Abstract
The reactivation of organophosphorus compound (OP)-inhibited acetylcholinesterase (AChE) by oximes is inadequate in case of different OP nerve agents. This fact led to the synthesis of numerous novel oximes by different research groups in order to identify more effective reactivators. In the present study, we investigated the reactivation kinetics of a homologous series of bispyridinium bis-oximes bearing a (E)-but-2-ene linker with tabun-, sarin-, and cyclosarin-inhibited human AChE. In part, marked differences in affinity and reactivity of the investigated oximes toward OP-inhibited human AChE were recorded. These properties depended on the position of the oxime groups and the inhibitor. None of the tested oximes was equally effective against all used OPs. In addition, the data indicate that a (E)-but-2-ene linker decreased in most cases the reactivating potency in comparison to oximes bearing an oxybismethylene linker, e.g., obidoxime and HI-6. The results of this study give further insight into structural requirements for oxime reactivators, underline the necessity to investigate the kinetic interactions of oximes and AChE with structurally different OP inhibitors, and point to the difficulty to develop an oxime reactivator which is efficient against a broad spectrum of OPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, acetylcholinesterase (AChE) reactivators, i.e., oximes, are the only causal therapy of poisoning by organophosphorus compounds (OP) (Eyer and Worek 2007). The primary mechanism of action of oximes is the removal of the phosphyl moiety from the active site serine at the bottom of the AChE gorge (Taylor et al. 1999) resulting in active AChE which is a prerequisite for the cessation of OP-induced cholinergic crisis. However, numerous in vitro and in vivo studies demonstrated that clinically used oximes, e.g., obidoxime, pralidoxime, TMB-4, are insufficient to reactivate AChE inhibited by different OP pesticides and nerve agents (Worek et al. 2004).
In the past six decades, a countless number of oximes has been synthesized by numerous research groups in order to identify compounds with improved efficiency (Bismuth et al. 1992; Eyer and Worek 2007; Hobbiger 1963). These efforts were mainly focused on the development of oximes against certain reactivation-resistant OP–AChE complexes, e.g., from soman, tabun, cyclosarin, or of oximes showing activity against a broad spectrum of structurally different OP.
With some exceptions, newly synthesized compounds were based on mono- or bispyridinium oximes bearing one or two oxime groups at different ring positions and having different linkers between the pyridinium rings (Schoene 1980; Reiner and Simeon-Rudolf 2006; Musilek et al. 2011; Gray 1984). The ability of new oximes to reactivate OP-inhibited AChE was investigated by the various research groups with a variety of experimental protocols, AChE sources, and OP inhibitors making a proper assessment of oxime effectiveness difficult (Worek et al. 2004).
Recently, a new group of bispyridinium bis-oximes bearing a (E)-but-2-ene linker was presented (Musilek et al. 2006), and initial reactivation studies were undertaken (Calic et al. 2008; Kuca et al. 2006a, b). Now it was tempting to determine the reactivation kinetics of a homologous series of these compounds at identical experimental conditions. For this purpose, three OP nerve agents with differential susceptibility toward reactivation by conventional oximes were selected as inhibitors, i.e., sarin, cyclosarin, and tabun. With this approach, it should be possible to get more insight into structural requirements for reactivation and to assess the ability of the tested oximes to reactivate human AChE inhibited by structurally different OP.
Materials and methods
Materials
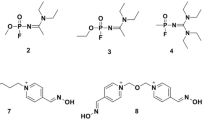
5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) and acetylthiocholine iodide (ATCh) were supplied by Sigma-Aldrich (Taufkirchen, Germany). Tabun (ethyl-N-dimethyl phosphoroamidocyanidate, GA), sarin (isopropylmethylphosphonofluoridate, GB), and cyclosarin (cyclohexylmethylphosphonofluoridate, GF) (>98 % by GC–MS, 1H NMR and 31P NMR) were made available by the German Ministry of Defence. The oximes K053, K068, K069, K075, and K105 (Table 1) were prepared as described before (Musilek et al. 2006). All other chemicals were from Merck (Darmstadt, Germany).

Nerve agent stock solutions (0.1 % v/v in acetonitrile) were stored at ambient temperatures, and working solutions were prepared in distilled water just before the experiment. Oxime stock solutions (200 mM) were prepared in distilled water, stored at −80 °C, and diluted as required in distilled water just before use. All solutions were kept on ice until use.
Hemoglobin-free human erythrocyte ghosts were prepared as described from heparinized human blood (Worek et al. 2002) and served as AChE source. Aliquots of the erythrocyte ghosts with an AChE activity adjusted to that found in whole blood were stored at −80 °C, and aliquots were homogenized prior to use to achieve a homogeneous matrix for the kinetic studies.
AChE assay
AChE activities were measured spectrophotometrically at 412 nm (Cary 50, Varian, Darmstadt, Germany) with a modified Ellman assay (Worek et al. 1999; Eyer et al. 2003) using polystyrol cuvettes and 0.45 mM ATCh as substrate and 0.3 mM DTNB as a chromogen in 0.1 M phosphate buffer (pH 7.4).
All experiments were performed at 37 °C and pH 7.4. All concentrations refer to final concentrations.
Reactivation of OP-inhibited AChE by oximes
A small volume (≤1 %, v/v) of tabun, sarin, or cyclosarin in distilled water was added to erythrocyte ghosts that were incubated with for 15 min at 37 °C to achieve an AChE inhibition by >95 %. To remove residual inhibitor, the treated ghosts were dialyzed (phosphate buffer, 0.1 M, pH 7.4) overnight at 4 °C, and the absence of inhibitory activity was tested by incubation of treated and control ghosts (30 min, 37 °C). Aliquots were stored at −80 °C until use.
One hundred and fifty microliters of inhibited AChE was mixed with 150 μl phosphate buffer containing 0.2 % gelatin, and at t = 0.5 μl oxime was added to initiate reactivation. Twenty microliter aliquots were transferred after specified time intervals (2–60 min) to tempered cuvettes containing 3,000 μl phosphate buffer and 100 μl DTNB for the measurement of AChE activity.
Six different oxime concentrations (10–1,000 μM) were used for the determination of the reactivation rate constants in duplicate experiments. For the reactivation of tabun-inhibited AChE, higher oxime concentrations (2,000 and 3,000 μM) were used in part, too. OP-inhibited AChE activity as well as AChE activity after time-dependent reactivation was referred to control AChE and % reactivation was calculated thereof.
Reactivation kinetics
The reactivation of OP-inhibited AChE by oximes can be quantified by the determination of reactivation rate constants. As shown in Scheme 1, oxime-induced reactivation of OP-inhibited AChE may be described by a two-step reaction.
In this scheme, [EP] is the OP-inhibited AChE, [EPOX] the Michaelis-type OP-AChE-oxime conjugate, [OX] the oxime, [E] the reactivated enzyme, and [POX] the phosphylated oxime. K D represents the dissociation constant which is inversely proportional to the affinity of the oxime to [EP], and k r the rate constant for removing the OP residue from [EPOX] by the oxime, quantifying the reactivity. The hybrid reactivation rate constant k r2 was calculated from the ratio of k r and K D , represents a second-order rate constant.
In case of complete reactivation and with [Ox] > [EP]0, a pseudo-first-order rate equation can be derived for the reactivation process (Eq. 1):
k obs was calculated by nonlinear regression analysis (Worek et al. 2010) using Eq. (2)
Finally, k r and K D were obtained by the nonlinear fit of the relationship between k obs versus [OX].
Data analysis
The experimental data were processed by nonlinear regression analysis using curve fitting programs (Prism™ vers. 4.03, GraphPad Software, San Diego, CA, USA) in order to calculate the reactivation rate constants.
Results
The reactivation of nerve agent-inhibited human AChE by a homologous series of bispyridinium oximes having a (E)-but-2-ene linker but bearing oxime groups at different positions (Table 1) revealed marked differences depending on the inhibitor and the oxime. Reactivation of inhibited AChE followed first-order kinetics with all investigated OP-oxime combinations. Figure 1 shows as an example the reactivation of cyclosarin-inhibited AChE by K075.
Time- and concentration-dependent reactivation of cyclosarin-inhibited human AChE by K075. GF-inhibited human AChE was incubated with 10–1,000 μM K075 and AChE activity was determined after 2–60 min (a). Data were analyzed by nonlinear regression to determine k obs. Plot of k obs versus [K075] enabled the calculation of K D and k r (b). Data are shown as means ± SE
All tested oximes had a low to negligible reactivity with tabun-inhibited AChE and a variable affinity (Table 2). Reactivity was lowest with K068 and K069, i.e., compounds bearing an oxime group at position 2 of the pyridinium ring and was higher with K053, K105, and K075, oximes having at least one oxime group at position 4. K075, bearing an oxime function at position 4 of both pyridinium rings, exhibited the highest affinity and reactivity and had a several-fold higher second-order reactivation rate constant compared with obidoxime and with its analogue TMB-4 (k r2 0.92 mM−1 min−1; unpublished data).
The reactivation of sarin-inhibited AChE by K-oximes resulted in comparable second-order reactivation rate constants but revealed large differences in affinity and reactivity (Table 3). K068 and K069 had a high affinity but low reactivity, while K053, K105, and K075 showed a higher reactivity but lower affinity. In comparison to obidoxime and HI-6, all tested oximes had a markedly lower reactivating potency.
Oximes having at least one oxime function in position 2, K068, K069, and K053, had a moderate to high affinity and reactivity toward cyclosarin-inhibited AChE resulting in a second-order reactivation rate constant between 3 and 10 mM−1 min−1, thus being 3–9 times lower compared with HI-6 (Table 4). Similar to obidoxime, the 4-oximes K105 and K075 exhibited a low affinity and reactivity resulting in a k r2 of 0.22 mM−1 min−1.
Discussion
The results of the present study demonstrate in part marked differences in affinity and reactivity of the investigated oximes toward OP-inhibited human AChE, depending on the position of the oxime groups and the inhibitor. In addition, the data indicate that a (E)-but-2-ene linker does in most cases decrease the reactivating potency in comparison to oximes bearing an oxybismethylene linker.
The use of a homologous series of bispyridinium bis-oximes bearing oxime groups at position 2, 3, and 4 at the pyridinium rings enabled the evaluation of the effect of the position of the oxime group(s) on the affinity and reactivity toward OP-inhibited AChE. By forming the ratio of reactivation constants between K075 (4,4′) and K068 (2,2′), K053 (4,3′) or K105 (4,3′), the impact of the position of the oxime group(s) could be quantified (Table 5). Hereby, it became evident that the structure–activity relationship was dependent on the inhibitor used. With tabun-inhibited AChE, a clear dependence of the reactivation kinetics on the position of the oxime groups could be determined (Tables 2 and 5). 2,2′- and 2,3′-oximes had an outstanding low second-order reactivation rate constant which was due to low affinity and low reactivity. Oximes bearing an oxime group at position 4 had a moderately higher reactivity and affinity, the 4,4′-oxime K075 being the most potent reactivator of tabun-inhibited AChE. These data are in line with previous results using 2- and 4-oximes with oxybismethylene linkers, e.g., obidoxime, HI-6, and HLö 7 (Worek et al. 2004; Worek et al. 2007; de Jong et al. 1989; Ekström et al. 2006). Interestingly, the position of the second oxime group of tested 4-oximes (K053, K105, K075) had a substantial impact on kinetic properties. Compounds bearing the second oxime group at position 2 or 3 had a lower affinity and reactivity in comparison to the 4,4′-oxime resulting in a 10- to 20-fold lower second-order reactivation rate constant. Hence, the ability of oximes to reactivate is not only determined by the position of the “first” oxime group but also by the substituent at the second pyridinium ring, a phenomenon which was already observed with bispyridinium monooximes bearing a aminocarbonyl group at the second ring (Schoene and Oldiges 1973; Musilek et al. 2007).
A different pattern was observed for the reactivation of sarin-inhibited AChE (Tables 3 and 5). 2,2′- and 2,3′-oximes had a substantially higher affinity and lower reactivity compared with 4,3′- and 4,4′-oximes, the 4,2′-oxime K053 being somewhere in between. The antidromic effect of the position of the oxime groups on K D and k r resulted in a comparable second-order reactivation rate constant k r2. Interestingly, such an effect on affinity and reactivity was not recorded with obidoxime and HI-6 (Table 2).
Previous studies indicated that bispyridinium oximes bearing an oxime function at position 2 exhibit a substantially higher affinity and reactivity toward cyclosarin-inhibited AChE compared with 4-oximes (Worek et al. 2004; Kuca et al. 2004; Luo et al. 2010). These data were confirmed by the present work demonstrating a substantially lower K D and k r of 4,4′- and 4,3′-oximes compared with 2-oximes, resulting in a 15- to 45-fold higher k r2 of the latter (Tables 4 and 5).
Structural studies indicate that the reactivation of phosphylated AChE by oximes is determined by the coordination of an oxime within the gorge of AChE and by the interaction of an oxime with components of the peripheral binding site and the active site (Ekström et al. 2006; Wong et al. 2000). These factors may provide an explanation for the differential kinetic properties of the tested oximes. In addition, it may not be ruled out that with the non-symmetrical oximes K069, K053, and K105, a differential orientation toward the phosphyl group occurs, e.g., partial binding of the 2- and 4-oxime group in case of K053.
The determination of the reactivation kinetics of the tested oximes allows an initial assessment of their potential as potential antidotes in nerve agent poisoning. The comparison of the reactivation constants of obidoxime and the tested oximes reveals that with the exception of K075, all test compounds had a substantially lower reactivating potency toward tabun-inhibited AChE (Fig. 2). Due to a lower affinity and reactivity of the K-oximes, their reactivating potency toward sarin-inhibited AChE was at least 10-fold lower compared with obidoxime (Fig. 3). A different pattern was observed with cyclosarin-inhibited AChE (Fig. 4). Here, the 2-oximes K068, K069, and K053 showed a 5- to 16-fold higher reactivating potency, while the 4-oximes K105 and K075 were slightly less potent. An additional comparison of the kinetic constants of HI-6 and K-oximes revealed that HI-6 was markedly superior to the 2-oximes K068, K069, and K053, primarily due to a higher reactivity. Hence, with the exception of K075, the tested oximes were found to be less potent reactivators than obidoxime and HI-6. K075 proved to be a promising reactivator of tabun-inhibited AChE but showed only moderate activity with sarin-inhibited AChE and low activity with cyclosarin-inhibited AChE and can therefore not be considered as a candidate for use as a nerve agent antidote.
Conclusions
The investigation of the reactivation kinetics of a homologous series of bispyridinium bis-oximes with tabun-, sarin-, and cyclosarin-inhibited human AChE gives further insight into structural requirements for oxime reactivators. The data indicate that oximes bearing an (E)-but-2-ene linker are not superior reactivators compared with bispyridinium oximes with an oxybismethylene bridge. It could be shown that the position of the oxime group(s) has differential effects on affinity and reactivity depending on the inhibitor used. In consequence, these and previous data underline the necessity to investigate the kinetic interactions of oximes and AChE with structurally different OP inhibitors and highlight the difficulty of developing an oxime reactivator which is efficient against a broad spectrum of OPs.
References
Bismuth C, Inns RH, Marrs TC (1992) Efficacy, toxicity and clinical use of oximes in anticholinesterase poisoning. In: Ballantyne B, Marrs TC (eds) Clinical and experimental toxicology of organophosphates and carbamates. Butterworth & Heinemann, Oxford, pp 555–577
Calic M, Bosak A, Kuca K, Kovarik Z (2008) Interactions of butane, but-2-ene or xylene-like linked bispyridinium para-aldoximes with native and tabun-inhibited human cholinesterases. Chem Biol Interact 175:305–308
de Jong LPA, Verhagen AAV, Langenberg JP, Hagedorn I, Löffler M (1989) The bispyridinium-dioxime HLö-7: a potent reactivator for acetylcholinesterase inhibited by the stereoisomers of tabun and soman. Biochem Pharmacol 38:633–640
Ekström F, Pang YP, Boman M, Artursson E, Akfur C, Börjegren S (2006) Crystal structures of acetylcholinesterase in complex with HI-6, Ortho-7 and obidoxime: structural basis for differences in the ability to reactivate tabun conjugates. Biochem Pharmacol 72:597–607
Eyer P, Worek F (2007) Oximes. In: Marrs TC, Maynard RL, Sidell FR (eds) Chemical warfare agents: toxicology and treatment. John Wiley & Sons Ltd., Chichester, pp 305–329
Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, Reiner E (2003) Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal Biochem 312:224–227
Gray AP (1984) Design and structure-activity relationships of antidotes to organophosphorus anticholinesterase agents. Drug Metab Rev 15:557–589
Hobbiger F (1963) Reactivation of phosphorylated acetylcholinesterase. In: Koelle GB (ed) Cholinesterases and anticholinesterase agents. Springer, Berlin, pp 921–988
Kuca K, Sevelova-Bartosova L, Krejcova-Kunesova G (2004) In vitro reactivation of acetylcholinesterase inhibited by cyclosarin using bisquaternary pyridinium aldoximes K005, K033, K027 and K048. Acta Med 47:107–109
Kuca K, Cabal J, Jun D, Bajgar J, Hrabinova M (2006a) Potency of new structurally different oximes to reactivate cyclosarin-inhibited human brain acetylcholinesterases. J Enzym Inhib 21:663–666
Kuca K, Cabal J, Jun D, Hrabinova M (2006b) In vitro evaluation of acetylcholinesterase reactivators as potential antidotes against tabun nerve agent poisonings. Drug Chem Toxicol 29:443–449
Luo C, Chambers C, Pattabiraman N, Tong M, Tipparaju P, Saxena A (2010) Y124 at the peripheral anionic site is important for the reactivation of nerve agent-inhibited acetylcholinesterase by H oximes. Biochem Pharmacol 80:1427–1436
Musilek K, Kuca K, Jun D, Dohnal V, Dolezal M (2006) Synthesis of the novel series of bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. Bioorg Med Chem Lett 16:622–627
Musilek K, Holas O, Jun D, Dohnal V, Gunn-Moore F, Opletalova V, Dolezal M, Kuca K (2007) Monooxime reactivators of acetylcholinesterase with (E)-but-2-ene linker—preparation and reactivation of tabun and paraoxon-inhibited acetylcholinesterase. Bioorg Med Chem 15:6733–6741
Musilek K, Dolezal M, Gunn-Moore F, Kuca K (2011) Design, evaluation and structure-activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med Res Rev 31:548–575
Reiner E, Simeon-Rudolf V (2006) Pyridinium, imidazolium and quinuclidinium compounds: toxicity and antidotal effects against the nerve agents tabun and soman. Arh Hig Rada Toksikol 57:171–179
Schoene K (1980) Pyridinium salts as organophosphate antagonists. Monogr Neural Sci 7:85–98
Schoene K, Oldiges H (1973) Die Wirkungen von Pyridiniumsalzen gegenüber Tabun- und Sarinvergiftungen in vivo und in vitro. Arch Int Pharmacodyn Ther 204:110–123
Taylor P, Wong L, Radic Z, Tsigelny I, Brüggemann R, Hosea N, Berman HA (1999) Analysis of cholinesterase inactivation and reactivation by systematic structural modification and enantiomeric selectivity. Chem Biol Interact 119–120:3–15
Wong L, Radic Z, Brüggemann JM, Hosea N, Berman HA, Taylor P (2000) Mechanism of oxime reactivation of acetylcholinesterase analyzed by chirality and mutagenesis. Biochemistry 39:5750–5757
Worek F, Mast U, Kiderlen D, Diepold C, Eyer P (1999) Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta 288:73–90
Worek F, Reiter G, Eyer P, Szinicz L (2002) Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch Toxicol 76:523–529
Worek F, Thiermann H, Szinicz L, Eyer P (2004) Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol 68:2237–2248
Worek F, Aurbek N, Koller M, Becker C, Eyer P, Thiermann H (2007) Kinetic analysis of reactivation and aging of human acetylcholinesterase inhibited by different phosphoramidates. Biochem Pharmacol 73:1807–1817
Worek F, Wille T, Aurbek N, Eyer P, Thiermann H (2010) Reactivation of organophosphate-inhibited human, cynomolgus monkey, swine and guinea pig acetylcholinesterase by MMB-4: a modified kinetic approach. Toxicol Appl Pharmacol 249:231–237
Acknowledgments
The study was funded by the German Ministry of Defence. Synthesis of tested compounds was supported by Czech project NT12062. The authors are grateful to T. Hannig for expert technical assistance.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Worek, F., von der Wellen, J., Musilek, K. et al. Reactivation kinetics of a homologous series of bispyridinium bis-oximes with nerve agent-inhibited human acetylcholinesterase. Arch Toxicol 86, 1379–1386 (2012). https://doi.org/10.1007/s00204-012-0842-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-012-0842-2