Abstract
Objective: Workers in various industries can be exposed to polycyclic aromatic hydrocarbons (PAHs). The relationship between biomarkers of genotoxic risk, PAH compounds in air (ambient monitoring) and PAH metabolites in urine (internal exposure) were studied in 17 workers exposed to PAHs in a fireproof-material producing plant before and 3 months after the PAH profile was altered in the binding pitch. Methods: Two biomarkers of exposure, specific DNA adducts of (±)-r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo[a]pyrene (anti-BPDE) and non-specific DNA adduct of 8-oxo-7,8-dihydro-2‘-deoxyguanosine (8-oxodGuo) were determined in white blood cells (WBCs). In addition, DNA strand breaks were analysed in lymphocytes by single-cell gel electrophoresis in a genotoxic risk assessment. Sixteen PAH compounds in air were determined by personal air sampling, and hydroxylated metabolites of phenanthrene, pyrene and naphthalene were determined in urine. Results: After substitution of the binding pitch the concentrations of benzo[a]pyrene in air decreased (P<0.01). No changes could be observed for pyrene, while levels of phenanthrene (P=0.0013) and naphthalene (P=0.0346) in air increased. Consequently, median DNA adduct rates of anti-BPDE decreased after alteration of the production material (from 0.9 to <0.5 adducts/108 nucleotides). No changes in the excretion of 1-hydroxypyrene in urine could be determined, whereas increased levels of 1-, 2+9-, 3- and 4-hydroxyphenanthrene (P<0.0001) and 1-naphthol and 2-naphthol (P=0.0072) were found in urine. In addition, a statistically significant increase in DNA strand break frequencies (P<0.01) and elevated 8-oxodGuo adduct levels (P=0.7819, not statistically significant) were found in the WBCs of exposed workers 3 months after the PAH profile in the binding pitch had been altered. Conclusion: The results presented here show that the increased concentration of naphthalene and/or phenanthrene in the air at the work place could induce the formation of DNA strand breaks and alkali-labile sites in WBCs of exposed workers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Workers in a fireproof-material producing plant are exposed to polycyclic aromatic hydrocarbons (PAHs) by inhalation of volatile PAHs and PAHs bound to respiratory particulate matter. An additional uptake of PAHs is caused by dermal contact with PAH-containing materials [International Agency for Research on Cancer (IARC) 1987]. Epidemiological studies have shown an increase in cancer incidence among workers exposed to PAHs, especially for the risk of developing lung, skin, bladder and prostate cancer (Boffetta et al. 1997; Costantino et al. 1995).
The mechanism by which PAHs produce cancer in humans is unclear. Benzo[a]pyrene (B[a]P), one of the most studied PAHs, becomes a powerful carcinogen after metabolic activation and has been shown to induce genotoxic effects and mutations. Exposure to B[a]P induces the formation of various types of macromolecular adducts, of which DNA adducts are most important. After exposure, B[a]P is metabolized predominantly to anti-benzo[a]pyrene diolepoxide (anti-BPDE), which can covalently bind to DNA, mainly to the exocyclic N2 position of guanine (Sayer et al. 1991). This BPDE-DNA adduct has been used as a suitable variable for the monitoring of biochemical effects of exposure to PAHs in various studies (Pavanello et al. 1999). Since B[a]P is the most intensively studied PAH, research on naphthalene has been reinforced since the US National Toxicology Program (2000) revealed clear evidence of a carcinogenic potential of naphthalene in rodents. Briefly, there are, currently, two main hypotheses concerning the mechanism by which the DNA could be affected after naphthalene exposure. Both hypotheses are based on the presence of naphthoquinones as ultimate carcinogenic intermediates. On the one hand, there are emerging indications that naphthoquinones lead to increased formation of reactive oxygen species (ROS). On the other, the second thesis assumes naphthoquinones as electrophiles that lead to increased covalent adduct formation with nucleophilic groups in DNA.
Three principal pathways of PAH activation have been proposed. These include the formation of anti-BPDE by the combined action of cytochrome P450 (CYP) 1A1 and epoxide hydrolase (Gelboin 1980; Conney 1982), the formation of radical cations by CYP peroxidase (Cavalieri and Rogan 1995) and the formation of reactive and redox-active o-quinones by dihydrodiol dehydrogenase members of the aldo-keto reductase superfamily (Penning et al. 1999). The mutagenic and carcinogenic role of radical–DNA interactions and DNA depurination is under discussion (Canova et al. 1998).
An increasing amount of evidence demonstrates the involvement of ROS in the production of tumours by PAHs (Frenkel et al. 1988; Wattenberg 1980). These oxygen species may lead to the formation of oxidative DNA damage. DNA damage (adducts and strand breaks) represents an early, detectable and critical step in the chemical carcinogenesis process and, thus, may serve as an internal dosimeter for carcinogens (van Delft et al. 1998). The induction of oxidative stress has been suggested as a possible mechanism of non-genotoxic chemical carcinogenesis and has been shown to participate in all stages of the carcinogenesis process, namely initiation, promotion and progression (Pryor 1997). The spectrum of oxidation products that involve DNA includes oxidized bases, DNA strand scission, apurinic/apyrimidinic (AP) sites and strand breaks. Within the latter group, emphasis has been focused on 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo), a major product with a clear mutagenic potential (Kasai 1997). The presence of 8-oxodGuo reveals a lower fidelity in the replication process and enhances the probability of adenine incorporation into the complementary strand, giving rise to G-to-T transversions (Grollman and Moriya 1993; Halliwell and Gutteridge 1999).
Studies examining DNA-specific adduct formation have shown a very low level of DNA binding for some PAH metabolites. Further in vivo studies, inquiring into PAH genotoxicity, have yielded inconclusive results. The DNA adduct formation induced by some PAHs does not appear to be the only mechanism that leads to cancer formation. At this stage, there is little information describing the levels of strand breaks and of oxidized bases in DNA from workers exposed to PAHs (Binkova et al. 1996; Carstensen et al. 1999; Popp et al. 1997). Previously published results (Marczynski et al. 2002) indicate that PAH exposure creates oxidative DNA damage, as measured by the production of one type of oxidative base modification, the 8-oxodGuo, and the production of DNA strand beaks and alkali-labile sites. Complete carcinogens, such as PAHs, probably exert their biological effect not only through DNA damage, equated with the initiation step of carcinogenesis, but also through production of ROS associated with the promotion and progression phases.
Taking into account the present knowledge about PAH metabolism pathways, we performed a study to analyse the levels of 8-oxodGuo- and DNA-adducts of anti-BPDE and the formation of DNA strand breaks and alkali-labile sites in the white blood cells (WBCs) of workers occupationally exposed to PAHs in a fireproof-material producing plant. These results were compared with data obtained from ambient B[a]P, pyrene, phenanthrene and naphthalene concentrations in the air of the workplace and with the results from biological monitoring [urinary metabolites of pyrene, 1-hydroxypyrene (1-OHP), sum of five metabolites of phenanthrene, 1-, 2+9-, 3- and 4-hydroxyphenanthrene (OHPH) as well as the sum of both major metabolites of naphthalene, 1-naphthol and 2-naphthol (NOL)]. 1-OHP, OHPHs and NOL are used for the biological monitoring of exposure to PAHs (Heudorf and Angerer 2001). Seventeen PAH-exposed workers were examined before and 3 months after the alteration of production material (binding pitch) containing different PAH concentrations.
Subjects and methods
Subjects
Seventeen PAH-exposed workers were examined before and 3 months after new binding pitch, containing different PAH concentrations, had been altered. All exposed workers were male, with a mean age of 44.1 years (range 30–58 years). Prior to blood sampling, a questionnaire was performed in order to elicit the workplace description, smoking habits, medical history, age, diet and use of personal protection devices for each exposed worker. Questionnaires were completed in a personal interview with the company physician. Smoking status differed between exposed workers: ten workers were smokers whereas seven subjects were non-smokers. The workers used protective gloves and took a shower immediately after work.
The study was approved by the ethics commission of the Ruhr University of Bochum and was conducted in accordance with the principles for human experience as defined by the Helsinki Declaration. All persons that were investigated gave their informed consent prior to their inclusion in the study.
Determination of PAHs in the air
Personal air sampling in the workers’ breathing zones was carried out according to method 5506 published by the National Institute for Occupational Safety and Health (NIOSH 1994). Briefly, particulate-bound PAHs and PAH vapours were collected on glass fibre filters and in sorbent tubes filled with polystyrene/divinyl benzene-based polymer (XAD-2), respectively. Until the analysis of the 16 PAHs (acenaphthene, acenaphthylene, anthracene, benz[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[ghi]perylene, benzo[a]pyrene, chrysene, dibenz[ah]anthracene, fluoranthene, fluorene, indeno[1,2,3- cd]pyrene, naphthalene, phenanthrene, and pyrene) the filters and sorbent tubes were stored at −20°C in the dark. The filters were mixed with 5 ml of acetonitrile (ACN) and shaken for 30 min, and the contents were extracted ultrasonically (60 min). Subsequently, XAD-2 material from sorbent tubes was extracted with ACN (2 ml) and dichloromethane (2 ml). The original filter extract and the combined extracts of the XAD-2 material were evaporated to dryness in a gentle stream of nitrogen. The residues were re-dissolved with ACN, and a 25 μl aliquot was analysed by high-performance liquid chromatography with diode array detection (HPLC-DAD). The HPLC-DAD analysis was carried out by an HPLC system from Merck (Pump L-6200, autosampler L-7200, column oven L-7350, diode array detector L-7450, computer with D-7000 HPLC system manager) using a reversed-phase column (LiChrospher PAH 250×3 mm, 5 μm) and a water/acetonitrile (w/acn) gradient for separation at 30°C (0–5 min: 60% ACN; 5–20 min: 60%–100% ACN; 20–30 min: 100% ACN; flow 0.5 ml/min). To detect the analytes, we monitored the wavelengths 250 nm, 266 nm and 286 nm. The detection limits for a 2-h sampling period varied between 7 ng/m3 and 513 ng/m3 for the different PAHs.
Determination of PAH metabolites in urine
The determination of the sum of five OHPHs and 1-OHP post-shift urine samples of the workers was carried out with a modified HPLC method developed by Lintelmann and Angerer (1999). Six millilitres of urine was buffered with 12 ml sodium acetate buffer (0.1 mol/l, pH 5.0) and hydrolyzed with 80 μl of β-glucuronidase/arylsulphatase for 16 h at 37°C in a waterbath. In order to separate the solution from particles, we centrifuged it at 1,500g for 10 min. From the supernatant 15 ml were transferred into a sampler vial from which a 3 ml aliquot was injected into an HPLC system with an autosampler. The HPLC system used has been described elsewhere in detail (Lintelmann and Angerer 1999). Briefly, the metabolites were enriched on a tailor-made pre-column consisting of copper phthalocyanine modified silica gel, separated on an RP-C18 (LiChrospher PAH 250×4 mm, 5μm) column and quantified by fluorescence detection (236/386 nm). The metabolites 2-OHPH and 9-OHPH could not be separated by HPLC. These two co-eluting metabolites were quantified by way of a calibration curve of 2-OHPH. The detection limit of the method ranged between 4 ng and 16 ng metabolite/l urine.
Two major naphthalene metabolites, 1-NOL and 2-NOL, were simultaneously determined by a column-switching HPLC method for on-line clean-up, according to Preuss and Angerer (2004). Briefly, aliquots of 2 ml urine were diluted with 4 ml sodium acetate buffer solution (0.1 mol/l, pH 5.0), and 25 μl β-glucuronidase/arylsulphatase were added for enzymatic hydrolysis (16 h at 37°C). Each sample was centrifuged at 1,500g for 10 min. Three hundred and fifty microlitres of the supernatant were injected into the HPLC system for quantitative analysis. The whole method, including the HPLC-system and quality assurance, has been described in detail elsewhere (Preuss and Angerer 2004). Eluting analytes were quantified by fluorescence detection (227/430 nm) after an external calibration. Within a total run time of 40 min both NOLs were separately quantified with detection limits of 1.5 μg/l and 0.5 μg/l for 1-NOL and 2-NOL, respectively. Results are given as the sum of 1-NOL and 2-NOL.
Creatinine measurements
Urinary creatinine was determined photometrically as picrate, according to the Jaffé method (Taussky 1954).
Determination of cotinine in urine
Urinary cotinine was determined by gas chromatography with nitrogen-specific detection after a liquid/liquid extraction of the urine samples, according to the procedure described by Scherer et al. (2001).
Determination of DNA adduct rates of anti-BPDE
Whole-blood samples (9 ml) were collected in EDTA-treated tubes and immediately frozen at −20°C. DNA from the WBCs was isolated and frozen at −80°C. DNA extraction was carried out with the procedure of Marczynski et al. (2002). We used HPLC separation and fluorescence detection to determine the r-7,c-10,t-8,t-9-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (B[a]P-tetrol I-1) that arose after acid hydrolysis of DNA adducts of anti-BPDE according to a modified method of Rojas et al. (1998).
Determination of DNA-adducts (8-oxodGuo)
Whole-blood samples (9 ml) were collected in EDTA-treated tubes and immediately frozen at −20°C. DNA from the WBCs was isolated and frozen at −80°C. DNA extraction and 8-oxodGuo adduct isolation were carried out with the procedure of Marczynski et al. (1997), with the modifications described later (Marczynski et al. 2002). The isolated DNA was dissolved in 200 μl of 10 mmol/l sodium acetate, pH 5.0. The following day, the DNA was incubated at 95°C for 5 min and placed on ice for 10 min. Twenty microlitres of 1 mmol/l deferoxamine mesylate was added, and the denatured DNA was digested with 20 μg of nuclease P1 for 30 min at 37°C, followed by 20 μl Tris-HCl, pH 7.5 and by 1.2 U alkaline phosphatase at 37°C for 60 min. The resulting hydrolysates were centrifuged for 30 min, with a Microcon YM-3 filter (Millipore, Bedford, USA), in order for us to separate the nucleosides from the enzymes.
For the analysis of nucleosides in WBC DNA, Shimadzu HPLC/UV apparatus, connected to a Coulochem II (model 5200) electrochemical detector (ED) (ESA, Chelmsford, Mass., USA) was used. The analysis was carried out blind. The presence of 8-oxodGuo adducts in WBC DNA was detected in accordance with the methods proposed by Pouget et al. (1999). The HPLC (with SIL-10A auto-injector and sample cooler), set at a flow rate of 0.8 ml/min, was used to introduce 20 μl of DNA hydrolysate into a column (C18, 250×4.6 mm, 5 μm particle size; Grom, Herrenberg-Kayh, Germany) in a CTO-10A oven at 37°C. The eluent consisted of 50 mmol/l monosodium phosphate in 8% methanol, pH 5.1. The determination of normal nucleosides was performed at 290 nm with a UV detector (SPD-10A). The oxidation potentials of the analytical cell (model 5011; ESA) of the ED were set at 150 mV and 350 mV for electrodes 1 and 2, respectively. The potential of the guard cell was set at 400 mV. For the recording and integration of the UV and ED responses, an integrator (CR 5A) was used.
Alkaline single-cell gel electrophoresis (comet assay)
Alkaline single-cell gel electrophoresis was used to study DNA strand breaks and alkali-labile sites. The previously published protocol (Östling and Johanson 1984; Singh et al. 1988) was modified in accordance with Pouget et al. (1999) as follows. Heparinized venous blood for the lymphocyte preparation was collected. Lymphocytes were isolated by the standard method of centrifugation on a Ficoll density gradient. Seven microlitres of whole blood from each subject was diluted 1:1 with a RPMI 1640 solution (pH 7.3) and kept on ice for 15 min. Lymphocytes were separated by centrifugation over 7 ml Lymphoprep at 200g for 30 min. Buffy coats were removed and washed twice with RPMI 1640. Lymphocytes suspended in the RPMI solution were counted in a haemocytometer and approximately 2×104 cells were used immediately for the comet assay. Cell viability, determined by the trypan blue exclusion technique, was constantly found to be over 96%.
As usual, 100 μl of 1% standard agarose, dissolved in PBS buffer, was allowed to solidify on to a microscope slide, kept at room temperature in a dry atmosphere. Another 10 μl of the lymphocyte suspension was mixed with 75 μl of 1.2% low melting-point agarose maintained at 37° C. Subsequently, the resulting solution was coated on to the first layer after removal of the cover glass. All the subsequent steps were performed under a red light to prevent the occurrence of additional DNA damage. The slides were then placed on ice for 15 min to allow the gel to solidify. Cover glasses were removed and the slides were immersed for 80 min at 4°C in a lysis buffer (1% triton X-100, 10% DMSO, 2.5 mmol/l NaCl, 100 mmol/l Na2EDTA, 10 mmol/l Tris, sodium lauroylsarcosinate 10%, pH 10).
To conduct the electrophoresis, we removed the cover glasses and transferred the slides to a horizontal electrophoresis tank where the slides were kept covered with an alkaline solution (1 mmol/l Na2EDTA, 300 mmol/l NaOH, pH 13) at 4°C for 40 min. Thereafter, the electrophoresis was performed at 25 V (0.8 V/cm) −300 mA for 45 min at 4°C. The slides were then washed three times with 0.4 mol/l of Tris–HCl, pH 7.4, and the nuclei were stained with 45 μl of 0.5 mg/ml ethidium bromide. The slides were placed at 4°C in a humidified, air-tight, container to prevent drying. Under these conditions, slides could be kept for several days prior to analysis.
The analysis was performed with a fluorescence microscope (Olympus BX60F-3, Olympus Optical, Tokyo, Japan). Using the computer image analysis software Komet, version 4.0 (Kinetic Imaging, Liverpool, UK), we examined each slide (51 cells per slide, using two different slides prepared for one subject) at 20× magnification under the fluorescence microscope equipped with both an excitation filter at 515–560 nm and a barrier filter at 590 nm. We randomly selected ‘Comets’ from each slide, avoiding the edges and damaged parts of the gel as well as the apparently dead cells (comets without a distinct ‘comet head’) and the superimposed comets. The image analysing program automatically calculated the total area of each tail, its absolute average intensity, and its distance to the centre position of the head. These data enabled the program to calculate several indicators of DNA damage, from which we have selected the Olive tail moment. This parameter was used to estimate the DNA break frequency, as it could express the migration of the various DNA fragments forming the tail and estimate the relative amounts of DNA in the tail, as one value.
Statistical analyses
All statistical analyses were performed with the SAS program, version 8e (SAS Institute, Cary, N.C., USA). Variables with a rate of more than 20% under the detection limit were dichotomized and tested by Fisher’s exact test for differences between pre-alteration and post-alteration. As the analysed data were paired, tests were performed on the difference between post-alteration and pre-alteration. This difference between post-alteration and pre-alteration results was calculated for the other variables (rate of values under detection limit <20%). We used QQ-plots and Kolmogorov–Smirnov tests to detect the normal distribution of the calculated differences to apply t-tests in addition. If the assumption of normal distribution did not hold, signed rank tests were used. We used Spearman rank correlation coefficients (rs) to describe the correlations between the different variables. Minima, lower and upper quartiles, medians, arithmetic means and maxima in different groups of exposure are presented as box-and-whisker plots. P<0.05 was set as the criterion for the statistical significance of a test.
Results
Ambient monitoring in comparison with biological monitoring
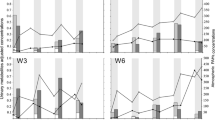
Two teams independently measured the parameters of internal exposure. A good correlation of the results between these two groups was found (results not shown). Seventeen PAH-exposed workers were examined before and 3 months after the production material had been altered to contain a different profile of PAHs. Personal monitoring devices were used to evaluate the ambient exposure of the workers to 16 PAHs during an 8-h working shift. The change in the production process led to a decrease in ambient B[a]P concentrations (P<0.0001, significantly different, n=16). For the 16 workers the median concentration of B[a]P was determined to be 0.165 μg/m3 (range <0.07–0.54 μg/m3) before alteration and <0.07 μg/m3 (range <0.07–16.43 μg/m3) 3 months later. Despite the B[a]P, the sum of 16 PAH concentrations in the air ranged from 6.55 to 149.22 (median 29.62) μg/m3 before and from 60.75 to 372.21 (median 120.11) μg/m3 3 months after alteration. Thus, a significantly increased sum of concentrations of 16 PAHs (P<0.0001, n=16, boxplots shown in Fig. 1) 3 months after the changing of the production material was determined, although the B[a]P concentration was significantly reduced after alteration of the production process.
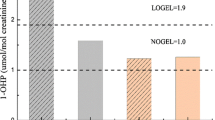
No changes in the ambient pyrene concentration were observed after the production material had been altered (median: 1.11 μg/m3 before and 1.23 μg/m3 3 months after alteration, P=0.8926, n=13). The same applied to the corresponding concentration of 1-OHP in the urine of the workers 3 months later (median: 6.73 μg/g before and 6.67 μg/g creatinine 3 months after, P=0.5744, n=17; data not shown in detail).
Increasing external exposure to phenanthrene and naphthalene 3 months later (P=0.0013, significantly different, n=16, for phenanthrene, Fig. 2, and P=0.0346, significantly different, n=16, for naphthalene, Fig. 3) was accompanied by rising sum of concentrations of five OHPHs and sum of 1-NOL and 2-NOL in the workers’ urine (P<0.0001, significantly different, n=17, for OHPHs, Fig. 4, and P<0.0072, significantly different, n=16, for 1-NOL and 2-NOL, Fig. 5).
Correlation between ambient B[a]P concentrations (marker of external exposure) and DNA adducts of anti-BPDE in WBCs (biomarker of exposure)
In 16 workers the rate ranged between 0.5 and 1.9 DNA adducts of anti-BPDE/108 nucleotides in WBC DNA before the conditions had changed and <0.5 to 0.9 adducts/108 nucleotides 3 months later (P<0.0001, significantly different, n=16, data not shown). Results lower than the analytical limit of detection (LOD) were set to three-quarters of the detection limit (3/4 LOD). Although ambient B[a]P concentration and DNA adduct level of anti-BPDE were in a low range, a good correlation between the airborne B[a]P concentration and the adduct level in WBC DNA was observed (rs=0.516, P=0.0029; n=31) (Table 1).
Correlation between markers of exposure and effect
The 8-oxodGuo/105 dGuo ratio in WBCs was not significantly higher in PAH-exposed workers 3 months after alteration of the binding pitch (P=0.7819, n=17, Fig. 6). The frequency of DNA strand breaks was measured as Olive tail moment (product of the percentage of DNA in the tail and the mean distance of migration in the tail). In our study, an increased frequency of DNA strand breaks was observed in PAH-exposed workers 3 months after exchange of the production material (P=0.0042, significantly different, n=17, Fig. 7). The Spearman rank correlation coefficient attributed to 8-oxodGuo/105 dGuo and Olive tail moment did not reveal any obvious association (rs=0.037, P=0.8335; n=34). No parallel increase in oxidative DNA adducts with elevated DNA strand breaks was observed (Table 1).
Olive tail moment showed a good association with the DNA adduct rates of anti-BPDE (rs=−0.478, P=0.0056; n=32). Rank correlation calculations did not reveal any obvious association between 8-oxodGuo adduct levels and DNA adduct rates of anti-BPDE (rs=−0.158, P=0.3891, n=32) (Table 1).
Biomarkers of exposure and effect in relation to smoking
Influence of smoking was assessed by medical history and by measurement of cotinine in the urine of the PAH-exposed workers. Because of the suspected role of smoking as a confounding factor, 8-oxodGuo adduct level and DNA strand break frequencies before and 3 months after binding pitch exchange were separately calculated with restriction to smokers (n=10) and non-smokers (n=7). The 8-oxodGuo/105 dGuo ratio in WBCs was not significantly higher (P>0.05) in smokers and significantly higher in non-smokers after alteration of the production material (P<0.05). Otherwise, no changes in the level of DNA strand breaks in non-smokers (P>0.05) and significant increase in frequency of DNA strand breaks in smokers (P=0.002) were measured 3 months later.
Correlation between markers of external exposure and biomarkers of exposure and effect
Spearman rank correlation coefficients were calculated for 8-oxodGuo and Olive tail moments in relation to ambient monitoring. Rank correlation calculations did not reveal any obvious association between 8-oxodGuo adduct levels and airborne B[a]P (rs=−0.020, P=0.9113, n=33); pyrene (rs=0.189, P=0.3186, n=30); phenanthrene (rs=0.122, P=0.4976, n=33); naphthalene (rs=0.311, P=0.0781, n=33) and the sum of 16 PAH (rs=0.173, P=0.3371, n=33) concentrations before and after alteration of the production material. The same applied to the association between formation of DNA strand breaks (Olive tail moment) and airborne B[a]P (rs=−0.046, P=0.7982, n=33) and pyrene (rs=−0.014, P=0.9414, n=30) concentrations in the air at the workplace before and after exchange of binding pitch. A good correlation between airborne phenanthrene (rs=0.391, P=0.0245, n=33); naphthalene (rs=0.401, P=0.0209, n=33), the sum of 16 PAH (rs=0.434, P=0.0115; n=33) concentrations and the production of DNA strand breaks was observed (Table 1).
Correlation between biomarkers of internal exposure and biomarkers of effect
With reference to the biological monitoring performed in this study, only a weak association was found between the concentrations of OHPHs in urine and the frequency of DNA strand breaks in comet assay (rs=0.314, P=0.0701, n=34). No association could be seen between concentration of 1-OHP and sum of 1-NOL and 2-NOL in urine and formation of DNA strand breaks (rs=−0.199, P=0.2578, n=34 for 1-OHP and (rs=0.087, P=0.6312, n=33 for 1-NOL and 2-NOL). The same applied to the association between formation of 8-oxodGuo adducts and 1-OHP (rs=−0.063, P=0.7255, n=34), sum of five OHPHs (rs=0.111, P=0.5322, n=34) and sum of 1-NOL and 2-NOL (rs=0.105, P=0.5629, n=33) concentrations in urine before and 3 months after exchange of binding pitch (Table 1).
Discussion
The relationship between genotoxic risk assessment in WBCs of occupationally exposed workers in a fireproof-material producing plant before and 3 months after the production material (binding pitch) had been altered to contain different concentrations of PAHs, on the one hand, and markers of external (ambient monitoring) and internal exposure (biomonitoring of urinary metabolites), on the other hand, was assessed. Two biomarkers of exposure, specific DNA adduct level of anti-BPDE and non-specific DNA adduct level of 8-oxodGuo, and the formation of DNA strand breaks (biomarker of effect) were studied in a genotoxic risk assessment.
Although the changes in the production process due to the alteration of the binding pitch led to a decrease in ambient B[a]P concentration, a significantly higher sum of concentrations of 16 PAHs was observed (Fig. 1). This was mainly attributable to a simultaneous and considerable increase in the ambient phenanthrene and naphthalene concentration (Figs. 2 and 3). The corresponding urinary metabolites of phenanthrene and naphthalene also exhibited significantly increased concentrations (Figs. 4 and 5). No changes in the pyrene concentration and the corresponding concentration of 1-OHP in the urine of the workers were observed 3 months later (data not shown).
Detailed comparisons of the levels of biomarkers with the concentration of PAH compounds revealed that, in WBC DNA of PAH-exposed workers, significantly increased frequencies of DNA strand breaks (P=0.0042, Fig. 7), together with a significant increase in the sum of 16 PAHs (P<0.0001, Fig. 1); phenanthrene (P=0.0013, Fig. 2); naphthalene (P=0.0346, Fig. 3) in the air at the workplace and sum of five OHPHs (P<0.0001, Fig. 4) and sum of both 1-NOL and 2-NOL (P=0.0072, Fig. 5) in urine, 3 months after alteration of the production material, were found.
Spearman rank correlation coefficients calculated for Olive tail moments in relation to ambient monitoring showed good correlation between airborne phenanthrene, naphthalene and sum of 16 PAH concentrations and the production of DNA strand breaks and alkali-labile sites (Table 1). However, in relation to urine metabolites, only a weak association was found between DNA strand breaks and the concentration of sum of OHPHs, and no association could be seen between the sum of 1-NOL and 2-NOL and the formation of DNA strand breaks (Table 1). The comparison between 8-oxodGuo level and external and internal exposure showed only a weak association between these adducts and airborne naphthalene concentration (Table 1). A good correlation between the ambient B[a]P concentration and DNA adduct level of anti-BPDE was found (Table 1). Because the B[a]P concentration in the air before alteration of the binding pitch was very low, and the change led to a further decrease in ambient B[a]P concentration, these results and their corresponding very low DNA adducts level of anti-BPDE do not correlate rationale with biomarkers of exposure and effect (Table 1). Spearman rank correlation coefficient between ambient and biological monitoring showed only good correlation between phenanthrene and sum of OHPHs. Surprisingly, no correlation could be observed between naphthalene and sum of 1-NOL and 2-NOL concentrations (Table 1). This could be due to an additional uptake of naphthalene caused by dermal contact with PAH-containing materials.
Smoking is assumed to influence both the formation of DNA strand breaks (Duthie et al. 1996) and the level of oxidative adducts (8-oxodGuo) (Asami et al. 1996). Therefore, distinct analyses in smokers and non-smokers were performed. Elevated 8-oxodGuo/105 dGuo ratio after alteration was found only in non-smokers (P<0.05). Otherwise, significant increase of DNA strand breaks after alteration was mostly observed in smokers (P=0.002). As only 17 workers were studied, our observations must be interpreted with caution. Until now, there has been no rationale to support such observations.
Our results are only in part similar to those previously obtained (Marczynski et al. 2002), who found positive correlations between the concentrations of pyrene and phenanthrene in the air at the workplace and of their metabolites 1-OHP and OHPH, respectively, in urine of PAH-exposed workers. They also observed higher levels of DNA damage (8-oxodGuo adducts and frequency of DNA strand breaks) in a group of workers in a graphite-electrode-producing plant than in coke-oven workers, for whom they also obtained the highest urinary concentrations of 1-OHP and sum of OHPHs.
The results based on alkaline single-cell gel electrophoresis include not only DNA strand breaks but also base modifications (Collins et al. 1996), as the oxidized purine bases (8-oxodGuo and others) and pyrimidine bases could be converted into additional DNA single-strand breaks (Boiteux 1993). The higher content of 8-oxodGuo in the cells could be expected to lead to a higher number of DNA strand breaks, although the steady-state level of damage can be modulated by DNA repair. On a steady-state level, the contribution of repair through enzyme-mediated DNA cleavage at the site of oxidized bases is very little with respect to the overall formation of DNA strand breaks (Gedik et al. 1998; Pflaum et al. 1997; Pouget et al. 1999, 2000). The level of •OH-induced base damage stays in the same range as the extent of radical reactions leading to DNA strand cleavage (Cadet et al. 1997; Pouget et al. 2000; von Sonntag 1987). However, the origin of the direct strand breaks and alkali-labile sites that may include modified sugar and base residues is difficult to establish with the alkaline comet assay, and, obviously, this strongly depends on the DNA modifying agent.
A statistically significant increase in the frequency of DNA strand breaks (P<0.01), and elevated 8-oxodGuo adduct levels (P=0.7819; however, not statistical significant), were found in WBCs of exposed workers 3 months after the PAH profile had been altered in the binding pitch. A computation of rank correlation coefficients revealed no significant association between 8-oxodGuo and Olive tail moment in workers of the fireproof-material producing plant (Table 1). The formation of DNA strand breaks does not seem to be very much influenced by base excision repair; otherwise a positive association of 8-oxodGuo and Olive tail moment would be expected.
Published results in vivo (Marczynski et al. 2002) and in vitro (Ohnishi and Kawanishi 2002; Yu et al. 2002) suggest that oxidative DNA damage may play an important role in the carcinogenic process of PAHs through ROS formation in addition to specific DNA adduct formation. Bagchi et al. (2001) demonstrated that naphthalene may induce manifestations of toxicity by enhanced production of reactive oxygen free radicals, resulting in lipid peroxidation and DNA damage, while pre-incubation with melatonin significantly suppressed cytotoxicity in macrophage cells. Bagchi et al. (1998) also showed that low-dose, chronic administration of naphthalene to rats induces oxidative stress resulting in DNA damage that may contribute to the toxicity and carcinogenicity of naphthalene. The ability to protect rat tissues against the toxic effect of naphthalene by the use of various antioxidants and free-radical scavengers had been demonstrated (Stohs et al. 2002). The results presented here show that the increased concentration of naphthalene and/or phenanthrene in the air at the work place could induce the formation of DNA strand breaks and alkali-labile sites in WBCs of exposed workers. This DNA damage may be, in part, related to naphthalene and/or phenanthrene-associated oxidative stress through the formation of ROS. Because the increase in the frequency of DNA strand breaks could also be attributed to compounds not assessed in the air sampling protocol, observations must be interpreted with caution.
In conclusion, this study provides evidence that exposure to higher concentrations of low-molecular-weight PAHs such as naphthalene and phenanthrene can result in WBC DNA damage. These findings reveal that investigations of ambient monitoring and both biomarkers of effect and internal exposure seem to be necessary for further surveillance studies of workers with high PAH exposure, especially when a change in the production process is carried out—as far as PAH exposure is concerned. Biomarker studies, with a large number of PAH-exposed workers, will be appropriate to improve statistical power. Although we investigated genotoxic risk assessment in only 17 PAH-exposed workers, before and 3 months after a binding pitch alteration, these in vivo studies are unique.
References
Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H (1996) Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res 56:2546–2549
Bagchi D, Bagchi M, Balmoori J, Vuchetich PJ, Stohs SJ (1998) Induction of oxidative stress and DNA damage by chronic administration of naphthalene to rats. Res Commun Mol Pathol Pharmacol 101:249–257
Bagchi M, Balmoori J, Ye X, Bagchi D, Ray SD, Stohs SJ (2001) Protective effect of melatonin on naphthalene-induced oxidative stress and DNA damage in cultured macrophage J774A.1 cells. Mol Cell Biochem 221:49–55
Binkova B, Lewtas J, Miskova I, Rossner P, Cerna M, Mrackova G, Peterkova K, Mumford J, Meyer S, Sram R (1996) Biomarker studies in Northern Bohemia. Environ Health Perspect 104 [Suppl 3]:591–597
Boffetta P, Jourenkova N, Gustavsson P (1997) Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 8:444–472
Boiteux S (1993) Properties and biological functions of the NTH and FPG proteins of Escherichia coli: two DNA glycosylases that repair oxidative damage in DNA. J Photochem Photobiol B 19:87–96
Cadet J, Berger M, Douki T, Ravanat J-L (1997) Oxidative damage to DNA: formation, measurement and biological significance. Rev Physiol Biochem Pharmacol 31:1–87
Canova S, Degan P, Peters LD, Livingstone DR, Voltan R, Venier P (1998) Tissue dose, DNA adducts, oxidative DNA damage and CYP1A-immunopositive proteins in mussels exposed to waterborne benzo[a]pyrene. Mutat Res 399:17–30
Carstensen U, Hou SM, Alexandrie AK, Hogstedt B, Tagesson C, Warholm M, Rannug A, Lambert B, Axmon A, Hagmar L (1999) Influence of genetic polymorphisms of biotransformation enzymes on gene mutations, strand breaks of deoxyribonucleic acid, and micronuclei in mononuclear blood cells and urinary 8-hydroxydeoxyguanosine in potroom workers exposed to polyaromatic hydrocarbons. Scand J Work Environ Health 25:351–360
Cavalieri El, Rogan EG (1995) Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica 25:677–688
Collins AR, Dusinska M, Gedik CM, Stetina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104 [Suppl 3]:465–469
Conney AH (1982) Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons. G H A Clowes Memorial Lecture. Cancer Res 42:4875–4917
Costantino JP, Redmond CK, Bearden A (1995) Occupationally related cancer risk among coke oven workers: 30 years of follow-up. J Occup Environ Med 37:597–604
van Delft JHM, Baan RA, Roza L (1998) Biological effect of markers for exposure to carcinogenic compound and their relevance for risk assessment. Crit Rev Toxicol 28:477–510
Duthie SJ, Ma A, Ross MA, Collins AR (1996) Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res 56:1291–1295
Frenkel K, Donahue JM, Banjeree S (1988) Benzo[a]pyrene-induced oxidative DNA damage: a possible mechanism for promotion by complete carcinogens. In: Cerutti P, Firdovich I, McCord J (eds) Oxy-radicals in molecular biology pathology. UCLA symposia on molecular and cellular biology, vol 82. Liss, New York, pp 509–524
Gedik CM, Wood SG, Collins AR (1998) Measuring oxidative damage to DNA; HPLC and the comet assay compared. Free Radic Res 29:609–615
Gelboin HV (1980) Benzo[a]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed function oxidases and related enzymes. Physiol Rev 60:1007–1166
Grollman AP, Moriya M (1993) Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet 9:246–249
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Heudorf U, Angerer J (2001) Internal exposure to PAHs of children and adults living in homes with parquet flooring containing high levels of PAHs in the parquet glue. Int Arch Occup Environ Health 74:91–101
International Agency for Research on Cancer (1987) Overall evaluations of carcinogenicity. An updating of IARC monographs on the evaluation of carcinogenic risks to humans. IARC Scientific Publications, nos. 1 to 42 [Suppl 7], IARC, Lyon
Kasai H (1997) Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 387:147–163
Lintelmann J, Angerer J (1999) PAH metabolites. In: Angerer J, Schaller K-H (eds) Analyses of hazardous substances in biological materials, vol 6. Wiley-VCH, Weinheim, pp 163–187
Marczynski B, Rozynek P, Elliehausen HJ, Korn M, Baur X (1997) Detection of 8-hydroxydeoxyguanosine, a marker of oxidative DNA damage, in white blood cells of workers occupationally exposed to styrene. Arch Toxicol 71:496–500
Marczynski B, Rihs HP, Rossbach B, Holzer J, Angerer J, Scherenberg M, Hoffmann G, Bräuning T, Wilhelm M (2002) Analysis of 8-oxo-7,8-dihydro-2′-deoxyguanosine and DNA strand breaks in white blood cells of occupationally exposed workers: comparison with ambient monitoring of urinary metabolites and enzyme polymorphisms. Carcinogenesis 23:273–281
National Toxicology Program (2000) Technical report on the toxicology and carcinogenesis studies of naphthalene (CAS No. 91-20-3) in F344/N rats (inhalation studies). DHHS, PHS, Technical Report Series 500, Rockville
NIOSH (1994) Polynuclear aromatic hydrocarbons by HPLC. In: Cassinelli ME, O’Conner PE (eds) NIOSH manual of analytical methods (NMAM), 4th edn. National Institute for Occupational Safety and Health, Washington
Ohnishi S, Kawanishi S (2002) Double base lesions of DNA by a metabolite of carcinogenic benzo[α]pyrene. Biochem Biophys Res Commun 290:778–782
Östling O, Johanson KJ (1984) Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun 123:291–298
Pavanello S, Gabbani G, Mastrangelo G, Brugnone F, Maccacaro G, Clonfero E (1999) Influence of GSTM1 genotypes and anti–BPDE-DNA adduct levels in mononuclear white blood cells of humans exposed to PAH. Int Arch Occup Environ Health 72:238–246
Penning TM, Burczynski ME, Hung C-F, McCoull KD, Palackal NT, Tsuruda LS (1999) Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox-active oquinones. Chem Res Toxicol 12:1–18
Pflaum M, Will O, Epe B (1997) Determination of steady-state levels of oxidative DNA base modifications in mammalian cells by means of repair endonucleases. Carcinogenesis 18:2225–2231
Popp W, Vahrenholz C, Schell C, Grimmer G, Dettbarn G, Kraus R, Brauksiepe A, Schmeling B, Gutzeit T, von Bulow J, Norpoth K (1997) DNA single strand breakage, DNA adducts, and sister chromatid exchange in lymphocytes and phenanthrene and pyrene metabolites in urine of coke oven workers. Occup Environ Med 54:176–183
Pouget J-P, Ravanat J-L, Douki T, Richard M-J, Cadet J (1999) Measurement of DNA base damage in cells exposed to low doses of γ-radiation: comparison between the HPLC-EC and comet assays. Int J Radiat Biol 75:51–58
Pouget J-P, Douki T, Richard M-J, Cadet J (2000) DNA damage induced in cells by γ and UVA radiation as measured by HPLC/GC-MS and HPLC-EC and comet assay. Chem Res Toxicol 13:541–549
Preuss R, Angerer J (2004) Simultaneous determination of 1- and 2-naphthol in human urine using on-line clean-up column-switching liquid chromatography-fluorescence detection. J Chromatogr B 801:307–316
Preuss R, Angerer J, Drexler H (2003): Naphthalene— an environmental and occupational toxicant. Int Arch Occup Environ Health 76:556–576
Pryor WA (1997) Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect 105 [Suppl 4]:875–882
Rojas M, Alexandrov K, Cascorbi I, Brockmoller J, Likhachev A, Pozharisski K, Bouvier G, Auburtin G, Mayer L, Kopp-Schneider A, Roots I, Bartsch H (1998) High benzo[a]pyrene diol epoxide DNA adduct levels in lung and blood cells from subjects with combined CYP1A1 MspI/Msp/I-GSTM1*0/*0 genotypes. Pharmacogenetics 8:109–118
Sayer JM, Chadha A, Agarwal SK, Yeh HJC, Yagi H, Jerina DM (1991) Covalent nucleoside adducts of benzo[a]pyrene 7,8-diol 9,10-epoxides: structural reinvestigation and characterization of a novel adenosine adduct on the ribose moiety. J Org Chem 56:20–29
Scherer G, Meger-Kossien I, Angerer J, Knecht U (2001) Cotinine. In: Angerer J, Schaller K-H (eds) Analyses of hazardous substances in biological materials, vol. 7. Wiley-VCH, Weinheim, pp 171–189
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
von Sonntag C (1987) The chemical basis of radiation biology. Taylor and Francis, New York, pp 117–166, 221–294
Stohs SJ, Ohia S, Bagchi D (2002) Naphthalene toxicity and antioxidant nutrients. Toxicology 180:97–105
Taussky HH (1954) A micro-colorimetric determination of creatinine in urine by the Jaffey’s reaction. J Biol Chem 208:853–861
Wattenberg LW (1980) Inhibition of chemical carcinogenesis by antioxidants. In: Slaga TJ (ed) Carcinogenesis: modifiers of chemical carcinogenesis, vol 5. Raven Press, New York, pp 85–98
Yu D, Berlin JA, Penning TM, Field J (2002) Reactive oxygen species generated by PAH oquinones cause change-in-function mutations in p53. Chem Res Toxicol 15:832–842
Acknowledgements
We thank Anja Bracht, Beate Engelhardt and Elke Schomberg (Bochum) for expert technical support and Drs. H.C. Broding, T. Merz, J. Müller and K. Meyer (Erlangen) for medical and technical assistance. This study was kindly sponsored by the “Hauptverband der gewerblichen Berufsgenossenschaften”, Sankt Augustin, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marczynski, B., Preuss, R., Mensing, T. et al. Genotoxic risk assessment in white blood cells of occupationally exposed workers before and after alteration of the polycyclic aromatic hydrocarbon (PAH) profile in the production material: comparison with PAH air and urinary metabolite levels. Int Arch Occup Environ Health 78, 97–108 (2005). https://doi.org/10.1007/s00420-004-0567-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-004-0567-5