI include azathioprine, oxaliplatin, thioguanine, mercaptopurine, antiretroviral agents and possibly methotrexate (Hartleb et al. 2011). Another vascular liver disease related to drugs is peliosis hepatis, where blood-filled cavities are found in liver histopathological examination. Drugs related to this particular phenotype are anabolic steroids, oral contraceptives, tamoxifen and thiopurines (Crocetti et al. 2015).
Hepatocellular adenoma and carcinoma have also been associated with drug therapy. Long term oral contraceptive use, is linked to hepatocellular adenoma and androgenic steroids to hepatocellular carcinoma. Regression or disappearance of adenomas after drug discontinuation has been described (Rooks et al. 1979; Velazquez and Alter 2004).
Diagnosis
An early and reliable diagnosis of DILI is essential. This is firstly, because the main therapeutic measure for hepatotoxicity is withdrawal of the culprit drug to limit further damage; secondly, to confidently rule out DILI (i.e. identify an incompatible temporal sequence) facilitates a correct diagnosis of other causes of liver injury; thirdly, identification and reporting of DILI cases can aid regulatory decisions including warnings and withdrawals of drugs from the market. However, the absence of specific tests makes the diagnosis of idiosyncratic DILI troublesome in the majority of cases. Thus, the DILI diagnosis relies heavily on clinical suspicion, temporal compatibility, and exclusion of alternative causes of liver damage. Bearing in mind that a hepatic adverse drug reaction might explain the patient’s symptoms, clinicians should search for a rapid improvement of liver tests after withdrawal of the culprit drug or “dechallenge”, the presence of drug-related special features, compatible histological findings, reappearance of the injury after unintentional re-exposure to the substance, referred to as “rechallenge”, as well as the presence of extrahepatic manifestations such as immunoalergic features (Andrade et al. 2004) (Fig. 2).

Modified from Andrade et al. (2004)
Step-by-step approach to DILI diagnosis.
Stepwise approach to diagnosis
The diagnostic process can be improved by making a stepwise approach, which starts with the suspicion of DILI when facing any liver disorder, either de novo or a worsening of a known hepatic disease, and is followed by a systematic clinical, laboratory and imaging evaluation intended to retrieve all the necessary information for DILI diagnosis (Fig. 2) (Andrade et al. 2004). This step by step diagnostic approach has become the standard method for evaluation of suspected DILI (EASL 2019).
-
1.
Physician awareness and pharmacological history
Physicians and health care providers should keep in mind the possibility of an adverse hepatic reaction when evaluating patients with a new onset of liver injury or worsening of a pre-existing liver disorder (i.e. acute on chronic liver disease). Therefore, a necessary step when liver injury is detected is a thorough investigation of prescribed and over-the-counter drugs, illegal products or HDS, taking into consideration doses, duration of treatment, the relationship between start/stop dates and the appearance of symptoms or laboratory abnormalities (Kullak-Ublick et al. 2017).
-
2.
Temporal eligibility
Evaluating the temporal relationship between drug intake and the start of liver symptoms, signs, or laboratory tests, whichever comes first, is essential. This latency period varies widely among different drugs and patients. The majority of idiosyncratic DILI reactions occur within the first 3 months after initiating therapy. However, DILI associated with some drugs such as amoxicillin-clavulanate can present with a delay after discontinuation of the drug (Kullak-Ublick et al. 2017).
-
3.
Hepatotoxicity information and phenotypes associated with drugs and HDS
Once a given drug or botanical compound is suspected, their hepatotoxic potential and liver injury signature must be assessed. Nevertheless, data on a drug’s DILI potential is not always available (Björnsson and Hoofnagle 2016). The information included in the summary of product characteristics of the drug may be incomplete for DILI, since it mostly includes data of transaminase elevations during clinical trials, but description of severe DILI cases that more frequently occur in the post-marketing phase is typically missing (EMA 2015). Published information about DILI can be found in different research sources. Although data from these resources can be helpful in cases of frequent or severe DILI, useful information in rare cases is more difficult to find (Björnsson and Hoofnagle 2016).
In order to collect and update information about hepatotoxic potential and features of drugs and HDS, a number of databases and apps have been developed. “LiverTox” is an open access website created by the National Institutes of Health that provides information on liver injury induced by medicines and HDS based on a rigorous literature research including all published DILI cases from the previous 50 years (NIDDK 2017). Based on LiverTox, a categorization of the drug DILI potential has been proposed, with the development of a “likelihood score” according to the probability of the association of a certain drug with DILI (Björnsson and Hoofnagle 2016). This seven-point categorization is based on the number of DILI cases published in the literature, thus it is more accurate for drugs that have been extensively used for a prolonged period and less for recently approved medications or for drugs and HDS that have been scarcely used (Björnsson and Hoofnagle, 2016). However, classification of drugs hepatotoxic potentials based on number of published case reports can be misleading as the number of publications may not accurately capture all elements of DILI risks (i.e., frequency, severity and causality) and regulatory actions taken on the drugs.
Other resources of DILI information are UpToDate (https://www.uptodate.com), Liver Toxicity Knowledge Base and DILIrank Dataset (https://www.fda.gov/ScienceResearch/BioinformaticsTools/default.htm), Toxicogenomics Project-Genomic Assisted Toxicity Evaluation System (https://toxico.nibiohn.go.jp/english), Natural Medicines Comprehensive Database (https://naturalmedicines.therapeuticresearch.com), Toxicology Data Network (https://toxnet.nlm.nih.gov), or MedWatch (https://www.fda.gov/safety/medwatch) (Isaacson and Babich 2020).
-
4.
Exclusion of other causes of liver injury: clinical, laboratory and complementary tests
The exclusion of alternative causes is mandatory in DILI adjudication. These include viral hepatitis, autoimmune liver diseases, metabolic hepatic diseases, alcoholic hepatitis, vascular disease, biliary obstruction, bacterial hepatitis, or tumors depending on the clinical context (Table 4). Indeed, a history of liver related risk factors such as alcohol intake, risk behaviors for transmission of infections or cardiovascular disorders could suggest other etiologies of liver disease. Blood test including blood count, coagulation, and liver biochemistry are necessary for the diagnosis and phenotyping of liver damage, along with metabolic status for the diagnosis of non-alcoholic steatohepatitis, ceruloplasmin and urine copper levels for the diagnosis of Wilson disease, autoantibodies and immunoglobulin levels for the exclusion of autoimmune disorders, viral serology for hepatitis A, B, C and E (including RNA-VHE and RNA-VHC), and abdominal imaging test (i.e. ultrasonography) are of help in the exclusion of the main causes of liver disease. The phenotype of the liver injury can further guide complementary diagnostic tests such as magnetic resonance cholangiopancreatography or endoscopic ultrasound in the evaluation of cholestatic damage to exclude biliopancreatic pathology (EASL 2019).
-
5.
Dechallenge
Withdrawal of the suspected causative agent is generally followed by a clinical and laboratory improvement over the next days or weeks in the majority of DILI cases. For acute hepatocellular or cholestatic injury the expected time to resolution is 2–6 months, with cholestatic damage generally taking longer time to resolve than hepatocellular damage (Lewis and Kleiner 2006). Although a rapid decline of aminotransferases after drug discontinuation supports the diagnosis, in some cases liver injury continues to progress leading to ALF.
-
6.
Rechallenge
A positive re-challenge is defined as the reappearance of a liver damage after re-administration of a drug previously involved in a DILI episode. Although generally inadvertent, it can be considered the nearest to a gold standard in the diagnosis of DILI (Andrade et al. 2009). Classically, a positive rechallenge has been defined as doubling of ALT or ALP in hepatocellular or cholestatic/mixed type of liver injury, respectively (Benichou 1990). More recently, an increase in the cutoff for rechallenge in ALT values to > 3 × ULN has been proposed based on antineoplastic and antituberculosis clinical studies (Hunt et al. 2017).
A number of host factors (HLA polymorphisms) and drug properties such as higher drug daily dose, production of reactive metabolites, mitochondrial dysfunction, BSEP inhibition, and more frequent ALT increases during clinical trials, have been related to the development of positive rechallenge (Hunt et al. 2017). Drugs most commonly associated with rechallenge are halothane (51%), lapatinib (55%), tolvaptan (55%), pazopanib (38%), tacrine (33%) and the antituberculous combination of isoniazid/rifampin/pyrazinamide (11–24%) (Hunt et al. 2017).
Given the risk of severe DILI development upon rechallenge, it has been classically considered as a dangerous practice (Mushin et al. 1971). Therefore, intentional re-exposure is not allowed as a diagnostic tool for evident ethical reasons. Besides, interpretation of such reactions can be difficult and potentially lead to erroneous assumptions (Andrade et al. 2007).
Hence, rechallenge is only justified in cases where essential and non-replaceable drugs for the treatment of life-threatening diseases are needed, as it is the case for antituberculous and oncological therapies (EASL 2019). Two prospective controlled clinical trials showed a variable rate (0–24%) of positive rechallenge to antituberculous drugs (Tahaoğlu et al. 2001; Sharma et al. 2010).
In oncology, rechallenge is increasingly tried both in clinical trials and daily practice, especially regarding new antitumor-targeted drugs with known DILI potential. An analysis of pazopanib phase II and III studies showed 5% of DILI development and 38% of rechallenged patients had ALT elevations recurrence but no patients developed severe liver injury (Powles et al. 2015).
Scarce information on rechallenge is available in clinical practice. An analysis of the Spanish DILI Registry yielded a 6% rechallenge rate, mostly inadvertent and with antibiotics being the most frequently involved agents. In this cohort, cases with a positive rechallenge had more frequently hepatocellular type of liver injury, a shorter time to onset than the initial episode, and a higher proportion of fatal cases (Fernández-Castañer et al. 2008).
-
7.
Extrahepatic manifestations
In rare instances, involvement of other organs aside from the liver can occur and constitute a strong clue for DILI diagnosis. These include pancreas, kidney and skin (Kardaun et al. 2007; Bastuji-Garin et al. 1993; Devarbhavi et al. 2016). In addition, any of the following hypersensitivity features such as fever, rash, lymphadenopathy, periorbital oedema, arthralgia, blood eosinophilia, and/or decreased lymphocyte count can be present in approximately 25% of idiosyncratic DILI patients, which suggest an immunologic-allergic idiosyncrasy operating in these cases (Andrade et al. 2005; Devarbhavi and Raj 2019). Drugs more frequently associated with immunological manifestations are dapsone, carbamazepine, or phenytoin (Devarbhavi et al. 2011, 2016, 2017; Sanabria-Cabrera et al. 2019).
-
8.
Liver biopsy
Liver biopsy is seldom useful for DILI diagnosis as histological findings are often unspecific and not conclusive. Thus it should only be considered when withdrawal of the suspected drug or HDS is followed by incomplete improvement or worsening of liver damage, in acute or chronic atypical presentation (i.e. non-alcoholic fatty liver disease, liver vascular disorders, chronic hepatitis with fibrosis), or when the case presents with features suggestive of AIH (EASL 2019). Although no pathognomonic findings for idiosyncratic hepatotoxicity exist, some histological features such as the presence of severe acute hepatitis, submassive or massive necrosis, zonal necrosis, cholestatic hepatitis, eosinophils, granulomatous hepatitis, microvesicular steatosis, or features of sinusoidal obstruction syndrome can be of help in hepatotoxicity diagnosis (Kleiner 2018). The key points to have in mind to make a proper diagnosis of DILI are listed in Table 5
Causality assessment methods
The diagnostic process described above, can be further strengthened by the use of causality assessment methods (García-Cortés et al. 2011) defined as structured and objective processes of evaluation of suspected DILI cases. Different tools for the evaluation of adverse drug reactions have been developed with the aim of conferring more objectivity and reproducibility to hepatotoxicity diagnosis. These methods can be classified into general or liver specific tools, and furthermore based on the type of tool into probabilistic methods, algorithms or scales, and expert opinion based systems (Arimone et al. 2006). The criteria generally included in these tools are the temporal relationship between drug and injury, known drug toxicity potential, exclusion of an alternative explanation for the liver disorder, improvement after withdrawal of the offending drug, or a positive rechallenge. Non-liver specific methods, such as the Naranjo adverse drug reaction scale (Naranjo et al. 1981), have not shown to have sufficient validity to assess DILI, thus it is no longer recommended in hepatotoxicity causality assessment (García-Cortés et al. 2008).
DILI specific causality assessment scales
In 1987, the Council for International Medical Sciences sponsored by the Roussel Uclaf pharmaceutical company coordinated several consensus meetings with a panel of experts on DILI, including hepatologists, members from the French Drug Surveillance Network, and from the International Drug Surveillance Department of Roussel Uclaf. The objectives of these meetings were to develop standardized definitions of DILI, define clinical and chronological criteria, and to describe the causes of liver injury to be excluded (Danan 1988). These meetings led to the publication of the CIOMS/RUCAM causality assessment criteria and the development of a scale (Benichou 1990; Danan and Benichou 1993). This method is based on a weighted scoring system according to 7 distinct domains. The sum of the individual scores is translated into different categories of probability. The reproducibility of the scale was evaluated by applying the method to 50 cases of suspected DILI by four experts. Agreement between 2 experts was 99%, 74% among three experts, and 37% of agreement was found when 4 experts evaluated the cases (Danan and Benichou 1993). The scale was validated using a cohort of 49 published DILI cases with positive re-challenge, as well as 28 non-DILI controls. The results from this study showed a sensitivity of 86%, a specificity of 89%, a positive predictive value of 93%, and a negative predictive value of 78% (Benichou et al. 1993).
In 1997, the doctors Maria and Victorino, published a less complex liver specific causality assessment scale called the Clinical Diagnostic Scale (CDS), also named the Maria and Victorino (M&V) scale (Maria and Victorino 1997). Unlike the CIOMS/RUCAM scale, this method does not differentiate between types of liver injury in the evaluation of chronological relationship, and does not include risk factors or concomitant medication. However, the CDS takes into consideration extrahepatic manifestations such as rash, fever, arthralgia, and haematologic immunoallergic features (García-Cortés et al. 2011). The sum of the results of each criterion is translated into five DILI probability categories: definite, probable, possible, unlikely, and excluded. This method was evaluated in cases of immunoallergic DILI, and was compared with experts’ opinion as gold standard reaching full agreement in 84% of cases, and 86% of agreement between raters. In spite of high validity and inter-rater reliability, the authors point out some limitations of the CDS such as a poorer reliability in cases with chronic evolution or long latency period (Maria and Victorino 1997).
Later on, the Digestive Disease Week-Japan (DDW-J) scale, a modified CIOMS/RUCAM scale, was proposed in Japan (Takikawa et al. 2003). Changes were made in the item concerning chronological criteria, removing “unrelated” when the reaction occurred more than 15 or 30 days after stopping the drug in the hepatocellular and cholestatic/mixed type, respectively. Besides, the domain of concomitant drugs was removed, and a domain of extrahepatic manifestations was included, where cases with positive drug-induced lymphocyte stimulation test (DLST), or eosinophilia score positive. The DDW-J scale has been shown to accurately diagnose DILI and was superior to the Maria and Victorino scale (Watanabe and Shibuya 2004). However, the DDW-J scale has not been generalized outside the country of origin because the drug-induced lymphocyte stimulation test is not available or standardized elsewhere.
In 2013, Cheetham et al. published an electronic version of the CIOMS/RUCAM scale named eRUCAM, which showed good concordance with the classic scale (Cheetham et al. 2014). More recently, an updated CIOMS/RUCAM was developed to overcome the limitations of the original scale. Modifications included new additional criteria, clarification of some ambiguous items, incorporation of herbal-induced liver injury (HILI) special features, and updating the exclusion criteria (Danan and Teschke 2016). However, a prospective validation of this scale has yet to be carried out. Finally, another CIOMS/RUCAM method variant was developed in the pharmacovigilance setting, the PV-RUCAM (Scalfaro et al. 2017). This scale was specially created for cases with incomplete information included in pharmacovigilance datasets and showed excellent sensitivity and negative predictive value, 91% specificity, good correlation with expert opinion, and high inter-rater agreement, but a low positive predictive value (25%). Although promising, prospective validation of these results is pending.
Expert opinion based methods
Expert opinion refers to the assessment of a DILI case by clinical experts based on available data. Experts should have experience in managing patients with liver disease and also understand the concept of DILI. Expert opinion is mainly used to assess causality when a significant DILI signal appears during clinical drug development. The key advantages of this approach over scales and algorithms are that experts may (1) have insights into the differential diagnosis of liver injuries that occur in study subjects, (2) take into account different or unusual DILI phenotypes and pathological mechanisms in their analysis; and (3) weight and synthesize relevant pre-clinical, treatment population and individual case-level data to provide a full picture of risk assessment. Furthermore, experts may have access to updated DILI information, which can improve causality assessment, especially with recently marketed drugs (Hayashi 2016).
Nevertheless, the main limitation of expert opinion based tools is subjectivity. As DILI clinical signatures vary considerably and no specific biomarkers are currently available, the inter rater variability among experts on a specific case may be noticeable (Arimone et al. 2006). To overcome this limitation, the DILIN group developed a structured expert opinion process for the assessment of suspected hepatotoxicity cases recorded in the DILIN registry. It consists in a method where expert hepatologists evaluate prospectively collected clinical and laboratory data from cases of suspected DILI. The likelihood of an event being DILI is described using both a percentage associated with a descriptive legal terminology (Rockey et al. 2010), classifying events as “definite” with a likelihood of DILI of more than 95% when the evidence for the drug causing the injury is beyond reasonable doubt; “highly likely” with a likelihood of an ADR between 75 and 95%, with evidence for the drug causing the injury being clear and convincing but not definite; “probable” with a likelihood from 50 to 74%, where the preponderance of the evidence supports the link between the drug and the liver injury; “possible” with a probability of 25% to 49%, with present but equivocal evidence of DILI; “unlikely” with a probability of ADR of less than 25% with evidence that alternative causes other than drugs caused the liver damage; and “not determinable” in the case of missing key data (Rockey et al. 2010; Hayashi 2016). Afterwards, three experts evaluate the cases, and if complete concordance is reached the results are accepted. However, if no agreement is reached, the causality assessment results are reviewed in monthly conference calls in an attempt to solve discrepancies. If there is still no accord reached, the full causality committee vote, and the majority vote is accepted as the final score (Rockey et al. 2010). While this approach may work for investigational purposes, it cannot be brought for obvious reasons to daily clinical practice.
Comparative studies between different causality assessment methods
Since the development of the above mentioned scales and the expert opinion method, different comparative studies have been carried out. Two studies evaluated the performance of the CDS compared with the International Consensus Criteria (Aithal et al. 2000) and with the CIOMS/RUCAM scale (Lucena et al. 2001). The earlier concluded that the CDS scoring correlates well with the International Consensus classification, and found that a cut-off score of 9 points in the CDS scale reliably identifies if liver injury is DILI related (Aithal et al. 2000). Lucena et al. evaluated reliability and validity of CDS compared with the CIOMS/RUCAM scale in 185 cases included in the Spanish DILI Registry previously evaluated by three DILI experts (Lucena et al. 2001). This study yielded poor agreement between the scales, especially in cholestatic cases and in cases with fatal outcome. The CIOMS/RUCAM scale demonstrated better discriminative power and more agreement with expert opinion than the CDS scale (Lucena et al. 2001).
The DILIN group also studied the reproducibility of the CIOMS/RUCAM using DILI cases included in the DILIN Registry (Rochon et al. 2008). The test–retest complete agreement was reached in only 26% of cases, with an inter-rater reliability of 0.45. This group concluded that the CIOMS/RUCAM has a “mediocre” reliability of DILI causality assessment. To improve the diagnosis of hepatotoxicity the DILIN suggested that the CIOMS/RUCAM scale should be modified, new drug-specific instruments should be developed or that the causality assessment should be based on expert opinion (Rochon et al. 2008). After developing the DILIN expert opinion process, the authors compared this method with the CIOMS/RUCAM scale reaching a modest agreement between the two methods (r = 0.42). Complete inter-rater agreement was found in only 27% of cases with expert opinion and in 19% with CIOMS/RUCAM. Although an important inter-observer variability was demonstrated with both methods, the expert opinion process produced higher inter-rater agreement and likelihood score (Rockey et al. 2010).
In conclusion, diagnostic scales serve to translate the clinician’s suspicion into a quantitative score and are designed for supporting and less for excluding causality. Furthermore, the objectives of causality assessment methods are to add consistency to the diagnostic process, to reduce inter-observer discrepancies, and to provide a guidance of the main features and information required for the evaluation of suspected hepatic adverse reactions. Among the scales, the CIOMS/RUCAM scale has demonstrated to be superior to other general or liver-specific methods such as the Naranjo scale, or the CDS (García-Cortés et al. 2008; Lucena et al. 2001). Advantages of the CIOMS/RUCAM scale are increased validity and that can remind physicians of the essential information to be retrieved, and therefore can act as a guide in the assessment of DILI cases (Agarwal et al. 2010; Kaplowitz 2001). Besides, this method is the most commonly used causality assessment scale in the majority of studies, and published literature. Even more, the American College of Gastroenterology Guidance, the European Association for the Study of the Liver (EASL) Clinical Practice Guidelines, and the Chinese Clinical practice guidelines, recommend this method for causality assessment in DILI (Chalasani et al. 2014; EASL 2019; Xiao et al. 2019).
However, the CIOMS/RUCAM scale was developed more than 25 years ago based on expert opinion and is not a perfect tool for DILI evaluation, since there is poor inter-rater reliability (Rochon et al. 2008). The reasons for discrepancies among raters are diverse (Roytman et al. 2018). Besides, there are some situations with special features that make evaluation by CIOMS/RUCAM more difficult such as fatal or chronic cases with negative dechallenge, atypical presentation, HDS-induced liver injury, or patients with underlying liver disease (García-Cortés et al. 2011; Suk et al. 2012). On the other hand, The DILIN expert opinion process has demonstrated more reproducibility than the CIOMS/RUCAM scale, especially in DILI induced by new marketed drugs without previous history of DILI (Rockey et al. 2010; Regev et al. 2014; EASL 2019). Nevertheless, limitations of the DILIN expert opinion process include absence of external validation, lack of expert panels in routine clinical practice (time consuming and costly), and the fact that the assessment is done retrospectively (Caines and Moonka 2020).
Briefly, to improve the CIOMS/RUCAM scale an international prospective collaboration has been set up to establish evidence-based cut-offs, to improve clarity, reliability and validity (Hoofnagle 2004; Molokhia and McKeigue 2006; Björnsson and Olsson 2005; Matheis et al. 2011; Slim et al. 2016). Refinement of the CIOMS/RUCAM scale is feasible using current cohorts of DILI cases collected in prospective Registries that would allow developing a more objective, evidence-based tool, simple to score, able to identify DILI across a range of DILI likelihood categories, and potentially an online application.
New biomarkers
Biomarkers in DILI can be classified according their context of use into predictive or susceptibility biomarkers (genetic) and monitoring/safety, mechanistic and prognostic biomarkers (soluble proteins). In addition, some in vitro tests are being used for supporting DILI causality assessment.
Genetic markers
In the last years the availability of bioinformatics and array platforms have made it feasible to undertake GWA studies in search of genetic variations, which have long been considered of crucial importance in DILI. GWA studie have identified some significant links to hepatotoxicity in the HLA region on chromosome 6 (e.g. haplotypes HLA-DRB1*15:01- DQB1*06:02 for amoxicillin-clavulanate, HLA-B*57:01 for flucloxacillin, and HLA_B*33:01 for terbinafine and fenofibrate) (Lucena et al. 2011; Nicoletti et al. 2017) as well as a polymorphism in the PTPN22 gene, the only genetic marker indicating a general risk for DILI regardless drugs (Cirulli et al. 2019). Genetic markers are primarily predictive. As for most polygenic disorders, the positive predictive value of individual associations is very low and of limited use in preventing idiosyncratic DILI. However, the high negative predictive values (> 95%) of most identified HLA alleles associated with DILI can be useful in case assessment (e.g. when the subject does not harbor the genetic risk allele an alternative etiology should be considered) (Andrade and Robles-Díaz 2020).
Soluble biomarkers
Current standard serum liver tests ALT, AST, ALP and bilirubin, although not specific for hepatotoxicity, have been used to identify and monitor DILI since the 1950s both in clinical practice and in drug development. However, the discovery and validation of more satisfactory liver safety biomarkers is clearly an unmet need that is further highlighted in the new therapeutic scenario with drugs such as checkpoint inhibitors, associated with a high hepatotoxicity risk, as well as for the increasing number of patients with underlying liver disease exposed to drugs.
Improved biomarkers compared with current standard serum liver tests should have any of the following advantages: (a) more specific for liver injury than ALT; (b) more sensitive in detecting hepatocellular function than TBL; (c) Be more informative on the mechanisms of DILI; (d) more accurate in predicting prognosis than currently available analytes for clinical outcomes in DILI patients. Recently, international collaborative efforts have been set-up to identify and validate new biomarkers able to address this improved performance (Church et al. 2019). More liver specific biomarkers include microRNA-122 (miR-122), which, nevertheless, in healthy volunteers showed substantial inter- and intra-subject variability in circulating levels (Church et al. 2019). This is possibly related to the fact that miR122 can be released from healthy liver cells and have physiological effects in other tissues (Chai et al. 2017). This may limit is future applicability. Another potential biomarker with liver specificity is glutamate dehydrogenase (GLDH) an enzyme present in the mitochondrial matrix and abundant in the liver. It had lower inter- and intra-subject variation than miR122 in healthy volunteers (Church et al. 2019). Elevation in GLDH levels can point to mitochondrial toxicity as a mechanism of DILI. In addition to this enzyme, other biomarkers that can provide additional insights into the mechanisms of liver injury include full-length cytokeratin (CK) 18 (Jaeschke and McGill 2013), (indicating necrosis), caspase cleaved CK 18 (a marker of apoptosis), macrophage colony stimulating factor receptor 1 (MCSFR1) (a receptor for a cytokine that controls the proliferation, differentiation, and function of macrophages indicating immune activation) and high-mobility group box 1 (HMGB1), (a nuclear protein that is released during necrosis of most cell types and can act as a DAMP to activate innate immune cells) a potential marker of “danger signals” involved in the initiation of DILI events. From the list of candidate biomarkers those that were found to have prognostic ability in a recent international collaborative study were osteopontin (OPN), CK18, and MCSFR1 (Church et al. 2019). Although INR had the highest predictive performance for liver failure, OPN (an extracellular matrix protein widely expressed in immune cells that contribute to inflammation) ranked first among candidate biomarkers, exceeding the predictive capacity of total bilirubin. In this study other candidate biomarkers (full-length CK 18 and MCSFR1) when incorporated were found to improve specificity of the Model of End-stage Liver Disease (MELD) to predict liver failure (Church et al. 2019).
Mechanistic and prognostic biomarkers although not yet fully qualified are further explored in the Translational Safety Biomarker Pipeline, TransBioLine consortium under the umbrella of the EU’s Innovative Medicines Initiative (https://transbioline.com/project/). Indeed, the new biomarkers are meant to complement the standard liver tests, which provide information only across two dimensions, i.e. injury (ALT, AST, ALP), and function (bilirubin, INR, albumin), shedding light onto the injury phenotype, mechanism of liver damage and risk of progression. Hopefully, these will be an important new addition to the interpretation of liver safety signals in clinical trials and for case assessment in the near future.
Biomarkers for causality assessment
In addition to measurement of soluble biomarkers, use of dedicated ex vivo or in vitro tests may help DILI causality assessment in specific cases.
Lymphocyte transformation test (LTT) was developed in the 1960s in Japan and uses in vitro proliferation of T-cells from DILI patients exposed to the suspected drug. Although it has been reported to have good sensitivity and specificity, the test is hardly reproducible: A modified version (mLTT) recently tested was similarly found to be unreliable for the diagnosis of DILI (Whritenour et al. 2017). Hence, neither the LTT nor the mLTT are currently recommended for routine application in DILI causality assessment.
The MetaHepsTM test is based on the culture of hepatocyte-like cells derived from monocytes of suspected DILI subjects. These are exposed in vitro to the suspicious agent, and release of lactate dehydrogenase was used to assess cytotoxicity. MetaHepsTM was compared with RUCAM and was able to diagnose DILI correctly in 29 of 31 patients in a study published by the inventors (Benesic et al. 2016). The assay will undergo further validation in the TransBioLine consortium.
Prognosis
DILI typically resolves upon discontinuation of the offending drug. However, in some cases DILI progress to ALF with fatal outcome or requiring liver transplantation. In addition, some episodes do not fully resolve resulting in chronic liver damage. Clinical features such as jaundice, ascites and encephalopathy but also more unspecific symptoms such as fatigue, vomiting or pruritus are associated with a poorer outcome (Agal et al. 2005).
Acute liver failure defined as an alteration in liver function with jaundice, coagulopathy and encephalopathy in patients without underlying chronic liver disease (Bernal and Wendon 2013) carries a mortality rate of 60% to 90% without liver transplantation depending on the etiology and center experience (Yantorno et al. 2007) with the poorer prognosis for idiosyncratic DILI patients (Ostapowicz et al. 2002). Different scoring systems for predicting prognosis (serious outcome with ALF) in DILI have been proposed (Table 6). In the 1960s, Hyman Zimmerman observed that patients with hepatocellular DILI and jaundice had an increased risk (10–50%) of acute liver failure (before liver transplantations were performed) (Zimmerman 1968). This observation was referred to as Hy´s law, an later defined as ALT > 3 × ULN and TBL > 2 × ULN in DILI patients excluding other causes of liver injury. The FDA currently uses this law for identifying drugs that can potential induce severe liver injury during drug development (FDA et al. 2009). Hy’s law was validated in cases from the Spanish DILI Registry (Andrade et al. 2005), Swedish Adverse Drug Reactions Advisory Committee retrospective database (Björnsson and Olsson 2005) and DILIN (Chalasani et al. 2008) with 11.7%, 9.2% and 15% of death/liver transplantations, respectively, in patients who met Hy’s law criteria.
An analysis of 771 DILI patients included in the Spanish DILI Registry led to a reformulation of Hy´s law. This group proposed the “new Hy’s Law” based on the definition of hepatocellular cases having new Ratio (nR) > 5 (nR = AST or ALT in ULN (whichever highest)/(ALP in ULN) and TBL > 2 × ULN. This new Hy´s law demonstrated similar sensitivity but higher specificity and area under the receiver operating characteristic (AUROC) than the traditional Hy’s law (Robles-Diaz et al. 2014). The new Hy’s law was later validated in an independent cohort from DILIN (Hayashi et al. 2017). In addition, the Spanish DILI Registry also developed a detection algorithm for predicting prognosis by establishing cut-off points of various liver parameters (AST, TBL and AST/ALT ratio) that were independently associated with risk of ALF in a logistic regression model. This algorithm showed slightly lower sensitivity but better specificity and AUROC in predicting ALF compared with traditional Hy´s law and nHy´s law (AUROC 0.80, 0.67 and 0.77, respectively) (Robles-Diaz et al. 2014).
The reported rate of liver-related death/liver transplantation in DILI patients varies across studies, in part probably due to methodological issues and differences in inclusion criteria. The reported rate in the Spanish DILI Registry was 4% (Robles-Diaz et al. 2014), 6% in DILIN (Hayashi et al. 2017), while being reported as 14% in an Indian study (Devarbhavi et al. 2018), 15% in a Korean center (Jeong et al. 2015) and only 0.2% in a study undertaken in California (Lo Re et al. 2015).
Other scores for predicting severity in DILI have been proposed (Table 6). In a study encompassing 15,353 DILI cases identified from the Kaiser Permanente database in California the authors constructed a model including TBL and platelets, whereby higher TBL values and lower platelet counts were predictors of worse outcome (Lo Re et al. 2015). The performance of MELD score, King’s college criteria (KCC) score and Acute Liver Failure Study Group (ALFSG) index (scores used for predicting ALF, regardless the cause of liver injury) have also been evaluated in DILI populations (Lo Re et al. 2015; Jeong et al. 2015; Rathi et al. 2017; Hayashi et al. 2017; Devarbhavi et al. 2018). In a cohort of 1089 DILI patients with 107 deaths/liver transplantations from the DILIN registry MELD (cutoff 19) demonstrated better accuracy for predicting death compared to nHy’s law and Hy’s law with C statistics of 0.83, 0.73 and 0.60, respectively (Hayashi et al. 2017). A study from India compared the utility of MELD, KCC score and ALFSG index in predicting ALF in a cohort of 905 DILI patients of whom 128 developed ALF. The MELD and ALFSG index had higher AUROC values than KCC with 0.76 for the former two vs 0.51 for KCC (Devarbhavi et al. 2018). Since these studies have different design and case definition criteria direct comparison between them to determine the best predictive score is not possible. Hence, prospective studies comparing all of these methods in the same DILI cohort are needed to achieve this aim.
The influence of pre-existing liver disease in DILI outcome was addressed by DILIN in 89 out of 899 patients enrolled in this prospective cohort. Higher mortality was observed in the group of subjects with underlying liver disease, but interestingly the liver-related mortality did not differ between the two groups (Chalasani et al. 2015). An analysis integrating comorbidity burden (estimated by the Charlson’s score) into the prediction of death risk at 6 months after DILI onset was recently reported by the DILIN group, using a discovery cohort from a single center comprising of 306 patients and a validation cohort including 247 patients from another center. Significant comorbidity, MELD and albumin were all independently associated with 6-month mortality. A model based on these 3 variables identified patients who died within 6 months with a C-statistic value of 0.89 (Ghabril et al. 2019).
In some instances, liver injury caused by medicinal agents or HDS does not fully resolve despite an initial improvement. The issue of chronicity in DILI is a matter of debate and the time of abnormal liver tests required to define chronic liver injury is still controversial. In the first international consensus meeting on DILI, in 1990, chronic DILI was defined as persistent liver damage after 3 months of DILI onset (Benichou 1990). In a later study from the Spanish DILI Registry (2006), chronic DILI was considered when liver damage did not resolve within 3 months from onset in hepatocellular cases and 6 months in cholestatic cases (Andrade et al. 2006). The DILIN however defined chronic DILI as persistent liver damage after 6 months from DILI onset regardless of the type of liver damage (Chalasani et al. 2008; Fontana et al. 2009). More recently, the Spanish DILI Registry undertook a 3-year prospective study and determined that 1 year after DILI onset is the best cut-off point for establishing chronicity in both hepatocellular and cholestatic/mixed cases (Medina-Caliz et al. 2016).
A number of studies using different definitions have reported on chronic DILI outcome. For example, in a retrospective study from the UK, 11 out of 33 patients who underwent a liver biopsy showed evidence of persistent liver damage with a minimum follow-up of 1-year after DILI onset (Aithal and Day 1999). In a Swedish database, 8 patients out of 685 were found to develop liver cirrhosis after a DILI episode in a mean follow-up of ten years (Björnsson and Davidsdottir 2009). The Spanish DILI Registry showed that in a cohort of 298 DILI patients, 8% met criteria for chronicity (Medina-Caliz et al. 2016).
Several drugs have been implicated in DILI chronicity. These include statins, fenofibrate, oral contraceptives, isoniazid, sulphonamides and trimethoprim, nitrofurantoin, ebrotidine, amoxicillin-clavulanate, methotrexate and terbinafine among others (Bessone et al. 2019). In addition, risk factors for chronicity identified across studies include older age, female gender, cholestatic damage, dyslipidemia and diabetes (Aithal and Day, 1999; Andrade et al. 2006; Björnsson et al. 2007; Fontana et al. 2015; Medina-Caliz et al. 2016).
Management
The most important step in DILI management is stopping the offending drug. However, DILI is sometimes not suspected in the initial assessment and the culprit drug is therefore not immediately withdrawn, which can result in worsening of the liver injury leading to liver failure or chronic damage (Medina-Caliz et al. 2016). Hence, clinicians facing any patient with new onset liver injury should keep in mind that DILI is always a potential etiology (EASL 2019).
Once the drug is identified and stopped, clinical and biochemical alterations usually improve over the next several days or weeks. However, in some cases liver function can deteriorate and the affected patients require close monitoring. The need for hospitalization or prolonged hospitalization depends on the liver function of each individual. Patients with jaundice need strict monitoring and probably hospitalization, particularly in conjunction with a hepatocellular phenotype, which is more prone to progress to ALF (EASL 2019). If the patient develops coagulopathy that might precede impending liver failure, referral to a liver transplant center is mandatory.
The treatment of idiosyncratic DILI is mainly supportive. Scientific evidence for use of specific medications in DILI is in general weak, as well powered and conducted randomized clinical trials are lacking in this area (EASL 2019). However, there are a few examples of medications advocated in DILI associated with specific drugs. Such is the case of cholestyramine (a bile-acid resin), which is recommended for DILI associated with the use of leflunomide under the rationale of accelerating the clearance of this agent that undergoes enterohepatic circulation and has a long-half-life (EASL 2019). Cholestyramine has also been tried to ameliorate cholestasis induced by terbinafine (EASL 2019; Mallat et al. 1997). Carnitine is an antidote for valproate-induced liver injury presumably because its ability to counteract the impairment in mitochondrial β-oxidation by up-regulating mitochondrial acetyl-CoA levels, which leads to enhanced fatty acid uptake by the mitochondria (Lheureux et al. 2005; Lheureux and Hantson 2009; Ohtani et al. 1982; Bohan et al. 2001).
Ursodeoxycholic acid has long been used in cholestatic DILI to shorten the time to resolution based on published anecdotal reports (Piotrowicz et al. 1995; Katsinelos et al. 2000; Studniarz et al. 2012; Agca et al. 2004) as well as in retrospective and prospective cohort studies (Wree et al. 2011; Lang et al. 2019; Asgarshirazi et al. 2015), although with important methodological limitations that preclude a generalization of the results. Recently, in a phase II controlled clinical trial magnesium isoglycyrrhizinate, a compound with antioxidant properties, was shown to significantly increase the rate of ALT normalization in patients with idiosyncratic DILI compared to trioponin, a standard therapy for DILI in China (Wang et al. 2019).
Corticosteroids are empirically used by many clinicians to treat drug-induced liver injury and, indeed, recent knowledge on the pathogenesis of DILI with the recognition of the importance of the immune system (Dara et al. 2016) gives further support to the use of immunosuppressant drugs. A study performed by the ALFSG evaluated the effects of corticosteroids on survival in 361 ALF patients divided into three groups according to liver damage etiology: autoimmune hepatitis, DILI and indeterminate ALF. The use of steroids did not demonstrated benefits in survival in any of the groups. In fact, steroid use was associated with increased mortality in patients with worse liver function (higher MELD) (Karkhanis et al. 2014). Corticosteroids have also been used for DI-AIH. In a study performed in the Mayo Clinic on 261 patients (24 DI-AIH compared with 237 AIH) demonstrated that the response to steroids in DI-AIH was similar to that of classical AIH, but with no relapse after discontinuation of immunosuppression (Bjornsson et al. 2010). Corticosteroids are widely used in the oncology area and recommended by experts to manage frequent liver injury caused by immune checkpoint inhibitors. However, the criteria for using immunosuppression in these patients, as well as the appropriate dose and duration of steroid therapy, has yet to be determined (De Martin et al. 2018; EASL 2019; Andrade and Robles-Díaz 2019).
N-Acetylcysteine has demonstrated benefits in transplant-free survival in subjects with non-acetaminophen ALF of varied etiology, including DILI but only in the subgroup of subjects with earlier stages of encephalopathy (Lee et al. 2009). Supporting detoxification system such as molecular adsorbents recirculation system or plasma exchange used in patients waiting for liver transplantation, has not demonstrated increased survival (Stine and Lewis 2016). Finally, drug-induced ALF is an established indication for liver transplantation, the rate of survival at 1 year after liver transplantation is around 80% (Fontana and Bari 2017).
It is clear that to make progress in the management of DILI there is an urgent need for well-designed randomized clinical trials evaluating the effectiveness of some marketed drugs with known potential benefits as well as for the proper clinical development of new molecules.
References
Adolph TE, Grander C, Moschen AR, Tilg H (2018) Liver-microbiome axis in health and disease. Trends Immunol 39(9):712–723. https://doi.org/10.1016/j.it.2018.05.002
Agal S, Baijal R, Pramanik S, Patel N, Gupte P, Kamani P et al (2005) Monitoring and management of antituberculosis drug induced hepatotoxicity. J Gastroenterol Hepatol 20(11):1745–1752. https://doi.org/10.1111/j.1440-1746.2005.04048.x
Agarwal VK, McHutchison JG, Hoofnagle JH, Network D-ILI (2010) Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol 8(5):463–470. https://doi.org/10.1016/j.cgh.2010.02.008
Agca E, Akcay A, Simsek H (2004) Ursodeoxycholic acid for terbinafine-induced toxic hepatitis. Ann Pharmacother 38(6):1088–1089. https://doi.org/10.1345/aph.1D420
Aithal PG, Day CP (1999) The natural history of histologically proved drug induced liver disease. Gut 44(5):731–735
Aithal PG, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H et al (2011) Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 89(6):806–815. https://doi.org/10.1038/clpt.2011.58
Aithal GP, Rawlins MD, Day CP (2000) Clinical diagnostic scale: a useful tool in the evaluation of suspected hepatotoxic adverse drug reactions. J Hepatol 33(6):949–952
Andrade RJ, Camargo R, Lucena MI, González-Grande R (2004) Causality assessment in drug-induced hepatotoxicity. Expert Opin Drug Saf 3(4):329–344
Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB et al (2019) Drug-induced liver injury. Nat Rev Dis Primers 5(1):58. https://doi.org/10.1038/s41572-019-0105-0
Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E et al (2005) Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 129(2):512–521. https://doi.org/10.1016/j.gastro.2005.05.006
Andrade RJ, Lucena MI, Kaplowitz N, Garcia-Munoz B, Borraz Y, Pachkoria K et al (2006) Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology 44(6):1581–1588. https://doi.org/10.1002/hep.21424
Andrade RJ, Robles M, Fernández-Castañer A, López-Ortega S, López-Vega MC, Lucena MI (2007) Assessment of drug-induced hepatotoxicity in clinical practice: a challenge for gastroenterologists. World J Gastroenterol 13(3):329–340
Andrade RJ, Robles M, Lucena MI (2009) Rechallenge in drug-induced liver injury: the attractive hazard. Expert Opin Drug Saf 8(6):709–714. https://doi.org/10.1517/14740330903397378
Andrade RJ, Robles-Díaz M (2019) Diagnostic and prognostic assessment of suspected drug-induced liver injury in clinical practice. Liver Int. https://doi.org/10.1111/liv.14271
Andrade RJ, Robles-Díaz M (2020) Diagnostic and prognostic assessment of suspected drug-induced liver injury in clinical practice. Liver Int 40(1):6–17. https://doi.org/10.1111/liv.14271
Arimone Y, Bégaud B, Miremont-Salamé G, Fourrier-Réglat A, Molimard M, Moore N et al (2006) A new method for assessing drug causation provided agreement with experts' judgment. J Clin Epidemiol 59(3):308–314. https://doi.org/10.1016/j.jclinepi.2005.08.012
Asgarshirazi M, Shariat M, Dalili H, Keihanidoost Z (2015) Ursodeoxycholic acid can improve liver transaminase quantities in children with anticonvulsant drugs hepatotoxicity: a pilot study. Acta Med Iran 53(6):351–355
Bagheri H, Michel F, Lapeyre-Mestre M, Lagier E, Cambus JP, Valdiguié P et al (2000) Detection and incidence of drug-induced liver injuries in hospital: a prospective analysis from laboratory signals. Br J Clin Pharmacol 50(5):479–484. https://doi.org/10.1046/j.1365-2125.2000.00282.x
Bao Q, Yu L, Chen D, Li L (2020) Variation in the gut microbial community is associated with the progression of liver regeneration. Hepatol Res 50(1):121–136. https://doi.org/10.1111/hepr.13424
Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC (1993) Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 129(1):92–96
Benesic A, Leitl A, Gerbes AL (2016) Monocyte-derived hepatocyte-like cells for causality assessment of idiosyncratic drug-induced liver injury. Gut 65(9):1555–1563. https://doi.org/10.1136/gutjnl-2015-309528
Benichou C (1990) Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 11(2):272–276
Benichou C, Danan G, Flahault A (1993) Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 46(11):1331–1336
Bernal W, Wendon J (2013) Acute liver failure. N Engl J Med 369(26):2525–2534. https://doi.org/10.1056/NEJMra1208937
Bessone F, Robles-Diaz M, Hernandez N, Medina-Caliz I, Lucena MI, Andrade RJ (2019) Assessment of serious acute and chronic idiosyncratic drug-induced liver injury in clinical practice. Semin Liver Dis 39(3):381–394. https://doi.org/10.1055/s-0039-1685519
Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S (2013) Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 144(7):1419–1425. https://doi.org/10.1053/j.gastro.2013.02.006(1425.e1-3; quiz e19–20)
Bjornsson ES, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S et al (2010) Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology 51(6):2040–2048. https://doi.org/10.1002/hep.23588
Björnsson E, Davidsdottir L (2009) The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol 50(3):511–517. https://doi.org/10.1016/j.jhep.2008.10.021
Björnsson E, Ismael S, Nejdet S, Kilander A (2003) Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis. Scand J Gastroenterol 38(1):86–94. https://doi.org/10.1080/00365520310000492
Björnsson E, Jacobsen EI, Kalaitzakis E (2012) Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol 56(2):374–380. https://doi.org/10.1016/j.jhep.2011.07.023
Björnsson E, Kalaitzakis E, Av Klinteberg V, Alem N, Olsson R (2007) Long-term follow-up of patients with mild to moderate drug-induced liver injury. Aliment Pharmacol Ther 26(1):79–85. https://doi.org/10.1111/j.1365-2036.2007.03355.x
Björnsson E, Olsson R (2005) Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 42(2):481–489. https://doi.org/10.1002/hep.20800
Björnsson ES, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S (2017) Drug-induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol 15(10):1635–1636. https://doi.org/10.1016/j.cgh.2017.05.027
Björnsson ES, Hoofnagle JH (2016) Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology 63(2):590–603. https://doi.org/10.1002/hep.28323
Bohan TP, Helton E, McDonald I, König S, Gazitt S, Sugimoto T et al (2001) Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology 56(10):1405–1409. https://doi.org/10.1212/wnl.56.10.1405
Bonkovsky HL, Severson T, Nicoletti P, Barnhart H, Serrano J, Chalasani N et al (2019) Genetic polymorphisms implicated in nonalcoholic liver disease or selected other disorders have no influence on drug-induced liver injury. Hepatol Commun 3(8):1032–1035. https://doi.org/10.1002/hep4.1382
Brownlie RJ, Zamoyska R, Salmond RJ (2018) Regulation of autoimmune and anti-tumour T-cell responses by PTPN22. Immunology 154(3):377–382. https://doi.org/10.1111/imm.12919
Caines A, Moonka D (2020) Drug hepatotoxicity: causality assessment. Clin Liver Dis 24(1):25–35. https://doi.org/10.1016/j.cld.2019.09.001
Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M (2019) Epidemiology of drug-induced liver injury in a University Hospital from Colombia: updated RUCAM being used for prospective causality assessment. Ann Hepatol 18(3):501–507. https://doi.org/10.1016/j.aohep.2018.11.008
Carrascosa MF, Salcines-Caviedes JR, Lucena MI, Andrade RJ (2015) Acute liver failure following atorvastatin dose escalation: is there a threshold dose for idiosyncratic hepatotoxicity? J Hepatol 62(3):751–752. https://doi.org/10.1016/j.jhep.2014.11.019
Chai C, Rivkin M, Berkovits L, Simerzin A, Zorde-Khvalevsky E, Rosenberg N et al (2017) Metabolic circuit involving free fatty acids, microRNA 122, and triglyceride synthesis in liver and muscle tissues. Gastroenterology 153(5):1404–1415. https://doi.org/10.1053/j.gastro.2017.08.013
Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J et al (2015) Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 148(7):1340–52.e7. https://doi.org/10.1053/j.gastro.2015.03.006
Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J et al (2008) Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 135(6):1924–1934. https://doi.org/10.1053/j.gastro.2008.09.011(1934.e1–4)
Chalasani N, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ (2014) ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 109(7):950–966. https://doi.org/10.1038/ajg.2014.131
Chan R, Benet LZ (2018) Measures of BSEP inhibition in vitro are not useful predictors of DILI. Toxicol Sci 162(2):499–508. https://doi.org/10.1093/toxsci/kfx284
Cheetham TC, Lee J, Hunt CM, Niu F, Reisinger S, Murray R et al (2014) An automated causality assessment algorithm to detect drug-induced liver injury in electronic medical record data. Pharmacoepidemiol Drug Saf 23(6):601–608. https://doi.org/10.1002/pds.3531
Chen M, Borlak J, Tong W (2013) High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology 58(1):388–396. https://doi.org/10.1002/hep.26208
Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena M (2015) Drug-Induced liver injury: interactions between drug properties and host factors. J Hepatol 63(2):503–514. https://doi.org/10.1016/j.jhep.2015.04.016
Church RJ, Kullak-Ublick GA, Aubrecht J, Bonkovsky HL, Chalasani N, Fontana RJ et al (2019) Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: an international collaborative effort. Hepatology 69(2):760–773. https://doi.org/10.1002/hep.29802
CIOMS (2020) 'Drug-induced liver injury (DILI): Current status and future directions for drug development and the post-market setting'. Geneva: Council for International Organizations of Medical Sciences (CIOMS), p 160. https://cioms.ch/publications/product/drug-induced-liver-injury/
Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N et al (2019) A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology 156(6):1707–1716.e2. https://doi.org/10.1053/j.gastro.2019.01.034
Corsini A, Ganey P, Ju C, Kaplowitz N, Pessayre D, Roth R et al (2012) Current challenges and controversies in drug-induced liver injury. Drug Saf 35(12):1099–1117. https://doi.org/10.1007/BF03261997
Crocetti D, Palmieri A, Pedullà G, Pasta V, D'Orazi V, Grazi GL (2015) Peliosis hepatis: personal experience and literature review. World J Gastroenterol 21(46):13188–13194. https://doi.org/10.3748/wjg.v21.i46.13188
Czaja AJ (2016) Factoring the intestinal microbiome into the pathogenesis of autoimmune hepatitis. World J Gastroenterol 22(42):9257–9278. https://doi.org/10.3748/wjg.v22.i42.9257
Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A et al (2009) HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet 41(7):816–819. https://doi.org/10.1038/ng.379
Danan G (1988) Causality assessment of drug-induced liver injury. Hepatology Working Group. J Hepatol 7(1):132–136
Danan G, Benichou C (1993) Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 46(11):1323–1330
Danan G, Teschke R (2016) RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci 17(1):14. https://doi.org/10.3390/ijms17010014
Dara L, Liu ZX, Kaplowitz N (2016) Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int 36(2):158–165. https://doi.org/10.1111/liv.12988
Darvin P, Toor SM, Sasidharan Nair V, Elkord E (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50(12):1–11. https://doi.org/10.1038/s12276-018-0191-1
Dash A, Figler RA, Sanyal AJ, Wamhoff BR (2017) Drug-induced steatohepatitis. Expert Opin Drug Metab Toxicol 13(2):193–204. https://doi.org/10.1080/17425255.2017.1246534
de Abajo FJ, Montero D, Madurga M, García Rodríguez LA (2004) Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol 58(1):71–80. https://doi.org/10.1111/j.1365-2125.2004.02133.x
De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O et al (2018) Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 68(6):1181–1190. https://doi.org/10.1016/j.jhep.2018.01.033
De Valle MB, Av Klinteberg V, Alem N, Olsson R, Björnsson E (2006) Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther 24(8):1187–1195. https://doi.org/10.1111/j.1365-2036.2006.03117.x
deLemos AS, Foureau DM, Jacobs C, Ahrens W, Russo MW, Bonkovsky HL (2014) Drug-induced liver injury with autoimmune features. Semin Liver Dis 34(2):194–204. https://doi.org/10.1055/s-0034-1375959
Devarbhavi H, Karanth D, Prasanna KS, Adarsh CK, Patil M (2011) Drug-Induced liver injury with hypersensitivity features has a better outcome: a single-center experience of 39 children and adolescents. Hepatology 54(4):1344–1350. https://doi.org/10.1002/hep.24527
Devarbhavi H, Patil M, Reddy VV, Singh R, Joseph T, Ganga D (2018) Drug-induced acute liver failure in children and adults: Results of a single-centre study of 128 patients. Liver Int 38(7):1322–1329. https://doi.org/10.1111/liv.13662
Devarbhavi H, Raj S (2019) Drug-induced liver injury with skin reactions: drugs and host risk factors, clinical phenotypes and prognosis. Liver Int 39(5):802–811. https://doi.org/10.1111/liv.14004
Devarbhavi H, Raj S, Aradya VH, Rangegowda VT, Veeranna GP, Singh R et al (2016) Drug-induced liver injury associated with Stevens-Johnson syndrome/toxic epidermal necrolysis: patient characteristics, causes, and outcome in 36 cases. Hepatology 63(3):993–999. https://doi.org/10.1002/hep.28270
Devarbhavi H, Raj S, Joseph T, Singh R, Patil M (2017) Features and treatment of dapsone-induced hepatitis, based on analysis of 44 cases and literature review. Clin Gastroenterol Hepatol 15(11):1805–1807. https://doi.org/10.1016/j.cgh.2017.05.031
Duh MS, Walker AM, Kronlund KH (1999) Descriptive epidemiology of acute liver enzyme abnormalities in the general population of central Massachusetts. Pharmacoepidemiol Drug Saf 8(4):275–283. https://doi.org/10.1002/(SICI)1099-1557(199907)8:4<275:AID-PDS427>3.0.CO;2-D
EASL (2019) EASL clinical practice guidelines: drug-induced liver injury. J Hepatol 70(6):1222–1261. https://doi.org/10.1016/j.jhep.2019.02.014
EMA (2015) European Medicines Agency. https://www.ema.europa.eu. Accessed 1 May 1 2015
FDA, (CDER), C. f. D. E. a. R., & (CBER), C. f. B. E. a. R. (2009) Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation. https://www.fda.gov/downloads/Guidances/UCM174090.pdf. Accessed 10 June 2020
Fernández-Castañer A, García-Cortés M, Lucena MI, Borraz Y, Peláez G, Costa J et al (2008) An analysis of the causes, characteristics, and consequences of reexposure to a drug or compound responsible for a hepatotoxicity event. Rev Esp Enferm Dig 100(5):278–284. https://doi.org/10.4321/s1130-01082008000500006
Fisher K, Vuppalanchi R, Saxena R (2015) Drug-induced liver injury. Arch Pathol Lab Med 139(7):876–887. https://doi.org/10.5858/arpa.2014-0214-RA
Fontana RJ, Bari K (2017) Acute liver failure. In: Schiff E, Maddrey W, Reddy K (eds) Schiff’s diseases of the liver. Wiley, Oxford, pp 411–431
Fontana RJ, Hayashi PH, Barnhart H, Kleiner DE, Reddy KR, Chalasani N et al (2015) Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol 110(10):1450–1459. https://doi.org/10.1038/ajg.2015.283
Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J et al (2010) Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology 52(2):730–742. https://doi.org/10.1002/hep.23696
Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J et al (2009) Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf 32(1):55–68
Fontana RJ (2014) Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology 146(4):914–928. https://doi.org/10.1053/j.gastro.2013.12.032
Fromenty B (2013) Drug-induced liver injury in obesity. J Hepatol 58(4):824–826. https://doi.org/10.1016/j.jhep.2012.12.018
Funk C, Ponelle C, Scheuermann G, Pantze M (2001) Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol 59(3):627–635
García Rodríguez LA, Ruigómez A, Jick H (1997) A review of epidemiologic research on drug-induced acute liver injury using the general practice research data base in the United Kingdom. Pharmacotherapy 17(4):721–728
García-Cortés M, Lucena MI, Pachkoria K, Borraz Y, Hidalgo R, Andrade RJ et al (2008) Evaluation of naranjo adverse drug reactions probability scale in causality assessment of drug-induced liver injury. Aliment Pharmacol Ther 27(9):780–789. https://doi.org/10.1111/j.1365-2036.2008.03655.x
García-Cortés M, Stephens C, Lucena MI, Fernández-Castañer A, Andrade RJ (2011) Causality assessment methods in drug induced liver injury: strengths and weaknesses. J Hepatol 55(3):683–691. https://doi.org/10.1016/j.jhep.2011.02.007
George N, Chen M, Yuen N, Hunt CM, Suzuki A (2018) Interplay of gender, age and drug properties on reporting frequency of drug-induced liver injury. Regul Toxicol Pharmacol 94:101–107. https://doi.org/10.1016/j.yrtph.2018.01.018
Ghabril M, Gu J, Yoder L, Corbito L, Ringel A, Beyer CD et al (2019) Development and validation of a model consisting of comorbidity burden to calculate risk of death within 6 months for patients with suspected drug-induced liver injury. Gastroenterology 157(5):1245–1252.e3. https://doi.org/10.1053/j.gastro.2019.07.006
Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR et al (2015) Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 148(7):1353–61.e3. https://doi.org/10.1053/j.gastro.2015.02.050
Gudnason HO, Björnsson HK, Gardarsdottir M, Thorisson HM, Olafsson S, Bergmann OM et al (2015) Secondary sclerosing cholangitis in patients with drug-induced liver injury. Dig Liver Dis 47(6):502–507. https://doi.org/10.1016/j.dld.2015.03.002
Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ et al (2013) Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci 34(4):243–253. https://doi.org/10.1016/j.tips.2013.01.009
Hartleb M, Gutkowski K, Milkiewicz P (2011) Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol 17(11):1400–1409. https://doi.org/10.3748/wjg.v17.i11.1400
Hayashi PH (2016) Drug-induced liver injury network causality assessment: criteria and experience in the United States. Int J Mol Sci 17(2):201. https://doi.org/10.3390/ijms17020201
Hayashi PH, Rockey DC, Fontana RJ, Tillmann HL, Kaplowitz N, Barnhart HX et al (2017) Death and liver transplantation within 2 years of onset of drug-induced liver injury. Hepatology 66(4):1275–1285. https://doi.org/10.1002/hep.29283
Hillman L, Gottfried M, Whitsett M, Rakela J, Schilsky M, Lee WM et al (2016) Clinical features and outcomes of complementary and alternative medicine induced acute liver failure and injury. Am J Gastroenterol 111(7):958–965. https://doi.org/10.1038/ajg.2016.114
Holman NS, Church RJ, Nautiyal M, Rose KA, Thacker SE, Otieno MA et al (2019) Hepatocyte-derived exosomes promote liver immune tolerance: possible implications for idiosyncratic drug-induced liver injury. Toxicol Sci 170(2):499–508. https://doi.org/10.1093/toxsci/kfz112
Hoofnagle JH (2004) Drug-induced liver injury network (DILIN). Hepatology 40(4):773. https://doi.org/10.1002/hep.20445
Hoofnagle JH, Björnsson ES (2019) Drug-induced liver injury—types and phenotypes. N Engl J Med 381(3):264–273. https://doi.org/10.1056/NEJMra1816149
Hunt CM, Papay JI, Stanulovic V, Regev A (2017) Drug rechallenge following drug-induced liver injury. Hepatology 66(2):646–654. https://doi.org/10.1002/hep.29152
Hunt CM, Yuen NA, Stirnadel-Farrant HA, Suzuki A (2014) Age-related differences in reporting of drug-associated liver injury: data-mining of WHO Safety Report Database. Regul Toxicol Pharmacol 70(2):519–526. https://doi.org/10.1016/j.yrtph.2014.09.007
Iorga A, Dara L, Kaplowitz N (2017) Drug-induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int J Mol Sci 18(5):1018. https://doi.org/10.3390/ijms18051018
Isaacson M, Babich M (2020) Drug-induced liver injury resources and reporting for the clinician. Clin Liver Dis 24(1):131–139. https://doi.org/10.1016/j.cld.2019.09.010
Jaeschke H, McGill MR (2013) Serum glutamate dehydrogenase–biomarker for liver cell death or mitochondrial dysfunction? Toxicol Sci 134(1):221–222. https://doi.org/10.1093/toxsci/kft087
Jennings JJ, Mandaliya R, Nakshabandi A, Lewis JH (2019) Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol 15(3):231–244. https://doi.org/10.1080/17425255.2019.1574744
Jeong R, Lee YS, Sohn C, Jeon J, Ahn S, Lim KS (2015) Model for end-stage liver disease score as a predictor of short-term outcome in patients with drug-induced liver injury. Scand J Gastroenterol 50(4):439–446. https://doi.org/10.3109/00365521.2014.958094
Kaliyaperumal K, Grove JI, Delahay RM, Griffiths WJH, Duckworth A, Aithal GP (2018) Pharmacogenomics of drug-induced liver injury (DILI): Molecular biology to clinical applications. J Hepatol 69(4):948–957. https://doi.org/10.1016/j.jhep.2018.05.013
Kaplowitz N (2001) Causality assessment versus guilt-by-association in drug hepatotoxicity. Hepatology 33(1):308–310. https://doi.org/10.1053/jhep.2001.21083
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M et al (2007) Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol 156(3):609–611. https://doi.org/10.1111/j.1365-2133.2006.07704.x
Karkhanis J, Verna EC, Chang MS, Stravitz RT, Schilsky M, Lee WM et al (2014) Steroid use in acute liver failure. Hepatology 59(2):612–621. https://doi.org/10.1002/hep.26678
Katsinelos P, Vasiliadis T, Xiarchos P, Patakiouta F, Christodoulou K, Pilpilidis I et al (2000) Ursodeoxycholic acid (UDCA) for the treatment of amoxycillin-clavulanate potassium (Augmentin)-induced intra-hepatic cholestasis: report of two cases. Eur J Gastroenterol Hepatol 12(3):365–368. https://doi.org/10.1097/00042737-200012030-00017
Kenna JG, Taskar KS, Battista C, Bourdet DL, Brouwer KLR, Brouwer KR et al (2018) Can bile salt export pump inhibition testing in drug discovery and development reduce liver injury risk? An international transporter consortium perspective. Clin Pharmacol Ther 104(5):916–932. https://doi.org/10.1002/cpt.1222
Kim SH, Saide K, Farrell J, Faulkner L, Tailor A, Ogese M et al (2015) Characterization of amoxicillin- and clavulanic acid-specific T cells in patients with amoxicillin-clavulanate-induced liver injury. Hepatology 62(3):887–899. https://doi.org/10.1002/hep.27912
Kleiner DE (2018) Histopathological challenges in suspected drug-induced liver injury. Liver Int 38(2):198–209. https://doi.org/10.1111/liv.13584
Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB et al (2014) Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology 59(2):661–670. https://doi.org/10.1002/hep.26709
Kubes P, Jenne C (2018) Immune responses in the liver. Annu Rev Immunol 36:247–277. https://doi.org/10.1146/annurev-immunol-051116-052415
Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL et al (2017) Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut 66(6):1154–1164. https://doi.org/10.1136/gutjnl-2016-313369
Kuna L, Bozic I, Kizivat T, Bojanic K, Mrso M, Kralj E et al (2018) Models of drug induced liver injury (DILI)—current issues and future perspectives. Curr Drug Metab 19(10):830–838. https://doi.org/10.2174/1389200219666180523095355
Lang SM, Ortmann J, Rostig S, Schiffl H (2019) Ursodeoxycholic acid attenuates hepatotoxicity of multidrug treatment of mycobacterial infections: a prospective pilot study. Int J Mycobacteriol 8(1):89–92. https://doi.org/10.4103/ijmy.ijmy_159_18
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. https://doi.org/10.1056/NEJMoa1504030
Lauschke VM, Vorrink SU, Moro SM, Rezayee F, Nordling Å, Hendriks DF et al (2016) Massive rearrangements of cellular MicroRNA signatures are key drivers of hepatocyte dedifferentiation. Hepatology 64(5):1743–1756. https://doi.org/10.1002/hep.28780
Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM et al (2009) Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 137(3):856–864. https://doi.org/10.1053/j.gastro.2009.06.006
Lewis J, Kleiner D (2006) Hepatic injury due to drugs, chemicals and toxins. In: Burt A, Portmann B, Ferrell L (eds) MacSween’s pathology of the liver. Churchill Livingstone Elsevier, Amsterdam
Lheureux PE, Hantson P (2009) Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila) 47(2):101–111. https://doi.org/10.1080/15563650902752376
Lheureux PE, Penaloza A, Zahir S, Gris M (2005) Science review: carnitine in the treatment of valproic acid-induced toxicity—what is the evidence? Crit Care 9(5):431–440. https://doi.org/10.1186/cc3742
Li M, Huang W, Jie F, Wang M, Zhong Y, Chen Q et al (2019) Discovery of Keap1-Nrf2 small-molecule inhibitors from phytochemicals based on molecular docking. Food Chem Toxicol 133:110758. https://doi.org/10.1016/j.fct.2019.110758
LiverTox (2012) Clinical and research information on drug-induced liver injury. NBK548900
Lo Re V, Haynes K, Forde KA, Goldberg DS, Lewis JD, Carbonari DM et al (2015) Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy's law and a new prognostic model. Clin Gastroenterol Hepatol 13(13):2360–2368. https://doi.org/10.1016/j.cgh.2015.06.020
Lomtadze N, Kupreishvili L, Salakaia A, Vashakidze S, Sharvadze L, Kempker RR et al (2013) Hepatitis C virus co-infection increases the risk of anti-tuberculosis drug-induced hepatotoxicity among patients with pulmonary tuberculosis. PLoS ONE 8(12):e83892. https://doi.org/10.1371/journal.pone.0083892
Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F (2001) Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology 33(1):123–130. https://doi.org/10.1053/jhep.2001.20645
Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ et al (2011) Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology 141(1):338–347. https://doi.org/10.1053/j.gastro.2011.04.001
Mallat A, Zafrani ES, Metreau JM, Dhumeaux D (1997) Terbinafine-induced prolonged cholestasis with reduction of interlobular bile ducts. Dig Dis Sci 42(7):1486–1488. https://doi.org/10.1023/a:1018870828038
Maria VA, Victorino RM (1997) Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology 26(3):664–669. https://doi.org/10.1002/hep.510260319
Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U et al (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339(6123):1084–1088. https://doi.org/10.1126/science.1233521
Matheis K, Laurie D, Andriamandroso C, Arber N, Badimon L, Benain X et al (2011) A generic operational strategy to qualify translational safety biomarkers. Drug Discov Today 16(13–14):600–608. https://doi.org/10.1016/j.drudis.2011.04.011
McGill MR, Jaeschke H (2019) Animal models of drug-induced liver injury. Biochim Biophys Acta Mol Basis Dis 1865(5):1031–1039. https://doi.org/10.1016/j.bbadis.2018.08.037
Medina-Caliz I, Robles-Diaz M, Garcia-Muñoz B, Stephens C, Ortega-Alonso A, Garcia-Cortes M et al (2016) Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol 65(3):532–542. https://doi.org/10.1016/j.jhep.2016.05.003
Medina-Cáliz I, Robles-Díaz M, Lucena MI, Andrade RJ (2017) Drug-induced liver and skin reactions: in need of a consensus definition. Hepatology 65(1):391. https://doi.org/10.1002/hep.28808
Meier Y, Cavallaro M, Roos M, Pauli-Magnus C, Folkers G, Meier PJ et al (2005) Incidence of drug-induced liver injury in medical inpatients. Eur J Clin Pharmacol 61(2):135–143. https://doi.org/10.1007/s00228-004-0888-z
Miller ED, Abu-Sbeih H, Styskel B, Nogueras Gonzalez GM, Blechacz B, Naing A et al (2020) Clinical characteristics and adverse impact of hepatotoxicity due to immune checkpoint inhibitors. Am J Gastroenterol 115(2):251–261. https://doi.org/10.14309/ajg.0000000000000398
Molokhia M, McKeigue P (2006) EUDRAGENE: European collaboration to establish a case-control DNA collection for studying the genetic basis of adverse drug reactions. Pharmacogenomics 7(4):633–638. https://doi.org/10.2217/14622416.7.4.633
Monshi MM, Faulkner L, Gibson A, Jenkins RE, Farrell J, Earnshaw CJ et al (2013) Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology 57(2):727–739. https://doi.org/10.1002/hep.26077
Mushin WW, Rosen M, Jones EV (1971) Post-halothane jaundice in relation to previous administration of halothane. Br Med J 3(5765):18–22. https://doi.org/10.1136/bmj.3.5765.18
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA et al (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30(2):239–245. https://doi.org/10.1038/clpt.1981.154
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284(20):13291–13295. https://doi.org/10.1074/jbc.R900010200
Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M et al (2017) Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology 152(5):1078–1089. https://doi.org/10.1053/j.gastro.2016.12.016
NIDDK (2017) LiverTox. Clinical and Research Information on Drug-Induced Liver Injury. https://www.ncbi.nlm.nih.gov/books/NBK547852/. Accessed 10 June 2020
Nooredinvand HA, Connell DW, Asgheddi M, Abdullah M, O'Donoghue M, Campbell L et al (2015) Viral hepatitis prevalence in patients with active and latent tuberculosis. World J Gastroenterol 21(29):8920–8926. https://doi.org/10.3748/wjg.v21.i29.8920
Ogimura E, Nakagawa T, Deguchi J, Sekine S, Ito K, Bando K (2017) Troglitazone inhibits bile acid amidation: a possible risk factor for liver injury. Toxicol Sci 158(2):347–355. https://doi.org/10.1093/toxsci/kfx091
Ohtani Y, Endo F, Matsuda I (1982) Carnitine deficiency and hyperammonemia associated with valproic acid therapy. J Pediatr 101(5):782–785. https://doi.org/10.1016/s0022-3476(82)80320-x
Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH et al (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 137(12):947–954
Perdices EV, Medina-Cáliz I, Hernando S, Ortega A, Martín-Ocaña F, Navarro JM et al (2014) Hepatotoxicity associated with statin use: analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev Esp Enferm Dig 106(4):246–254
Pessayre D, Fromenty B, Berson A, Robin MA, Lettéron P, Moreau R et al (2012) Central role of mitochondria in drug-induced liver injury. Drug Metab Rev 44(1):34–87. https://doi.org/10.3109/03602532.2011.604086
Piotrowicz A, Polkey M, Wilkinson M (1995) Ursodeoxycholic acid for the treatment of flucloxacillin-associated cholestasis. J Hepatol 22(1):119–120. https://doi.org/10.1016/0168-8278(95)80272-x
Powles T, Bracarda S, Chen M, Norry E, Compton N, Heise M et al (2015) Characterisation of liver chemistry abnormalities associated with pazopanib monotherapy: a systematic review and meta-analysis of clinical trials in advanced cancer patients. Eur J Cancer 51(10):1293–1302. https://doi.org/10.1016/j.ejca.2015.03.019
Pérez Gutthann S, García Rodríguez LA (1993) The increased risk of hospitalizations for acute liver injury in a population with exposure to multiple drugs. Epidemiology 4(6):496–501
Ramachandran R, Kakar S (2009) Histological patterns in drug-induced liver disease. J Clin Pathol 62(6):481–492. https://doi.org/10.1136/jcp.2008.058248
Rathi C, Pipaliya N, Patel R, Ingle M, Phadke A, Sawant P (2017) Drug induced liver injury at a tertiary hospital in India: etiology, clinical features and predictors of mortality. Ann Hepatol 16(3):442–450. https://doi.org/10.5604/16652681.1235488
Regev A, Seeff LB, Merz M, Ormarsdottir S, Aithal GP, Gallivan J et al (2014) Causality assessment for suspected DILI during clinical phases of drug development. Drug Saf 37(Suppl 1):S47–56. https://doi.org/10.1007/s40264-014-0185-4
Reuben A, Koch DG, Lee WM, Group ALFS (2010) Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 52(6):2065–2076. https://doi.org/10.1002/hep.239377
Robles-Diaz M, Garcia-Cortes M, Medina-Caliz I, Gonzalez-Jimenez A, Gonzalez-Grande R, Navarro JM et al (2015) The value of serum aspartate aminotransferase and gamma-glutamyl transpetidase as biomarkers in hepatotoxicity. Liver Int 35(11):2474–2482. https://doi.org/10.1111/liv.12834
Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A et al (2014) Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology 147(1):109–118.e5. https://doi.org/10.1053/j.gastro.2014.03.050
Rochon J, Protiva P, Seeff LB, Fontana RJ, Liangpunsakul S, Watkins PB et al (2008) Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology 48(4):1175–1183. https://doi.org/10.1002/hep.22442
Rockey DC, Seeff LB, Rochon J, Freston J, Chalasani N, Bonacini M et al (2010) Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology 51(6):2117–2126. https://doi.org/10.1002/hep.23577
Rodrigues S, Kollipara L, Chaudhari U, Sachinidis A, Zahedi RP, Sickmann A et al (2018) Omics-based responses induced by bosentan in human hepatoma HepaRG cell cultures. Arch Toxicol 92(6):1939–1952. https://doi.org/10.1007/s00204-018-2214-z
Rodrigues S, Lopes S, Magro F, Cardoso H, Horta e Vale AM, Marques M et al (2015) Autoimmune hepatitis and anti-tumor necrosis factor alpha therapy: a single center report of 8 cases. World J Gastroenterol 21(24):7584–7588. https://doi.org/10.3748/wjg.v21.i24.7584
Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP et al (1979) Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 242(7):644–648
Roytman MM, Poerzgen P, Navarro V (2018) Botanicals and hepatotoxicity. Clin Pharmacol Ther 104(3):458–469. https://doi.org/10.1002/cpt.1097
Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE et al (2014) Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology 60(2):679–686. https://doi.org/10.1002/hep.27157
Saha A, Shanthi FXM, Winston AB, Das S, Kumar A, Michael JS et al (2016) Prevalence of hepatotoxicity from antituberculosis therapy: a five-year experience from South India. J Prim Care Community Health 7(3):171–174. https://doi.org/10.1177/2150131916642431
Sanabria-Cabrera J, Medina-Cáliz I, Stankevičiūtė S, Rodríguez-Nicolás A, Almarza-Torres M, Lucena MI et al (2019) Drug-induced liver injury associated with severe cutaneous hypersensitivity reactions: a complex entity in need of a multidisciplinary approach. Curr Pharm Des 25(36):3855–3871. https://doi.org/10.2174/1381612825666191107161912
Scalfaro E, Streefkerk HJ, Merz M, Meier C, Lewis D (2017) Preliminary results of a novel algorithmic method aiming to support initial causality assessment of routine pharmacovigilance case reports for medication-induced liver injury: the PV-RUCAM. Drug Saf 40(8):715–727. https://doi.org/10.1007/s40264-017-0541-2
Selwyn FP, Cheng SL, Bammler TK, Prasad B, Vrana M, Klaassen C et al (2015) Developmental regulation of drug-processing genes in livers of germ-free mice. Toxicol Sci 147(1):84–103. https://doi.org/10.1093/toxsci/kfv110
Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C et al (2002) Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology 36(2):451–455. https://doi.org/10.1053/jhep.2002.34857
Sharma SK, Singla R, Sarda P, Mohan A, Makharia G, Jayaswal A et al (2010) Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis 50(6):833–839. https://doi.org/10.1086/650576
Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M et al (2019) Incidence and etiology of drug-induced liver injury in Mainland China. Gastroenterology 156(8):2230–2241.e11. https://doi.org/10.1053/j.gastro.2019.02.002
Slim M, Stephens C, Robles-Daz M, Medina-Caliz I, Grove JI, Ortega-Alonso A et al (2016) Pro-Euro-DILI Registry: a collaborative effort to enhance the understanding of DILI. J Hepatol 64(2):S293–S294
Stepan AF, Walker DP, Bauman J, Price DA, Baillie TA, Kalgutkar AS et al (2011) Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: a perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem Res Toxicol 24(9):1345–1410. https://doi.org/10.1021/tx200168d
Stephens C, Andrade RJ (2020) Genetic predisposition to drug-induced liver injury. Clin Liver Dis 24(1):11–23. https://doi.org/10.1016/j.cld.2019.08.003
Stevens JL, Baker TK (2009) The future of drug safety testing: expanding the view and narrowing the focus. Drug Discov Today 14(3–4):162–167. https://doi.org/10.1016/j.drudis.2008.11.009
Stine JG, Lewis JH (2016) Current and future directions in the treatment and prevention of drug-induced liver injury: a systematic review. Expert Rev Gastroenterol Hepatol 10(4):517–536. https://doi.org/10.1586/17474124.2016.1127756
Studniarz M, Czubkowski P, Cielecka-Kuszyk J, Jankowska I, Teisseyre M, Kamińska D et al (2012) Amoxicillin/clavulanic acid-induced cholestatic liver injury after pediatric liver transplantation. Ann Transpl 17(1):128–131. https://doi.org/10.12659/aot.882646
Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS et al (2012) A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol 107(9):1380–1387. https://doi.org/10.1038/ajg.2012.138
Sun Q, Zhang Q, Gu J, Sun WW, Wang P, Bai C et al (2016) Prevalence, risk factors, management, and treatment outcomes of first-line antituberculous drug-induced liver injury: a prospective cohort study. Pharmacoepidemiol Drug Saf 25(8):908–917. https://doi.org/10.1002/pds.3988
Sundaram V, Björnsson ES (2017) Drug-induced cholestasis. Hepatol Commun 1(8):726–735. https://doi.org/10.1002/hep4.1088
Tahaoğlu K, Ataç G, Sevim T, Tärün T, Yazicioğlu O, Horzum G et al (2001) The management of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis 5(1):65–69
Takikawa H, Takamori Y, Kumagi T, Onji M, Watanabe M, Shibuya A et al (2003) Assessment of 287 Japanese cases of drug induced liver injury by the diagnostic scale of the International Consensus Meeting. Hepatol Res 27(3):192–195
Toda T, Ohi K, Kudo T, Yoshida T, Ikarashi N, Ito K et al (2009) Ciprofloxacin suppresses Cyp3a in mouse liver by reducing lithocholic acid-producing intestinal flora. Drug Metab Pharmacokinet 24(3):201–208. https://doi.org/10.2133/dmpk.24.201
Urban TJ, Shen Y, Stolz A, Chalasani N, Fontana RJ, Rochon J et al (2012) Limited contribution of common genetic variants to risk for liver injury due to a variety of drugs. Pharmacogenet Genom 22(11):784–795. https://doi.org/10.1097/FPC.0b013e3283589a76
Vega M, Verma M, Beswick D, Bey S, Hossack J, Merriman N et al (2017) The incidence of drug- and herbal and dietary supplement-induced liver injury: preliminary findings from gastroenterologist-based surveillance in the population of the state of delaware. Drug Saf 40(9):783–787. https://doi.org/10.1007/s40264-017-0547-9
Velazquez I, Alter BP (2004) Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol 77(3):257–267. https://doi.org/10.1002/ajh.20183
Vuppalanchi R, Liangpunsakul S, Chalasani N (2007) Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol 102(3):558–562. https://doi.org/10.1111/j.1572-0241.2006.01019.x(quiz 693)
Wang Y, Wang Z, Gao M, Zhong H, Chen C, Yao Y et al (2019) Efficacy and safety of magnesium isoglycyrrhizinate injection in patients with acute drug-induced liver injury: a phase II trial. Liver Int 39(11):2102–2111. https://doi.org/10.1111/liv.14204
Watanabe M, Shibuya A (2004) Validity study of a new diagnostic scale for drug-induced liver injury in Japan-comparison with two previous scales. Hepatol Res 30(3):148–154. https://doi.org/10.1016/j.hepres.2004.08.005
Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S et al (2007) Acute liver failure in Sweden: etiology and outcome. J Intern Med 262(3):393–401. https://doi.org/10.1111/j.1365-2796.2007.01818.x
Whritenour J, Ko M, Zong Q, Wang J, Tartaro K, Schneider P et al (2017) Development of a modified lymphocyte transformation test for diagnosing drug-induced liver injury associated with an adaptive immune response. J Immunotoxicol 14(1):31–38. https://doi.org/10.1080/1547691X.2016.1254305
Wilson ID, Nicholson JK (2017) Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res 179:204–222. https://doi.org/10.1016/j.trsl.2016.08.002
Wree A, Dechêne A, Herzer K, Hilgard P, Syn WK, Gerken G et al (2011) Steroid and ursodesoxycholic Acid combination therapy in severe drug-induced liver injury. Digestion 84(1):54–59. https://doi.org/10.1159/000322298
Xiao X, Tang J, Mao Y, Li X, Wang J, Liu C et al (2019) Guidance for the clinical evaluation of traditional Chinese medicine-induced liver injuryIssued by China Food and Drug Administration. Acta Pharm Sin B 9(3):648–658. https://doi.org/10.1016/j.apsb.2018.12.003
Yantorno SE, Kremers WK, Ruf AE, Trentadue JJ, Podestá LG, Villamil FG (2007) MELD is superior to King's college and Clichy's criteria to assess prognosis in fulminant hepatic failure. Liver Transpl 13(6):822–828. https://doi.org/10.1002/lt.21104
Zimmerman HJ (1968) The spectrum of hepatotoxicity. Perspect Biol Med 12(1):135–161
Zimmerman HJ (1999) Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. Lippincott Williams & Wilkins, Philadelphia
Funding
The present study has been supported by grants of the Instituto de Salud Carlos III co-founded by Fondo Europeo de Desarrollo Regional-FEDER (contract numbers: PT17/0017/0020, PI 18-01804, PI 18-00901, UMA-18 FEDERJA-193), the Consejería de Salud de la Junta de Andalucía (PI-0274/2016, PI-0285/2016) and by the Agencia Española del Medicamento. MRD holds an Acción B de refuerzo investigador research contract from the Servicio Andaluz de Salud (Expediente B-0002–2019). CIBERehd is funded by Instituto de Salud Carlos III. We acknowledge the support from European Cooperation in Science & Technology (COST) Action CA17112 Prospective European Drug-Induced Liver Injury Network. MGC, CS, AOA, MIL, MRD and RJA, are member of COST Action CA17112.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garcia-Cortes, M., Robles-Diaz, M., Stephens, C. et al. Drug induced liver injury: an update. Arch Toxicol 94, 3381–3407 (2020). https://doi.org/10.1007/s00204-020-02885-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02885-1