Abstract
Sulfur mustard (SM) is a highly toxic war chemical that causes significant morbidity and mortality and lacks any effective therapy. Rats exposed to aerosolized CEES (2-chloroethyl ethyl sulfide; 10% in ethanol), an analog of SM, developed acute respiratory distress syndrome (ARDS), which is characterized by increased inflammation, hypoxemia and impaired gas exchange. We observed elevated levels of extracellular nucleic acids (eNA) in the bronchoalveolar lavage fluid (BALF) of CEES-exposed animals. eNA can induce inflammation, coagulation and barrier dysfunction. Treatment with hexadimethrine bromide (HDMBr; 10 mg/kg), an eNA neutralizing agent, 2 h post-exposure, reduced lung injury, inhibited disruption of alveolar–capillary barrier, improved blood oxygenation (PaO2/FiO2 ratio), thus reversing ARDS symptoms. HDMBr treatment also reduced lung inflammation in the CEES-exposed animals by decreasing IL-6, IL-1A, CXCL-1 and CCL-2 mRNA levels in lung tissues and HMGB1 protein in BALF. Furthermore, HDMBr treatment also reduced levels of lung tissue factor and plasminogen activator inhibitor-1 indicating reduction in clot formation and increased fibrinolysis. Fibrin was reduced in BALF of the HDMBr-treated animals. This was further confirmed by histology that revealed diminished airway fibrin, epithelial sloughing and hyaline membrane in the lungs of HDMBr-treated animals. HDMBr completely rescued the CEES-associated mortality 12 h post-exposure when the survival rate in CEES-only group was just 50%. Experimental eNA treatment of cells caused increased inflammation that was reversed by HDMBr. These results demonstrate a role of eNA in the pathogenesis of CEES/SM-induced injury and that its neutralization can serve as a potential therapeutic approach in treating SM toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to hazardous chemicals like sulfur mustard (SM; bis-2-chloroethyl ethyl sulfide) can result in significant morbidity and mortality (Henretig et al. 2019). The threat of accidental or intentional exposures to these chemicals is ever increasing, because large stockpiles of these still exist and effective therapies are lacking (Jett and Laney 2019). SM and its analog CEES (2-chloroethyl ethyl sulfide; aka: half mustard) are highly reactive alkylating and vesicating agents (Ahmad and Ahmad 2016). Our understanding of its pathophysiology is derived from human victims and experimental animal exposures to SM or CEES. Inhalation of SM causes direct insult to the lungs leading to injury, acute effects of which are characterized as acute respiratory distress syndrome (ARDS). Most acute inhalation exposures affect both the upper and lower airways (McClintock et al. 2006; Pant and Vijayaraghavan 1999) causing injury, impaired gas exchange and epithelial sloughing (Calvet et al. 1994; Chevillard et al. 1992). Acute exposures to SM/CEES also cause severe lung inflammation, airway hyperreactivity, pulmonary edema and activation of the coagulation cascade resulting in formation of fibrin-rich bronchial plugs causing airway obstruction and death (Eisenmenger et al. 1991; Veress et al. 2010). Increased morbidity and mortality are also attributed to the fact that exposure to SM/CEES causes multiple organ failure (Balali-Mood and Hefazi 2006; Kehe and Szinicz 2005; Kehe et al. 2009; Sinclair 1948). Inhalation exposures to SM have, therefore, great parallels with ARDS. In some preclinical models of acute lung injury/ARDS, inhibition of fibrin formation mitigated injury (Schultz et al. 2006). However, anti-coagulation therapies do not attenuate ARDS in humans and may even increase mortality (Standiford and Ward 2016).
After acute exposure to SM/CEES, several factors may activate the coagulation cascade and promote clot formation in the bloodstream and within airspaces of the lungs (Ahmad and Ahmad 2016). Extracellular NA (eNA) is one of the factors that can activate the coagulation cascade and also prevent fibrinolysis thereby increasing the stability of fibrin clots (Preissner and Herwald 2017). Extracellular NA have traditionally been used as diagnostic and prognostic marker of various diseases (O'Driscoll 2007). However, the role of eNA in the pathogenesis of ARDS is unknown. Extracellular NAs are also increasingly being recognized as important mediators of inflammation and injury (Preissner and Herwald 2017). Extracellular NAs released from the cells as a result of injury or through the normal apoptotic/necrotic process can activate multiple signaling pathways (Preissner and Herwald 2017). NA released from cells can also stimulate the pattern-recognition receptors and are potent activators of the inflammatory pathway (Pisetsky et al. 2012). They are, therefore, unique molecules that can accelerate the development of inflammatory response and also activate the coagulation cascade. Since both the coagulation and inflammatory pathways are activated in CEES/SM-induced lung injury, we hypothesized that eNAs play an important mechanistic role in the pathogenesis of this injury.
The current study utilizes aerosolized CEES, an analog of SM, that manifests many of the pathophysiologic features’ characteristic of SM poisoning and also shows significant morbidity and mortality within 12 h of exposure. This study also investigates the role of eNAs in causing injury and the use of nucleic acid scavengers as rescue agents.
Materials and methods
Chemicals
2-Chloroethyl ethyl sulfide (CEES, C4H9ClS) and hexadimethrine bromide (HDMBr) were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO). Antibodies for HMGB1 and anti-human fibrinogen were obtained from Abcam and DAKO, respectively.
Animals
All experiments involving animals were conducted according to protocols approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Adult male (275–300 g) Sprague–Dawley rats from Envigo Co., (Indianapolis, IN) were used. Animals were provided food and water ad libitum and maintained at 25 °C in a 12-h light/dark cycle room.
CEES exposure, animal monitoring and treatment protocol
Animals were randomly assigned to one of four groups: Ethanol (aerosolized ethanol as diluent for CEES), Ethanol + HDMBr (aerosolized ethanol + HDMBr), CEES (10% aerosolized CEES in ethanol) or CEES + HDMBr (10% aerosolized CEES in ethanol + HDMBr). The exposure system (nose-only inhalation, CH Technologies, NJ) has been described previously (Ahmad et al. 2019). Briefly, rats were anesthetized with a mixture of ketamine (75 mg/kg), xylazine (7.5 mg/kg), and acepromazine (1.5 mg/kg). They were then placed in sealed restraint tubes for containment and attached to the exposure chamber operated under negative pressure. A solution of CEES (10% in ethanol) was injected through a syringe pump and the contents aerosolized for 15 min using a bioaerosol nebulizing generator (BANG). A gravimetric sampler and a seven-stage Mercer impactor provided the necessary measurements to characterize the particle size. After 15 min of exposure, rats were returned to their cages and observed until they had fully recovered from anesthesia. Two hours later, 10 mg/kg hexadimethrine bromide (HDMBr) or saline was administered intraperitoneally. Animals were monitored continuously and mean clinical distress scores were recorded. Heart rates and percent oxygen saturations were also recorded every 2 h using a MouseOx pulseoximeter. Survival times were measured for each animal as the time from CEES exposure till the animals died or till they met the euthanasia criterion and were euthanized as described previously (Veress et al. 2013). After 12 h of CEES exposure, animals were euthanized and bronchoalveolar lavage fluid (BALF) and blood were collected from the lung and descending aorta, respectively. Samples were also collected from animals that met euthanasia criterion earlier. Lungs were perfused through the pulmonary artery and tissues were snap-frozen in liquid nitrogen. Separate animals were used for fixed tissues. Lungs were inflation fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) with a pressure resembling 20 cm of water.
Isolation of extracellular nucleic acid and in vitro studies
The total extracellular nucleic acids were isolated from 200 μl of bronchoalveolar lavage fluid (BALF) supernatant using a commercially available kit (Cat #56300, Norgen BIOTEK CORP., Ontario, CA) according to the manufacturer's instructions. Nucleic acid concentration was measured using a microvolume spectrophotometer (DeNovix Inc. Wilmington, DE, USA). The effect of CEES BALF-derived extracellular nucleic acid was tested on lung epithelial cells in the presence or absence of HDMBr. Extracellular nucleic acid was preincubated with HDMBr (10 mg/ml) or vehicle for 30 min and added to human bronchial epithelial cells (16HBE). After 6 h incubation in a cell culture incubator, cells were harvested, RNA-isolated and real-time RT-PCR was carried out using specific primers and probes.
Clinical distress scoring
Respiratory quality, stridor and activity levels were assessed for every 2 h after CEES exposure with each variable scored at 0–3, with higher numbers indicating the greatest distress. Briefly, a score of 0 is given to an animal demonstrating normal respiratory quality, no stridor and normal activity. Similarly, a score of 1 is given for ‘mild’, 2 for ‘moderate’ and 3 for ‘severe’ for each variable. The three category scores were added to obtain a cumulative score (maximum 9). Thus, an animal demonstrating mild loss of respiratory quality (score = 1), moderate stridor (score = 2) and mild loss of activity (score = 1) will have a cumulative clinical score of 4. Criteria for early euthanasia were oxygen saturation less than 70% plus respiratory distress score of 7 or greater, as described previously (Veress et al. 2010). These observations made by a minimum of two investigators at the indicated time. The criterion for early euthanasia was based on consensus from at least two investigators.
Pulse oximetry
Oxygen saturation and heart rate were monitored using the MouseOx pulseoximeter (Starr Life Science, Pittsburg, PA) in un-anesthetized rats before CEES exposure, and every 2 h following CEES exposure. Three measurements per time point were taken.
Arterial blood gas measurements
To assess pulmonary gas exchange, blood gas analyses were performed by obtaining blood from the descending aorta. Heparinized whole blood was analyzed using calibrated test cards (EPOC-BGEM) and a Heska EPOC blood gas and electrolyte analyzer (Loveland, CO). Partial pressure of arterial carbon dioxide (PaCO2), bicarbonate (HCO3−), calculated oxygen saturation (cSO2), partial pressure of arterial oxygen (PaO2) and pH was measured. Fraction of inspired oxygen (FiO2) was the room air oxygen fraction (21%), which was used to calculate the PaO2/ FiO2 ratio, an indicator of hypoxemia.
Protein and IgM measurement
BALF supernatant was collected as previously described (Rancourt et al. 2013; Veress et al. 2010). To obtain BAL fluid, the lungs were lavaged two times with 5 ml of PBS solution. Collected lavage fluid was centrifuged at 800 g for 10 min; aliquots of supernatants were frozen at − 80 °C until further measurements. Total protein concentration was measured in the BALF supernatants using the Bio-Rad DC method with bovine serum albumin (BSA) as a standard. All samples were assayed in duplicate. IgM was measured using a standard ELISA kit (Bethyl Lab Inc, Montgomery, TX).
Differential cell counts
BALF was centrifuged, and the pellet was re-suspended in 2 ml of PBS. From this, 100 μl of BALF was centrifuged in a Cytospin (Shandon Inc.). Cells on the cytospin slide were then air-dried and stained to count neutrophils and macrophages using the Hema 3 differential stain (Fisher Diagnostics, Middle town, VA).
Measurement of blood parameters
Blood samples were collected at euthanasia for analysis of complete blood count (CBC). An aliquot of whole blood was transferred to EDTA-coated vials and analyzed using hematology analyzer (HEMAVET 950 FS; Drew Scientific, Dallas, TX).
Gene expression
Total RNA was extracted using an RNeasy Mini Kit (Cat #74106, Qiagen Co, USA). RNA quality was assessed using Agilent Bioanalyser 2100 (Agilent). For quantitative RT-PCR, first-strand cDNA was reverse transcribed from 1 μg of total RNA using the iScript reverse transcription super mix (Cat #1708840, Bio-Rad laboratories Inc.). For each sample, 50 ng cDNA (total RNA equivalent) was amplified in a 25 μl reaction volume using the Bio-Rad CFX 96 real-time PCR machine (Bio-Rad laboratories Inc.) as per the instructions specified by the manufacturer. Taqman primers specific for IL-1A (Rn00566700_m1), IL-6 (Rn01410330_m1), CCL-2 (Rn00580555), CXCL-1 (Rn00578225_m1), PAI-1(Rn01481341_m1) and TF(Rn00564925_m1) were used for gene expression analysis. Results were normalized to the housekeeping genes, β-actin (Rn00667869_m1) or 18S rRNA (4310893-E) and calculated as a ratio of gene expression to the expression of the reference gene, β-actin or 18S. All primer/probe sets for cytokines/chemokines and coagulation genes were procured from Applied Biosystems (Foster City, CA).
Histology and immunohistochemistry
The lung was inflated for 30 min at a constant hydrostatic pressure of 20 cm with 4% buffered formalin and immersed in the same solution for 24 h. The fixed lung was trimmed, embedded in paraffin, and cut into 5 μm sections. The sections were stained with hematoxylin and eosin (H&E) for morphological examination.
For immunohistochemistry, lung sections were processed and stained using specific antibodies. Immunostaining for fibrin was performed using a polyclonal rabbit anti-human fibrinogen antibody (Cat# A0080; DAKO corporation, Carpinteria, CA) at a 1:1000 dilution for 60-min. Rabbit IgG control (DAKO) was used at the same specifications. The stains were developed using a peroxidase-based Envision detection system (DAKO corporation; Carpinteria, CA). The counterstaining was performed using hematoxylin.
Western blotting
Western blotting for HMGB1 and fibrin were carried out in BALF supernatant. Forty microliters of BALF supernatant was resolved in a 4–20% SDS-PAGE gradient gel, transferred to a nitrocellulose membrane and probed separately with polyclonal antibodies against HMGB1 (Cat# ab18256; Abcam, Cambridge, MA) at a dilution of 1:1000, and mouse monoclonal antibody against Fibrin(ogen) (Cat# GTX-19079, GeneTex Inc. Irvine, CA) at a dilution of 1:1000. Blots were developed using a chemiluminescent substrate and imaged in a Chemidoc Imager (BioRAD). The bands were quantitated and plotted.
Statistical analysis
Prism 8.0 software (GraphPad Prism, La Jolla, CA) was used, with one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons test, unless otherwise indicated. Data reported are mean values with standard error of the mean (SEM). A p value of < 0.05 was considered significant.
Results
Rats exposed to aerosolized 2-chloroethyl ethyl sulfide (CEES), an analog of sulfur mustard, caused severe lung injury and significant mortality (~ 50% in 12 h) and resembled a pathophysiology similar to SM exposures and clinical ARDS. Aerosolization produced a particle size of around 1 micron (Online Resource 1), suggesting potential for delivery to both upper and lower airways.
Increased extracellular nucleic acids in CEES-exposed rats are reduced by HDMBr
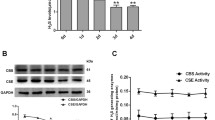
Extracellular nucleic acids can increase inflammation, disrupt the alveolar–capillary barrier and enhanced coagulation (Lee et al. 2011), which are also manifestations of SM poisoning. We assessed levels of eNAs in the BALF samples from rats exposed to CEES. CEES exposure caused an increase in eNA levels in the BALF of rats (Fig. 1a). These eNAs comprised of dsDNA, RNA and miRNA that were also increased in the BALF (Fig. 1b–d). To understand the role of increased levels of these eNAs and whether they contribute to the pathogenesis, we sought to use scavengers of NA in vivo. HDMBr, a known scavenger of NA (Lee et al. 2011), when injected 2 h post-CEES exposure decreased levels of eNAs at 12 h post-exposure (Fig. 1a). HDMBr also reduced levels of dsDNA, RNA and miRNA in BALF (Fig. 1b–d).
Extracellular nucleic acid is increased in the bronchoalveolar lavage fluid of rats exposed to CEES and nucleic acid neutralizing agent reduces these levels (a) Sprague Dawley rats were exposed to aerosolized CEES. Two hours later, hexadimethrine bromide (HDMBr), a nucleic acid neutralizing agent was administered intraperitoneally at a dose of 10 mg/kg body weight. Twelve hours post-CEES exposure, animals were euthanized, bronchoalveolar lavage fluid (BALF) supernatant was collected and total extracellular nucleic acids were isolated and quantified. Nucleic acid subtypes were further analyzed and quantitated using the Quant-iT kit (Invitrogen) for b double stranded DNA (dsDNA), c RNA (> 1000 bases) and d miRNA using standard curves for each. In in vitro studies total nucleic acid isolated from the BALF supernatant of CEES-exposed animals were pooled and added exogenously to cultured human airway epithelial cells. After 6 h total RNA was isolated from cells and analyzed for mRNA levels of pro-inflammatory cytokines by Real-time RT-PCR. e IL-6 mRNA levels and f CXCL-1 mRNA levels were measured following exposure of cells to varying concentrations of eNA. g Extracellular nucleic acid (2.5 mg/ml) in presence or absence of 10 mg/ml HDMBr was added to cells and CXCL-1 mRNA levels were determined. Values with their mean ± SEM are shown. h Rhodamine labeled poly (I:C) was added to cells in presence or absence of HDMBr. Arrows in images show aggregation of eNA in presence of HDMBr. Values represent mean ± SEM
Nucleic acid scavenger reduces eNA-induced inflammatory cytokines in lung epithelial cells
Nucleic acids can interact with cell surface receptors and activate inflammatory pathways. To explore the role of eNA in causing airway inflammation, we isolated total eNA from BALF of CEES-exposed rats and added it exogenously to airway epithelial cell cultures. Extracellular NA dose dependently increased mRNA levels of IL-6 and CXCL-1 (Fig. 1e, f). Interestingly, CCL-2 was not increased in these cells following eNA addition (data not shown). Pre-incubation with hexadimethrine bromide (HDMBr) to scavenge nucleic acids resulted in diminished eNA-induced CXCL-1 (Fig. 1g). To investigate mechanisms by which HDMBr influences nucleic acid interaction with cells, we used rhodamine labeled poly(I:C). HDMBr bound extracellular poly(I:C) and formed aggregates. Due to the polymeric nature of HDMBr, binding to poly(I:C) caused aggregation, which made visualization possible and demonstrated specificity (Fig. 1h). Labeled poly(I:C) without the drug was barely detectable. This suggested that HDMBr binding to eNA reduced its access at the cell surface.
HDMBr treatment reduces clinical distress and improves oxygen saturations in CEES-exposed rats
CEES exposure affects physical activity, respiratory quality and stridor, which can be cumulatively expressed numerically as clinical distress scores (Veress et al. 2013). Compared to the control groups, which showed no clinical distress, the CEES-exposed animals showed a progressive increase in the clinical distress scores, which was partly reduced with HDMBr treatment (Fig. 2a). Non-invasive pulse oximetry can be employed to effectively assess oxygenation criterion in ARDS longitudinally (Rice et al. 2007). Compared to the control groups, exposure to aerosolized CEES caused progressive decrease in SpO2 (Fig. 2b). SpO2 significantly improved after HDMBr administration 2 h after CEES exposure.
Nucleic acid scavenger mitigates clinical distress and oxygen saturations in rats exposed to aerosolized CEES. Sprague Dawley rats were exposed to aerosolized CEES (10%) in ethanol for 15 min using a nose-only inhalation system. Two hours later, the nucleic acid scavenger, HDMBr, was administered intraperitoneally at a dose of 10 mg/kg body weight. Rats were continuously monitored for 12 h (Study endpoint) and a clinical distress scores were recorded and b noninvasively acquired oxygen saturations (SpO2) were measured using a MouseOx pulseoximeter. Values represent mean ± SEM with a minimum of n > 11 per group. In the CEES group, n = 14 at 0 h and n = 6 at 12 h due to mortality. A * indicates p< 0.05, CEES vs CEES + HDMBr
HDMBr increases arterial blood oxygenation in rats exposed to CEES
Arterial blood gas (ABG) measurements were carried out to evaluate extent of lung injury, impairment in gas exchange and subsequent hypoxemia. Arterial blood pH was significantly decreased in CEES-exposed rats compared to ethanol(vehicle)-exposed rats (Fig. 3a). HDMBr treatment reversed the drop in pH (Fig. 3a). Similarly, partial pressure of arterial CO2 (PaCO2) increased following CEES exposure and was significantly reduced by HDMBr treatment (Fig. 3b). Bicarbonate levels though not significant indicated partial compensation (Fig. 3c). Moreover, while CEES exposure decreased cSO2 and PaO2, HDMBr treatment improved cSO2 and arterial PaO2 levels (Fig. 3d, e). The PaO2/FiO2 ratio decreased to about 150 in the CEES-exposed group, which is consistent with ARDS (Thompson et al. 2017). Treatment with HDMBr significantly improved this ratio to above 300 (Fig. 3f).
Effect of HDMBr on arterial blood gas (ABGs) measurements in rats exposed to aerosolized CEES. Rats were exposed to CEES (10%) in ethanol for 15 min using a nose-only inhalation system. Two hours later HDMBr was administered intraperitonially at a dose of 10 mg/kg body weight. At the end of study (12 h for CEES), blood was collected from the descending aorta and analyzed for ABGs using the EPOC-Vet Blood Analysis System. Data show a arterial pH, b partial pressure of arterial carbon dioxide (PaCO2), c bicarbonate (HCO3−), d calculated oxygen saturation (cSO2), e partial pressure of arterial oxygen (PaO2), and f PaO2/FiO2 ratio. Individual values with their mean ± SEM are shown
HDMBr treatment decreases protein leakage in the airway of CEES-exposed rats
Increased permeability of the alveolar–capillary barrier is characteristic of acute inflammation and is a key pathophysiological feature of SM poisoning and ARDS (Matthay et al. 2012; Weinberger et al. 2011). This leads to accumulation of protein-rich plasma exudates in the airspaces. A single dose of HDMBr reduced CEES-induced increase in BALF total protein and IgM (Fig. 4a, b). Thus, the nucleic acid scavenging agent HDMBr mitigated disruption of alveolar–capillary membrane.
CEES-induced disruption of alveolar–capillary barrier is mitigated by treatment with HDMBr. Rats were exposed to aerosolized CEES. To assess the alveolar–capillary barrier, BALF supernatants were analyzed for total protein concentration (a) and IgM using ELISA (b). Individual values with their mean ± SEM are shown
HDMBr alters cell counts and reduces inflammation in rats exposed to CEES
CEES exposure in rats caused increases in BALF neutrophils. Treatment with HDMBr did not reduce neutrophil counts from the CEES-only exposed group (Fig. 5a). The macrophage numbers decreased in the CEES-exposed animals but did not change upon HDMBr treatment (Fig. 5b). Serial measurements of complete blood counts (CBC) are useful in assessing general health including inflammation. In the blood, exposures to CEES did not alter the number of neutrophils. However, treatment with HDMBr increased neutrophils (Fig. 5c). The WBC counts, on the other hand, decreased in the CEES-exposed group. The WBC numbers tended to increase in the CEES + HDMBr group (Fig. 5d).
Effect of HDMBr treatment on cell counts in bronchoalveolar lavage fluid and blood following exposure to CEES. Rats were exposed to CEES (10%). To assess inflammation, total neutrophil (a) and macrophage (b) counts in BALF were made in all treatment groups by microscopic analysis of at least 200 nucleated cells in each group. At the study end, blood was collected from the descending aorta and analyzed for complete blood cell count using a Hemavet differential cell count analyzer. c Neutrophil number and d white blood cell (WBC) counts were determined. Individual values with their mean ± SEM are shown
Inflammation is an integral part of CEES/SM-induced injury and inflammatory cytokines are significantly increased in humans exposed to SM (Ahmad and Ahmad 2016; Attaran et al. 2010; White et al. 2016). There was a significant increase in mRNA levels of pro-inflammatory cytokines IL-6, IL-1A, CXCL-1 and CCL-2 12 h post-CEES exposure (Fig. 6a–d). HDMBr treatment significantly reversed these levels. Thus, CEES-mediated inflammation was significantly attenuated by HDMBr. HMGB1, a common marker of inflammatory response in ARDS (Yang et al. 2002), was also significantly attenuated by HDMBr treatment in the BALF of CEES-exposed rats (Fig. 6e).
HDMBr reduces lung inflammation in CEES-exposed animals. Rats were exposed to CEES (10%) and lung tissues were harvested at 12 h. Real-time RT-PCR was carried out to determine steady-state mRNA levels of inflammation related genes in the lung tissue using Taqman primers and probes. Relative changes in mRNA levels for IL-6 (a), IL1A (b), CXCL-1 (c) and CCL-2 (d), are shown after normalization with β-actin. Individual values with their mean ± SEM are shown. e HMGB1, a pro-inflammatory molecule, was quantified in BALF by western blot analysis. Representative immunoblot showing changes in HMGB1 protein levels. Densitometry analysis on blots from two independent experiments are presented as mean ± SEM
HDMBr reduces pulmonary edema and airway obstruction in experimental ARDS
In H&E-stained sections of lungs from rats exposed to CEES, there was evidence of significant peribronchial and perivascular edema (Fig. 7a). The airways showed epithelial sloughing and obstruction (Fig. 7a). Airway obstruction due to formation of fibrin-rich casts is frequently observed in CEES/SM-exposed animals (Rancourt et al. 2014). We, therefore, assessed airway casts using fibrin staining of serial lung sections (Fig. 7a). Besides intense fibrin staining of airway casts, there was also significant peribronchial and perivascular staining for fibrin. More than 50% of the airways stained positive for fibrin casts indicative of obstruction (Fig. 7b). In the HDMBr-treated group, the extent of cast formation was reduced (Fig. 7a, b). However, there was still some residual perivascular and peribronchial edematous fluid that also contained fibrin. To further quantify levels of fibrin in the airways, we performed western blots in the BALF supernatant. As shown in Fig. 7c, there was marked decrease in fibrin in the BALF supernatant of HDMBr-treated group when compared to the CEES-exposed group.
HDMBr reduces airway obstruction and mortality in rats exposed to aerosolized CEES. CEES-exposed rats were treated with HDMBr 2 h post-exposure. 12 h post-exposure rats were euthanized, and lungs inflation fixed with 4% paraformaldehyde in PBS. Airway obstruction was assessed by staining sections of right middle and right lower lobes with antibodies against fibrin. a Serial sections from each group were stained with either hematoxylin and eosin dyes or immunohistochemically for fibrin (brown). Yellow arrows indicate fibrin-rich casts in the airway. Black arrowheads indicate peribronchial edema whereas the green arrowheads indicate perivascular edema. Epithelial sloughing is indicated by green arrows. b Airways were scored for fibrin casts by counting for the presence or absence of positive staining at 5 × magnification. A minimum of five fields per section were counted. Percent of airways positive for fibrin casts were plotted. Values represent mean ± SEM. A * indicates p < 0.05 from the untreated CEES-exposed animals. c Representative western blot and densitometric quantification (n = 5–6 per group) of BALF fibrin from CEES-exposed rats. Real-time RT-PCR was carried out to determine steady-state mRNA levels of coagulations genes in lung tissues using Taqman primers and probes. Changes in mRNA levels for tissue factor (d) and PAI-1 (e) are shown. Values represent mean ± SEM. f Animals were continuously monitored over a 12 h period and survival assessed using a Kaplan–Meier curve and groups compared using the Mantel–Cox model. (*p < 0.0001)
HDMBr decreases pro-coagulation genes in CEES-exposed rats
Like in SM exposures, CEES-exposed animals showed a hypercoagulable phenotype. An essential component in the initiation of coagulation is tissue factor (TF), expression of which can increase in the lung under certain pro-coagulant states (Idell 2003; Mackman et al. 1993). As shown in Fig. 7d, CEES-exposed rats had increased levels of TF mRNA. Following treatment with HDMBr, these levels were significantly reduced. We also showed an increase in the fibrinolytic inhibitors, plasminogen activator inhibitor (PAI-1) after CEES exposure (Rancourt et al. 2014). PAI-1 gene expression was significantly increased in lung tissues 12 h following CEES inhalation in this study (Fig. 7e). These increases were significantly attenuated by HDMBr.
HDMBr decreases mortality in CEES-exposed rats
Exposure to SM or CEES is known to be fatal where death is mainly due to respiratory distress. CEES exposure caused more than 50% mortality in rats. Treatment with HDMBr rescued animals from mortality (Fig. 7f).
Discussion
Extracellular NA can activate cell surface receptors and increase pro-inflammatory cytokines. Our demonstration that eNAs from the BALF of CEES-exposed animals, when added to airway epithelial cells directly increased levels of inflammatory cytokines in a dose-dependent manner clearly underscores their significance in inflammation. Prevention of eNA-induced cytokine production by HDMBr further confirmed a role for eNA in lung injury and inflammation. Perhaps, HDMBr prevents eNA interaction with cells by aggregating them and hindering their signaling ability (Fig. 8).
Inhalation of aerosolized CEES causes acute respiratory failure that captures multiple pathological features of acute SM poisoning observed in humans. There are no approved pharmacologic therapies against such injuries (Ahmad and Ahmad 2016). Hypercoagulation, an exacerbated inflammatory response, hypoxemia and disruption of the alveolar–capillary barrier are some of the cardinal features of SM poisoning in humans that are also manifested in CEES-induced lung injury. Recent studies in other disease models have indicated that host-derived eNAs can activate the coagulation cascade and cause the development of an inflammatory response (Ahmad and Ahmad 2016; Idell 2003; Jain et al. 2012; Komissarov et al. 2011; Preissner 2007). Our study demonstrated that inhaled CEES causes increase in eNAs in BALF. This is consistent with previous reports in other non-pulmonary disease models (Barrat et al. 2005; Garcia-Olmo et al. 2004; Hajizadeh et al. 2003; Sozzi et al. 2003; Stieger et al. 2017). These increases reflected steady-state levels at the 12 h time point. Given the fact that extracellular/circulating NAs are constantly being degraded by nucleases or cleared by the liver (Gauthier et al. 1996), the high BALF eNA levels suggest either an increased production and/or release as a result of insult, release due to cell death, a decrease in nuclease activity, a decrease in clearance or a combination of all. Previous studies have shown that NA scavengers can mitigate injury at least in sub-acute and non-lethal conditions. In acute settings, sensitized mice when challenged with synthetic nucleic acid poly(I:C) had significant liver injury and mortality. The effects were, however, mitigated by NA scavengers only when co-administered with the toxin (Lee et al. 2011). Our study demonstrating that post-treatment with the NA scavenger HDMBr, reduced BALF eNA levels to baseline and rescued from acute mortality, indicates a more vital role of eNAs in the pathogenesis of CEES/SM-induced respiratory failure.
CEES-exposed animals exhibit progressive decrease in SpO2 and stridor (Veress et al. 2013). A decrease in SpO2/FiO2 ratio, an alternative indicator for diagnosing ALI/ARDS, was also observed (Chen et al. 2015; Rice et al. 2007). Respiratory acidosis was also evident. While there was a consistent increase in PaCO2 along with decreases in blood pH and PaO2, levels of HCO3− were variable indicating partial compensation. Impaired gas exchange due to compromised alveolar–capillary barrier along with airway obstruction can contribute towards decreased oxygenation (Rice et al. 2007). A PaO2/FiO2 (P/F) ratio of < 200 indicates hypoxemia, which along with a mortality of 50–60% within 12 h, is consistent with moderate-to-severe ARDS (Thompson et al. 2017). The fact that HDMBr improves SpO2 and P/F ratio and reverses respiratory acidosis suggests that there is improved gas exchange possibly due to decrease in pulmonary edema and fibrin formation in the air spaces. Accumulation of fibrin-rich exudates can occlude the airways by forming casts. Furthermore, pulmonary edema observed in our studies is consistent with previous reports that also showed CEES-induced edema (Das et al. 2003; McClintock et al. 2002). Therefore, these studies reinforce the role of eNAs in causing ARDS-like pathology and that they are important targets for injury mitigation.
Cells are primed to tolerate low levels of circulating NAs. In conditions where prolonged increases in eNAs exist, activation of endosomal toll-like receptors (TLRs) and a subsequent surge in the pro-inflammatory cytokine signaling occurs. Although inhibition of specific NA-sensing receptors mitigates injury in some disease models (Gao et al. 2017; Gay et al. 2014; Lawton and Ghosh 2003), heterogeneity in the released NAs and activation of multiple receptors may limit efficacy of this approach, at least in the context of ALI/ARDS. NAs can promote inflammation in a number of ways either by itself or in association with other inflammatory mediators such as HMGB1 (Yanai et al. 2009; Yanai and Taniguchi 2014). HMGB1, released from cells, is a known mediator of lung inflammation and injury (Abraham et al. 2000; Ogawa et al. 2006). It is conceivable that increased HMGB1 in the BALF of CEES-exposed rats could bind to eNAs and exacerbate lung inflammation through TLRs. Binding of HMGB1 to eNAs has been documented in other instances (Yanai et al. 2009). Therefore, scavenging of eNAs with HDMBr, likely reduced HMGB1 levels and mitigated inflammation. This is also evident from decreases in IL-6, CXCL-1 and CCL-2 gene expression in the lung of HDMBr-treated CEES-exposed rats.
Previous studies have also reported that HMGB1 impairs alveolar–capillary barrier (Wolfson et al. 2011). Disruption of alveolar–capillary barrier has also been demonstrated with eNAs (Fischer et al. 2007). It is possible that both HMGB1 and eNA contribute independently towards barrier disruption and, therefore, scavenging NAs by HDMBr did not completely reverse leaks.
CEES-induced lung injury is characterized by profound histopathological alterations in the lung including the recruitment of inflammatory cell. In agreement with other studies (Gao et al. 2011; Veress et al. 2010), our results also show depletion of macrophages in the BALF of CEES-exposed animals. Decrease in WBCs in the blood of CEES-exposed rats is consistent with SM-mediated immune suppression and exacerbation of injury and death (Hassan et al. 2006; McElroy et al. 2016; Vijayaraghavan et al. 2005). A decrease in blood WBC count was reported in an accidental inhalation exposure of nitrogen mustard in human (Wang and Xia 2007). CEES exposure also caused increased neutrophils in the BALF. Interestingly, HDMBr treatment caused increased neutrophil count both in the BALF as well as in the blood that did not correlate with lung levels of chemoattractants CXCL-1 and CCL-2, both of which were reduced in the HDMBr-treated group. Increased neutrophil may influence resolution of inflammation and injury (Campbell et al. 2014; Neudecker et al. 2017; Zemans et al. 2011).
Activation of the coagulation cascade, extravascular fibrin deposition and inhibition of fibrinolysis are observed following SM exposures and in ARDS (Gunther et al. 2000; Idell 2003; White et al. 2016). Autopsy of a patient exposed to SM in the Iran-Iraq conflict showed fibrin-rich pseudomembranes and casts in the airways (Eisenmenger et al. 1991). Exposure to CEES caused both an activation and increase in pro-coagulant factors along with a decrease in fibrinolysis. eNAs can influence the coagulation cascade by activating Factor VII leading to recruitment of TF activation of the extrinsic coagulation pathway (Nakazawa et al. 2005). Increased TF in the lung of CEES-exposed animals is also consistent with a pro-coagulant response (Idell 2003). This is supported by increased fibrin deposition in the airways and in the BALF of the CEES-exposed animals. NA can also bind to PAI-1 and stabilize it (Wygrecka et al. 2007). NA neutralizers like HDMBr can, therefore, be effective in both impeding the activation of the coagulation cascade and also in promoting fibrinolysis. Fibrin staining in the peribronchial area of the HDMBr-treated group, possibly indicates repair onset (Erjefalt et al. 1994). Overall, HDMBr decreased fibrin content and also decreased TF levels in the lungs of CEES-exposed animals further confirming the role of NA scavenger in reducing coagulation. These studies demonstrate a vital role for eNAs in the pathogenesis of CEES-induced lung injuries. While these studies underscore the significance of an activated coagulation cascade in CEES/SM-induced poisoning, previous attempts of anti-coagulation therapies have shown limited success possible due to increased risk of bleeding (Houin et al. 2015). The current investigation highlights the significance of eNA in reducing coagulation and support the premise that blocking of eNA signaling can mitigate injury in conditions such as CEES/SM exposures and ARDS where coagulation is not the only factor causing morbidity and mortality.
Nucleic acids are known to activate TLRs and increase pro-inflammatory cytokines. Our demonstration that eNAs from the BALF of CEES-exposed animals, when added to airway epithelial cells directly increase levels of cytokines further underscores their significance in CEES-induced inflammation. Furthermore, the fact that HDMBr prevented this increase in an in vitro system demonstrated a plausible role for eNA in CEES-induced lung injury and inflammation. It is possible that different cell types in the lung upon activation by NAs can increase different cytokines. It is, therefore, not surprising that CCL-2 levels were not altered in these cells with eNAs.
In summary, our findings demonstrate that CEES-induced lung injury closely resembles the pathophysiology of injury induced by SM and ARDS. The efficacy of NA neutralization in a severe model of injury underscores the significance of eNAs in the pathogenesis of SM/CEES-induced poisoning. Thus, NA neutralization reflects an effective strategy in treating chemical exposures like sulfur mustard as well as by other risk factors, which are associated with increased eNA and increased mortality.
References
Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ (2000) HMG-1 as a mediator of acute lung inflammation. J Immunol 165(6):2950–2954
Ahmad S, Ahmad A (2016) Emerging targets for treating sulfur mustard-induced injuries. Ann N Y Acad Sci 1374(1):123–131. https://doi.org/10.1111/nyas.13095
Ahmad S, Zafar I, Mariappan N et al (2019) Acute pulmonary effects of aerosolized nicotine. Am J Physiol Lung Cell Mol Physiol 316(1):L94–L104. https://doi.org/10.1152/ajplung.00564.2017
Attaran D, Lari SM, Towhidi M et al (2010) Interleukin-6 and airflow limitation in chemical warfare patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 5:335–340. https://doi.org/10.2147/COPD.S12545
Balali-Mood M, Hefazi M (2006) Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin Pharmacol Toxicol 99(4):273–282
Barrat FJ, Meeker T, Gregorio J et al (2005) Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med 202(8):1131–1139. https://doi.org/10.1084/jem.20050914
Calvet JH, Jarreau PH, Levame M et al (1994) Acute and chronic respiratory effects of sulfur mustard intoxication in guinea pig. J Appl Physiol 76(2):681–688. https://doi.org/10.1152/jappl.1994.76.2.681
Campbell EL, Bruyninckx WJ, Kelly CJ et al (2014) Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40(1):66–77. https://doi.org/10.1016/j.immuni.2013.11.020
Chen W, Janz DR, Shaver CM, Bernard GR, Bastarache JA, Ware LB (2015) Clinical characteristics and outcomes are similar in ARDS diagnosed by oxygen saturation/Fio2 ratio compared With Pao2/Fio2 Ratio. Chest 148(6):1477–1483. https://doi.org/10.1378/chest.15-0169
Chevillard M, Lainee P, Robineau P, Puchelle E (1992) Toxic effects of sulfur mustard on respiratory epithelial cells in culture. Cell Biol Toxicol 8(2):171–181
Das SK, Mukherjee S, Smith MG, Chatterjee D (2003) Prophylactic protection by N-acetylcysteine against the pulmonary injury induced by 2-chloroethyl ethyl sulfide, a mustard analogue. J Biochem Mol Toxicol 17(3):177–184. https://doi.org/10.1002/jbt.10076
Eisenmenger W, Drasch G, von Clarmann M, Kretschmer E, Roider G (1991) Clinical and morphological findings on mustard gas [bis(2-chloroethyl)sulfide] poisoning. J Forensic Sci 36(6):1688–1698
Erjefalt JS, Erjefalt I, Sundler F, Persson CG (1994) Microcirculation-derived factors in airway epithelial repair in vivo. Microvasc Res 48(2):161–178. https://doi.org/10.1006/mvre.1994.1047
Fischer S, Gerriets T, Wessels C et al (2007) Extracellular RNA mediates endothelial-cell permeability via vascular endothelial growth factor. Blood 110(7):2457–2465. https://doi.org/10.1182/blood-2006-08-040691
Gao X, Anderson DR, Brown AW et al (2011) Pathological studies on the protective effect of a macrolide antibiotic, roxithromycin, against sulfur mustard inhalation toxicity in a rat model. Toxicol Pathol 39(7):1056–1064. https://doi.org/10.1177/0192623311422079
Gao W, Xiong Y, Li Q, Yang H (2017) Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front Physiol 8:508. https://doi.org/10.3389/fphys.2017.00508
Garcia-Olmo DC, Ruiz-Piqueras R, Garcia-Olmo D (2004) Circulating nucleic acids in plasma and serum (CNAPS) and its relation to stem cells and cancer metastasis: state of the issue. Histol Histopathol 19(2):575–583. https://doi.org/10.14670/HH-19.575
Gauthier VJ, Tyler LN, Mannik M (1996) Blood clearance kinetics and liver uptake of mononucleosomes in mice. J Immunol 156(3):1151–1156
Gay NJ, Symmons MF, Gangloff M, Bryant CE (2014) Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol 14(8):546–558. https://doi.org/10.1038/nri3713
Gunther A, Mosavi P, Heinemann S et al (2000) Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Criti Care Med 161(2 Pt 1):454–462
Hajizadeh S, DeGroot J, TeKoppele JM, Tarkowski A, Collins LV (2003) Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res Ther 5(5):R234–R240. https://doi.org/10.1186/ar787
Hassan ZM, Ebtekar M, Ghanei M, Taghikhani M, Noori Daloii MR, Ghazanfari T (2006) Immunobiological consequences of sulfur mustard contamination. Iran J Allergy Asthma Immunol 5(3):101–108
Henretig FM, Kirk MA, McKay CA Jr (2019) Hazardous Chemical Emergencies and Poisonings. N Engl J Med 380(17):1638–1655. https://doi.org/10.1056/NEJMra1504690
Houin PR, Veress LA, Rancourt RC et al (2015) Intratracheal heparin improves plastic bronchitis due to sulfur mustard analog. Pediatr Pulmonol 50(2):118–126. https://doi.org/10.1002/ppul.23043
Idell S (2003) Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med 31(4 Suppl):S213–S220
Jain S, Pitoc GA, Holl EK et al (2012) Nucleic acid scavengers inhibit thrombosis without increasing bleeding. Proc Natl Acad Sci USA 109(32):12938–12943. https://doi.org/10.1073/pnas.1204928109
Jett DA, Laney JW (2019) Civilian research on chemical medical countermeasures. Toxicol Mech Methods. https://doi.org/10.1080/15376516.2019.1669250
Kehe K, Szinicz L (2005) Medical aspects of sulphur mustard poisoning. Toxicology 214(3):198–209
Kehe K, Thiermann H, Balszuweit F, Eyer F, Steinritz D, Zilker T (2009) Acute effects of sulfur mustard injury–Munich experiences. Toxicology 263(1):3–8
Komissarov AA, Florova G, Idell S (2011) Effects of extracellular DNA on plasminogen activation and fibrinolysis. J Biol Chem 286(49):41949–41962. https://doi.org/10.1074/jbc.M111.301218
Lawton JA, Ghosh P (2003) Novel therapeutic strategies based on toll-like receptor signaling. Curr Opin Chem Biol 7(4):446–451
Lee J, Sohn JW, Zhang Y, Leong KW, Pisetsky D, Sullenger BA (2011) Nucleic acid-binding polymers as anti-inflammatory agents. Proc Natl Acad Sci USA 108(34):14055–14060. https://doi.org/10.1073/pnas.1105777108
Mackman N, Sawdey MS, Keeton MR, Loskutoff DJ (1993) Murine tissue factor gene expression in vivo. Tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol 143(1):76–84
Matthay MA, Ware LB, Zimmerman GA (2012) The acute respiratory distress syndrome. J Clin Invest 122(8):2731–2740. https://doi.org/10.1172/JCI60331
McClintock SD, Till GO, Smith MG, Ward PA (2002) Protection from half-mustard-gas-induced acute lung injury in the rat. J Appl Toxicol 22(4):257–262. https://doi.org/10.1002/jat.856
McClintock SD, Hoesel LM, Das SK et al (2006) Attenuation of half sulfur mustard gas-induced acute lung injury in rats. J Appl Toxicol 26(2):126–131. https://doi.org/10.1002/jat.1115
McElroy CS, Min E, Huang J et al (2016) From the cover: catalytic antioxidant rescue of inhaled sulfur mustard toxicity. Toxicol Sci 154(2):341–353. https://doi.org/10.1093/toxsci/kfw170
Nakazawa F, Kannemeier C, Shibamiya A et al (2005) Extracellular RNA is a natural cofactor for the (auto-)activation of Factor VII-activating protease (FSAP). Biochem J 385(Pt 3):831–838. https://doi.org/10.1042/BJ20041021
Neudecker V, Brodsky KS, Clambey ET et al (2017) Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aah5360
O'Driscoll L (2007) Extracellular nucleic acids and their potential as diagnostic, prognostic and predictive biomarkers. Anticancer Res 27(3A):1257–1265
Ogawa EN, Ishizaka A, Tasaka S et al (2006) Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med 174(4):400–407. https://doi.org/10.1164/rccm.200605-699OC
Pant SC, Vijayaraghavan R (1999) Histomorphological and histochemical alterations following short-term inhalation exposure to sulfur mustard on visceral organs of mice. Biomed Environ Sci 12(3):201–213
Pisetsky DS, Lee J, Leong KW, Sullenger BA (2012) Nucleic acid-binding polymers as anti-inflammatory agents: reducing the danger of nuclear attack. Expert Rev Clin Immunol 8(1):1–3. https://doi.org/10.1586/eci.11.82
Preissner KT (2007) Extracellular RNA. A new player in blood coagulation and vascular permeability. Hamostaseologie 27(5):373–377
Preissner KT, Herwald H (2017) Extracellular nucleic acids in immunity and cardiovascular responses: between alert and disease. Thromb Haemost 117(7):1272–1282. https://doi.org/10.1160/TH-16-11-0858
Rancourt RC, Veress LA, Ahmad A et al (2013) Tissue factor pathway inhibitor prevents airway obstruction, respiratory failure and death due to sulfur mustard analog inhalation. Toxicol Appl Pharmacol 272(1):86–95. https://doi.org/10.1016/j.taap.2013.05.020
Rancourt RC, Ahmad A, Veress LA, Rioux JS, Garlick RB, White CW (2014) Antifibrinolytic mechanisms in acute airway injury after sulfur mustard analog inhalation. Am J Respir Cell Mol Biol 51(4):559–567. https://doi.org/10.1165/rcmb.2014-0012OC
Rice TW, Wheeler AP, Bernard GR et al (2007) Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 132(2):410–417. https://doi.org/10.1378/chest.07-0617
Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS (2006) Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia–a review. Crit Care Med 34(3):871–877
Sinclair DC (1948) The clinical features of mustard-gas poisoning in man. Br Med J 2(4570):290–294
Sozzi G, Conte D, Leon M et al (2003) Quantification of free circulating DNA as a diagnostic marker in lung cancer. J Clin Oncol 21(21):3902–3908. https://doi.org/10.1200/JCO.2003.02.006
Standiford TJ, Ward PA (2016) Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res 167(1):183–191. https://doi.org/10.1016/j.trsl.2015.04.015
Stieger P, Daniel JM, Tholen C et al (2017) Targeting of extracellular RNA reduces edema formation and infarct size and improves survival after myocardial infarction in mice. J Am Heart Assoc 6(6):e004541. https://doi.org/10.1161/JAHA.116.004541
Thompson BT, Chambers RC, Liu KD (2017) Acute Respiratory Distress Syndrome. N Engl J Med 377(6):562–572. https://doi.org/10.1056/NEJMra1608077
Veress LA, O'Neill HC, Hendry-Hofer TB, Loader JE, Rancourt RC, White CW (2010) Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am J Respir Crit Care Med 182(11):1352–1361. https://doi.org/10.1164/rccm.200910-1618OC
Veress LA, Hendry-Hofer TB, Loader JE, Rioux JS, Garlick RB, White CW (2013) Tissue plasminogen activator prevents mortality from sulfur mustard analog-induced airway obstruction. Am J Respir Cell Mol Biol 48(4):439–447. https://doi.org/10.1165/rcmb.2012-0177OC
Vijayaraghavan R, Kulkarni A, Pant SC et al (2005) Differential toxicity of sulfur mustard administered through percutaneous, subcutaneous, and oral routes. Toxicol Appl Pharmacol 202(2):180–188. https://doi.org/10.1016/j.taap.2004.06.020
Wang GQ, Xia ZF (2007) Tissue injury by hot fluid containing nitrogen mustard. Burns 33(7):923–926. https://doi.org/10.1016/j.burns.2006.11.004
Weinberger B, Laskin JD, Sunil VR, Sinko PJ, Heck DE, Laskin DL (2011) Sulfur mustard-induced pulmonary injury: therapeutic approaches to mitigating toxicity. Pulm Pharmacol Ther 24(1):92–99. https://doi.org/10.1016/j.pupt.2010.09.004
White CW, Rancourt RC, Veress LA (2016) Sulfur mustard inhalation: mechanisms of injury, alteration of coagulation, and fibrinolytic therapy. Ann N Y Acad Sci 1378(1):87–95. https://doi.org/10.1111/nyas.13130
Wolfson RK, Chiang ET, Garcia JG (2011) HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res 81(2):189–197. https://doi.org/10.1016/j.mvr.2010.11.010
Wygrecka M, Morty RE, Markart P et al (2007) Plasminogen activator inhibitor-1 is an inhibitor of factor VII-activating protease in patients with acute respiratory distress syndrome. J Biol Chem 282(30):21671–21682. https://doi.org/10.1074/jbc.M610748200
Yanai H, Taniguchi T (2014) Nucleic acid sensing and beyond: virtues and vices of high-mobility group box 1. J Intern Med 276(5):444–453. https://doi.org/10.1111/joim.12285
Yanai H, Ban T, Wang Z et al (2009) HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462(7269):99–103. https://doi.org/10.1038/nature08512
Yang H, Wang H, Czura CJ, Tracey KJ (2002) HMGB1 as a cytokine and therapeutic target. J Endotoxin Res 8(6):469–472. https://doi.org/10.1179/096805102125001091
Zemans RL, Briones N, Campbell M et al (2011) Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci USA 108(38):15990–15995. https://doi.org/10.1073/pnas.1110144108
Acknowledgements
This work is supported by National Institutes of Health Office of the Director (NIH OD), the National Institute of Environmental Health Sciences (NIEHS) grant numbers U01ES025069 (AA) and U01ES028182 (SA) and the National Heart Lung and Blood Institute (NHLBI) grant numbers R01HL114933 (AA). VS and KGS are supported by the CAAP program of the Department of Anesthesiology and Perioperative Medicine of the University of Alabama at Birmingham. The authors would also like to thank Stacy Miller, Tara Hendry-Hofer, Jacqueline Rioux, Rhonda Garlick and Joan Loader from the University of Colorado Denver for help in setup of initial studies. The authors are also grateful to Dr. Carl White and Dr. Raymond Rancourt for their valuable advice.
Author information
Authors and Affiliations
Contributions
AA conceived the idea and designed experiments. NM, MH, IZ, VS, SA and AA carried out the experiments, acquired and analyzed data and wrote the manuscript. KS and JFP analyzed data and wrote the manuscript. DRC acquired and analyzed data.
Corresponding author
Ethics declarations
Conflict of interest
A patent application for the use of nucleic acid scavengers in chemical-induced ARDS has been filed (AA). All other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mariappan, N., Husain, M., Zafar, I. et al. Extracellular nucleic acid scavenging rescues rats from sulfur mustard analog-induced lung injury and mortality. Arch Toxicol 94, 1321–1334 (2020). https://doi.org/10.1007/s00204-020-02699-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02699-1