Abstract
Hydrogen sulfide (H2S) is a toxic gaseous substance, and accidental exposure to high concentrations of H2S has been reported to be lethal to humans. Inhaled and absorbed H2S is partially dissolved within the circulation and causes toxic effects on lymphocytes. However, the mechanisms involved in H2S toxicity have not been well documented. In this study, we examined the cellular uptake and injury of sulfide-exposed human T lymphocytes (Jurkat). Cells were exposed to a H2S donor, sodium hydroxysulfide (NaHS), at pH 6.0, 7.0, or 8.0 for 1 h at 37 °C in a sealed conical tube to avoid the loss of dissolved H2S gas. Cytotoxicity and cellular sulfide concentrations increased dramatically as the pH of the NaHS solution decreased. Sulfide enhanced the cleavage of caspase-3 and poly (ADP-ribose) polymerase and induced early cellular apoptosis. A pan-caspase inhibitor reduced sulfide-induced apoptosis. These results indicate that sulfide induces pH-dependent and caspase-dependent apoptosis. We also found that blebbing of the plasma membrane occurred in sulfide-exposed cells. Both ROCK-1 and ROCK-2 (Rho kinases) were activated by sulfide, and sulfide-induced cell blebbing was suppressed by a ROCK inhibitor, suggesting that a Rho pathway is involved in sulfide-induced blebbing in lymphocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S) is a toxic gas with a characteristic rotten egg odor. Exposure to H2S is widespread owing to the numerous industrial processes and natural sources that emit the gas (Dorman et al. 2002). The immediately dangerous to life or health (IDLH) for H2S was determined to be 100 ppm based on the maximum concentration that can be endured for 1 h without serious consequences (Centers for Disease Control and Prevention, NIOSH). Accidental acute exposure to high concentrations of H2S (≥500 ppm) causes respiratory paralysis, unconsciousness, and eventually death within minutes (Beauchamp et al. 1984). H2S is absorbed in the upper respiratory tract mucosa (Hildebrandt and Grieshaber 2008) and is rapidly metabolized from sub-lethal concentrations of sulfide into thiosulfate by both hemoglobin and hepatic enzymes (Curtis et al. 1972; Hildebrandt and Grieshaber 2008; Li and Moore 2008). An in vivo experiment using sodium 35S-sulfide indicated that orally administered or inhaled sulfide appears in the circulation transiently (Curtis et al. 1972). One of the mechanisms for H2S toxicity has been shown to be due to an inhibition of cytochrome c oxidase in vivo (Dorman et al. 2002) and in vitro (Roberts et al. 2006; Thompson et al. 2003). Conversely, H2S is synthesized endogenously by two pyridoxal-5′-phosphate-dependent enzymes responsible for the metabolism of l-cysteine in mammalian cells, namely, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) (Hughes et al. 2009; Li et al. 2011). Endogenous H2S has many physiological effects in mammalian tissues. Recent studies suggest that H2S is a unique gasotransmitter with regulatory roles in immune system. Endogenous H2S modulates immune responses, such as neutrophil migration (Dal-Secco et al. 2008) and leukocyte-mediated inflammation (Zanardo et al. 2006). Exogenous H2S has been reported to reduce local inflammatory responses of peripheral blood lymphocytes (Mirandola et al. 2007). H2S has also been identified as an autocrine immunomodulatory molecule in T lymphocytes (Miller et al. 2012). However, it is unclear how H2S changes the function of T lymphocytes.
Apoptosis, a genetically programed cell death, is an essential physiological process required for the normal development and maintenance of tissue homeostasis. It is reported that low levels of H2S prevent the apoptosis of human polymorphonuclear cells via the inhibition of p38 mitogen-activated protein kinase (MAPK) and caspase-3 (Rinaldi et al. 2006). Yin et al. reported that sodium hydroxysulfide (NaHS) protected 1-methyl-4-phenyl-pyridinium (MPP+)-induced cytotoxicity and apoptosis in pheochromocytoma cells (Abe and Kimura 1996; Yin et al. 2009). Conversely, it has been reported that H2S induced apoptosis in various types of cells, including human lung fibroblasts (Baskar et al. 2007), pancreatic acinar cells through the activation of Bax (Cao et al. 2006), and insulin-secreting β cells via p38 MAPK activation (Yang et al. 2007). During apoptosis, cells undergo distinct morphological changes, including cell contraction, membrane blebbing, and nuclear disintegration. Contractile forces generated by actin–myosin cytoskeletal structures lead to cell contractions and membrane blebbing (Coleman et al. 2001).
Rho kinases (ROCK-1 and ROCK-2) are the effectors of Rho GTPase and have a molecular mass of ~160 kDa (Ishizaki et al. 1996; Leung et al. 1995). Activated ROCK-1 induces myosin light chain (MLC) phosphorylation and cellular F-actin and activates the actin–myosin contractile system (Coleman et al. 2001). MLC phosphorylation leads to the formation of membrane blebbing during apoptosis (Orlando et al. 2006). ROCK-2 has been also reported to implicate as a mediator of the morphological changes associated with apoptosis (Song et al. 2002). However, it has not reported whether ROCKs contribute to morphological changes during sulfide-induced apoptosis.
Since H2S is a weak acid (\( {\text{p}}K_{{{\text{a}}_{1}}} = 6.76\)), it dissociates into [HS−] and [H+] with a trace of [S2−] in physiological liquids, including plasma (pH 7.4, 37 °C), according to the Handerson–Hasselbach equation (Dombkowski et al. 2004). The proportion of [H2S]/[HS−] is greatly affected by the pH of a solution, and thus, the loss of H2S from the culture solution in the form of a gas also depends on its pH. It is reported that about 25 % of 1 mM H2S escapes from the cell-free medium within 2 h (Oh et al. 2006). Thus, exposure of cells to an exact amount of sulfide would not be completed using a conventional in vitro culture system. The membrane transport systems of sulfide are not well understood. It is reported that the membrane transport of sulfide occurs by simple diffusion as H2S form using planar lipid membrane (Mathai et al. 2009). However, it is not clear until now which species of sulfide are permeable to transport cell membrane and responsible for cell injury. In the present study, we briefly exposed cells to buffered solution in a capped tube to prevent the loss of H2S and adjust the total amount of sulfur species (H2S, HS−, or S2−) in the exposure solution. Herein, we report on the cellular concentration of sulfide, sulfide-induced apoptosis, and involvement of ROCKs during the apoptotic process in Jurkat cells. The term “sulfide” used in this study refers to H2S, HS−, and S2−.
Materials and methods
Chemicals
NaHS, Y-27632, cytochalasin D, and nocodazole were purchased from Sigma (St. Louis, MO). Z-VAD-fmk and Z-VDVAD-fmk were purchased from BioVision (Mountain View, CA). Two-amino-5-N,N-diethylaminotoluene hydrochloride was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). Sodium sulfide nonahydrate (Na2S·9H2O), zinc acetate, iron chloride (FeCl3), and sodium 1-octansulfonate were purchased from Wako (Osaka, Japan). The solution containing NaHS was prepared immediately before use. Cytochalasin D or nocodazole stock solutions (1 mg/ml) were prepared in dimethyl sulfoxide (DMSO), and these stock solutions were used at a final DMSO concentration of 0.1 %. Y-27632 stock solution (5 mM) was prepared in Hanks’ Balanced Salt Solution (HBSS, Life Technologies, Carlsbad, CA, USA). All chemicals were of analytical grade.
Cell culture
A human T lymphocyte cell line, Jurkat, and a human bronchial epithelial cell line, BEAS-2B, were obtained from the American Type Culture Collection (ATCC). Jurkat and BEAS-2B cells were cultured at 37 °C in 5 % CO2 atmosphere in RPMI1640 medium (Life Technologies) and Dulbecco’s modified minimum essential medium (DMEM, Life Technologies), respectively. The culture media contain 10 % heat-inactivated fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 μg/ml of streptomycin. Unless otherwise specified, the pH of the culture medium was adjusted to 7.4. For experiments using cytochalasin D or nocodazole, Jurkat cells were exposed to 5 mM of NaHS in the presence or absence of 1 μg/ml of cytochalasin D, 1 μg/ml of nocodazole, or 0.1 % DMSO (as control) in HBSS at pH 6.0 for 1 h. After washing, the cells were cultured in fresh medium in the presence or absence of 1 μg/ml of cytochalasin D, 1 μg/ml of nocodazole, or 0.1 % DMSO at the indicated time in each experiment.
For experiments using Y-27632, Jurkat cells were pre-cultured with or without 20 μM of Y-27632 for 2 h. The cells were exposed to 5 mM of NaHS in HBSS at pH 6.0 in the presence or absence of 20 μM of Y-27632 for 1 h. After washing, the cells were cultured in fresh medium in the presence or absence of 20 μM of Y-27632 at the indicated time in each experiment.
Cytotoxicity
Jurkat cells were exposed to 0–5 mM of NaHS in HBSS adjusted to pH 6.0, 7.0, or 8.0 for 1 h in polypropylene tubes (BD Biosciences, Franklin Lakes, NJ, USA) in an incubator set at 37 °C with gentle shaking. In the NaHS exposure of Jurkat cells, the cell suspension was placed in an airtight capped tube, and the air was removed by filling the tube with HBSS to avoid a loss of dissolved H2S gas from HBSS. After exposure, cells were washed twice with phosphate-buffered saline (PBS, Life Technologies). The cells were plated into a 96-well culture dish and cultured in fresh medium. After 24 h of culture, cell viability was evaluated using AlamarBlue® (Life Technologies), according to the manufacturer’s instruction. The fluorescence intensity of the medium was measured with a spectrofluorometer (POLARstar OPTIMA, BMG Labtach, Offenburg, Germany) using the excitation and emission wavelengths of 540 and 588 nm, respectively.
BEAS-2B cells were cultured to early confluence in a 96-well culture plate and exposed to 0–5 mM of NaHS in HBSS adjusted to pH 6.0, 7.0, or 8.0 for 1 h. The plate was sealed using Heat Sealer (Eppendorf, Hamburg, Germany) to prevent the escape of gas. The cell monolayers were washed twice with PBS and further cultured in fresh medium without sealing. After 24 h of culture, cell viability of BEAS-2B cells was evaluated using AlamarBlue®.
Analysis of sulfide
Sulfide was extracted from cells according to a method commonly used for the measurement of blood sulfide concentrations (Nagashima et al. 1995) with minor modifications. The reaction solution was prepared by dissolving 2-amino-5-N,N-diethylaminotoluene hydrochloride in 0.35 M of sulfuric acid at final concentration of 2 %. Intracellular concentrations of sulfide were measured by an HPLC-fluorescence detector (FLD). The analytical column was an ODS column (Inertsil, ODS-3, 3 μm, 150 mm × 4.6 mm i.d., GL Science, Tokyo, Japan). The HPLC system consists of a pump (LC-10AD) equipped with a UV-FLD (SPD-10A) (Shimadzu, Kyoto, Japan). Fluorescence of the eluate was monitored with the excitation set at 640 nm and emission at 675 nm. A 20-μl sample of the organic layer obtained from the sulfide extraction was injected into the HPLC. Under this condition, the thionine derivative of sulfide was eluted at 4.8 min.
A standard sulfide solution was prepared by dissolving Na2S·9H2O. Sulfide concentrations were determined from the liner regression (r 2 = 0.998) of a calibration curve ranging from 0 to 2.5 μM. The recovery rate of 1 μM was 78.2 ± 1.7 %.
Cell morphology
Jurkat cells were exposed to 0, 1, or 5 mM of NaHS in HBSS at pH 6.0, 7.0, or 8.0 for 1 h. After washing twice with PBS, cells were plated into a 24-well culture dish and cultured in fresh medium. After 3–6 h, cell suspensions were prepared using the cytospin apparatus. Cells were cytocentrifuged on a slide glass at 1,200 rpm for 5 min and subsequently air-dried. Lastly, cells were stained with Diff-Quik® (International Reagents, Kobe, Japan).
Caspase-3/7 activity assay
Jurkat cells were exposed to 0, 1, or 5 mM of NaHS in HBSS at pH 6.0, 7.0, or 8.0 for 1 h. After washing twice with PBS, cells were plated into a 96-well culture dish and cultured in fresh medium in the presence or absence of 2 μM of Z-VAD-fmk or 2 μM of Z-VDVAD-fmk for 6 h. Caspase-3/7 activities were measured with a Caspase-Glo™ 3/7 Assay kit (Promega, Madison, MI, USA), according to the manufacturer’s instruction. The luminescence was measured with a microplate luminometer (GLOMAX, Promega) at 565 nm.
Western blot analysis
Jurkat cells were exposed to 0, 1, or 5 mM of NaHS in HBSS at pH 6.0. After washing twice with PBS, cells were plated into a 6-well culture dish and cultured in fresh medium in the presence or absence of 2 μM of Z-VAD-fmk for 4 h (for ROCK-1 and ROCK-2) or 6 h [for caspase-3 and poly (ADP-ribose) polymerase (PARP)]. Cells were lysed with RIPA buffer containing the protease inhibitor, PMSF, and sodium orthovanadate (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The lysate was centrifuged at 10,000g for 5 min at 4 °C. Proteins in the supernatant were resolved on SDS-PAGE under reducing conditions and electroblotted onto a PVDF membrane. The membrane was blocked with PVDF blocking reagent (TOYOBO, Osaka, Japan) and probed with anti-PARP, anti-caspase-3, anti-ROCK-1, or anti-ROCK-2 (BD Biosciences), followed by peroxidase (POD)-tagged anti-mouse IgG antibody (Santa Cruz Biotechnology). The immunoreactions on the membrane were visualized by using enhanced chemiluminescence (GE Healthcare, Buckinghamshire, UK). After probing with anti-ROCK-1 or anti-ROCK-2, the membrane was incubated with stripping buffer (2 % SDS and 100 mM of 2-mercaptoethanol in tween-PBS) at 56 °C for 20 min to remove the antibodies and reprobed with POD-tagged anti-α-tubulin antibody (MBL, Nagoya, Japan). The intensities of the bands were quantified using a chemiluminescence densitometer (Lumino Imaging Analyzer, FAS-1100, TOYOBO, Osaka, Japan).
Flow cytometric analysis of apoptotic cells
The detection of apoptotic cells was conducted using an Annexin V-FITC Apoptosis Detection Kit (BioVision). Jurkat cells were exposed to 5 mM of NaHS for 1 h at pH 6.0. After washing twice with PBS, cells were plated into a 24-well culture dish and cultured in fresh medium in the presence or absence of 2 μM of Z-VAD-fmk for 4 h. Cells were then collected by centrifugation at 1,200g for 2 min. Then, cells were resuspended with binding buffer and stained with FITC-labeled annexin V and propidium iodide (PI) for 5 min at room temperature in the dark, according to the manufacturer’s instruction, and analyzed with a flow cytometer (Guava EasyCyte™ 8HT, MILLIPORE, Hayward, CA, USA).
Statistical analysis
Data are presented as mean ± SEM. Statistical analyses were performed by ANOVA followed by Bonferroni/Dunn’s post hoc analysis. The statistical significance level was set at p < 0.05.
Results
Sulfide-induced changes in cell viability
Figure 1 and supplemental Fig. 1 demonstrate the cytotoxic effects of NaHS on Jurkat and BEAS-2B cells in HBSS at pH 6.0, 7.0, or 8.0, respectively. Cytotoxicity of Jurkat cells due to NaHS was markedly enhanced with decreases in the pH of the culture solution. When a curve of the secondary degree was fitted to the dose–effect relationship, the effective concentration of 50 % (EC50) for lethality of NaHS at pH 6.0 was calculated to be 2.5 mM, whereas the EC50 of NaHS at pH 7.0 and 8.0 was >5 mM. Similar results of cytotoxicity were also observed in BEAS-2B cells. Cytotoxicity was reduced at alkaline pHs and enhanced at acidic pHs when BEAS-2B cells were exposed to 5 mM of NaHS. However, the EC50 of NaHS at all pHs was >5 mM. In the absence of NaHS, changes in pH did not affect the viability of both Jurkat and BEAS-2B cells.
Effects of pH on cytotoxicity induced by sulfide in Jurkat cells. Jurkat cells were exposed to 0–5 mM of NaHS in HBSS at pH 6.0, 7.0, or 8.0 for 1 h. After washing twice with PBS, cells were plated into a 96-well culture dish and cultured in fresh RPMI1640 complete medium. After 24 h of culture, cell viability was evaluated using AlamarBlue®. Data are presented as mean ± SEM (N = 5)
Intracellular concentrations of sulfide
Jurkat cells were exposed to 0, 1, or 5 mM of NaHS, and the intracellular concentrations of sulfide were measured immediately after exposure using HPLC-FLD. As shown in Fig. 2, the intracellular concentrations of sulfide increased dramatically as the pH decreased, when the cells were exposed to 5 mM of NaHS. Moreover, the intracellular concentrations of sulfide were still detectable by the current HPLC-FLD method without NaHS treatment.
Effects of pH on cellular uptake of sulfide. Jurkat cells were exposed to 0, 1, or 5 mM of NaHS in HBSS at pH 6.0, 7.0, or 8.0 for 1 h. The cells were washed twice with 10 ml of HBSS, resuspended in 100 μl of fresh HBSS, and transferred into an airtight glass tube. The cell suspension was diluted to a final volume of 4.0 ml with 0.5 % of zinc acetate in H2O to trap the sulfide, and then, the mixture was sonicated for 5 min to disrupt the cells. A 0.4 ml of the reaction solution and 0.2 ml of 0.1 mM FeCl3 solution in 5 mM of sulfuric acid were added to the sample solution and diluted the solution with H2O to 5 ml. The mixture was incubated for 60 min at 25 °C in the dark. After the incubation, 0.5 ml of 0.1 M sodium 1-octansulfonate and 0.5 ml of 2-octanol were added to the mixture. The extraction was performed by shaking the tube for 10 min and centrifuging at 3,000 rpm for 10 min to separate the aqueous and organic layers. A 20-μl sample of the organic layer was injected into the HPLC. Analytical column: an ODS column, mobile phase: a mixture of 0.1 M of sodium 1-octansulfonate solution and acetonitrile (1:9, v/v), HPLC analysis condition: isocratically at a flow rate of 0.5 ml/min at 40 °C. *p < 0.05, compared to control; #p < 0.05, compared to 1 mM
Effects of sulfide on cell blebbing
Figure 3a–d show photomicrographs of cells stained with Diff-Quik®. Morphological changes were not observed immediately after exposure (data not shown). Cells cultured for 6 h after the cessation of exposure to 5 mM of NaHS at pH 6.0 showed fragmented nuclei and plasma membrane blebbing (Fig. 3b). Conversely, no morphological changes were observed in cells exposed to 5 mM of NaHS at either pH 7.0 (Fig. 3c) or 8.0 (Fig. 3d). Furthermore, 1 mM of NaHS did not affect cell morphology even at pH 6.0 (data not shown).
Photomicrographs of sulfide-exposed Jurkat cells stained with Diff-Quik®. Jurkat cells were exposed to 0 or 5 mM of NaHS in HBSS at pH 6.0, 7.0, or 8.0 for 1 h. After washing twice with PBS, cells were plated into a 24-well culture dish and cultured in fresh RPMI1640 complete medium. After 6 h of culture, the cell suspension was cytocentrifuged and stained with Diff-Quik®. The lower panel is a higher magnification of each upper panel. Arrows and arrow heads indicate plasma membrane blebbing and fragmented nuclei, respectively
Cleavage of caspase-3 and PARP in sulfide-exposed cells
The activation of caspase-3 is a typical apoptotic event. We investigated the activation of caspase-3 and caspase-3/7 in NaHS-exposed cells, and the effect of Z-VAD-fmk, a pan-caspase inhibitor, by Western blotting and chemiluminescence method. Figure 4 demonstrates that the inactive form of pro-caspase-3 at 32 kDa was decreased and the cleaved active form of caspase-3 at 17 kDa was increased with NaHS exposure. Additionally, Z-VAD-fmk suppressed the conversion of pro-caspase-3 to the active form of caspase-3. Apoptotic responses in NaHS-exposed cells were further confirmed by detecting the degradation of PARP, which is known to be cleaved by activated caspase-3. PARP at 116 kDa and cleaved PARP fragment at 89 kDa were detected in 5-mM NaHS-exposed cells, and this was also inhibited by Z-VAD-fmk.
Western blot analyses for the detection of caspase-3 and PARP cleavage in sulfide-exposed Jurkat cells. Jurkat cells were exposed to 5 mM of NaHS in HBSS at pH 6.0 for 1 h. After washing twice with PBS, cells were plated into a 6-well culture dish and cultured in fresh RPMI1640 complete medium in the presence or absence of 2 μM of Z-VAD-fmk for 6 h. The lysate was centrifuged, and the proteins in the supernatant were resolved by SDS-PAGE and electroblotted onto a PVDF membrane. The blot was probed with anti-PARP or anti-caspase-3 followed by a corresponding POD-tagged secondary antibody
As shown in Fig. 5, caspase-3/7 activity was increased with 5 mM of NaHS at both pH 6.0 and 7.0, but not pH 8.0. The caspase activities induced by 5 mM of NaHS at both pH 6.0 and 7.0 were completely inhibited by Z-VAD-fmk. In the absence of NaHS, the changes in pH did not affect caspase-3/7 activity. Furthermore, Z-VDVAD-fmk, a caspase-2-specific inhibitor, inhibited caspase-3/7 activity induced by 5 mM of NaHS at pH 6.0 (see supplemental Fig. 2).
Changes in caspase-3/7 activity in sulfide-exposed Jurkat cells. Jurkat cells were exposed to 0 (control) or 5 mM of NaHS in HBSS at pH 6.0, 7.0, or 8.0 for 1 h. After washing twice with PBS, cells were plated into a 96-well culture dish and cultured in fresh RPMI1640 complete medium in the presence (open column) or absence (closed column) of 2 μM of Z-VAD-fmk. After 6 h, caspase-3/7 activities were measured with a Caspase-Glo™ 3/7 Assay Kit. Data are presented as means ± SEM (N = 3). *p < 0.05, compared to control at each pH; #p < 0.05, compared to the corresponding Z-VAD-fmk group
Flow cytometric analyses of sulfide-exposed cells
Figure 6 shows that the proportion of cells in the upper left quadrant (annexin V positive and PI negative), which denotes early apoptotic cells, was increased about 17 % after exposure to NaHS. The proportion of NaHS-induced early apoptotic cells was decreased by treatment with Z-VAD-fmk. These results indicate that NaHS-induced cell death was caused by caspase-mediated apoptosis.
Flow cytometric analyses of sulfide-exposed Jurkat cells. Jurkat cells were exposed to 0 (control) or 5 mM of NaHS in HBSS at pH 6.0 for 1 h. After washing twice with PBS, cells were plated into a culture dish and cultured in fresh RPMI1640 complete medium in the presence or absence of 2 μM of Z-VAD-fmk for 4 h. Cells were centrifuged, resuspended with binding buffer, stained with annexin V and PI for 5 min at room temperature in dark, and analyzed with a flow cytometer. The number inside each quadrant represents the percentage of intact (lower left, LL), apoptotic (upper left, UL), and necrotic (lower and upper right, LR and UR) cells
Effects of cytochalasin D or nocodazole on cell blebbing
As described above, cell blebbing occurred in NaHS-exposed cells at 6 h after exposure, as examined by microscopic examination (Fig. 3). It is known that membrane blebbing is dependent on intracellular force generated by the actin–myosin cytoskeleton (Croft et al. 2005). The depolymerization of microtubules is also reported to contribute to apoptotic events (Ndozangue-Touriguine et al. 2008). Thus, we further investigated the effects of actin filament-destabilizing or microtubule-destabilizing agents to examine the mechanisms of cell blebbing in NaHS-exposed cells. Microscopic examination revealed that cytochalasin D, but not nocodazole, suppressed cell blebbing induced by 5 mM of NaHS (Fig. 7). Neither cytochalasin D nor nocodazole alone without NaHS treatment changed cell morphology as observed by photomicroscopy (data not shown). Conversely, caspase-3/7 activity was not suppressed by these agents significantly (see supplemental Fig. 3). These results suggest that the disruption of actin filaments, but not microtubule structures, prevents cell blebbing without affecting caspase activity.
Effects of cytochalasin D or nocodazole on morphological changes in sulfide-exposed Jurkat cells. Jurkat cells were exposed to 0 (control) or 5 mM of NaHS in the presence or absence of 1 μg/ml of cytochalasin D or 1 μg/ml of nocodazole in HBSS at pH 6.0 for 1 h. After washing twice with PBS, cells were plated into a culture dish and cultured in fresh RPMI1640 complete medium in the presence or absence of cytochalasin D or nocodazole. After 3 h of culture, the cell suspension was cytocentrifuged and stained with Diff-Quik®. The lower panel is a higher magnification of each upper panel. Arrows indicate plasma membrane blebbing
Cleavage of ROCK-1 and ROCK-2 in sulfide-exposed cells
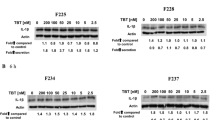
To elucidate whether ROCK-1 and -2 are involved in the NaHS-induced apoptotic process, we examined the cleavage of ROCKs and the effects of Z-VAD-fmk by Western blotting. As shown in Fig. 8a, b, both ROCK-1 and ROCK-2 were cleaved by 5 mM of NaHS, respectively. The amount of cleaved ROCK-1 following exposure to 5 mM of NaHS decreased with Z-VAD-fmk treatment, however, not completely (Fig. 8a). Conversely, the amount of cleaved ROCK-2 was suppressed completely following Z-VAD-fmk treatment (Fig. 8b). The relative amount of cleaved ROCK-2 was lower than that of ROCK-1.
Western blot analyses for the detection of cleavage of ROCK-1 (a) and ROCK-2 (b) in sulfide-exposed Jurkat cells. Jurkat cells were exposed to 5 mM of NaHS in HBSS at pH 6.0 for 1 h in the presence or absence of 2 μM of Z-VAD-fmk. After washing twice with PBS, cells were plated into a 6-well culture dish and cultured in fresh RPMI1640 complete medium in the presence or absence of Z-VAD-fmk for 4 h. The lysate was centrifuged, and proteins in the supernatant were resolved by SDS-PAGE and electroblotted onto a PVDF membrane. The blot was probed with anti-ROCK-1 or anti-ROCK-2 followed by a corresponding POD-tagged secondary antibody. The membranes were stripped and reprobed with POD-tagged anti-tubulin antibody. The intensity of the bands was quantified with chemiluminescence densitometry
Effects of a ROCK inhibitor on cell blebbing
We examined the effects of Y-27632, a ROCK inhibitor, on cell blebbing. Microscopic examination revealed that Y-27632 suppressed cell blebbing induced by 5 mM of NaHS (Fig. 9). Conversely, caspase-3/7 activity was not affected by Y-27632 (see supplemental Fig. 5). These results indicate that this particular ROCK inhibitor prevents cell blebbing without affecting caspase activity.
Effects of ROCK inhibitor on cell morphological changes in sulfide-exposed Jurkat cells. Jurkat cells were pre-cultured with or without 20 μM of Y-27632 for 2 h. Cells were exposed to 5 mM of NaHS in HBSS at pH 6.0 in the presence or absence of Y-27632 for 1 h. After washing twice with PBS, cells were plated into a 24-well culture dish and cultured in fresh RPMI1640 complete medium in the presence or absence of Y-27632. After 3 h of culture, the cell suspension was cytocentrifuged and stained with Diff-Quik®. The lower panel (b) is a higher magnification of each upper panel (a). Arrows indicate plasma membrane blebbing
Discussion
H2S is a harmful gas and is rapidly metabolized in mitochondria and converted to thiosulfate (Li and Moore 2008). When human body is accidentally inhaled H2S, H2S is absorbed by the upper respiratory tract mucosa (Hildebrandt and Grieshaber 2008). Therefore, bronchial epithelium is one of the targets affected by H2S as a gaseous material directly. Next, inhaled and absorbed H2S is partially dissolved H2S within the circulation. On the other hand, sulfide is known to be present in normal human blood at concentrations of 10–100 μM (Richardson et al. 2000). H2S dissociates to HS− and S2− depending on pH, and, therefore, we examined cytotoxicity of sulfide in Jurkat cells (Fig. 1) and also in BEAS-2B cells (supplemental Fig. 1) at different pHs. The cytotoxicity of sulfide was markedly enhanced with decrease in pH of the solution in both Jurkat and BEAS-2B cells. The pH-dependent remarkable change in cytotoxicity was not limited to sulfide. It was reported that the toxicity of sodium fluoride in macrophages was enhanced when the pH of the culture medium was decreased and the generation of hydrogen fluoride (HF) in acidic condition was responsible for the entry of fluoride into the cells (Hirano and Ando 1997). Thus, it is plausible that cytotoxicity of sulfide depends on entry of H2S, which is generated in the culture medium at acidic condition, into the cells.
We, then, measured the intracellular concentrations of sulfide immediately after exposure using the HPLC-FLD method, as we speculated that cytotoxicity may be due to an intracellular accumulation of sulfide. The morphological changes in NaHS-exposed cells were not observed at this point (data not shown). Interestingly, the sulfide concentration in cells exposed to 5 mM of NaHS increased dramatically as the pH of the culture solution decreased (Fig. 2). The entry systems through cell membrane of H2S are not well understood. It has been reported that H2S is a highly lipophilic molecule and readily crosses the cell membrane by diffusion (Hughes et al. 2009). It is suggested that HS− is protonated, and only the uncharged H2S permeates the planar lipid membrane (Mathai et al. 2009). In physiological solution (pH 7.4), 18.5 % of sulfide is in the form of H2S and 81.5 % is in the form of HS− and more than 90 % of the sulfide is present as H2S at pH 6.0, as predicted by the Handerson–Hasselbach equation (Dombkowski et al. 2004). At pH 6.0, the proportion of H2S, which is permeable through the cell membrane, to less permeable HS− and S2− forms is higher compared to that at pHs 7.0–8.0. Therefore, the pH-dependent cytotoxicity in NaHS-exposed cells was probably caused by an increased uptake of sulfide at lower pH. Flow cytometric analyses revealed that pH-dependent cell death caused by NaHS was apoptotic rather than necrotic, if any (Fig. 6).
It has been reported that blood sulfide concentration obtained from victims who had died of acute exposure to H2S was up to 0.995 ppm (Maebashi et al. 2011). It is plausible that the function of T lymphocytes is deteriorated after exposure to lethal level of H2S.
In this study, we observed cell blebbing in cells treated with NaHS (Figs. 3, 7 and 9) and staurosporine (data not shown) by microscopic examination and flow cytometry. Jurkat cells have been used to investigate the release of microvesicles (MVs) and cell blebbing from during apoptosis induced by a variety of chemotherapeutic agents or protein kinase C inhibitors, such as staurosporine and 7-hydroxy staurosporine (Azuma et al. 2011; Ullal and Pisetsky 2010). The cell blebbing in NaHS-exposed cells suppressed following treatment with cytochalasin D, an actin filament-destabilizing agent, but not nocodazole, a microtubule-destabilizing agent (Fig. 7). Similar inhibitory effects on cell blebbing by these agents were observed in staurosporine-treated Jurkat cells (data not shown). On the other hand, caspase-3/7 activity and ROCK cleavage were not affected by either agent (supplemental Figs. 3 and 4). It has been reported that cytochalasin D blocks bleb formation, but not apoptosis, as the two processes do not occur in the same linear cascade (Huot et al. 1998), and disruption of only actin filament, but not microtubule structure, prevents membrane blebbing or apoptotic nuclear breakdown (Croft et al. 2005). Our results are consistent with those of previous reports. Only actin filaments, but not microtubule structure, were involved in blebbing without affecting caspase activity and ROCK cleavage.
One of the most interesting findings in the present study is that both ROCK-1 and -2 were cleaved to generate a truncated active form during the apoptotic process in 5-mM NaHS-exposed cells (Fig. 8a, b). To our knowledge, this is the first report to describe both ROCK-1 and -2 cleavage are involved in apoptosis via caspases, with the exception of ouabain-induced apoptosis (Ark et al. 2010). ROCK-1 is cleaved to generate active form by activated caspase-3 during apoptosis (Sebbagh et al. 2001). It has been shown that caspase-3-cleaved ROCK-1 induces membrane blebbing during apoptosis (Coleman et al. 2001; Sebbagh et al. 2005). In this study, ROCK-1 cleavage induced by NaHS was suppressed by Z-VAD-fmk, a pan-caspase inhibitor (Fig. 8a), indicating that ROCK-1 caused cell blebbing following activation by caspases. On the other hand, the cleavage of ROCK-2 induced by NaHS was completely suppressed by Z-VAD-fmk (Fig. 8b). ROCK-2 has been reported to be cleaved primarily by either granzyme B (Sebbagh et al. 2005), which is a serine protease derived from cytotoxic T lymphocytes, or caspase-2 (Ark et al. 2010). Our current results indicate that ROCK-2 may be cleaved by caspases in NaHS-exposed cells. ROCK-1 and ROCK-2 have been proposed to perform the same functions that induce membrane blebbing and chromatin condensation (Sebbagh et al. 2005). However, ezrin–radixin–moesin phosphorylation mediated by ROCK-1, but not ROCK-2, is involved in anti-Fas-induced apoptosis in Jurkat cells (Hebert et al. 2008). The activation of ROCK-2 by cleavage is a prerequisite for the generation of MVs, which are small cell fragments released from membrane blebs during apoptosis, in response to thrombin (Sapet et al. 2006). Though further studies are needed to understand the functions of each ROCK in sulfide-induced apoptosis, ROCKs may have sulfide-specific physiological roles involving cell blebbing. In this study, Z-VAD-fmk suppressed caspase-3/7 activity (Fig. 5) and the cleavage of ROCK-1 and ROCK-2 (Fig. 8a, b), and accordingly, suppressed cell blebbing (data not shown). Conversely, Y-27632, a ROCK inhibitor, suppressed cell blebbing (Fig. 9) without affecting caspase-3/7 activity (supplemental fig. 5). These results indicate that sulfide-induced cell blebbing requires ROCK activity (Fig. 10).
Schematic representation of the sulfide-induced apoptotic pathway in Jurkat cells. A larger amount of sulfide is accumulated at lower pH. Intracellular sulfide triggers caspase-dependent apoptosis. Activated caspases induce cell blebbing through both ROCK-1 and ROCK-2 cleavages and DNA fragmentation. The sulfide-induced cell blebbing was suppressed by a ROCK inhibitor, indicating that sulfide-induced cell blebbing requires ROCK activity
Sulfide-induced caspase-3/7 activity was completely blocked by Z-VDVAD-fmk, a caspase-2 inhibitor (supplemental Fig. 2), suggesting that caspase-2 acts upstream of caspase-3/7 in the apoptotic pathway. In a recent study, caspase-2 was implicated in the release of cytochrome c from mitochondria (Guo et al. 2002). It is reported that NaHS induces the release of cytochrome c from mitochondria via the apoptotic pathway in human lung fibroblast cells (Baskar et al. 2007). These previous reports and our current results indicate that the activation of caspase-2 might result in a similar apoptotic pathway in NaHS-exposed Jurkat cells.
This study was designed to examine which species of sulfide are permeable to transport cell membrane and responsible for cell injury. The pH adjustments of NaHS-containing buffered solution to pH 6.0, 7.0 or 8.0 resulted in changing only proportion of sulfur species (H2S, HS−, or S2−) without changing total amount of sulfide. However, exposure of cells to an exact amount of sulfide would not be completed using a conventional in vitro culture system because H2S is easy to escape from culture solution. Thus, we briefly exposed cells to NaHS-containing buffered solution in a capped tube to prevent the loss of H2S and adjust the total amount of sulfur species (H2S, HS−, or S2−) in the exposure solution. The measurement of intracellular sulfide concentrations revealed pH-dependent cytotoxicity correlated with the proportion of H2S in culture solution. High concentration of H2S uptaken into the cells at acidic condition caused apoptosis in Jurkat cells, whereas low concentration of H2S did not affect cytotoxicity.
In summary, we demonstrate that the intracellular accumulation of sulfide was caused by a penetration of H2S through the cell membrane in a pH-dependent manner, and sulfide-induced cytotoxicity is due to caspase-dependent apoptosis. Furthermore, we revealed that sulfide induces both ROCK-1 and ROCK-2 cleavage and causes ROCK-dependent cell blebbing during apoptosis.
References
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071
Ark M, Ozdemir A, Polat B (2010) Ouabain-induced apoptosis and Rho kinase: a novel caspase-2 cleavage site and fragment of Rock-2. Apoptosis 15:1494–1506
Azuma Y, Nakagawa H, Dote K, Higai K, Matsumoto K (2011) Decreases in CD31 and CD47 levels on the cell surface during etoposide-induced Jurkat cell apoptosis. Biol Pharm Bull 34:1828–1834
Baskar R, Li L, Moore PK (2007) Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J 21:247–255
Beauchamp RO Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA (1984) A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol 13:25–97
Cao Y, Adhikari S, Ang AD, Moore PK, Bhatia M (2006) Mechanism of induction of pancreatic acinar cell apoptosis by hydrogen sulfide. Am J Physiol Cell Physiol 291:C503–C510
Centers for Disease Control and Prevention. Documentation for immediately dangerous to life or health concentrations. http://wwwcdcgov/niosh/idlh/7783064
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3:339–345
Croft DR, Coleman ML, Li S et al (2005) Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol 168:245–255
Curtis CG, Bartholomew TC, Rose FA, Dodgson KS (1972) Detoxication of sodium 35 S-sulphide in the rat. Biochem Pharmacol 21:2313–2321
Dal-Secco D, Cunha TM, Freitas A et al (2008) Hydrogen sulfide augments neutrophil migration through enhancement of adhesion molecule expression and prevention of CXCR2 internalization: role of ATP-sensitive potassium channels. J Immunol 181:4287–4298
Dombkowski RA, Russell MJ, Olson KR (2004) Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol 286:R678–R685
Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF (2002) Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci 65:18–25
Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES (2002) Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem 277:13430–13437
Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J (2008) Rho-ROCK-dependent ezrin–radixin–moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol 181:5963–5973
Hildebrandt TM, Grieshaber MK (2008) Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275:3352–3361
Hirano S, Ando M (1997) Fluoride mediates apoptosis in osteosarcoma UMR 106 and its cytotoxicity depends on the pH. Arch Toxicol 72:52–58
Hughes MN, Centelles MN, Moore KP (2009) Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med 47:1346–1353
Huot J, Houle F, Rousseau S, Deschesnes RG, Shah GM, Landry J (1998) SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J Cell Biol 143:1361–1373
Ishizaki T, Maekawa M, Fujisawa K et al (1996) The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 15:1885–1893
Leung T, Manser E, Tan L, Lim L (1995) A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem 270:29051–29054
Li L, Moore PK (2008) Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci 29:84–90
Li L, Rose P, Moore PK (2011) Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187
Maebashi K, Iwadate K, Sakai K et al (2011) Toxicological analysis of 17 autopsy cases of hydrogen sulfide poisoning resulting from the inhalation of intentionally generated hydrogen sulfide gas. Forensic Sci Int 207:91–95
Mathai JC, Missner A, Kugler P et al (2009) No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci USA 106:16633–16638
Miller TW, Wang EA, Gould S et al (2012) Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem 287:4211–4221
Mirandola P, Gobbi G, Sponzilli I et al (2007) Exogenous hydrogen sulfide induces functional inhibition and cell death of cytotoxic lymphocytes subsets. J Cell Physiol 213:826–833
Nagashima K, Fukushima K, Kamaya M (1995) Determination of trace amounts of sulfide in human serum by high-performance liquid chromatography with fluorometric detection after derivatization with 2-amino-5-N,N-diethylaminotoluene and iron(III). J Liq Chromatogr 18:515–526
Ndozangue-Touriguine O, Hamelin J, Breard J (2008) Cytoskeleton and apoptosis. Biochem Pharmacol 76:11–18
Oh GS, Pae HO, Lee BS et al (2006) Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med 41:106–119
Orlando KA, Stone NL, Pittman RN (2006) Rho kinase regulates fragmentation and phagocytosis of apoptotic cells. Exp Cell Res 312:5–15
Richardson CJ, Magee EA, Cummings JH (2000) A new method for the determination of sulphide in gastrointestinal contents and whole blood by microdistillation and ion chromatography. Clin Chim Acta 293:115–125
Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M (2006) Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest 86:391–397
Roberts ES, Wong VA, McManus BE, Marshall MW, Lancianese S, Dorman DC (2006) Changes in intracellular pH play a secondary role in hydrogen sulfide-induced nasal cytotoxicity. Inhal Toxicol 18:159–167
Sapet C, Simoncini S, Loriod B et al (2006) Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 108:1868–1876
Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J (2001) Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 3:346–352
Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J (2005) Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med 201:465–471
Song Y, Hoang BQ, Chang DD (2002) ROCK-II-induced membrane blebbing and chromatin condensation require actin cytoskeleton. Exp Cell Res 278:45–52
Thompson RW, Valentine HL, Valentine WM (2003) Cytotoxic mechanisms of hydrosulfide anion and cyanide anion in primary rat hepatocyte cultures. Toxicology 188:149–159
Ullal AJ, Pisetsky DS (2010) The release of microparticles by Jurkat leukemia T cells treated with staurosporine and related kinase inhibitors to induce apoptosis. Apoptosis 15:586–596
Yang G, Yang W, Wu L, Wang R (2007) H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem 282:16567–16576
Yin WL, He JQ, Hu B, Jiang ZS, Tang XQ (2009) Hydrogen sulfide inhibits MPP(+)-induced apoptosis in PC12 cells. Life Sci 85:269–275
Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL (2006) Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20:2118–2120
Acknowledgments
We would like to thank Ms. M. Tadano for the technical support in performing the HPLC-FLD. This work was, in part, supported by JSPS KAKENHI (Grant Number 24590868).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanno, S., Hirano, S., Sagi, M. et al. Sulfide induces apoptosis and Rho kinase-dependent cell blebbing in Jurkat cells. Arch Toxicol 87, 1245–1256 (2013). https://doi.org/10.1007/s00204-013-1027-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1027-3