Abstract
Exposure to polycyclic aromatic hydrocarbons (PAH) and DNA damage were analyzed in coke oven (n = 37), refractory (n = 96), graphite electrode (n = 26), and converter workers (n = 12), whereas construction workers (n = 48) served as referents. PAH exposure was assessed by personal air sampling during shift and biological monitoring in urine post shift (1-hydroxypyrene, 1-OHP and 1-, 2 + 9-, 3-, 4-hydroxyphenanthrenes, ΣOHPHE). DNA damage was measured by 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) and DNA strand breaks in blood post shift. Median 1-OHP and ΣOHPHE were highest in converter workers (13.5 and 37.2 μg/g crea). The industrial setting contributed to the metabolite concentrations rather than the air-borne concentration alone. Other routes of uptake, probably dermal, influenced associations between air-borne concentrations and levels of PAH metabolites in urine making biomonitoring results preferred parameters to assess exposure to PAH. DNA damage in terms of 8-oxo-dGuo and DNA strand breaks was higher in exposed workers compared to referents ranking highest for graphite-electrode production. The type of industry contributed to genotoxic DNA damage and DNA damage was not unequivocally associated to PAH on the individual level most likely due to potential contributions of co-exposures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAH) are produced by incomplete combustion or pyrolysis of organic materials and are important occupational and environmental pollutants. Epidemiological studies have shown an increased incidence of lung cancer in various industrial settings with high exposure to PAH (IARC 1984; Boffetta et al. 1997). Exposures during coal gasification, coke production, coal tar distillation, as a chimney sweep, during paving and roofing with coal tar pitch and during aluminum production were categorized by the International Agency for Research on Cancer (IARC) as carcinogenic to humans (Group 1), whereas exposures during graphite-electrode manufacture were categorized as probably carcinogenic to humans (Group 2A) (IARC 2009).
PAH are a complex mixture of more than 100 compounds (Boström et al. 2002). Sixteen PAH compounds (ΣPAH) are recommended for monitoring of air-borne exposure by the US Environmental Protection Agency. ΣPAH comprises of seven carcinogenic PAH (c-PAH) recently categorized by IARC in group 1 (benzo[a]pyrene, B[a]P), group 2A (dibenz[a,h]anthracene) and group 2B (possibly carcinogenic in humans: benz[a]anthracene, benzo[b]fluoranthene benzo[k]fluoranthene, chrysene, and indeno[1,2,3-cd]pyrene) (Straif et al. 2005). PAH exposure is also assessed by biological monitoring of the internal dose taking into account all routes of uptake rather than inhalation only. The most prominent biomarker is 1-hydroxypyrene (1-OHP) in urine (Jongeneelen 1997). In addition, metabolites of other PAH including phenanthrene such as isomers of hydroxylated phenanthrenes (1-, 2-, 3-, 4-, and 9-hydroxyphenanthrenes, ΣOHPHE) in urine have also been suggested as biomarkers of exposure (Seidel et al. 2008; Hecht 2002).
PAH require metabolic activation in order to induce carcinogenic effects. A major pathway includes the activation of PAH to diol epoxides by CYP450 and epoxide hydrolase. Diolepoxides serve as ultimate carcinogens with tumor-initiating properties by directly forming covalently bound DNA adducts with N2 of purines (Dipple et al. 1999). A second pathway involves the induction of oxidative stress including the formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dGuo) as a by-product of redox cycling between PAH-dihydrodiols and PAH-o-chinons (Burczynski et al. 1999). DNA adducts formed by both the direct and the indirect pathway can lead to DNA damage such as DNA strand breaks and mutations and are associated with subsequent cancer (Luch 2005; Klaunig et al. 1998).
The purpose of the study presented here was to analyze biomarkers of PAH exposure and DNA damage by type of industry and at various workplaces with high PAH exposures. The data presented here are part of a nationwide cross-sectional study in Germany. Results on statistical approaches for dose–response modeling and the influence of polymorphic genes on the excretion of PAH metabolites in urine already have been published earlier (Pesch et al. 2007; Rihs et al. 2005).
Materials and methods
Study subjects
A cross-sectional study was conducted in male workers from different PAH industries in Germany. Face-to-face interviews were performed with all workers using a structured questionnaire on characteristics of the workplace, smoking habits, medical history, and use of personal protection devices during work. Air-borne PAH exposure was measured during shift with personal devices, whereas urine and blood samples were collected on the same day post shift. A complete set of data consisting of personal monitoring of air-borne PAH, biological monitoring of PAH metabolites, level of DNA damage, and questionnaire information were available for 171 employees. Forty-eight construction workers without occupational exposure to PAH served as referents. Exposed workers were enrolled from coke (n = 37), refractory (n = 96), and graphite-electrode production (n = 26). In addition, converter workers (n = 12) employed in steel industry and performing brickwork lining and repair of converters were studied. Coke-oven workers included top-oven workers (n = 11), side-oven workers (n = 15), and persons working close to the coke-oven batteries in the side-product area (n = 11). Employees from the manufacture of refractory materials were producing clay and non-clay refractories in terms of formed objects and unformed granulated compositions. Workers from the production of graphite electrodes were recruited from all workplaces including the production of green electrodes, impregnation, graphitization, and finishing. All persons gave their informed consent. The study was conducted under the provisions of the World Medical Association declaration of Helsinki.
Exposure assessment
Personal air sampling of ΣPAH in the workers’ breathing zone was carried out during shift. Samples were analyzed according to NIOSH method 5506 (NIOSH 1994). Depending on the compound the limit of detection (LOD) varied between 0.01 and 0.05 μg/m3. 1-OHP and ΣOHPHE were analyzed in spot urine samples post shift according to a method developed by Lintelmann and Angerer (1999). LODs were between 0.02 and 0.09 μg/L depending on the respective metabolite. Volume-related concentrations of PAH metabolites in urine were adjusted to creatinine (crea). Creatinine was determined according to a colorimetric method (Jaffé reaction) published by Taussky (1954). Urinary cotinine was determined in post-shift samples according to a method published by Scherer et al. (2001) with a LOD of 1.0 μg/L. All methods were fully validated prior to use using external quality control material of the German External Quality Assessment Scheme (G-EQUAS, Schaller et al. 2002).
DNA damage
Blood samples were collected at the end of the shift. White blood cells (WBC) were isolated for the analysis of 8-oxo-dGuo according to a procedure previously published by Marczynski et al. (2005). Nucleosides were analyzed according to the methods proposed by Floyd et al. (1986) and Pouget et al. (1999) with a LOD of 0.1 adduct/106 nucleotides. The method has been recommended by the European Standards Committee on Oxidative DNA Damage (ESCODD 2003). DNA strand breaks in lymphocytes were determined with the Comet assay using a modified protocol of the original description by Östling and Johanson (1984) and Singh et al. (1988). All quality control measures in accordance with the guidelines of the In Vivo Comet Assay Workgroup of the International Conference on Harmonization (ICH) were taken into account (Burlinson et al. 2007). Clean up steps were performed under red light to prevent additional DNA damage and artifact formation. Final analysis was performed by fluorescence microscopy and automated image analysis. The Olive tail moment (OTM) was selected to quantify DNA damage of each cell.
Statistical analyses
Statistical analyses were performed with SAS v. 9.1 (SAS Institute Inc., Cary, NC, USA). c-PAH were defined as sum of the concentrations of B[a]P, dibenz[a,h]anthracene, benz[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, chrysene, and indeno[1,2,3,-cd]pyrene. Values below LOD were set half of the LOD. The distributions of the values are presented by type of industry with median and inter-quartile range. Spearman rank correlation coefficients (r S) were calculated to describe the associations between the various variables. In order to determine the variation by type of industry and to estimate the influence of air-borne PAH on biomarkers, potential confounders such as smoking were included in the statistical model. Due to highly skewed distributions, air-borne PAH and biomarker concentrations were log-transformed in order to apply a linear model. PAH exposure was implemented as continuous and independent variable. Type of workers (coke, refractory material, graphite electrodes, and converter workers), current smoking (yes, no), German nationality (yes, no), and age (<30, 30–49, ≥50 years) were modeled as categorical variables. PAH concentrations were centered for exposed workers by the corresponding median concentration. Occupational PAH exposure of the referents was set to zero for statistical analyses. Quantitative PAH effects were presented as exp(β), which corresponds to the factor of alteration of a biomarker if air-borne PAH concentrations doubles. All tests were conducted two-sided with a significance level of 5%.
Results
Characteristics of the study populations
Table 1 depicts the socio-demographic characteristics of the various study groups. Median age of exposed workers was 39 years (range 19–62 years) and 36 years in the reference subjects (range 19–61 years). Self-reported smoking status was assessed by questionnaire for all persons. The percentage of current smokers differed between exposed workers (67.8%) and referents (41.7%) but not between workers in the various PAH industries (P = 0.65). Self-reported smoking status was used for statistical analyses. For 114 out of 171 exposed workers, urinary cotinine was available for correction. In these workers, current smoking was re-categorized based on cotinine in urine using a cut-off level of 100 μg/l. The comparison between self-reported smoking status and measured urinary cotinine revealed a high concordance of 93%.
Air-borne PAH exposure
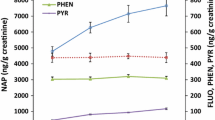
Table 2 shows the distribution of air-borne PAH exposure by type of industry. Median ΣPAH was 30.2 μg/m3 for all exposed workers. The highest median concentrations were observed for converter workers (576 μg/m3), followed by workers in the production of refractory materials (30.0 μg/m3), coke (22.5 μg/m3), and graphite electrodes (13.5 μg/m3). Similar results were obtained for pyrene (PYR) and phenanthrene (PHE). Concentrations of ΣPAH were strongly associated to PYR (r S = 0.80; P < 0.0001) and PHE (r S = 0.88; P < 0.0001) (Table 3). The association between c-PAH and ΣPAH was significant but weaker (r S = 0.51; P < 0.0001). With particular focus on c-PAH including B[a]P, the highest median concentrations were observed for converter workers (12.7 and 2.4 μg/m3). c-PAH and B[a]P concentrations were about 2–3 times higher than those measured in coke production (4.7 and 1.0 μg/m3). The lowest concentrations of c-PAH and B[a]P were found in the production of refractory materials (0.9 and 0.1 μg/m3) and graphite electrodes (1.0 and 0.1 μg/m3). The association between c-PAH and ΣPAH was significant (r S = 0.51; P < 0.0001). ΣPAH contained high proportions of 2–4-ring PAH such as naphthalene, PHE, and PYR, whereas only low concentrations of 5- and 6-ring PAH such as B[a]P or benzo[ghi]perylene were detected. Naphthalene was the most abundant PAH detected in coke-oven and converter emissions, whereas phenanthrene was the most abundant PAH in the manufacture of graphite electrodes and refractory materials. PYR and PHE could be measured in 85.4 and 99.4% of all air samples, whereas c-PAH including B[a]P (73.1%), dibenz[a,h]anthracene (47.9%), benz[a]anthracene (80.7), benzo[b]fluoranthene (75.4%), benzo[k]fluoranthene (67.2%), chrysene (81.3%), and indeno[1,2,3-cd]pyrene (58.5%) were quantified in less samples and were frequently close to their respective LODs. Concentrations of c-PAH and naphthalene ranked among the highest in converter and in coke-oven emissions.
Internal PAH exposure
Median levels of 1-OHP and ΣOHPHE were 7.8 and 12.4 μg/g crea in exposed workers compared to 0.18 and 1.0 μg/g crea in referents (Table 2). Median concentrations of 1-OHP and ΣOHPHE were highest in converter workers (13.5 and 37.2 μg/g crea), followed by the production of graphite electrodes (9.7 and 15.8 μg/g crea), refractory materials (8.4 and 13.0 μg/g crea), and coke (3.8 and 6.8 μg/g crea). An association was found between 1-OHP and ΣOHPHE in urine (r S = 0.66; P < 0.0001, Table 3). Considerable differences in exposures depending on the site of the workplace were observed in coke-oven workers with top-oven workers (n = 11) having higher urinary concentrations of 1-OHP (median 17.4 μg/g crea) than side-oven workers (n = 15, median 5.7 μg/g crea). Coke-oven workers in other areas of the plant but adjacent to the coke-oven batteries (n = 11) had a median 1-OHP level of 5.4 μg/g crea.
The correlations between air-borne PAH exposure and internal PAH exposure are shown in Table 3. With the exception of c-PAH and ΣOHPHE (r S = 0.11; P = 0.15) external exposure and internal dose were associated with each other. Highly significant associations were found between ΣPAH and ΣOHPHE (r S = 0.67; P < 0.0001) and PHE with ΣOHPHE (r S = 0.73; P < 0.0001), while other associations were of weak to moderate significance with Spearman correlation coefficients of r S ≤ 0.54. After adjusting for smoking and type of industry, a doubling of PYR was associated with a 1.31-fold increase of 1-OHP (95% CI 1.24–1.39; P < 0.0001), whereas a doubling of PHE resulted in a 1.40-fold increase of ΣOHPHE (95% CI 1.32–1.48; P < 0.0001) (Table 4). Type of industry (workplace-specific factors) appeared to be a strong additional predictor of 1-OHP levels rather than external exposure to PYR alone and resulted in 17.8- (coke production) to 51.8-fold (graphite-electrode production) higher concentrations when compared with referents after taking into account the association of 1-OHP with its parent compound PYR. Similarly, the type of industry was a significant and strong predictor of ΣOHPHE levels but with less pronounced differences between the industries. Current smoking was associated with a 1.77-fold higher concentration of 1-OHP (95% CI 1.36–2.31) but did not significantly influence ΣOHPHE (P = 0.72). Occupational exposure to PAH in all settings was the dominant source of PAH exposure rather than smoking. Non-smoking PAH-exposed workers had 20-fold higher median levels of 1-OHP than smoking referents (5.85 vs. 0.29 μg/g crea).
DNA damage
Table 2 shows the distribution of 8-oxo-dGuo and DNA strand breaks between exposed workers and referents as well as by type of industry. The median level of 8-oxo-dGuo in exposed workers was 5.7/106 dGuo nucleotides and significantly elevated compared to the referents (3.5/106 dGuo). DNA strand breaks in terms of the OTM also were higher in exposed workers (2.3 vs. 1.3). Levels of 8-oxo-dGuo and DNA strand breaks were highest in graphite-electrode production (11.3/106 dGuo and 3.0) followed by those in the manufacture of refractory materials (5.4/106 dGuo and 2.1). Coke-oven workers had significantly elevated levels of DNA strand breaks (2.2) but not of adducts (4.9/106 dGuo), whereas effects in form of 8-oxo-dGuo (4.4/106 dGuo) and DNA strand breaks (1.4) were less pronounced in converter workers. DNA strand breaks were weakly associated with the air-borne concentration of ΣPAH in the regression model (P = 0.03), whereas no associations between 8-oxo-dGuo with exposure to PAH were observed (Table 5). All other associations between exposure and DNA damage were found to be negative on the individual level suggesting that the positive finding between ΣPAH and OTM can be a result from multiple testing. Current smoking was not associated with 8-oxo-dGuo or DNA strand breaks. No association was observed between 8-oxo-dGuo and DNA strand breaks (r S = 0.13; P = 0.08).
Discussion
PAH are known to be genotoxic and carcinogenic in humans. Epidemiological studies have shown an excess of lung cancer in certain PAH industries including coke production (IARC Group 1). However, limited data on PAH exposure as well as genotoxic and carcinogenic effects have been available for graphite-electrode production and other occupational settings with potentially high exposures to PAH (Boffetta et al. 1997; IARC 2009). Here, a nationwide cross-sectional study in Germany was conducted to analyze exposure to PAH and DNA damage. Previous reports in our cohort focused on dose–response associations and the influence of polymorphic genes (Pesch et al. 2007; Rihs et al. 2005). We focused on PAH exposure and effects by occupational setting (type of industry). The results allow a direct comparison of PAH exposure and DNA damage across typical settings of which one of them, coke production, is well studied and categorized as carcinogenic in humans by IARC. However, only limited data on graphite-electrode, refractory, and converter workers are available in the literature.
PAH exposure
The highest levels of PAH exposure were observed in workers recruited from the steel industry who performed brickwork lining of converters where pig iron is converted into crude steel. Graphite and other unwanted residues were burnt off by the injection of air. Workers were also required to clean the insides of the converters, a confined environment, by scratching off burned residues by hand. They were wearing overalls, leather gloves, and dust masks. Converter workers in steel industry experienced 20–40-fold higher concentrations of ΣPAH (576 μg/m3) and three- to tenfold higher concentrations of c-PAH (12.7 μg/m3) than workers recruited from the production of coke, refractory materials, and graphite electrodes. Internal dose in terms of 1-OHP (13.5 μg/g crea) and ΣOHPHE (37.2 μg/g crea) also ranked highest in converter workers compared to the other PAH industries. However, the differences of metabolite levels (two- to fourfold) were far less pronounced compared to those observed for air-borne exposures pointing to other routes of uptake rather than inhalation only. In addition, particle-bound PAH might be efficiently filtered by the dust masks. Available data on converter workers in steel industry exposure could not be traced in the literature, making it impossible to compare the present results with previous studies.
Workers in coke production revealed a wide variation of individual PAH exposures. Air-borne c-PAH exposure (4.7 μg/m3) ranked second highest and concentrations were fivefold higher than those observed in the production of graphite-electrodes and refractory materials. However, no significant differences in ΣPAH concentrations between these settings were observed. Median internal exposures in terms of 1-OHP (3.8 μg/g crea) and ΣOHPHE (6.8 μg/g crea) in coke-oven workers were lower than those observed in all other industrial settings. The selection of certain areas at the coke plant for exposure monitoring highly influenced the levels observed, e.g., median 1-OHP in top-oven workers (17.4 μg/g crea) were about threefold higher than those in side-oven workers (5.7 μg/g crea) in agreement with previous publications (Yang et al. 2007; Lin et al. 2006). No differences between side-oven workers and employees working in the side-product area (e.g., ammonium sulfate production) were observed (5.4 μg/g crea). Similar results as described for 1-OHP were found for ΣOHPHE. The results indicate that coke-oven emissions are first directed upwards (leading to higher exposures in top-oven workers), and are then evenly distributed over the area while affecting both, side-oven and side-product workers to a similar extent. Median personal PAH exposures at the German workplaces reported in this study (ΣPAH 22.5 μg/m3; 1-OHP 3.8 μg/g crea; ΣOHPHE 6.8 μg/g crea) were at the lower end of those concentrations previously reported in coke production. Van Delft et al. (2001) provided an overview of PAH exposure in the production of coke from European plants with average levels ranging from 15.9 to 241 μg/m3 and internal exposures in terms of 1-OHP between 0.8 and 11.6 μg/g crea. In recent years, studies were also carried out in Chinese coke-oven plants. There, median and average concentrations of ΣPAH were higher and reported to be between 63 and 515 μg/m3 (Chen et al. 1999; Zhang et al. 2000; Wu et al. 2002, 2003). Results also have shown higher internal exposures and 1-OHP concentrations in urine between 17 and 23.8 μg/g crea (Serdar et al. 2003; Lu et al. 2002; Leng et al. 2004, 2005). Technical and other reasons might explain the observed differences between exposures at European plants and those in China, because the methods of assessing exposures were similar between all studies. Only a few studies are available on the excretion of ΣOHPHE in coke-oven workers. Mean concentrations of 16.5 μg/g crea were reported from spot urine samples post shift and a total of 34 μg in 24-h-urine samples from German plants (Strunk et al. 2002; Grimmer et al. 1993), whereas volume-related exposures of 7.2 μg/l for the sum of 3- and 9-hydroxyphenanthrenes were observed in a Chinese plant (Serdar et al. 2003).
IARC recently assessed occupational exposures during carbon-electrode manufacturing for the first time and classified graphite-electrode production as probably carcinogenic to humans (Straif et al. 2005). Workers in the production of graphite electrodes were exposed to lower concentrations of air-borne ΣPAH but to similar levels of c-PAH as measured in the production of refractory materials. Concentrations of c-PAH, in both the production of graphite electrodes and refractory materials, were about 1.0 μg/m3 and close to the limits of detection of the individual compounds. Therefore, results on c-PAH exposure remain inconclusive. Relatively high 1-OHP levels were observed in production of graphite electrodes in our study (9.7 μg/g crea) when compared with coke production (3.8 μg/g crea). Earlier investigations in a German plant also revealed high levels of 1-OHP (approximately 20 μg/g crea) in the baking and impregnation area but levels below 3.0 μg/g crea in graphitization and conditioning (Angerer et al. 1997). Median concentrations measured in German plants, including those presented here, were higher than reported for an Italian graphite-electrode plant with 1.4 μg/g crea where protective measures were implemented (dell’Omo et al. 1998). The concentrations measured in our study were also at the upper end compared to graphite-electrode workers in a Dutch cohort in 1992 where urinary concentrations of 1-OHP could be observed in the range between 1.7 and 9.8 μg/g crea (Buchet et al. 1992). The corresponding average exposure to ΣPAH in Dutch workers was 223 μg/m3 and thus far higher than in the German settings presented here (13.5 μg/m3). Our results indicate dermal uptake of PAH in graphite-electrode production as previously assumed (Angerer et al. 1997). Although air-borne PYR (~1 μg/m3) and PHE (~3 μg/m3) were similar in the production of coke and graphite electrodes, more than twice the amounts of urinary 1-OHP and ΣOHPHE were measured in graphite-electrode workers (9.7 and 15.8 μg/g crea) compared to coke-oven workers (3.8 and 6.8 μg/g crea). Similar results are seen for workers in the production of refractory materials although less pronounced for PHE. Therefore, our study also confirms additional dermal uptake of PAH in workers producing refractory materials as previously reported (Gündel et al. 2000). Because the concentrations of PAH metabolites were moderately correlated with their parent compounds and varied by type of industry, our study overall supports additional routes of PAH uptake rather than by inhalation alone. Additional routes of uptake are of particular importance in graphite-electrode and refractory production as well as converter workers but less in coke-oven workers. We observed that workers in refractory and graphite-electrode production often were wiping sweat off their skin (with or without using gloves) due to hot working environments, while this behavior was less observed in coke-oven workers (who mainly worked outside). Therefore, biological monitoring has to be considered an important tool to accurately assess exposure in PAH industries. The association between PHE and ΣOHPHE is stronger (r = 0.73) than between PYR and 1-OHP (r = 0.54). In addition, current smoking did not influence ΣOHPHE, whereas it turned out to significantly confound internal exposure in terms of 1-OHP in our study and in line with literature (Qiu et al. 2007). Therefore, ΣOHPHE might be a more accurate predictor of occupational exposure to high PAH levels rather than 1-OHP. A potential cause might be the higher volatility of PHE compared to PYR and the relative amounts of PYR and PHE taken up by smoking and via workplace exposure. Uptake of PHE and PYR by smoking (pure inhalation) and therefore the ratio between PHE and PYR can be considered constant for a particular smoker. However, at the workplace there is an additional uptake of PHE and PYR by inhalation on top of smoking. This additional uptake by inhalation is better represented by PHE rather than PYR because PHE shows higher volatility than PYR. Consequently, occupational exposure by inhalation can be assessed more suitable by ΣOHPHE in urine rather than 1-OHP.
The median level of 1-OHP of the general population in Germany (smokers and non-smokers, n = 573) is 0.10 μg/g crea (Human Commission 2005). No data on ΣOHPHE is available. Concentrations in exposed workers were found to be 100-fold higher for 1-OHP (7.8 μg/g), whereas concentrations in the referents (0.18 μg/g) were approximately twofold higher than those observed in the general population of Germany. The latter can be explained by a higher percentage of smokers (41 vs. 32%) and a potential background exposure to traffic exhaust in our reference group compared to the general population in Germany.
DNA damage
PAHs have been demonstrated to damage DNA. 8-oxo-dGuo, a highly mutagenic DNA lesion, and the Comet assay have been used in vitro to show that oxidative stress contributes to PAH-induced genotoxicity and lung carcinogenicity (Seike et al. 2004; Thielen et al. 2006; Park et al. 2008a, b). Previously published studies in humans reported significantly higher levels of DNA strand breaks and 8-oxo-dGuo in WBC of exposed workers than in referents (Zhang et al. 2003; Marczynski et al. 2006). In our study, significantly higher levels of both 8-oxo-dGuo and DNA strand breaks were found also in WBC of PAH-exposed workers compared to unexposed workers. However, the results varied by type of industry. Although PAH exposure was highest in converter workers, DNA damage was found at lower than average levels in these workers compared to other industries. Decreased bioactivation and tumor initiation of PAH by inhibiting CYP1A1 and CYP1B1 in a dose-dependent manner after high co-exposures to particulate matter as recently observed in animal studies might serve as an explanation (Courter et al. 2007). These authors concluded that complex mixtures can alter PAH-induced carcinogenesis and directly influence PAH-induced genotoxic effects. Although we did not measure dust load in the different types of industries, high co-exposures to dust were observed at the workplaces, in particular for converter workers in steel industry and, to a lesser extend, in the production of refractory materials.
Highest levels of DNA damage in terms of DNA strand breaks and 8-oxo-dGuo in WBC were found in the manufacture of graphite electrodes and were about twice as high (3.0 and 11.3/106) compared to the other industries. A recent study in Italian graphite-electrode workers employing the Comet assay also revealed an increased genotoxic risk (Moretti et al. 2007). However, again co-exposures to silica and carbon dusts were observed in those workers and excess mortality was high for silicosis (Merlo et al. 2004). Similar as for converter workers and in refractory material industry, the results for graphite-electrode workers show the significance of exposures to multiple compounds, their interactions, and the importance to discuss DNA damaging effects dependent on particular exposure circumstances at different workplaces.
In contrast to converter workers and employees in the production of refractory materials and graphite electrodes, more data could be traced in the literature for coke-oven workers. As previously reported in several studies in Chinese workers, our study employees in coke production had significantly elevated levels of DNA strand breaks compared to referents. Current opinion is that data from different laboratories cannot be compared with one another, but it has been shown that levels of DNA strand breaks, in terms of OTM, are similar between those studies where appropriate methods recommended by ESCODD are used (ESCODD 2003). Based on these protocols mean OTM levels of 2.6 (95% CI 2.1–3.3) in exposed and 1.0 (95% CI 0.8–1.2) in non-exposed workers were observed by Leng and co-workers (2004), whereas OTM levels of 2.5 ± 0.75 in high-exposed workers compared to 1.7 ± 0.69 and 1.6 ± 0.46 in intermediate and low-exposed workers were found by Yang et al. (2007). The differences between all three groups using a multivariate analysis of covariance with adjustment for age, length of work, pack-years smoked, and alcohol use were significant (P = 0.019). Although reports in coke-oven workers consistently show increased DNA strand breaks, there were no differences between exposed workers and referents for 8-oxo-dGuo in WBC in our study or in a previous study by Zhang et al. (2003).
The majority of previous publications on PAH exposure reported significantly higher levels of DNA damage in terms of DNA strand breaks and 8-oxo-dGuo but usually at group level only and in line with our study (Leng et al. 2004; Marczynski et al. 2006; Yang et al. 2007). These levels were representative of grouped data. Together with previously published data from studies in vitro (Seike et al. 2004; Thielen et al. 2006; Park et al. 2008a, b) our results provide evidence that oxidative DNA damage is a relevant mechanism of PAH-induced genotoxicity in occupationally exposed workers and in the overall network of different mechanisms of PAH-induced genotoxicity.
However, group levels are difficult to interpret and can hardly be used to draw accurate conclusions of PAH-induced genotoxicity for an individual worker. Group levels also cannot be used to provide evidence whether or not a particular compound or group of compounds such as PAH specifically and exclusively cause genotoxic stress in humans. Therefore, we also studied the dose–response association on an individual level because statistical analyses of dose–response relations with larger sets of individual data from these industries are scarce. We could not find quantitative correlations between DNA strand breaks or 8-oxodGuo in WBC with any single shift measure of air-borne PAH exposure at the individual level with the exception of a weak correlation between OTM and ΣPAH. No associations were also detected with internal exposure levels. Similar to our data, no association between 1-OHP in urine and 8-oxo-dGuo in blood could be found by Zhang et al. (2003). There are several reasons for the observed lack of associations between PAH exposure and DNA damage on an individual level in our study but also the study published by Zhang et al. (2003). The cross-sectional study design does not necessarily account for variability in exposures and long-term effects. A single shift measurement of air-borne PAH might not be sufficient to assess genotoxic effects. The assessment of internal exposure and effect also is based on single time points (all post shift) although post-shift sampling must be considered an appropriate time point to study oxidative DNA damage of PAH exposure because studies in vitro have shown an immediate increase of oxidative DNA damage (within hours) in terms of 8-oxo-dGuo and DNA strand breaks after exposure to PAH (Seike et al. 2004; Thielen et al. 2006; Park et al. 2008b). Nevertheless, a slight delay in response due to the in vivo situation cannot be ruled out completely. In addition, 1-OHP and ΣOHPHE in urine—although highly sensitive and specific—are derived by non-carcinogenic PAH and do not represent the internal dose of carcinogenic PAH compounds such as B[a]P. PAH are a complex mixture, several of them are complete carcinogens and their overall mode-of-action must be considered a conglomeration of different pathways within the exposure-disease continuum rather than a “one-way street” in terms of a particular mechanism such as oxidative stress. The complexity of the individual exposure circumstances at the workplace, including co-exposures and varying routes of exposures (e.g., dermal exposure) are also possible reasons for the lacking association with individual data. With regard to co-exposures Courter et al. (2007) studied the confounding influence of particulate matter on CYP450 enzymes in a controlled animal experiment with PAH exposure. This experiment is of particular importance for the interpretation of epidemiological studies because high co-exposures to dust were observed at the workplaces in our study and previously in graphite-electrode workers by Merlo et al. (2004). Enzyme activities of CYP1A1, an important enzyme responsible for biochemical activation of PAH, were found to be inhibited in the presence of dust in a dose-dependent manner. This finding is remarkable keeping in mind that Courter et al. (2007) studied one single confounding factor only, whereas workers are exposed to a huge variety of different compounds which either can amplify or attenuate individual biological pathways of PAH-induced genotoxicity such as oxidative stress. Path analysis directly confirms the interpretation of our experimental results from a biological point of view (Qiu et al. 2007). Air-borne exposure to PAH is contributing about 60% to internal dose in terms of 1-OHP, whereas 1-OHP itself is contributing 9% only to the occurrence of DNA strand breaks in blood. The results by Qiu et al. (2007) and our results show that PAH exposure contributes to oxidative DNA damage but cannot be used exclusively as sensitive and specific predictor of oxidative DNA damage in humans and in varying types of industries. Co-exposures, as discussed above for converter and graphite-electrode workers, together with potential confounders increase the variation of the biological data and impede the assessment of associations between exposure to particular substances and biological effects on an individual level.
Summary
In conclusion, our analyses revealed high levels of external and internal PAH exposure in coke, refractory material, and graphite-electrode industry as well as in converter workers. Exposure at the workplaces by far exceeded exposure due to tobacco smoking. Internal dose in terms of 1-OHP and ΣOHPHE in relation to ΣPAH exposures indicated additional routes of exposure at various workplaces rather than inhalation only, in particular in the production of graphite electrodes, refractory materials, and in converter workers. We also observed increased levels of DNA damage in blood in exposed workers that were highest in the graphite-electrode production but no quantitative dose–response relations could be found on an individual level. The present study suggests the significance of exposures to multiple compounds, their interactions, and the importance to discuss DNA damaging effects dependent on particular exposure circumstances at different workplaces. In addition, the underlying biological processes which are capable to influence pathways such as oxidative stress must be taken into account.
References
Angerer J, Mannschreck C, Gündel J (1997) Occupational exposure to polycyclic aromatic hydrocarbons in a graphite-electrode producing plant: biological monitoring of 1-hydroxypyrene and monohydroxylated metabolites of phenanthrene. Int Arch Occup Environ Health 69:323–331
Boffetta P, Jourenkova N, Gustavsson P (1997) Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 8:444–472
Boström CE, Gerde P, Hanberg A et al (2002) Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 110(Suppl 3):451–488
Buchet JP, Gennart JP, Mercado-Calderon F et al (1992) Evaluation of exposure to polycyclic aromatic hydrocarbons in a coke production and a graphite electrode manufacturing plant: assessment of urinary excretion of 1-hydroxypyrene as a biological indicator of exposure. Br J Ind Med 49:761–768
Burczynski ME, Lin HK, Penning TM (1999) Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAH), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Cancer Res 59:607–614
Burlinson B, Tice RR, Speit G et al (2007) In vivo comet assay workgroup, part of the fourth international workgroup on genotoxicity testing. Fourth international workgroup on genotoxicity testing: results of the in vivo Comet assay workgroup. Mutat Res 627:31–35
Chen ML, Mao IF, Wu MT et al (1999) Assessment of coke oven emissions exposure among coking workers. Am Ind Hyg Assoc J 60:105–110
Courter LA, Musafia-Jeknic T, Fischer K et al (2007) Urban dust particulate matter alters PAH-induced carcinogenesis by inhibition of CYP1A1 and CYP1B1. Toxicol Sci 95:63–73
dell’Omo M, Muzi G, Marchionna G et al (1998) Preventive measures reduce exposure to polycyclic aromatic hydrocarbons at a graphite electrode plant. Occup Environ Med 55:401–406
Dipple A, Khan QA, Page JE et al (1999) DNA reactions, mutagenic action and stealth properties of polycyclic aromatic hydrocarbon carcinogens. Int J Oncol 14:103–111
European Standards Committee on Oxidative DNA Damage (ESCODD) (2003) Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic Biol Med 34:1089–1099
Floyd RA, Watson JJ, Wong PK et al (1986) Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun 1:163–172
Grimmer G, Dettbarn G, Jacob J (1993) Biomonitoring of polycyclic aromatic hydrocarbons in highly exposed coke plant workers by measurement of urinary phenanthrene and pyrene metabolites (phenols and dihydrodiols). Int Arch Occup Environ Health 65:189–199
Gündel J, Schaller KH, Angerer J (2000) Occupational exposure to polycyclic aromatic hydrocarbons in a fireproof stone producing plant: biological monitoring of 1-hydroxypyrene, 1-, 2-, 3- and 4-hydroxyphenanthrene, 3-hydroxybenz(a)anthracene and 3-hydroxybenzo(a)pyrene. Int Arch Occup Environ Health 73:270–274
Hecht SS (2002) Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis 23:907–922
Human Biomonitoring Commission (HBM) (2005) 1-Hydroxypyrene in urine as an indicator of internal exposure to polycyclic aromatic hydrocarbons (PAH)—reference level of 1-hydroxypyrene in urine (in German). Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 48:1194–1206
IARC (1984) Industrial exposures in aluminium production, coal gasification, coal production, and iron and steel founding. IARC Monogr Eval Carcinog Risks Hum 34
IARC (2009) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum 92 (in press)
Jongeneelen FJ (1997) Methods for routine biological monitoring of carcinogenic PAH-mixtures. Sci Total Environ 199:141–149
Klaunig JE, Xu Y, Isenberg JS et al (1998) The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect 106(Suppl 1):289–295
Leng S, Cheng J, Pan Z et al (2004) Associations between XRCC1 and ERCC2 polymorphisms and DNA damage in peripheral blood lymphocyte among coke oven workers. Biomarkers 9:395–406
Leng S, Cheng J, Zhang L et al (2005) The association of XRCC1 haplotypes and chromosomal damage levels in peripheral blood lymphocyte among coke-oven workers. Cancer Epidemiol Biomarkers Prev 14:1295–1301
Lin YC, Pan CH, Chen CJ et al (2006) Associations between exposure to polycyclic aromatic hydrocarbons and temporal change of urinary 1-hydroxypyrene levels in Taiwanese coke-oven workers. J Occup Environ Med 48:930–936
Lintelmann J, Angerer J (1999) PAH metabolites. In: Angerer J, Schaller KH (eds) Analysis of hazardous substances in biological materials, vol 6. Wiley-VCH, Weinheim, pp 163–187
Lu PL, Chen ML, Mao IF (2002) Urinary 1-hydroxypyrene levels in workers exposed to coke oven emissions at various locations in a coke oven plant. Arch Environ Health 57:255–261
Luch A (2005) Nature and nurture—lessons from chemical carcinogenesis. Nat Rev Cancer 5:113–125
Marczynski B, Preuss R, Mensing T et al (2005) Genotoxic risk assessment in white blood cells of occupationally exposed workers before and after alteration of the polycyclic aromatic hydrocarbon (PAH) profile in the production material: comparison with PAH air and urinary metabolite levels. Int Arch Occup Environ Health 78:97–108
Marczynski B, Raulf-Heimsoth M, Preuss R et al (2006) Assessment of DNA damage in WBCs of workers occupationally exposed to fumes and aerosols of bitumen. Cancer Epidemiol Biomarkers Prev 15:645–651
Merlo DF, Garattini S, Gelatti U et al (2004) A mortality cohort study among workers in a graphite electrode production plant in Italy. Occup Environ Med 61:e9
Moretti M, dell’Omo M, Villarini M et al (2007) Primary DNA damage and genetic polymorphisms for CYP1A1, EPHX and GSTM1 in workers at a graphite electrode manufacturing plant. BMC Public Health 7:270–279
NIOSH (1994) Polynuclear aromatic hydrocarbons by HPLC. In: Casinelli ME, O’Connor PE (eds) NIOSH manual of analytical methods, 4th edn. National Institute for Occupational Safety and Health, Washington
Östling O, Johanson KJ (1984) Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun 123:291–298
Park JH, Gelhaus S, Vedantam S et al (2008a) The pattern of p53 mutations caused by PAH o-quinones is driven by 8-oxo-dGuo formation while the spectrum of mutations is determined by biological selection for dominance. Chem Res Toxicol 21:1039–1049
Park JH, Mangal D, Tacka KA et al (2008b) Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proc Natl Acad Sci USA 105:6846–6851
Pesch B, Kappler M, Straif K et al (2007) Dose-response modeling of occupational exposure to polycyclic aromatic hydrocarbons with biomarkers of exposure and effect. Cancer Epidemiol Biomarkers Prev 16:1863–1873
Pouget JP, Ravanat JL, Douki T et al (1999) Measurement of DNA base damage in cells exposed to low doses of gamma-radiation: comparison between the HPLC-EC and comet assays. Int J Radiat Biol 75:51–58
Qiu L, Leng S, Wang Z et al (2007) Path analysis of biomarkers of exposure and early biological effects among coke-oven workers exposed to polycyclic aromatic hydrocarbons. Cancer Epidemiol Biomarkers Prev 16:1193–1199
Rihs HP, Pesch B, Kappler M et al (2005) Occupational exposure to polycyclic aromatic hydrocarbons in German industries: association between exogenous exposure and urinary metabolites and its modulation by enzyme polymorphisms. Toxicol Lett 157:241–255
Schaller KH, Angerer J, Drexler H (2002) Quality assurance of biological monitoring in occupational and environmental medicine. J Chromatogr B Analyt Technol Biomed Life Sci 778:403–417
Scherer G, Meger-Kossien I, Angerer J (2001) Cotinine. In: Angerer J, Schaller KH et al (eds) Analysis of hazardous substances in biological materials, vol 7. Wiley-VCH, Weinheim, pp 171–189
Seidel A, Spickenheuer A, Straif K et al (2008) New biomarkers of occupational exposure to polycyclic aromatic hydrocarbons. J Toxicol Environ Health A 71:734–745
Seike K, Murata M, Hirakawa K et al (2004) Oxidative DNA damage induced by benz[a]anthracene dihydrodiols in the presence of dihydrodiol dehydrogenase. Chem Res Toxicol 17:1445–1451 2004
Serdar B, Waidyanatha S, Zheng Y et al (2003) Simultaneous determination of urinary 1- and 2-naphthols, 3- and 9-phenanthrols, and 1-pyrenol in coke oven workers. Biomarkers 8:93–109
Singh NP, McCoy MT, Tice RR et al (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Straif K, Baan R, Grosse Y et al (2005) Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol 6:931–932
Strunk P, Ortlepp K, Heinz H et al (2002) Ambient and biological monitoring of coke plant workers—determination of exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health 75:354–358
Taussky HH (1954) A micro-colometric determination of creatinine in urine by the Jaffé reaction. J Biol Chem 208:853–861
Thielen S, Baum M, Hoffmann M et al (2006) Genotoxicity of glycidamide in comparison to (+/−)-anti-benzo[a]pyrene-7, 8-dihydrodiol-9, 10-epoxide and alpha-acetoxy-N-nitroso-diethanolamine in human blood and in mammalian V79-cells. Mol Nutr Food Res 50:430–436
Van Delft JH, Steenwinkel MS, van Asten JG et al (2001) Biological monitoring the exposure to polycyclic aromatic hydrocarbons of coke oven workers in relation to smoking and genetic polymorphisms for GSTM1 and GSTT1. Ann Occup Hyg 45:395–408
Wu MT, Simpson CD, Christiani DC et al (2002) Relationship of exposure to coke-oven emissions and urinary metabolites of benzo(a)pyrene and pyrene in coke-oven workers. Cancer Epidemiol Biomarkers Prev 11:311–314
Wu MT, Pan CH, Huang YL et al (2003) Urinary excretion of 8-hydroxy-2-deoxyguanosine and 1-hydroxypyrene in coke-oven workers. Environ Mol Mutagen 42:98–105
Yang X, Zheng J, Bai Y et al (2007) Using lymphocyte and plasma Hsp70 as biomarkers for assessing coke oven exposure among steel workers. Environ Health Perspect 115:1573–1577
Zhang J, Ichiba M, Feng Y et al (2000) Aromatic DNA adducts in coke-oven workers, in relation to exposure, lifestyle and genetic polymorphism of metabolic enzymes. Int Arch Occup Environ Health 73:127–135
Zhang J, Ichiba M, Hanaoka T et al (2003) Leukocyte 8-hydroxydeoxyguanosine and aromatic DNA adduct in coke-oven workers with polycyclic aromatic hydrocarbon exposure. Int Arch Occup Environ Health 76:499–504
Acknowledgments
We would like to thank all workers and companies for their help and support. Additional thanks are due to Dr. Holger M. Koch, Dr. Sabine Plöttner, and Dr. Rosemarie Marchan for internal review and editorial comments prior submission. This work was core-supported by the German Social Accident Insurance (DGUV).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marczynski, B., Pesch, B., Wilhelm, M. et al. Occupational exposure to polycyclic aromatic hydrocarbons and DNA damage by industry: a nationwide study in Germany. Arch Toxicol 83, 947–957 (2009). https://doi.org/10.1007/s00204-009-0444-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-009-0444-9